Abstract
Introduction
Pancreatic intraductal papillary mucinous neoplasms (IPMN) are now identified with increasing frequency. The detection of carcinoma in IPMN is difficult and suffers from high false-positive and false-negative rates, often resulting in inappropriate treatment decisions. Improved detection of malignancy using novel biomarkers may therefore improve diagnostic accuracy. One such promising novel biomarker is Plectin-1 (Plec-1).
Methods
Using immunohistochemistry, Plec-1 expression was assayed in benign (low and moderate dysplasia, n=6) as well as malignant IPMN (high-grade dysplasia and invasive carcinoma, n=31) and lymph node metastases from carcinoma arising in IPMN (n=12). Furthermore, cyst fluids from benign (n=3) and malignant IPMN (n=4) were evaluated for Plec-1 expression.
Results and discussion
Twenty-six of 31 malignant IPMN and all 12 lymph node metastases were Plec-1 positive. In contrast, only one of six benign IPMN expressed Plec-1. The specificity of Plec-1 in distinguishing malignant IPMN from benign IPMN was 83% and its sensitivity 84%. Furthermore, all (four out of four) cyst fluids from malignant IPMN, but none of the three benign IPMN, were Plec-1 positive. These data support Plec-1 as an excellent biomarker for the early detection of carcinoma arising in IPMN.
Keywords: Plectin-1, Biomarker, Malignant IPMN, Benign IPMN
Introduction
Over the past decades, intraductal papillary mucinous neoplasms (IPMN) of the pancreas have been identified with increasing frequency; they now account for up to 20% of all resected pancreatic specimens in large referral centers.1–6 IPMNs are thought to progress from low-grade dysplasia (adenoma) to high-grade dysplasia (carcinoma in situ) and invasive carcinoma through moderate-grade dysplasia (borderline malignancy). Invasive carcinoma in IPMN is present in 12.5–57% of all IPMN cases.3,4,7,8 The 5-year survival rate of all patients with surgically resected malignant IPMN is up to 70%3,4,7,8 and thus much better than the corresponding rate for pancreatic ductal adenocarcinoma, which is less than 20%.9–11
Clinically, IPMNs are classified as lesions of the main pancreatic duct (MD-IPMN), the branch ducts (BD-IPMN), or both (combined-type IPMN).5 MD-IPMN and combined-type IPMN have a high risk of malignancy (57–92%) and frequently of invasive carcinoma (23–57%), and surgery is therefore recommended.5,6,12,13 BD-IPMN has a much lower risk of malignancy, 6–46%5,6,12,13 and indications for surgery are less clear. The only way to stratify the risk of malignancy in IPMNs at present is by clinical symptoms, high-resolution cross-sectional abdominal imaging, endoscopic ultrasound with fine needle aspiration biopsies (FNA), cytology, and cyst fluid analysis for carcinoembryonic antigen and carbohydrate antigen 19.9.14–19 Current international consensus management guidelines5 recommend following small BDIPMN (<3 cm) in asymptomatic patients with high-resolution cross-sectional abdominal imaging studies. Mural nodules on radiologic studies, a dilated main pancreatic duct (>6 mm), positive cytology, or a cyst size larger than 3 cm all correlate with malignancy, and resection is therefore recommended when these signs and symptoms are present. However, the predictive value of these guidelines to correctly distinguish benign from malignant cysts remains low, as not all small and asymptomatic BD-IPMN are nonmalignant20 and up to 85% of surgically treated patients have no malignancy despite the presence of the aforementioned signs and thus undergo unnecessary resection.21
Recently, in an effort to improve diagnostic accuracy, analyses of genetic changes in cyst fluid have been used, but these suffer from limited clinical validation and high cost.22 No other reliable biomarkers are presently available to clearly distinguish malignant from nonmalignant IPMN.
Novel biomarkers that improve the accuracy of detection of malignancy in IPMN, especially in BD-IPMN, are therefore much needed. One such novel and promising biomarker may be Plectin-1 (Plec-1). Plec-1 was initially identified in a phage display screen for unique markers of pancreatic ductal adenocarcinoma23 and found to be highly specific and sensitive for early and invasive cancer. Plec-1 expression intensity increases during pancreatic carcinogenesis; strong and specific staining has been identified in 60% of PanIN-3 lesions (ductal carcinoma in situ; Bausch et al., manuscript in preparation). Based on these findings, the aim of this study was to evaluate whether Plec-1 is a potential specific biomarker for malignant IPMNs, whether Plec-1 expression can be exploited to identify metastatic foci in lymph nodes, and whether cyst fluid analysis for Plec-1 allows the discrimination of malignant from benign cysts.
