Abstract
Objective
To examine the surgical indications and clinical outcomes of a large cohort of patients with necrotizing pancreatitis.
Summary Background Data
Mortality after debridement for necrotizing pancreatitis continues to be inordinately high. The clinical experience with patients who underwent uniform surgical treatment for necrotizing pancreatitis at the Massachusetts General Hospital over a 15-year period is described.
Methods
Retrospective review of 167 patients with necrotizing pancreatitis who required intervention and were treated with single stage debridement and a closed packing technique. Particular emphasis was placed on the indication for surgery and the presence of infected necrosis. Multiple logistic regression models were used to identify predictors of mortality.
Results
The primary preoperative indication for operation was infected necrosis (51%), but intraoperative cultures proved that 72% of the entire cohort was infected. The rate of reoperation was 12.6%, and 29.9% of patients required percutaneous interventional radiology drainage after initial debridement. Overall operative mortality was 11.4% (19/167), but higher in patients who were operated upon before 28 days (20.3% vs. 5.1%, P = 0.002). Other important predictors of mortality included organ failure ≥3 (OR = 2.4, P = 0.001), postoperative intensive care unit stay ≥6 days (OR = 15.9, P = 0.001), and female gender (OR = 5.41, P = 0.02).
Conclusions
Open, transperitoneal debridement followed by closed packing and drainage results in the lowest reported mortality and reoperation rates, and provides a standard for comparing other methods of treatment. A negative FNA does not reliably rule out infection. The clinical status of the patients and not proof of infection should determine the need for debridement.
Necrosis of the pancreas and/or peripancreatic tissues after an attack of acute pancreatitis develops in approximately 10% to 20% of patients, and their management continues to present a challenge.1 As contemporary literature has favored nonoperative management in patients in whom infected necrosis cannot be proven, infection is said to be the only absolute indication, in effect, the sine qua non, for operative debridement.2–4 In those patients that do undergo surgery for debridement, mortality continues to be inordinately high,5,6 but significantly higher in those with infected necrosis compared with sterile necrosis. Since 1980, we have adopted a uniform surgical approach to postpancreatitis necrosis, comprising a single-stage debridement with closed packing.7 Short-term clinical outcomes on 64 patients were described in 19988. The purpose of the present study, in which we are able to add experience with 103 additional patients, is to reassess the indications for surgical intervention and to provide evidence that it is untenable and unsafe to require proof of infection as the only indication for debridement.
PATIENTS AND METHODS
During the 15-year period from 1990 to 2005, 2449 patients were admitted to the Massachusetts General Hospital with a primary diagnosis of acute pancreatitis. Of these, 167 (6.8%) had necrotizing pancreatitis that required surgical intervention. Their hospital and office charts were reviewed and demographic data and variables relevant to their clinical course were collected. Trends over time were analyzed and the characteristics of those patients who died were analyzed to evaluate predictors of mortality.
Surgical Technique
The operation used for debridement was blunt necrosectomy via a transmesocolic approach followed by closed packing with gauze-stuffed Penrose (Bard, Covington, GA) and closed suction drains. This technique was initially reported by us in 1985 and has been described in detail elsewhere.7,8 In brief, operations were typically performed via an upper midline incision, and using a recent computed tomography (CT) scan as a “road map,” the necrosis was approached through the transverse mesocolon and debrided bluntly, with the goal of removing all necrotic tissue and particulate debris. As necessary, the right and left colic gutters and the retroduodenal space were also opened and debrided. The resulting cavity was then packed with gauze-stuffed Penrose drains and closed-suction drains to aspirate fluid and channel a potential fistula. Fluid and tissue obtained at operation were cultured (aerobic and anaerobic). Cholecystectomy was performed if indicated. A tube gastrostomy was used occasionally. The Penrose drains were removed one at a time, beginning 1 week later to allow residual particulate matter to egress and the cavity to close behind an established tract. The closed-suction drains remained in place until the drainage volume was minimal and all fistulae had closed.
