Abstract
Objective
Mucinous cystic neoplasms (MCNs) of the pancreas have often been confused with intraductal papillary mucinous neoplasms. We evaluated the clinicopathologic characteristics, prevalence of cancer, and prognosis of a large series of well-characterized MCNs in 2 tertiary centers.
Methods
Analysis of 163 patients with resected MCNs, defined by the presence of ovarian stroma and lack of communication with the main pancreatic duct.
Results
MCNs were seen mostly in women (95%) and in the distal pancreas (97%); 25% were incidentally discovered. Symptomatic patients typically had mild abdominal pain, but 9% presented with acute pancreatitis. One hundred eighteen patients (72%) had adenoma, 17 (10.5%) borderline tumors, 9 (5.5%) in situ carcinoma, and 19 (12%) invasive carcinoma. Patients with invasive carcinoma were significantly older than those with noninvasive neoplasms (55 vs. 44 years, P = 0.01). Findings associated with malignancy were presence of nodules (P = 0.0001) and diameter ≥60 mm (P = 0.0001). All neoplasms with cancer were either ≥40 mm in size or had nodules. There was no operative mortality and postoperative morbidity was 49%. Median follow-up was 57 months (range, 4 –233); only patients with invasive carcinoma had recurrence. The 5-year disease-specific survival for noninvasive MCNs was 100%, and for those with invasive cancer, 57%.
Conclusions
This series, the largest with MCNs defined by ovarian stroma, shows a prevalence of cancer of only 17.5%. Patients with invasive carcinoma are older, suggesting progression from adenoma to carcinoma. Although resection should be considered for all cases, in low-risk MCNs (≤4 cm/no nodules), nonradical resections are appropriate.
Mucinous cystic neoplasms (MCNs) usually are large, septated, thick-walled mucinous cysts that lack communication with the ductal system, and occur almost exclusively in the pancreatic body and tail of middle-aged women.1– 6 Histologically, they are characterized by 2 distinct histologic components: an inner epithelial layer composed of tall mucin-secreting cells, and a dense cellular ovarian-type stroma. The latter was first described by Compagno and Oertel in 1978, who made a clear distinction between these neoplasms and serous cystic tumors.7 However, the presence of ovarian stroma was not considered a specific diagnostic criterion for MCNs, and as a consequence, for many years MCNs and intraductal papillary mucinous neoplasms (IPMNs) were frequently confused.1–14 It was not until 1996 that the World Health Organization (WHO) clearly defined and distinguished IPMNs and MCNs,15 and not until 2000 when a new WHO classification emphasized the presence of ovarian stroma in MCNs.4 More recently, at a consensus conference held in Sendai, Japan, the International Association of Pancreatology put forward guidelines requiring the presence of ovarian stroma to establish the diagnosis of MCNs.2
The distinction between MCNs and IPMNs is important in clinical practice. Lacking clear diagnostic criteria, many series of MCNs were actually “contaminated” by IPMNs, especially by branch-duct IPMNs.1–14 As our knowledge of pancreatic mucinous tumors has increased, MCNs and IPMNs appear to be 2 distinct neoplasms, having different biologic behavior and pathologic features, including prevalence of invasive cancer, recurrence rate after radical resection, and presence of multifocal lesions.1– 4
The aim of this study is to analyze the combined experience of the Massachusetts General Hospital (MGH) and the University of Verona (UV) with resected MCNs defined by the presence of ovarian stroma to elucidate their specific demographic and clinicopathological characteristics, as well as their long-term outcomes.
PATIENTS AND METHODS
The MGH and UV Institutional Review Boards approved this study. Patients who underwent pancreatic resection between January 1988 and October 2005 for pathologically confirmed MCNs were identified from prospectively collected databases. Both presence of ovarian stroma and lack of communication with the main pancreatic duct were used as criterion to distinguish MCNs from IPMNs.2,4
In the study period, 567 patients underwent surgery for mucinous tumors of the pancreas (305 MGH and 262 UV). Of these 163 (29%), 102 from UV and 61 from MGH, were determined to have MCNs, whereas the remaining patients had main-duct, branch-duct, or combined IPMNs, or indeterminate mucinous neoplasms. Information including demographics, clinical history, diagnostic work-up, type of surgery, postoperative course, pathology, and long-term follow-up were recorded. Perioperative mortality was defined as in-hospital or 30-day death.
Tumors were classified according to the WHO criteria as MCNs with mild dysplasia (adenoma), with moderate dysplasia (borderline neoplasm), with high-grade dysplasia (carcinoma in situ), and MCN with invasive carcinoma.4 In short, in MCN adenoma, the epithelium shows basally located nuclei with no increase in mitosis. In the borderline MCNs, the epithelium may exhibit papillary projections or crypt-like invagination, some nuclear pseudostratification with crowding and slightly enlarged nuclei. Mitoses can be observed. MCN with noninvasive carcinoma demonstrate high-grade dysplastic epithelial changes. The epithelium often forms papillae and irregular budding, as well as branching with nuclear stratification, severe nuclear atypia, and frequent mitoses.
MCNs with invasive carcinoma were characterized by the presence of malignant neoplastic cells beyond the epithelial lining of the cyst,1,4 and 2 degrees of invasion were defined: (1) intracapsular, if neoplastic invasion did not go beyond the outer layer of the wall; (2) extracapsular, if it extended into the surrounding pancreatic and extrapancreatic tissue.1 For the analysis MCNs with adenoma or borderline neoplasms were grouped as “benign,” and MCNs with carcinoma in situ and invasive carcinoma as “malignant.”