Material and Methods
Tissue Samples
All tissues and biologic samples were collected with the approval and in accordance with the requirements of the Institutional Review Board of the Massachusetts General Hospital, Boston, MA, USA. Paraffin-embedded tissue samples were obtained from the files of the Department of Pathology of the Massachusetts General Hospital, Boston, MA, USA. All specimens had an established diagnosis at the time of assessment. A total of 37 cases of IPMN were obtained, six benign cases and 31 malignant IPMN.
The six benign IPMN were low- and moderate-grade dysplasia (adenoma and borderline malignancy). Ten of the malignant cases were high-grade dysplasia (carcinoma in situ); six of the invasive carcinomas arising in IPMN were of colloid and 15 of ductal phenotype. Three colloid and nine ductal adenocarcinomas were noted to have lymph node metastases and were also analyzed for Plec-1 expression. Cyst fluids were from benign (n=3) and malignant (n=4) IPMN and were also analyzed for Plec-1 expression.
Immunostaining
Paraffin-embedded sections were deparaffinized, hydrated with Tris-buffered saline (TBS) and blocked with H2O2. Antigen retrieval was achieved by boiling tissue in Retrievit (BioGenex, San Ramon, CA, USA). After blocking with avidin/biotin (Vector Laboratories, Burlingame, CA, USA) and 5% goat serum in TBS, slides were incubated overnight at 4°C with 1:250 Plec-1 antibody (Abcam). Sections were washed three times in TBST, followed by incubation with biotinylated antirabbit goat secondary antibody (Vector Laboratories, Burlingame, CA, USA), then developed using 3,3′-diaminobenzidine tetrachloride (Invitrogen, Carlsbad, CA, USA), and counterstained with hematoxylin.
Histological Assessment
Nerves were noted to have moderate staining intensity for Plec-1 and were present on all slides. Expression of Plec-1 in nerves within each slide was therefore used as a staining control and reference for staining intensity. Staining intensity was recorded by two independent observers and, in case of discrepant results, evaluated by a third observer. Specific focal staining of abnormal epithelial cells was considered positive if it was noted to be at least as strong as nerves.
Immunoprecipitation and Western Blot Analysis of Cyst Fluids