Statistical Methods
To detect changes or trends in practice over time, we divided the study into 2 time periods. Period I included patients operated from 1990 to 1997 and Period II from 1998 to 2005. For continuous variables, mean values ± standard deviations are presented unless otherwise specified. For categorical variables, Fisher exact test and χ2 was used for univariate comparison. A P value ≤0.05 was considered statistically significant. Multiple logistic regression models were constructed to evaluate predictors of mortality using stepwise selection. These included variables that were considered significant from univariate analysis and those identified a priori as being related to mortality.
RESULTS
Patient Characteristics
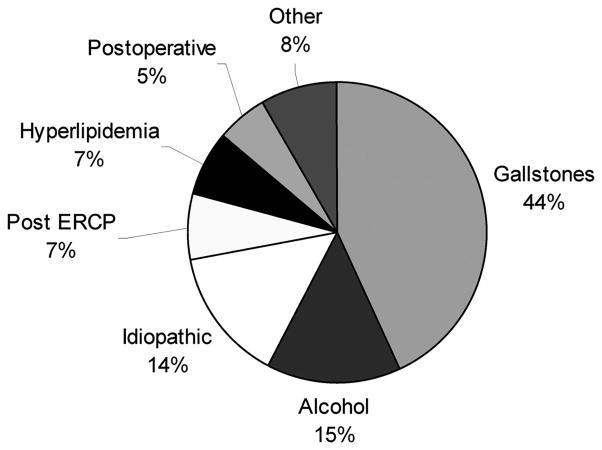
The mean and median age of patients were 57 and 59 years, respectively (range, 20–88), and 62% were male. The etiology of the pancreatitis is described in Figure 1. Sixty-eight percent of patients were transferred from another hospital to our institution after a mean of 18 days (range, 1–84). The mean acute physiological and chronic health evaluation (APACHE) II score in the 24 hours preceding surgery was 9.5 (range, 0–31). Forty percent of the patients had organ failure of at least one type as defined by the Atlanta classification,9 and 31% of patients required intensive care unit (ICU) care preoperatively. Antibiotic prophylaxis was administered in 84% of patients. Forty-four percent of patients underwent preoperative fine needle aspiration (FNA), and 28% had preoperative placement of a percutaneous drain.
FIGURE 1.
Etiology of pancreatitis in 167 patients with necrotizing pancreatitis requiring debridement.
Indications for Surgery
Surgical debridement occurred at a mean of 52.4 days (±57.9) [median of 33 days (range, 1–360)] after the onset of symptoms. The principal indication for operation was proven or presumed infected necrosis (51%). This was shown by FNA in 34% of cases and indicated by the presence of gas on CT in 17%. The second most common indication for operation was “persistent unwellness” in 53 patients (32%). These were patients in whom infected necrosis was not proven, but whose symptoms and signs failed to resolve with prolonged conservative management alone.10 They exhibited some combination of inability to tolerate oral feedings, hyperamylasemia, pain, nausea, vomiting, or persistent low grade fever. The third most common indication for operation was sepsis syndrome in 28 patients, (17%), defined as patients who progressively deteriorated beyond the first week with failure of one or more organs or systems, usually with leukocytosis and fever: the appearance of infection but without culture-positive proof.
Microbiology
Overall, 72% of patients had infected necrosis based on intraoperative cultures. Sixty-four percent of the sepsis syndrome group and, unexpectedly, 42% of the “persistent unwellness” group, had infected necrosis. In the latter group, 19% of patients were subjected to at least 1 preoperative FNA as were 43% of patients in the sepsis syndrome group. Thus FNA yielded a false negative rate of 20% and 25%, respectively, in these groups.
Intraoperative cultures revealed predominantly gram-positive cocci bacteria (50%) followed by gram-negative bacilli (28%) and yeast (13%) (Table 1). Patients with infected necrosis had higher preoperative APACHE II scores (10.5 vs. 7.9, P = 0.014) and a higher incidence of postoperative wound infections (21% vs. 4%, P = 0.009). They also trended toward older age (59.2 vs. 53.9, P = 0.065) and surgery earlier in the course of their illness (44 vs. 64.5, P = 0.082). Nevertheless, differences in reoperation rates (13% vs. 9%, P = 0.6) and mortality (15% vs. 4%, P = 0.1) did not reach statistical significance (Table 2).
TABLE 1.