Follow-Up
All patients had periodic follow-up evaluations consisting of clinical examination, blood tests including fasting glucose levels, and imaging procedures such as contrast-enhanced abdominal ultrasound, computer tomography, or magnetic resonance imaging. Recurrences were confirmed histologically whenever possible. Most patients with benign MCNs had yearly follow-up evaluation for the first 5 years, whereas patients with invasive carcinoma were seen every 6 months for the first 2 years and yearly thereafter.
Worsening diabetes was defined as deterioration in the metabolic control of previously diagnosed diabetes requiring modification of the medical treatment. No specific exocrine function tests were performed. New onset of exocrine insufficiency was defined as steatorrhea and weight loss requiring pancreatic enzyme supplementation.
Statistical Analysis
Statistical analysis was performed with the SPSS statistical software package (SPSS, Chicago, Ill). Results are presented as median (range). Categorical variables were compared using a Pearson χ2 test and Fisher exact test when cell counts were <5. Normally distributed continuous variables were compared using a 2-sample Student t test; the Mann-Whitney U test was used for non-normally distributed variables. Survival analysis was done using the Kaplan-Meier function, comparing patients with different histotype with the log rank test. A P value of less than 0.05 was considered statistically significant.
RESULTS
Of the 163 patients with resected MCNs, 118 had adenoma (72%), 17 borderline neoplasms (10.5%), 9 carcinoma in situ (5.5%), and 19 invasive carcinoma (12%). Invasive carcinoma was intracapsular in 13 cases (8%) and extracapsular in 6 (4%). One hundred thirty-five (82.5%) patients had benign MCNs (adenoma and borderline), and 28 (17.5%) malignant MCNs (carcinoma in situ and invasive carcinoma).
Demographic and Clinical Presentation
Demographic and clinical characteristics are shown in Table 1. Most patients with MCNs were middle-aged women. Only 8 male patients were identified in this series (5%), and they were significantly older than their female counterparts (63 vs. 44 years, P = 0.011). No statistically significant differences were found when comparing the age of the 28 patients with malignant tumors (carcinoma in situ and invasive carcinoma) with those with benign tumors (49.5 vs. 44 years, P = 0.129); however, patients with invasive carcinoma alone were significantly older than those with noninvasive neoplasms (55 vs. 44 years, P = 0.01).
TABLE 1.
Clinical Characteristics of 163 Patients With MCN
| Benign (n = 135) | Malignant (n = 28) | Total (n = 163) | P (Benign vs. Malignant) | |
|---|---|---|---|---|
| Male (%) | 7 (5.2) | 1 (3.6) | 8 (5) | NS |
| Female (%) | 128 (94.8) | 27 (96.4) | 155 (95) | NS |
| Median age, yr (range) | 44 (16–79) | 49.5 (27–82) | 45 (16–82) | NS |
| History of smoking (%) | 56 (42.7) | 7 (25) | 63 (39.6) | NS |
| Previous pancreatic surgery (%) | 10 (7.4) | 4 (14.3) | 14 (8.6) | NS |
| Diabetes (%) | 8 (5.9) | 4 (14.3) | 12 (7.4) | NS |
| Symptoms (%) | 97 (72) | 21 (75) | 118 (72.5) | NS |
| Abdominal pain (%) | 84 (62.2) | 14 (50) | 98 (60) | NS |
| Median duration of pain in wk (range) | 13 (1–102) | 9 (1–57) | 12 (1–102) | NS |
| Fatigue (%) | 12 (8.9) | 5 (17.9) | 17 (10.4) | NS |
| Weight loss (%) | 15 (11) | 4 (14.3) | 19 (11.7) | NS |
| Acute pancreatitis (%) | 13 (9.6) | 2 (13.3) | 15 (9.2) | NS |
| Abdominal mass (%) | 15 (11) | 5 (17.9) | 20 (12.3) | NS |
| Asymptomatic (%) | 38 (28) | 7 (25) | 45 (27.5) | NS |
| CA 19–9 >37 U/L | 22 (16.5) | 6 (21.5) | 28 (17) | NS |
| History of other neoplasms | 19 (14) | 4 (14.5) | 23 (14) | NS |
Fourteen (8.6%) patients, (10 with benign and 4 with malignant MCNs), had had previous surgery. In 11 of these, MCNs were misdiagnosed as pseudocysts, and had undergone cystjejunostomy or cystgastrostomy. In these cases, the correct diagnosis was not reached until a mean of 77 ± 32 (SD) months from the initial surgery.
Overall, 118 patients (72.5%) were symptomatic, complaining mostly of mild or vague abdominal pain. Fifteen patients (9%) presented with acute pancreatitis. There was a nonsignificant higher incidence of abdominal pain in patients with benign tumors and of weight loss, fatigue, and diabetes in those with cancer.
Prevalence of Other Neoplasms
Twenty-three patients (14%) had a history of previous (n = 20) or metachronous (n = 3) neoplasms. They were 21 females and 2 males, and they were significantly older than the remaining patients (51 vs. 43 years, P = 0.01). Of 155 female patients 10 (6.5%) had breast carcinoma (mean age ± SD at breast cancer diagnosis: 51 ± 9 years), diagnosed before pancreatic MCNs in 9 cases; of those patients with breast cancer, 2 also had papillary thyroid carcinoma, and 1 renal cell carcinoma. Two additional patients had breast fibroadenoma and 3 ovarian neoplasms (2 mucinous cystadenoma and 1 unspecified carcinoma).
Tumor Location and Size
Anatomic locations and radiologic features are reported in Table 2. Practically all MCNs were located in the body-tail of the pancreas (97%). Lesions were in the pancreatic head in only 5 patients. No patient had multifocal MCNs or neoplasms located in the uncinate process. The median diameter of the lesions, as determined by preoperative imaging, was 50 mm, and was significantly larger in malignant neoplasms (82.5 vs. 45 mm, P = 0.001). All malignant MCNs were located in the body or tail of the pancreas.
TABLE 2.