Plec-1 expression was evaluated by Immunoprecipitation and Western Blot analysis of cyst fluids from nonmalignant and malignant IPMN. After the addition of Triton X-100 to a final concentration of 1% (v/v) in combination with a protease inhibitor cocktail (Halt™, Thermo Scientific, Rockford, IL, USA), fluids were incubated at 4°C and cleared by centrifugation. 0.1 to 1 ml of fluid was incubated together with 10 μg mouse monoclonal antibody against human Plec-1 (Santa Cruz Biotechnology, La Jolla, CA, USA) and 50 μl protein G Sepharose (Amersham Biosciences, NJ, USA). The beads were then washed thrice with washing buffer (20 mM Tris, pH 7.4, 137 mM NaCl, 1% Triton X-100). Bound protein was eluted by boiling in sodium dodecyl sulfate (SDS) sample buffer. Proteins were separated via SDS-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. Antigen detection was performed using a rabbit monoclonal antibody against human Plec-1 (Abcam, Cambridge, MA, USA). The secondary antibody was an horseradish peroxidase-coupled goat antirabbit polyclonal antibody (Sigma-Aldrich, St. Louis, MO, USA). Bands were visualized with enhanced chemiluminescence. Rat brain lysate (Santa Cruz Biotechnology, La Jolla, CA, USA) was used as positive control.24
Results
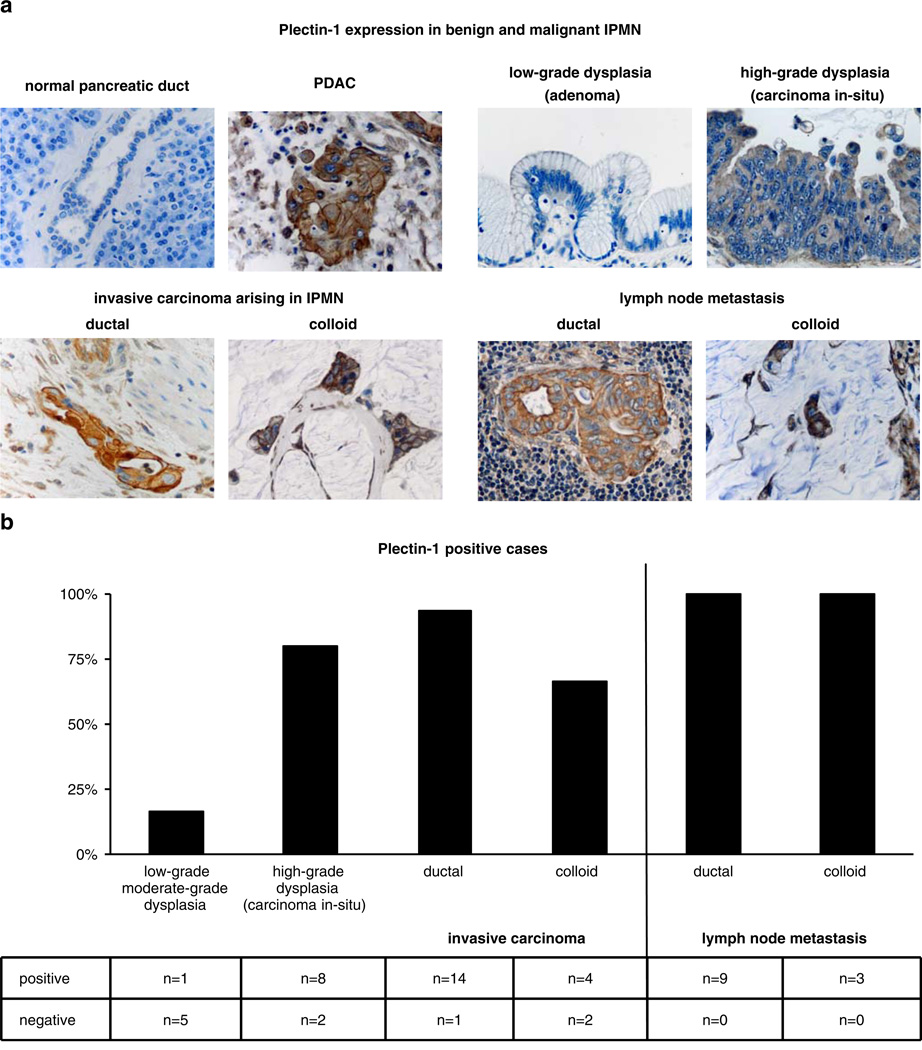
Plec-1 was identified in 84% (26 out of 31) of malignant IPMNs. Eighty percent (eight out of ten) of the high-grade dysplasia (carcinoma in situ) samples expressed Plec-1 and 86% (18 out of 21) of the invasive carcinomas were Plec-1 positive. Two distinct types of invasive carcinoma occur in IPMN: colloid carcinoma (CC) and ductal adenocarcinoma (DA). DA is characterized histologically by infiltrating small tubular units and a marked desmoplastic host reaction, while CC is characterized by dissecting nodules of mucin that contain scant numbers of malignant cells. Fifteen of the 21 invasive carcinomas were classified as DA; the remaining six were classified as CC. Fourteen of the 15 DA samples (93%), but only 66% (four out of six) of CC samples, expressed Plec-1 (Fig. 1a, b). In contrast to malignant IPMNs, only one out of six of the benign IPMN was Plec-1 positive (Fig. 1a, b). The positive benign IPMN was identified as moderate-grade dysplasia.
Figure 1.
Plectin-1 immunohistochemistry. a Representative images of evaluated normal pancreata, PDAC, low-grade and high-grade dysplasia IPMN, ductal and colloid carcinoma arising in the background of IPMN as well as lymph node metastases from ductal and colloid carcinoma arising in the background of IPMN. Normal pancreas and the majority of benign IPMN do not express Plec-1. PDAC, as well as most high-grade dysplasia IPMN, ductal and colloid carcinomas arising in the background of IPMN and their lymph node metastases, are Plec-1 positive. b Distribution of staining for Plec-1 in the specimens. The majority of benign IPMN are Plectin-1 negative. Most high-grade dysplasia IPMN, ductal and colloid carcinomas arising in the background of IPMN, as well as their lymph node metastases, are Plec-1 positive.