Microbiologic Classification of Organisms Cultured From Infected Necrosis in 167 Patients Who Underwent Surgery for Pancreatic Necrosis
| Organism | % |
|---|---|
| Gram positive cocci | 50 |
| Gram negative bacilli | 28 |
| Yeast | 13 |
| Other* | 5 |
| Anaerobes | 4 |
Lactobacillus, Haemophilus.
TABLE 2.
Characteristics and Complications of Patients With Infected Versus Sterile Necrosis*
| Infected (N = 113) | Sterile (N = 45) | P | |
|---|---|---|---|
| Males | 70 (61.9%) | 27 (60.0%) | 0.821 |
| Age | 59.2 (±14.4) | 53.9 (±16.5) | 0.065 |
| APACHE II | 10.5 (±6.4) | 7.9 (±5.6) | 0.014 |
| Organ failure | 49 (43.4%) | 17 (37.8%) | 0.521 |
| Preoperative antibiotic therapy | 98 (86.7%) | 36 (80.0%) | 0.288 |
| Days from onset of symptoms to surgery | 44 (±44.8) | 64.5 (±72.2) | 0.082 |
| Postoperative ICU | 72 (63.7%) | 20 (44.4%) | 0.027 |
| Pancreatic fistula | 45 (39.8%) | 15 (33.3%) | 0.448 |
| Enteric fistula | 21 (18.6%) | 3 (6.7%) | 0.84 |
| Intra-abdominal hemorrhage | 6 (5.3%) | 0 (0.0%) | 0.184 |
| Wound infection | 24 (21.2%) | 2 (4.4%) | 0.009 |
| Other major complication | 7 (6.2%) | 1 (2.2%) | 0.442 |
| Postoperative IR drain | 34 (30.1%) | 14 (31.1%) | 0.9 |
| Reoperation early | 15 (13.3%) | 4 (8.9%) | 0.591 |
| Survivors postoperative LOS† | 31.5 (±30.9) | 25.9 (±20.9) | 0.221 |
| Mortality | 17 (15.0%) | 2 (4.4%) | 0.101 |
N=158 Patients for whom intraoperative culture data were available.
Survivors N =145.
Early and Late Complications
Mean operative time was 137 minutes. Forty-two percent of patients required a blood transfusion (mean of 1.25 units), and 57% of the patients required postoperative admission to the ICU for a mean of 14.8 days (range, 3– 49).
Although, the median postoperative length of stay was only 19 days, the range varied from 4 to 195 days. The reoperation rate during the index hospitalization was 15%, and 30% of patients required placement of at least one percutaneous drain for a residual or recurrent collection in the postoperative period. The indications for reoperation were incomplete debridement (14 patients, 8%), hemorrhage (4 patients, 2.4%), intestinal necrosis (3 patients, 1.8%), wound dehiscence (2 patients, 1.2%), and enteric fistula (2 patients, 1.2%).
A postoperative pancreatic fistula via the drains occurred in 68 patients (41%). Only 4 of these required surgical intervention undertaken at a mean of 4.7 months after the initial debridement; the other 64 (94%) closed spontaneously. Enteric fistulae developed in 15% of patients. Permanent endocrine and exocrine insufficiency occurred in 16% and 20% of patients, respectively.
Mortality
Nineteen patients (11.4%) died in the perioperative period a median of 21 days (range, 1–240) after the initial surgery. The mortality rate increased in proportion to the number of failed organs (Fig. 2). Univariate analysis identified APACHE II >10, organ failure ≥1, early surgery, age >65, female gender, and the need for ICU care to be significantly associated with the likelihood of death (Table 3).
FIGURE 2.
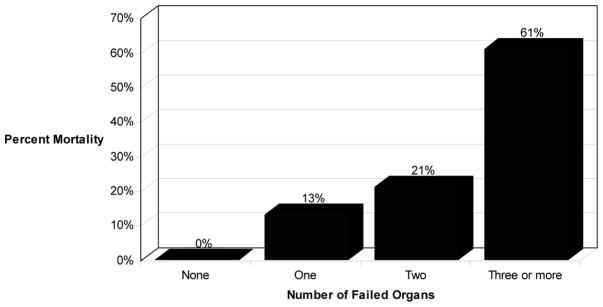
Mortality as a function of pre-operative organ failure in 167 patients.