Location of the Tumor and Radiologic Diameter of the Lesions
| Benign (n = 135) | Malignant (n = 28) | Female (n = 155) | Male (n = 8) | Total (n = 163) | |
|---|---|---|---|---|---|
| Head (%) | 5 (3.7) | 0 | 5 (3.2) | 0 | 5 (3) |
| Uncinate process | 0 | 0 | 0 | 0 | 0 |
| Body (%) | 34 (25.2) | 3 (10.7) | 33 (21.3) | 4 (50) | 37 (22.7) |
| Tail (%) | 75 (55.6) | 9 (32.1.5) | 80 (51.6) | 4 (50) | 84 (51.5) |
| Body-tail (%) | 21 (15.6) | 16 (57.1) | 37 (23.9) | 0 | 37 (22.7) |
| Multifocal lesions | 0 | 0 | 0 | 0 | 0 |
| Median radiologic size (mm)* | 45 (10–200) | 82.5 (20–120) | 50 (10–150) | 43 (34–70) | 50 (10–200) |
Radiologic size was available in 150 patients.
Surgical Procedures
In keeping with MCNs anatomic location, the most common surgical procedure was a distal pancreatic resection, performed in 153 cases (94%). Standard distal pancreatectomy (DP) with splenectomy was carried out in 119 patients (73%), spleen-preserving DP in 28 (17%) and an extended DP in 6 (4%). Other surgical resections included 4 (2.5%) pancreaticoduodenectomies, 5 (3%) atypical resections (3 enucleations and 2 middle pancreatectomies), and 1 total pancreatectomy for a large MCN involving almost the entire gland. Fourteen patients (8.5%) underwent laparoscopic DP, with splenic preservation in 4 cases.
Operative Outcomes
There was no mortality in this series of 163 patients, although overall postoperative morbidity was 49%. Postoperative complications for the entire cohort, as well as the comparison of patients treated at MGH or at UV, are described in Table 3. The rate of postoperative complications was almost the same in the 2 Institutions. The postoperative hospital stay was longer at UV, but the rate of readmission was higher at MGH. Seven (4.5%) patients required reoperation because of intra-abdominal collections (n = 5), bleeding (n = 1), or splenic infarction after spleen-preserving DP (n = 1).
TABLE 3.
Surgical Complications and Postoperative Course in 163 Patients Who Underwent Pancreatic Resections for MCN
| Total (n = 163) | University of Verona (n = 102) | MGH (n = 61) | P (MGH vs. UV) | |
|---|---|---|---|---|
| Overall morbidity (%) | 81 (49) | 51 (50) | 30 (49) | NS |
| Abdominal (%) complications* | 54 (33) | 33 (32.5) | 21 (34.5) | NS |
| Overall pancreatic fistula (%) | 39 (24) | 26 (25.5) | 13 (21) | NS |
| Grade B–C pancreatic fistula† (%) | 15 (9) | 12 (12) | 3 (5) | NS |
| Intra-abdominal (%) collections/abscess | 19 (12) | 12 (12) | 7 (11.5) | NS |
| Extra-abdominal (%) complications | 39 (24) | 30 (29.5) | 9 (15) | 0.025 |
| Perioperative readmission (%) | 12 (7.5) | 4 (4) | 8 (13) | 0.033 |
| Need for interventional radiology (%) | 13 (8) | 9 (9) | 4 (6.5) | NS |
| Reoperation (%) | 7 (4.5) | 5 (5) | 2 (3.5) | NS |
| Mortality | 0 | 0 | 0 | |
| Postoperative stay (median), d | 9 | 10 | 7 | 0.001 |
Abdominal complications is defined as the occurrence of a pancreatic or biliary fistula, abscess, collection, hemorrhage or delayed gastric emptying using previously defined criteria.
According to the pancreatic fistula classification by the International Study Group on pancreatic fistula.43
Pathology
The pathologic characteristics of the tumors are shown in Table 4. Multiple samples were obtained from neoplastic tissue and surrounding non-neoplastic tissue. A mean of 16 slides (range, 3–94) were examined per patient. The median diameter, as evaluated by the pathologist, was 50 mm (mean ± SD: 55 ± 30; range, 8–150), and benign neoplasms were significantly smaller than malignant ones (median, 45 vs. 80 mm; mean ± SD: 50 ± 28 vs. 83 ± 25; P = 0.0001). Pathologic examination confirmed the absence of multifocal lesions in all specimens.
TABLE 4.
Pathological Characteristics of 163 MCNs
| Benign (n = 135) | Malignant (n = 28) | Total (n = 163) | P (Benign vs. Malignant) | |
|---|---|---|---|---|
| Size >60 mm (%)* | 41 (30) | 24 (92)* | 65 (40.5)* | 0.0001 |
| Presence of denudation >50%† (%) | 55 (40.7) | 13 (46.4) | 68 (41.7) | NS |
| Presence of nodules/papillae (%) | 6 (4.4) | 18 (64.3) | 24 (14.7) | 0.0001 |
| Presence of macroscopic septae (%) | 50 (37) | 12 (42.9) | 62 (38) | NS |
| Single lesions (%) | 135 (100) | 28 (100) | 163 (100) | |
| Median size (mm)* | 45 (8–150) | 80 (20–130) | 50 (8–150) | 0.0001 |
Tumor size was not available in 2 specimens.
Denudation was defined as lack of lining epithelium in the cyst wall.
Significant factors associated with malignancy were the presence of nodules (P = 0.0001) and a diameter equal to or greater than 60 mm (P = 0.0001). Nodules were identified in 6 of 118 (5%) adenomas, in none of 17 borderline tumors, in 2 of 9 carcinomas in situ (22%), and in 16 of 19 invasive carcinomas (84%). The presence of septae and epithelial denudation were not significantly different between benign and malignant MCNs. Figure 1 is a scatter plot of radiologic tumor size as a function of histotype and nodules. All neoplasms with cancer were either greater than 40 mm in size or had nodules.