Taken together, the specificity of Plec-1 in distinguishing malignant from benign IPMN was 83% and its sensitivity was 84%. Sensitivity for high-grade dysplasia (in situ carcinoma) was 80% and for invasive carcinoma was 86%.
Plec-1 was also reliably identified in lymph node metastases. Twelve of the 31 malignant IPMN evaluated had metastases to lymph nodes. Nine were of DA and three of CC differentiation. Metastases in all nine lymph node metastases deriving from DA-IPMN and all three lymph nodes from CC-IPMN stained for Plec-1 (Fig. 1a, b).
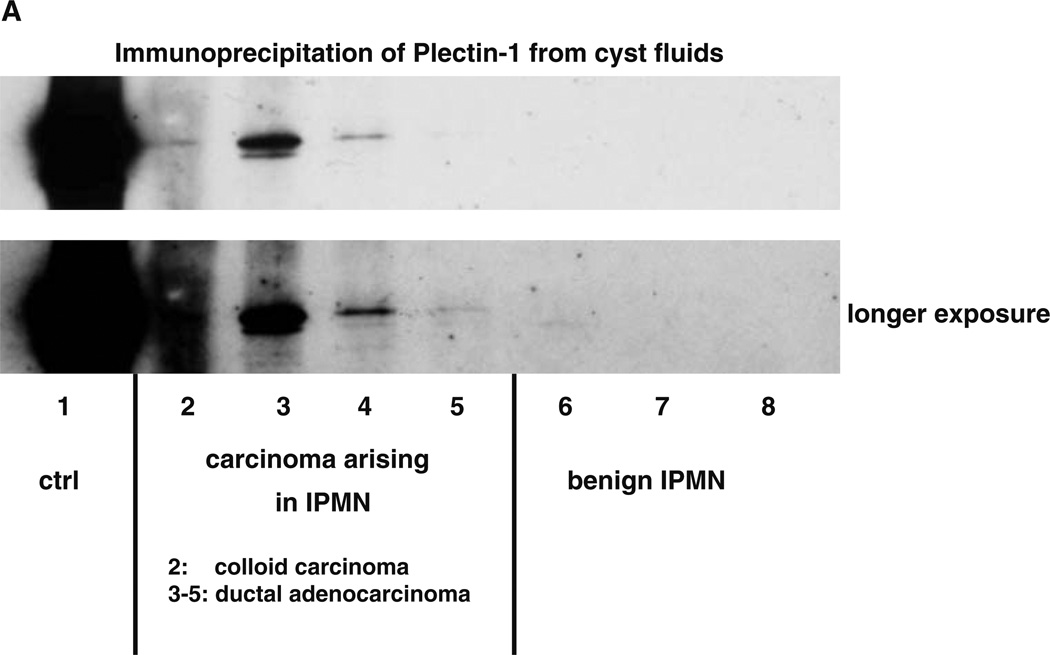
To determine whether there is sufficient Plec-1 present in IPMN cyst fluids to allow the detection of malignancy, Plec-1 immunoprecipitation of cyst fluids from benign and malignant IPMN was performed. Plec-1 was found in 100% (four out of four) of the cyst fluids from malignant IPMN. Three of the Plec-1-positive cyst fluids were from DA and one was from CC. In contrast, cyst fluid from all three benign IPMN contained no detectable Plec-1 (Fig. 2).
Figure 2.
Plectin-1 immunoprecipitation. a Plec-1 in cyst fluid alone is sufficient to distinguish malignant from benign IPMN. Plec-1 was found in 100% (four out of four) of the cyst fluids from malignant IPMN, whereas the cyst fluid from all three benign IPMN did not contain detectable amounts. The Plec-1-positive malignant IPMN cyst fluids were from three ductal adenocarcinomas and one colloid carcinoma.
Discussion
Presently, there are no available biomarkers to assist accurately in distinguishing benign from malignant IPMN. Here, we identify Plec-1 as a potential novel biomarker for carcinoma arising in IPMN. Plec-1 was specifically expressed in the vast majority of carcinomas arising in IPMN as well as in its lymph node metastases, whereas most benign IPMN did not express the protein. In addition to these findings, we were able to demonstrate specific Plec-1 detection in cyst fluid from malignant IPMN. This allows the incorporation of Plec-1 expression analysis into the routine clinical analysis of cyst fluid as an additional screening measure for cancer.