TABLE 3.
Univariate Analysis Comparing Clinical Variables With Mortality
| Survived (N = 148) | Expired (N = 19) | P | |
|---|---|---|---|
| Males | 97 (65.5%) | 7 (36.8%) | 0.015 |
| Age ≥65 | 53 (35.8%) | 12 (63.2%) | 0.021 |
| APACHE II ≥10 | 59 (39.9%) | 17 (89.5%) | <0.001 |
| Organ failure | 49 (31.1%) | 18 (94.7%) | <0.001 |
| Early surgery | 55 (37.2%) | 14 (73.7%) | 0.002 |
| Preoperative ICU | 37 (25.0%) | 15 (78.9%) | <0.001 |
| Infected necrosis | 96 (69.1%) | 17 (89.5%) | 0.101 |
| Postoperative ICU | 77 (52.0%) | 18 (94.7%) | <0.001 |
| Fungal infection | 17 (12.2%) | 3 (15.8%) | 0.712 |
| Hemorrhage | 5 (3.4%) | 1 (5.3%) | 0.521 |
| Reoperation | 18 (12.2%) | 3 (15.8%) | 0.712 |
Logistic regression analysis identified female gender (adjusted OR = 5.41, 95% CI 1.4–21.4; P = 0.02), organ failure ≥3 (OR = 2.4, 95% CI 2.6–42.4; P = 0.001), early surgery (≤28 days) (OR = 4.06, 95% CI 1.06–15.6; P = 0.04), and postoperative ICU stay ≥6 days (OR = 15.9, 95% CI 3.26–77.06; P = 0.001) as independent predictors of mortality.
Timing of Surgery
Comparisons were made between patients who underwent debridement early in their illness (<28 days) and those whose operation occurred later (≥28 days) (Table 4). Patients in the early intervention group had a significantly higher mortality (20.3% vs. 5.1%, P =0.002). These patients also had higher preoperative APACHE II scores (11.2 vs. 8.3, P =0.003).
TABLE 4.
Characteristics and Complications of Patients Who Underwent Early Surgery (<28 days) Versus Delayed Surgery
| Early (N = 69) | Delay (N = 98) | P | |
|---|---|---|---|
| Males | 43 (62.3%) | 61 (62.2%) | 0.992 |
| Age | 58.75 (±15.5) | 56.29 (±15.1) | 0.306 |
| APACHE II | 11.19 (±6.3) | 8.29 (±5.9) | 0.003 |
| Organ failure | 34 (49.3%) | 33 (33.7%) | 0.043 |
| Days from onset of symptoms to surgery | 17.5 (±6.8) | 77.1 (±65) | <0.001 |
| Postoperative ICU | 49 (71.0%) | 46 (46.9%) | 0.002 |
| Postoperative TPN | 59 (86.8%) | 67 (68.4%) | 0.011 |
| Pancreatic fistula | 30 (44.1%) | 38 (40%) | 0.542 |
| Enteric fistula | 11 (15.9%) | 14 (14.3%) | 0.768 |
| Intra-abdominal hemorrhage | 4 (5.8%) | 2 (2%) | 0.232 |
| Wound infection | 13 (18.8%) | 15 (15.3%) | 0.547 |
| Fungal infection | 10 (15.5%) | 6 (6.5%) | 0.076 |
| Other major complication | 4 (5.8%) | 5 (5.1%) | 1.0 |
| Postoperative IR drain | 22 (31.9%) | 28 (28.6%) | 0.645 |
| Reoperation early | 13 (18.8%) | 8 (8.2%) | 0.047 |
| Postoperative length of stay | 34.02 (±33.3) | 26.8 (±24.1) | 0.231* |
| Mortality | 14 (20.3%) | 5 (5.1%) | 0.002 |
Survivors N = 145.
Trends Over Time
Comparisons of patients who underwent surgery during the first time period (1990–1997) with those who were operated on during the second period (1998–2005) are shown in Table 5. The most notable difference between the 2 groups is a greater than 2-fold increase in the proportion of patients operated upon for proven infected necrosis (31% vs. 66%, P < 0.001), although the overall prevalence of infection was similar (67% vs. 75%, P = 0.234). Stated another way, during the second period there was a significant decrease in the proportion of patients with sterile necrosis selected to undergo debridement.