FIGURE 1.
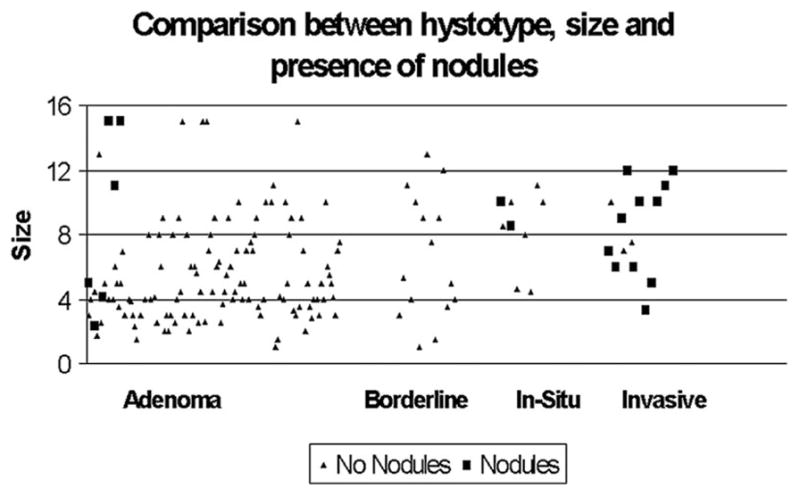
Scatter plot of radiologic tumor size as a function of histologic subtype, and presence of nodules.
Data on lymph nodes status were not available in 2 patients with invasive carcinoma. None of the remaining 17 patients with invasive carcinoma had positive lymph nodes; the mean number of resected nodes in this subgroup was 14. Of the 19 patients with invasive carcinoma, microvascular and perineural invasion were present in 2 (10.5%) and 3 (16%) patients, respectively. Five (26%) patients had extracapsular neoplastic invasion, and the other 14 (74%) intracapsular. In 7 cases (37%) the invasive component was focal, with limited invasion of the stroma. Among patients with invasive cancer, only 1 had a lesion with diameter ≤40 mm, as well as a nodule (Fig. 1). In this patient the cancer was intracapsular and only focally invasive.
Long-Term Follow-Up and Survival
Follow-up with a median duration of 57 months (mean ± SD: 71 ± 54; range, 4 –204) was available in 97% of patients.
Seven patients (4.5%) developed tumor recurrence after a mean of 32.5 months (range, 4 –99). All these patients had invasive carcinoma at the initial resection, accounting for a recurrence rate of 37% in the invasive cancer subgroup. Four patients had carcinoma with extracapsular invasion and 3 had intracapsular growth but with diffuse infiltration of the stroma. No patient with focally invasive carcinoma developed recurrence (P = 0.017). The recurrence was intraperitoneal in 2 patients, and distant (liver metastases) in 5.
All patients with tumor recurrence died of disease after a mean of 6.5 months (range, 2–17), and another 7 patients died during follow-up for other causes.
Overall, 12 (7.5%) patients developed new onset of exocrine insufficiency requiring oral enzyme supplements, and 37 (22.5%) developed new onset (n = 30) or worsening (n = 7) diabetes. The development of both exocrine and endocrine insufficiency was higher in patients with malignant neoplasms [exocrine insufficiency: 21.5% vs. 4.5% (P = 0.007); endocrine insufficiency: 43% vs. 18.5% (P = 0.005)].
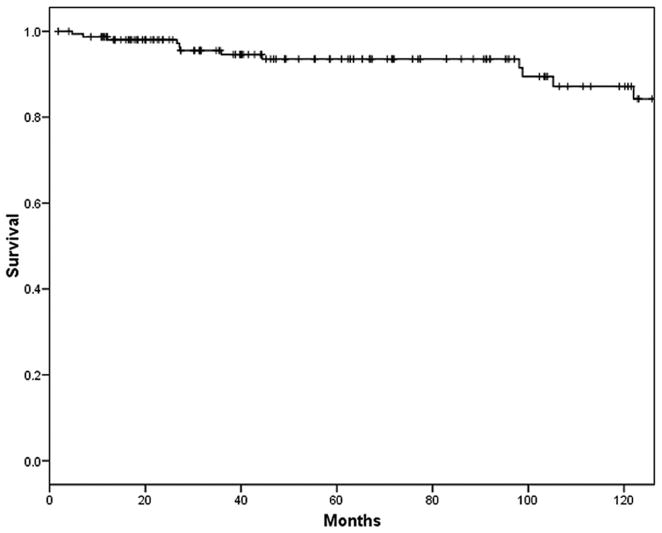
Overall 5- and 10-year actuarial survival for the entire series was 93% and 84%, respectively (Fig. 2). The 5-year disease-specific survival for noninvasive MCNs was 100%, and for those with invasive cancer 57% (Fig. 3).
FIGURE 2.
Overall 5- and 10-year actuarial survival for the entire series (n = 163) was 93% and 84%, respectively.
FIGURE 3.
The 5-year disease-specific survival was 100% for patients affected by adenoma, borderline neoplasm, or carcinoma in situ (black line), and 57% for those with invasive carcinoma (hatched line).