Two distinct types of invasive carcinoma occur in IPMN, CC and DA. It is estimated that 25% to 50% of carcinomas arising in IPMNs are CC,1,4,8,25–27 and in our series, CC accounted for 29% of the invasive carcinomas. In contrast to DA, where Plec-1 was almost always (93%) expressed, CC showed somewhat fewer Plec-1-positive cases (66%). This may be due in part to difficulty in detecting Plec-1 in the abundant intracellular mucin content of CC cells. However, genetic differences between DA and CC may also account for the lower expression rate of Plec-1 in CC compared to DA.
IPMN follow a classical adenoma–carcinoma sequence, progressing from low-grade and moderate-grade dysplasia to carcinoma in situ (high-grade dysplasia) and finally to invasive carcinoma. Based on our data, Plec-1 expression is acquired during the transition from moderate-grade dysplasia to carcinoma in situ (high-grade dysplasia). A small fraction of moderate-grade dysplasia IPMN (17%) expressed Plec-1, while the majority of carcinoma in situ cases (80%) were Plec-1 positive. Invasive DA had an even higher rate of positive samples (93%). It appears that Plec-1 overexpression may begin to appear at the stage of moderate dysplasia even before histological progression becomes evident.
Overall, Plec-1 expression analysis offers improved specificity over present methods of detecting malignancy in IPMN. In one series of 84 patients, the overall sensitivity of the international consensus guidelines5 for predicting malignancy in BD-IPMN was 97.3%; however, their specificity was only 29.8%.14
FNA with cytology can identify malignancy with high specificity and sensitivity when a relevant tissue sample can be obtained.17,19 However, the reliability of this diagnostic method depends on an experienced gastrointestinal cytopathologist, and its utility for risk stratification and therapeutic approach is limited.28
In contrast, Plec-1 in this study of 37 patients had a sensitivity of 84% and specificity of 83% in distinguishing malignant from benign IPMN and was thus equivalent to or better than other diagnostic approaches. The sensitivity of Plec-1 staining for high-grade dysplasia was a somewhat lower (80%), but it was excellent for invasive carcinoma (86%), especially the more common DA (93%).
Evaluation of cyst fluid from FNA for Plec-1 may greatly improve diagnostic accuracy and add valuable additional information to the clinical diagnostic panel at little additional cost. Additionally, in contrast to cytology, it is technically easy to perform and not operator dependent. The technical feasibility of cyst fluid analysis for Plec-1 was successfully demonstrated in this study, in which the protein was detectable in all cyst fluids from malignant IPMN. Validation in a larger data set is in progress.
We conclude that Plec-1 is a sensitive and specific biomarker for the early detection of malignant IPMN. Plec-1 expression analysis can be easily incorporated into routine clinical cyst fluid analyses and holds promise of contributing substantially to improving diagnostic accuracy for the detection of malignancy arising in IPMN.
Appendix
Discussant
Dr. Edward Whang (BWU, Boston): Congratulations for a nice study, and congratulations on picking an excellent mentor with whom to work.
I have some tough questions, but I am sure you will be able to handle them.
First, some methodology questions: How did you pick the cases for inclusion in the study? Surely this is a very small subset of IPMNs available to study at MGH.
Also, I noticed the results you presented today are somewhat different from what you wrote in the abstract. In the abstract, you wrote that one of the colloid carcinomas expressed Plectin-1, whereas today, you said four of them expressed Plectin-1. Is there difficulty in interpretation of the immunohistochemistry in the examples that account for that difference?
Now for some philosophical questions: The sensitivity and specificity associated with using Plectin-1 expression status as a basis for distinguishing benign and malignant IPMNs are each less than 85%. Are those performance characteristics sufficient for clinical application?
Lastly, why is it important to differentiate benign from malignant IPMNs preoperatively? Is not the goal of surgery to prevent cancer from developing? Maybe what you really should seek is a biomarker that differentiates benign IPMNs that are destined to become cancer from benign IPMNs that are destined to remain benign for the remainder of the patient’s life.