TABLE 5.
Characteristics of Patients Who Had Surgery During Period I Versus Period II
| Period I (1990–1997) N = 78 |
Period II (1998–2005) N = 89 |
P | |
|---|---|---|---|
| Males | 53 (67.9%) | 51 (57.3%) | 0.157 |
| Age | 56.2 (±15.1) | 58.3 (±15.4) | 0.383 |
| APACHE II | 9.0 (±6.1) | 9.9 (±6.4) | 0.346 |
| Organ failure | 26 (33.3%) | 41 (46.1%) | 0.094 |
| Preoperative antibiotic | 64 (82.1%) | 76 (85.4%) | 0.558 |
| Days from onset of symptoms to surgery | 56.8 (±68.7) | 48.6 (±46.5) | 0.378 |
| Early surgery | 34 (43.6%) | 35 (39.3%) | 0.577 |
| Postoperative ICU | 36 (46.2%) | 59 (66.3%) | 0.009 |
| Pancreatic fistula | 44 (56.4%) | 24 (28.2%) | <0.001 |
| Enteric fistula | 15 (19.2%) | 10 (11.2%) | 0.149 |
| Intra-abdominal hemorrhage | 3 (3.8%) | 3 (3.4%) | 1 |
| Wound infection | 13 (16.7%) | 15 (16.9%) | 0.974 |
| Other major complication | 6 (7.7%) | 3 (3.4%) | 0.307 |
| Postoperative IR drain placed | 20 (25.6%) | 30 (33.7%) | 0.256 |
| Indication for surgery Infected necrosis | 24 (30.8%) | 59 (66.3%) | <0.001 |
| Intraoperative cultures recorded | 69 (88.5%) | 89 (100%) | <0.001* |
| Intraoperative culture proven infected necrosis | 46 (66.7%) | 67 (75.3%) | 0.234 |
| Fungal infection | 6 (8.7%) | 14 (15.7%) | 0.187 |
| Early reoperation | 12 (15.4%) | 9 (10.1%) | 0.305 |
| Postoperative length of stay | 28.8 (±22.0) | 30.1 (±32.7) | 0.782† |
| Mortality | 5 (6.4%) | 14 (15.7%) | 0.058 |
Introperative cultures recorded N = 158.
Survivors N = 145.
DISCUSSION
The management (surgical or otherwise) of patients with necrotizing pancreatitis continues to evolve.11 There is uncertainty regarding the optimal timing of intervention and controversy regarding whether it is necessary to operate on patients without proven infection.12,13 Furthermore, recent studies have shown the feasibility of performing debridement of pancreatic necrosis with minimally invasive approaches, including laparoscopic,14 percutaneous retroperitoneal,15 and endoscopic routes.16
This large series of patients, treated with a uniform surgical technique, confirms the safety and efficacy of a transabdominal, open technique of debridement and closed packing for necrotizing pancreatitis, an approach used at our institution for over 25 years.8 Our very low mortality and reoperation rates of 11.4% and 15%, should serve as a standard for future assessment of variations in the treatment of pancreatic necrosis. Our outcomes compare favorably with other recent series from pancreatic specialty centers in which the mortality rates utilizing other techniques, including minimally invasive approaches, range from 11% to 28%.5,6,17
It is well known that the severity of illness in patients with acute necrotizing pancreatitis differs widely. The mean preoperative APACHE II score of 9.5 in the present cohort is commensurate with other series.5 It is notable that in the second period of the study, the APACHE II score increased by almost a full point (from 9 to 9.9), indicating more severe illness and perhaps explaining the increase in mortality in the second half of the study.
Over time we have observed a reduction in the proportion of patients with sterile necrosis whom we selected for debridement, consistent with the consensus that nonoperative management suffices in most of these patients.18 Notwithstanding, it is clear that spontaneous reabsorption of necrotic tissue and progressive resolution of symptoms does not occur in a timely manner in all patients with sterile necrosis and that some of these patients eventually benefit from surgical help to recovery. Our data supports the contention that the clinical condition of the patients may be sufficient in and of itself to justify a more aggressive stance, and that unrecognized infection will often underlie the failure to heal.