DISCUSSION
The pancreas has been known to harbor neoplastic cysts since the 19th century, but for more than a century these were referred to simply as cystadenomas and cystadenocarcinomas.12,13,17,18 It was not until 1978 that Compagno and Oertel more clearly characterized these lesions, made a distinction between serous and mucinous cystadenomas, and emphasized the benign course of serous and the malignant potential of mucinous lesions.7 A few years later Ohashi et al made the first description of what now we refer to as IPMNs,19 although it took another decade for wide recognition of this tumor.2 For these reasons, many patients with IPMN in the past were wrongly diagnosed as MCNs. Even through most of the 1990s, the distinction between MCNs and branch-duct IPMNs was not apparent, because IPMNs were thought to be a disease mainly affecting the main pancreatic duct.12,20,21 It was not until 1996 and later on in 2000,4,15 that the WHO classified IPMNs and MCNs as 2 distinct diseases. However, even subsequently, many reports continued not to require ovarian stroma as a diagnostic criterion for MCNs. As a result, there has been significant confusion in the analysis of these 2 entities.6,8,11–14 To complicate the situation further, the ovarian stroma can be partially fibrotic, hypocellular, and identifiable in only a few areas1; many series of MCNs did not even describe the percent of neoplasms with this characteristic.1,8
A review of the most important series of MCNs reported in the literature shows wide discrepancy in the clinical characteristics and malignancy rates (Table 5). When the WHO definition of MCNs is applied (group A), these neoplasms have unique characteristics: lesions are found almost exclusively in the distal pancreas of middle-aged women, and the rate of invasive cancer is generally low.1,3,6 The present series, which is the largest cohort of MCNs defined by presence of ovarian stroma reported in the literature to date, confirms these findings. We found that 95% of the patients were women with a median age of 45 years, and that 97% of the lesions were seen in the distal pancreas. Our data also show that a single MCN can harbor different degrees of dysplasia, form adenoma to invasive carcinoma. The prevalence of invasive cancer was only 12%, which is less than previously reported.1,3,5,10 However, the average size of our tumors (5.5 cm) was much smaller than the series published by Thompson et al (10.5 cm)5 and Zamboni et al (8.4 cm),1 and more in line with the series of Reddy et al, who reported prevalence of invasive cancer of 7% and an average tumor size of only 5 cm.3 In our experience, the proportion of adenomas in MCNs (72%) is much higher than in IPMNs (46% in branch-duct IPMN, and 12% in main-duct IPMN), and conversely the rate of overall malignancy was less (17.5% in MCNs, 22% in branch-duct IPMNs, and 60% in main-duct IPMNs).21,22
TABLE 5.
Review of the Largest Series of MCNs (>30 Patients) Published From 1990 to Present
| Study | Yr | No. Patients | Ovarian Stroma (%) | Age (yr) | Female (%) | Distal Lesions (%) | Invasive Cancer (%) | Mean Tumor Size (cm) |
|---|---|---|---|---|---|---|---|---|
| Group A | ||||||||
| Zamboni et al1 | 1999 | 56 | 86 | 45 | 100 | 93 | 27 | 8.4 |
| Thompson et al5 | 1999 | 130 | 100 | 45 | 100 | 94 | 36 | 10.5 |
| Izumo et al9 | 2003 | 34 | 100 | 44 | 100 | 100 | 3 | 8.4 |
| Kosmahl et al10 | 2004 | 32 | 100 | 47 | 100 | 84 | 44* | 9.8 |
| Reddy et al3 | 2004 | 56 | 100 | 48 | 98 | 94 | 7 | 5 |
| Present series | 2006 | 163 | 100 | 45 | 95 | 97 | 12 | 5.5 |
| Group B | ||||||||
| Warshaw et al12 | 1990 | 42 | Not reported | 59 | 76 | 52 | 15 | 5 |
| Le Borgne et al13 | 1999 | 228 | Not reported | 50 | 78 | 53 | 6 | 6 |
| Sarr et al14 | 2000 | 84 | Not reported | 48 | 83 | 78 | 8 | |
| Willentz et al8 | 1999 | 61 | Not reported | 49 | 70.5 | 57 | 33 | 4 |
| Suzuki et al11 | 2004 | 179 | 73 | 56 | 100 | 72 | 31* | 5.9 |
Group A included studies where the presence of ovarian stroma was a diagnostic criteria for MCN, whereas in Group B studies it was not mandatory for MCN diagnosis.
Including invasive carcinoma and carcinoma in situ.
In those series in which the presence of ovarian stroma is not considered a prerequisite to diagnose MCNs (group B of Table 5), patients are older; there is a higher proportion of males; and the lesions are more often located in the head.8,11–14 Wilentz et al8 reported that, of 20 patients with invasive carcinoma, 45% were male, the median age was 60 years, and 63% of lesions were in the proximal pancreas, which is much more likely the profile of IPMNs rather than MCNs. In doubtful cases (absence of ovarian stroma but no demonstrable communication with the main duct), it is better to classify these mucinous lesions as “indeterminate,” rather than to include them with MCNs.2
The observation that MCNs of the pancreas predominantly affect women is in keeping with the fact that MCNs of the liver and retroperitoneum are also diagnosed mainly in females23–27 and that MCNs can frequently be seen in ovaries28,29 but rarely in the testis.30 Zamboni et al1 suggested that pancreatic MCNs may develop from endodermal immature stroma stimulated by female hormones31 or from primary yolk cells implanted in the pancreas during embryogenesis.32 The expression of estrogen receptors in MCNs also supports the putative role of female hormones in pathogenesis.6,9
In the present series MCNs presented with a wide variety of symptoms, but 27.5% were asymptomatic. No individual symptom was significantly associated with a likelihood of malignancy. Sixty percent of patients complained of mild and vague abdominal pain, which was more frequent in benign than in malignant neoplasms. Interestingly, 9% of patients had acute pancreatitis, which is probably due to compression with extrinsic obstruction of the pancreatic duct by large MCNs. Fatigue, weight loss, palpable abdominal mass, and diabetes were more likely associated with malignant MCNs, but because of the low prevalence of these symptoms this was not statistically significant.