Closing Discussant
Dr. Dirk Bausch: Invasive carcinoma arising in IPMN is a rare occurrence. Therefore, only a limited number of cases were available to us and included in the study. Benign IPMN and noninvasive malignant IPMN are much more common. For the purpose of the study, an equal number of main duct and branch duct IPMN were assayed. The relatively small number of benign cases compared to malignant cases assayed is a limitation of the study.
Colloid carcinoma cells contain a high amount of mucin, replacing most of the cytoplasm where Plectin-1 is normally identified. This made the evaluation of these samples exceedingly difficult. To accommodate for these difficulties, two independent observers evaluated all slides.
Sensitivity and specificity of Plectin-1 to detect malignant IPMN were about 85%. Currently employed screening methodology to detect malignancy in branch duct IPMNs, such as the international consensus criteria, have a sensitivity of only about 30%. Therefore, the clinical use of Plectin-1 as an additional screening modality may improve the sensitivity to detect malignancy in IPMN substantially.
The distinction between benign and malignant IPMN is important in the case of branch duct IPMNs, which have a relatively low risk of malignancy. Here, the risk associated with surgical therapy can outweigh the risk of malignancy, especially in the elderly population with small IPMNs.
However, it is important that a biomarker for IPMN identifies preinvasive carcinoma in situ, i.e., high-grade dysplasia lesions, whose prognosis is excellent after surgical resection. Plectin-1 identified about 80% of these cases.
Discussant
Dr. Joe Hines (UCLA): Let me ask the goal of this would be to take cyst aspirate to determine if it the cyst benign or malignant?
Closing Discussant
Dr. Dirk Bausch: The aim is to determine if a cyst is benign or malignant by assessing Plectin-1 expression in a cyst fluid aspirate. The goal is to exclude the operator dependency cytology suffers from and to substitute or augment it with an objective assay for Plectin-1.
Discussant
Dr. Joe Hines (UCLA): But my question is, is Plectin-1 shed into the fluid or does the analysis actually require cells? Because, as you said, the ability to access cytologic aspects for these types of lesion is highly unreliable.
Closing Discussant
Dr. Dirk Bausch: In this study, we used cyst fluids obtained from surgical specimens. Since all cyst fluids were centrifuged before being assayed, they should not contain cells. Therefore, Plectin-1 is most likely shed into the fluid itself. However, Plectin-1 content is very low in cyst fluid. Therefore, enrichment by immunoprecipitation was required prior to detection.
Discussant
Dr. Marc Basson (Michigan State University, East Lansing, MI): Do you think that you would you improve on your sensitivity and specificity if you combined the Plectin-1 reactivity with the other clinical criteria that are already in use. Have you gone back and looked at the numbers? I realize they are small.
Closing Discussant
Dr. Dirk Bausch: No such comparison was made in the current study. Our long-term goal is to improve overall sensitivity and specificity by using Plectin-1 together with clinical criteria.
Contributor Information
Dirk Bausch, Department of Surgery, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114-2622, USA.
Mari Mino-Kenudson, Department of Pathology, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114-2622, USA.
Carlos Fernández-del Castillo, Department of Surgery, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114-2622, USA.