Our data demonstrates that persistent unwellness is an important but under-appreciated indication for surgery, in part because 42% of these patients proved, after all the evidence was in, to be infected. When deciding whether or not to operate, the overall clinical status of the patient must be considered along with other determinants. Perhaps one of the most important messages of this experience is that neither the failure of FNA to demonstrate infection nor the lack of clinical signs of sepsis rules out clandestine infection of the necrotic tissues. In this series >20% of patients with a negative FNA were infected.
In searching for predictors of mortality, we found that APACHE II, organ failure, and early surgical debridement all correlated with a higher likelihood of death. As in our earlier report, we demonstrate that presence of infection in the necrosis did not significantly impact mortality in comparison with sterile necrosis. Even though the higher mortality in the early surgery group is confounded by the higher disease severity index, it is clear from the contemporary literature and our data, that outcomes are better if intervention is delayed (>28 days) until demarcation of the dead tissue is well defined.19 Most centers, including our own, use the alternative of percutaneous radiologic drainage of infected collections in unstable patients as a bridge to surgery.13,20,21 Although it was not the objective of our study to demonstrate this, nearly 30% of the patients in our series were temporized with a preoperative percutaneous drain placed with radiologic guidance.
An unsuspected finding in this series was that female gender emerged as an independent risk factor for mortality. To our knowledge, this is the first report identifying gender as an independent risk factor for mortality in patients undergoing surgery for necrotizing pancreatitis, although female gender has been identified previously as an independent predictor of increased mortality in critically ill surgical patients with documented infection.22 The only previous information regarding female gender and severity of pancreatitis is a prospective epidemiologic study from Germany which found longer total hospital stays, higher Imrie scores, more frequent pseudocysts and significantly more pancreatic necrosis in women, but did not find a difference in mortality.23 In fact, a well validated prognostic severity scoring system, the Mannhein Peritonitis Index, incorporates female gender as a risk factor of prognostic relevance and weighs it according to its predictive power.24 Nevertheless, it is important to recognize that our results are only suggestive of a higher gender risk, given the low number of deaths in the series.
In summary, this cohort of patients shows that infection of pancreatic necrosis is unexpectedly present in a significant number of patients who lack signs of infection or who have had a negative FNA. It also reconfirms that higher preoperative APACHE II scores, organ failure, and early surgery are all independent predictors of mortality, and suggests for the first time that female gender is an independent risk factor as well. Our technique of open single-stage debridement and closed packing has the same very low mortality and acceptable complication rates in both infected and sterile necrosis. In the era of increasing options for treating patients with necrotizing pancreatitis including minimally invasive15,25 and nonoperative12,13 approaches, this series can serve as a standard for other treatment options.
Acknowledgments
Supported by the Claude Welch Surgical Research Fellowship.
References
- 1.Beger HG, Rau B, Isenmann R. Natural history of necrotizing pancreatitis. Pancreatology. 2003;3:93–101. doi: 10.1159/000070076. [DOI] [PubMed] [Google Scholar]
- 2.Bradley EL, III, Allen K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg. 1991;161:19–24. doi: 10.1016/0002-9610(91)90355-h. discussion-5. [DOI] [PubMed] [Google Scholar]