One interesting finding of this study is a seemingly high rate of extrapancreatic neoplasms in patients with both benign (14%) and malignant (14.5%) MCNs. In particular we found a prevalence of breast carcinoma of 6.5%, which is greater than the 4.2% expected rate in the general population in this age group,33 although this could be related to increased surveillance in patients with a prior diagnosis of neoplasm. In this series no patient had a history of previous pancreatic tumor, although IPMNs have been reported to be associated with pancreatic cancer or endocrine tumors of the pancreas.34,35 Moreover in IPMNs the prevalence of extrapancreatic neoplasms is higher than in MCNs, ranging from 27% to 39%.2,36 These may represent further differences between MCNs and IPMNs.
Patients with malignant tumors were found to be 5.5 years older than those with adenoma and borderline neoplasms, and patients with invasive carcinoma were 11 years older than those with noninvasive MCNs (P = 0.01). Sarr et al14 and Zamboni et al1 have also reported similar data. Moreover, MCNs with invasive carcinoma frequently contain areas of adenoma, borderline neoplasm, and carcinoma in situ. These findings suggest that benign MCNs may progress to malignancy,2 although the low prevalence of invasive carcinoma indicates a lesser biologic aggressiveness of MCNs and that malignant transformation may not be inevitable in all patients.
Areas of denudation of the epithelial lining were a common finding both in benign and malignant MCNs, especially in large lesions. Extensive denudation as well as the possible coexistence of different degrees of dysplasia makes thorough sampling of MCNs essential to detect even small foci of invasive carcinoma.1,3 In this study a mean of 16 slides (range, 3–94) was examined per patient.
An unexpected observation in this cohort is the complete absence of lymph node metastases in patients with invasive carcinoma, despite sampling of an average of 14 nodes in 17 of 19 patients. No specific data with regard to node status are mentioned in previous reports of MCNs defined by ovarian stroma.1,3,5 This represents a crucial difference with respect to IPMNs, in which lymph node metastases are present in 30% to 46% of invasive main-duct IPMNs,2,21,37 and, in our experience, in 19% of invasive branch-duct lesions.22 Interestingly, ovarian mucinous neoplasms show a similar biologic behavior, with distinctly different clinicopathologic characteristics compared with other ovarian histotypes.28,38,39 Ovarian mucinous carcinomas usually spread to the peritoneum and to other organs through the bloodstream, but lymph node metastases are reported in less than 10% of the cases.16,40
Another observation of the present series is the strong correlation between larger size and nodules with malignancy: 92% of malignant MCNs were either equal to or greater than 60 mm in size, and 64% of them had nodules. No cancer was diagnosed in neoplasms less than 40 mm in size unless nodules were present. There were also no cases of multifocal MCNs.
One of the important findings of this study is that no patient with adenoma, borderline neoplasm, or carcinoma in situ had a recurrence after a median follow-up of 57 months: their 5-year disease-specific survival is 100%. The actuarial 5-year survival for patients with invasive cancer is 57%, which is similar to that reported in invasive main-duct (60%) and branch-duct (63%) IPMNs, but much higher than that of ductal adenocarcinoma of the pancreas. Although the number of patients with invasive carcinoma is small (19 cases), our data suggest that only patients with invasive cancer diffusely invading into or beyond the tumor wall are at substantial risk of distant or local recurrence, whereas those with intracapsular foci of invasive carcinoma have a much better prognosis (in fact none of these patients had a recurrence). Unfortunately, once recurrence is diagnosed, the prognosis is very poor: all patients died after a mean of 6.5 months from the diagnosis of recurrence.
Reddy et al3 proposed nonoperative management for small (<3 cm) MCNs without mural nodules, especially in elderly patients with comorbidities. We agree that in such cases nonoperative management can be considered because our data show that all MCNs <4 cm with no nodules were either adenoma or borderline neoplasms. However, our age distribution indicates that such patients are very uncommon: in this series only 3 patients older than 70 years had tumors that were less than 4 cm and with no nodules. Because most patients with MCNs are middle-aged women with a long life expectancy, nonoperative management based on periodic computer tomography scan or magnetic resonance imaging would require years of careful follow-up with imaging at high cost. Because patients with invasive carcinoma were significantly older (by 11 years) than those with noninvasive MCNs, and MCNs with invasive cancer frequently contain areas of adenoma, borderline neoplasms, or carcinoma in situ, we believe that benign MCNs can progress to invasive carcinoma. It is also likely that some of these lesions could remain as adenomas. Because at present we are unable to identify those that will progress, our recommendation is to resect all MCNs, regardless of size, in patients who are fit candidates for surgery. This is in agreement with the recommendations of the International Association of Pancreatology in the Sendai consensus.2
The great majority of MCNs are located in the body or tail of the pancreas, and are therefore amenable to DP, which is a safe procedure in high-volume centers.41– 43 Because the probability of malignancy is very low in patients with small MCNs without nodules, lymphadenectomy can be avoided and parenchyma-sparing procedures such as middle pancreatectomy44 and perhaps enucleations45,46 should be performed more often, because they decrease the rate of postoperative pancreatic insufficiency and they have been proven to be safe in the treatment of well-selected patients with MCNs.44,47 If tumors are removed when they are smaller, the expected loss of pancreatic parenchyma should be low, thus decreasing the probability of developing endocrine and exocrine insufficiency during follow-up.47
When these operations are not feasible because of tumor location and/or size, spleen-preserving or limited DP is a valid alternative.48,49 In small neoplasms (<5 cm) a minimally invasive approach should be considered. Recent experiences from high-volume centers show that laparoscopic resection of neoplasms in the body and tail of the pancreas is feasible and safe in patients with benign tumors, and this approach can shorten the postoperative hospital length of stay, increase the rate of spleen-preserving DP, and minimize the cosmetic impact of the surgical wound.50 –52
Finally, considering the relatively low frequency of MCNs, the importance of distinguishing MCNs from IPMNs, and the improved surgical outcomes for pancreatic resections in high-volume hospitals, we believe that patients with MCNs should preferentially be managed in specialized centers.