Andrew L. Warshaw, Department of Surgery, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114-2622, USA
Kimberly A. Kelly, Department of Biomedical Engineering, University of Virginia, P.O. Box 800759, Health System, Charlottesville, VA 22908, USA
Sarah P. Thayer, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, 15 Parkman St., WAC 460, Boston, MA 02114-2622, USA sthayer@partners.org
References
- 1.Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, Clain JE, Norton IA, Pearson RK, Petersen BT, Wiersema MJ, Farnell MB, Sarr MG. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 2.Kobari M, Egawa S, Shibuya K, Shimamura H, Sunamura M, Takeda K, Matsuno S, Furukawa T. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–1136. doi: 10.1001/archsurg.134.10.1131. [DOI] [PubMed] [Google Scholar]
- 3.Salvia R, Fernandez-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 6.Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, Pederzoli P. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56:1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernard P, Scoazec JY, Joubert M, Kahn X, Le Borgne J, Berger F, Partensky C. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137:1274–1278. doi: 10.1001/archsurg.137.11.1274. [DOI] [PubMed] [Google Scholar]
- 8.Yamao K, Ohashi K, Nakamura T, Suzuki T, Shimizu Y, Nakamura Y, Horibe Y, Yanagisawa A, Nakao A, Nimuara Y, Naito Y, Hayakawa T. The prognosis of intraductal papillary mucinous tumors of the pancreas. Hepatogastroenterology. 2000;47:1129–1134. [PubMed] [Google Scholar]
- 9.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 11.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Thayer SP, Lauwers GY, Capelli P, Mino-Kenudson M, Razo O, McGrath D, Pederzoli P, Fernandez-Del Castillo C. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79. doi: 10.1053/j.gastro.2007.05.010. quiz 309–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, Bernades P, Belghiti J, Ruszniewski P, Flejou JF. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–1377. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Nagai K, Doi R, Ito T, Kida A, Koizumi M, Masui T, Kawaguchi Y, Ogawa K, Uemoto S. Single-institution validation of the international consensus guidelines for treatment of branch duct intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2009;16:353–358. doi: 10.1007/s00534-009-0068-8. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa H, Itoh S, Ikeda M, Suzuki K, Naganawa S. Intraductal papillary mucinous neoplasm of the pancreas: assessment of the likelihood of invasiveness with multisection CT. Radiology. 2008;248:876–886. doi: 10.1148/radiol.2482071578. [DOI] [PubMed] [Google Scholar]
- 16.Sahani DV, Kadavigere R, Blake M, Fernandez-Del Castillo C, Lauwers GY, Hahn PF. Intraductal papillary mucinous neoplasm of pancreas: multi-detector row CT with 2D curved reformations— correlation with MRCP. Radiology. 2006;238:560–569. doi: 10.1148/radiol.2382041463. [DOI] [PubMed] [Google Scholar]
- 17.Pais SA, Attasaranya S, Leblanc JK, Sherman S, Schmidt CM, DeWitt J. Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: correlation with surgical histopathology. Clin Gastroenterol Hepatol. 2007;5:489–495. doi: 10.1016/j.cgh.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Maire F, Voitot H, Aubert A, Palazzo L, O'Toole D, Couvelard A, Levy P, Vidaud M, Sauvanet A, Ruszniewski P, Hammel P. Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol. 2008;103:2871–2877. doi: 10.1111/j.1572-0241.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 19.Wiesenauer CA, Schmidt CM, Cummings OW, Yiannoutsos CT, Howard TJ, Wiebke EA, Goulet RJ, Jr, McHenry L, Sherman S, Lehman GA, Cramer H, Madura JA. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Arch Surg. 2003;138:610–617. doi: 10.1001/archsurg.138.6.610. discussion 617–8. [DOI] [PubMed] [Google Scholar]
- 20.Pitman MB, Michaels PJ, Deshpande V, Brugge WR, Bounds BC. Cytological and cyst fluid analysis of small (< or =3 cm) branch duct intraductal papillary mucinous neoplasms adds value to patient management decisions. Pancreatology. 2008;8:277–284. doi: 10.1159/000134276. [DOI] [PubMed] [Google Scholar]
- 21.Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, Pearson RK, Petersen BT, Topazian MD, Vege SS, Kendrick M, Farnell MB. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759–1764. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 22.Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, Brugge WR, Edmundowicz SA, Hawes RH, McGrath KM. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095–1102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 23.Kelly KA, Bardeesy N, Anbazhagan R, Gurumurthy S, Berger J, Alencar H, DePinho RA, Mahmood U, Weissleder R. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008;5:e85. doi: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Errante LD, Wiche G, Shaw G. Distribution of plectin, an intermediate filament-associated protein, in the adult rat central nervous system. J Neurosci Res. 1994;37:515–528. doi: 10.1002/jnr.490370411. [DOI] [PubMed] [Google Scholar]
- 25.Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, Wilentz RE, Argani P, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol. 2000;157:755–761. doi: 10.1016/S0002-9440(10)64589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adsay NV, Conlon KC, Zee SY, Brennan MF, Klimstra DS. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- 27.Seidel G, Zahurak M, Iacobuzio-Donahue C, Sohn TA, Adsay NV, Yeo CJ, Lillemoe KD, Cameron JL, Hruban RH, Wilentz RE. Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol. 2002;26:56–63. doi: 10.1097/00000478-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Maker AV, Lee LS, Raut CP, Clancy TE, Swanson RS. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol. 2008;15:3187–3192. doi: 10.1245/s10434-008-0110-0. [DOI] [PubMed] [Google Scholar]