- 3.Buchler MW, Gloor B, Muller CA, et al. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619– 626. doi: 10.1097/00000658-200011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- 5.Connor S, Alexakis N, Raraty MG, et al. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137:499–505. doi: 10.1016/j.surg.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Rau B, Bothe A, Beger HG. Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19-year, single-center series. Surgery. 2005;138:28–39. doi: 10.1016/j.surg.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Warshaw AL, Jin GL. Improved survival in 45 patients with pancreatic abscess. Ann Surg. 1985;202:408– 417. doi: 10.1097/00000658-198510000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-del Castillo C, Rattner DW, Makary MA, et al. Debridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg. 1998;228:676– 684. doi: 10.1097/00000658-199811000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley EL., III A clinically based classification system for acute pancreatitis. Arch Surg; Summary of the International Symposium on Acute Pancreatitis; Atlanta, GA. September 11 through 13, 1992; 1993. pp. 586–590. [DOI] [PubMed] [Google Scholar]
- 10.Warshaw AL. Pancreatic necrosis: to debride or not to debride-that is the question. Ann Surg. 2000;232:627– 629. doi: 10.1097/00000658-200011000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nathens AB, Curtis JR, Beale RJ, et al. Management of the critically ill patient with severe acute pancreatitis. Crit Care Med. 2004;32:2524–2536. doi: 10.1097/01.ccm.0000148222.09869.92. [DOI] [PubMed] [Google Scholar]
- 12.Adler DG, Chari ST, Dahl TJ, et al. Conservative management of infected necrosis complicating severe acute pancreatitis. Am J Gastroenterol. 2003;98:98–103. doi: 10.1111/j.1572-0241.2003.07162.x. [DOI] [PubMed] [Google Scholar]
- 13.Connor S, Raraty MG, Neoptolemos JP, et al. Does infected pancreatic necrosis require immediate or emergency debridement? Pancreas. 2006;33:128–134. doi: 10.1097/01.mpa.0000234074.76501.a6. [DOI] [PubMed] [Google Scholar]
- 14.Parekh D. Laparoscopic-assisted pancreatic necrosectomy: a new surgical option for treatment of severe necrotizing pancreatitis. Arch Surg. 2006;141:895–902. doi: 10.1001/archsurg.141.9.895. discussion-3. [DOI] [PubMed] [Google Scholar]
- 15.Connor S, Ghaneh P, Raraty M, et al. Minimally invasive retroperitoneal pancreatic necrosectomy. Dig Surg. 2003;20:270–277. doi: 10.1159/000071184. [DOI] [PubMed] [Google Scholar]
- 16.Charnley RM, Lochan R, Gray H, et al. Endoscopic necrosectomy as primary therapy in the management of infected pancreatic necrosis. Endoscopy. 2006;38:925–928. doi: 10.1055/s-2006-944731. [DOI] [PubMed] [Google Scholar]
- 17.Howard TJ, Patel JB, Zyromski N, et al. Declining morbidity and mortality rates in the surgical management of pancreatic necrosis. J Gastrointest Surg. 2007;11:43– 49. doi: 10.1007/s11605-007-0112-4. [DOI] [PubMed] [Google Scholar]
- 18.Uhl W, Warshaw A, Imrie C, et al. IAP Guidelines for the Surgical Management of Acute Pancreatitis. Pancreatology. 2002;2:565–573. doi: 10.1159/000071269. [DOI] [PubMed] [Google Scholar]
- 19.Hartwig W, Maksan SM, Foitzik T, et al. Reduction in mortality with delayed surgical therapy of severe pancreatitis. J Gastrointest Surg. 2002;6:481– 487. doi: 10.1016/s1091-255x(02)00008-2. [DOI] [PubMed] [Google Scholar]
- 20.Szentkereszty Z, Kerekes L, Hallay J, et al. CT-guided percutaneous peripancreatic drainage: a possible therapy in acute necrotizing pancreatitis. Hepatogastroenterology. 2002;49:1696–1698. [PubMed] [Google Scholar]
- 21.Olah A, Belagyi T, Bartek P, et al. Alternative treatment modalities of infected pancreatic necrosis. Hepatogastroenterology. 2006;53:603– 607. [PubMed] [Google Scholar]
- 22.Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg. 1999;134:1342–1347. doi: 10.1001/archsurg.134.12.1342. [DOI] [PubMed] [Google Scholar]
- 23.Lankisch PG, Assmus C, Lehnick D, et al. Acute pancreatitis: does gender matter? Dig Dis Sci. 2001;46:2470–2474. doi: 10.1023/a:1012332121574. [DOI] [PubMed] [Google Scholar]
- 24.Billing A, Frohlich D, Schildberg FW. Prediction of outcome using the Mannheim peritonitis index in 2003 patients. Peritonitis Study Group. Br J Surg. 1994;81:209–213. doi: 10.1002/bjs.1800810217. [DOI] [PubMed] [Google Scholar]
- 25.Connor S, Raraty MG, Howes N, et al. Surgery in the treatment of acute pancreatitis–minimal access pancreatic necrosectomy. Scand J Surg. 2005;94:135–142. doi: 10.1177/145749690509400210. [DOI] [PubMed] [Google Scholar]