CONCLUSIONS
MCNs defined by the presence of ovarian stroma are a distinct entity with characteristic demographics and clinicopathologic features. The prevalence of invasive cancer is low (12%), and in noninvasive tumors surgery is routinely curative, with no recurrence during follow-up. MCNs have a low biologic aggressiveness, and notably no lymph node metastases were found in invasive cancer. Although the prognosis of MCNs with invasive cancer is much better than ductal adenocarcinoma and is comparable to that of patients with invasive IPMNs, all patients who had recurrence died soon after diagnosis. The 11-year difference in age between patients with invasive versus noninvasive MCNs supports the hypothesis of transformation from benign MCNs to invasive carcinomas. For these reasons, and considering the relatively young age and long life expectancy of these patients, resection remains the treatment of choice. In small MCNs without nodules, parenchyma/spleen-sparing and minimally invasive procedures are preferable.
Acknowledgments
Supported by the International Hepato-Pancreato-Biliary Association (2007, Warren Fellowship Award) (to S.C.). Fundación México en Harvard A.C. (to I.D.), and grants from NIH and ACS (to S.P.T.). This study was supported by Cariverona, Fondazione Giorgio Zanotto, and COFIN 2005060715_004, MIUR (to R.S., C.B., M.F., P.P.).
The authors thank Hang Lee, PhD, of the Massachusetts General Hospital Biostatistics Center for his support with the statistical analysis, Freddi Denitto, MD, Silvia Germenia, MD, and Deborah McGrath, RN for their assistance with follow-up data, and Esther Oliva, MD and Michael V. Seiden, MD, PhD, for their helpful insight regarding mucinous neoplasms of the ovary.
References
- 1.Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–422. doi: 10.1097/00000478-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 3.Reddy RP, Smyrk TC, Zapiach M, et al. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol. 2004;2:1026–1031. doi: 10.1016/s1542-3565(04)00450-1. [DOI] [PubMed] [Google Scholar]
- 4.Zamboni G, Kloppel G, Hruban RH, et al. Mucinous cystic neoplasms of the pancreas. World Health Organization classification of tumors. In: Hamilton SRAL, editor. Pathology and Genetics of Tumors of the Digestive System. Lyon: IARC Press; 2000. pp. 237–240. [Google Scholar]
- 5.Thompson LD, Becker RC, Przygodzki RM, et al. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: a clinicopathologic study of 130 cases. Am J Surg Pathol. 1999;23:1–16. doi: 10.1097/00000478-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Goh BKP, Tan YM, Chung YFA, et al. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg. 2006;30:2236–2245. doi: 10.1007/s00268-006-0126-1. [DOI] [PubMed] [Google Scholar]
- 7.Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol. 1978;69:573–580. doi: 10.1093/ajcp/69.6.573. [DOI] [PubMed] [Google Scholar]
- 8.Wilentz RE, Albores-Saavedra J, Zahurak M, et al. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 1999;23:1320–1327. doi: 10.1097/00000478-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Izumo A, Yamaguchi K, Eguchi T, et al. Mucinous cystic tumor of the pancreas: immunohistochemical assessment of “ovarian-type stroma”. Oncol Rep. 2003;10:515–525. [PubMed] [Google Scholar]
- 10.Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–178. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Atomi Y, Sugiyama M, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004;28:241–246. doi: 10.1097/00006676-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Warshaw AL, Compton CC, Lewandrowski K, et al. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg. 1990;212:432–443. doi: 10.1097/00000658-199010000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partensky C, Le Borgne J, de Calan L French Surgical Association. Cystadenomas and cystadenocarcinomas of the pancreas. A multiinstitutional retrospective study of 398 cases. Ann Surg. 1999;230:152–61. doi: 10.1097/00000658-199908000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas. Can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231:205–212. doi: 10.1097/00000658-200002000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloppel G, Solcia E, Longnecker DS, et al. World Health Organization. International Histological Classification of Tumors. Berlin, Heidelberg, New York: Springer; 1996. Histological typing of tumors of the exocrine pancreas. [Google Scholar]
- 16.Rodriguez IM, Prat J. Mucinous tumors of the ovary. A clinicopathologic analysis of 75 borderline tumors (of intestinal type) and carcinomas. Am J Surg Pathol. 2002;26:139–152. doi: 10.1097/00000478-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Klebs AE. Textbook of Pathologic Anatomy. Vol. 2. Berlin: Hirschwald; 1867. [Google Scholar]
- 18.Kerlin DL, Frey CF, Bodai BI, et al. Cystic neoplasms of the pancreas. Surg Gynecol Obstet. 1987;165:475–478. [PubMed] [Google Scholar]
- 19.Ohashi M, Murakami I, Takekoshi T. Four cases of mucous secreting cancer of the pancreas [Abstract] Prog Dig Endosc. 1982;20:348–351. [Google Scholar]
- 20.Bastid C, Bernard JP, Sarles H, et al. Mucinous ductal ectasia of the pancreas: a premalignant disease and a cause of obstructive pancreatitis. Pancreas. 1991;6:15–22. doi: 10.1097/00006676-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Mainduct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez JR, Salvia R, Crippa S, et al. Branch duct intraductal papillary mucinous neoplasms of the pancreas (IPMNs): confirmed findings in 145 resected patients [Abstract] Pancreas. 2006;33:493. [Google Scholar]
- 23.Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma. A light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18:1078–1091. [PubMed] [Google Scholar]
- 24.Vogt DP, Henderson JM, Chmielewski E. Cystadenoma and cystadeno-carcinoma of the liver: a single center experience. J Am Coll Surg. 2005;200:727–733. doi: 10.1016/j.jamcollsurg.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Rizzardi C, Brollo A, Thomann B, et al. Intra-abdominal ovarian-type mucinous cystadenoma associated with fallopian tube-like structure and aberrant epididymal tissue in a male patient. Hum Pathol. 2005;36:927–931. doi: 10.1016/j.humpath.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Tenti P, Romagnoli S, Pellegata NS, et al. Primary retroperitoneal mucinous cystoadenocarcinomas: an immunohistochemical and molecular study. Virchows Arch. 1994;424:53–57. doi: 10.1007/BF00197393. [DOI] [PubMed] [Google Scholar]
- 27.Shiono S, Suda K, Nobukawa B, et al. Pancreatic, hepatic, splenic, and mesenteric mucinous cystic neoplasms (MCN) are lumped together as extra ovarian MCN. Pathol Int. 2006;56:71–77. doi: 10.1111/j.1440-1827.2006.01926.x. [DOI] [PubMed] [Google Scholar]
- 28.Hart WR. Mucinous tumors of the ovary: a review. Int J Gynecol Pathol. 2005;24:4–25. [PubMed] [Google Scholar]
- 29.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18:S19–S32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 30.Ulbright TM, Young RH. Primary mucinous tumors of the testis and paratestis: a report of nine cases. Am J Surg Pathol. 2003;27:1221–1228. doi: 10.1097/00000478-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Ganepola GA, Gritsman AY, Asimakopulos N, et al. Are pancreatic tumors hormone dependent? A case report of unusual, rapidly growing pancreatic tumor during pregnancy, its possible relationship to female sex hormones, and review of the literature. Am Surg. 1999;65:105–111. [PubMed] [Google Scholar]
- 32.Erdogan D, Lamers WH, Offerhaus GJ, et al. Cystadenomas with ovarian stroma in liver and pancreas: an evolving concept. Dig Surg. 2006;23:186–191. doi: 10.1159/000094488. [DOI] [PubMed] [Google Scholar]
- 33.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi K, Ohuchida J, Ohtsuka T, et al. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology. 2002;2:484–490. doi: 10.1159/000064716. [DOI] [PubMed] [Google Scholar]
- 35.Marrache F, Cazals-Hatem D, Kianmanesh R, et al. Endocrine tumor and intraductal papillary mucinous neoplasm of the pancreas: a fortuitous association? Pancreas. 2005;31:79–83. doi: 10.1097/01.mpa.0000164453.46394.07. [DOI] [PubMed] [Google Scholar]
- 36.Choi MG, Kim SS, Han SS, et al. High incidence of extrapancreatic neoplasms in patients with intraductal papillary mucinous neoplasms. Arch Surg. 2006;141:51–56. doi: 10.1001/archsurg.141.1.51. [DOI] [PubMed] [Google Scholar]
- 37.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hess V, A’Hern R, Nasiri N, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol. 2004;22:1040–1044. doi: 10.1200/JCO.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 39.Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, et al. A distinct molecular profile associated with mucinous epithelial ovarian cancer. Br J Cancer. 2006;94:904–913. doi: 10.1038/sj.bjc.6603003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morice P, Joulie F, Camatte S, et al. Lymph node involvement in epithelial ovarian cancer: analysis of 276 pelvic and paraortic lymphadenectomies and surgical implications. J Am Coll Surg. 2003;197:198–205. doi: 10.1016/S1072-7515(03)00234-5. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez JR, Germes SS, Pandharipande PV, et al. Implications and cost of pancreatic leak following distal pancreatic resection. Arch Surg. 2006;141:361–365. doi: 10.1001/archsurg.141.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lillemoe KD, Kaushal S, Cameron JL, et al. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693–698. doi: 10.1097/00000658-199905000-00012. discussion 698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Crippa S, Bassi C, Warshaw AL, et al. Middle pancreatectomy: indications, short and long-term operative outcomes. Ann Surg. doi: 10.1097/01.sla.0000262790.51512.57. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talamini MA, Moesinger R, Yeo CJ, et al. Cystadenomas of the pancreas: is enucleation an adequate operation? Ann Surg. 1998;227:896–903. doi: 10.1097/00000658-199806000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crippa S, Bassi C, Salvia R, et al. Outcome of pancreatic neoplasms enucleation. Br J Surg. doi: 10.1002/bjs.5833. In press. [DOI] [PubMed] [Google Scholar]
- 47.Falconi M, Mantovani W, Crippa S, et al. Pancreatic insufficiency after different resections for benign tumors. Br J Surg. doi: 10.1002/bjs.5652. In press. [DOI] [PubMed] [Google Scholar]
- 48.Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg. 1988;123:550–553. doi: 10.1001/archsurg.1988.01400290032004. [DOI] [PubMed] [Google Scholar]
- 49.Shoup M, Brennan MF, McWhite K, et al. The value of splenic preservation with distal pancreatectomy. Arch Surg. 2002;137:164–168. doi: 10.1001/archsurg.137.2.164. [DOI] [PubMed] [Google Scholar]
- 50.Melotti G, Butturini G, Piccoli M, et al. Laparoscopic distal pancreatectomy: results on a consecutive series of 58 patients. Ann Surg. doi: 10.1097/01.sla.0000258607.17194.2b. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mabrut JY, Fernandez-Cruz L, Azagra JS, et al. Laparoscopic pancreatic resection: results of a multicenter European study of 127 patients. Surgery. 2005;137:597–605. doi: 10.1016/j.surg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Cruz L, Martinez L, Gilabert R, et al. Laparoscopic distal pancreatectomy combined with preservation of the spleen for cystic neoplasms of the pancreas. J Gastrointest Surg. 2004;8:493–501. doi: 10.1016/j.gassur.2003.11.014. [DOI] [PubMed] [Google Scholar]