Abstract
Myocilin and optineurin are two genes linked to glaucoma, a major blinding disease characterized by progressive loss of retinal ganglion cells and their axons. This review describes the characteristics of myocilin and optineurin protein products and summarizes the consequences of ectopically expressed wild type and mutant myocilin and optineurin in trabecular meshwork and/or neuronal cells. Myocilin and optineurin exhibit differential characteristics and have divergent functional consequences. They contribute to the development of glaucoma likely via distinct mechanisms.
Keywords: Glaucoma, Gene, Myocilin, Optineurin, Mutation
Introduction
Glaucoma is one of the leading causes of irreversible blindness worldwide. This disease is characterized by progressive loss of retinal ganglion cells (RGCs) and accompanying axons. Primary open-angle glaucoma (POAG), the most common form of glaucoma, is frequently associated with increased intraocular pressure (IOP). The IOP is controlled by a balance between the production and outflow of the aqueous humor contained in the anterior chamber. The trabecular meshwork (TM), a specialized tissue, is the major site for regulation of the aqueous humor outflow.1,2
Studies over the past decades have revealed that POAG is genetically heterogeneous, caused by several susceptibility genes and environmental factors.3,4 To date, a total of 15 chromosomal loci have been mapped and designated as GLC1A to GLC1O.3-7 Four candidate genes have been identified that include myocilin as the GLC1A,6 optineurin as the GLC1E,7 WDR36 as the GLC1G,3,4 and neurotrophin-4 (NTF-4) as the GLC1O3,4 gene.
Myocilin is the first identified gene for both juvenile- and adult-onset POAG.6 More than 70 myocilin mutations have been found in a number of families.3,5 Glaucoma patients with myocilin mutation tend to have high IOP.5 Among the myocilin mutations, the Gln368Stop (Q368X) mutation is the most frequent8 and the Pro370Leu (P370L) mutation is responsible for one of the most severe glaucoma phenotypes.
Optineurin is a gene that links principally to normal tension glaucoma (NTG), a subtype of POAG.7 Optineurin mutations are noted to vary with ethnic background.9 The Glu50Lys (E50K) mutation, found in Caucasian and Hispanic populations,9 seems to be associated with a more progressive and severe disease in NTG patients.10
Gene structure and expression
The myocilin gene contains three exons and two introns that span 17 kilo base pairs.11 The protein product encoded by the human gene contains 504 amino acids. There is a hydrophobic signal peptide sequence (amino acids or aa 1-32) and non-muscle myosin-like domain near the amino (N)-terminus and an olfactomedin-like domain (aa 326-501) at the carboxyl (C)-terminus. The N-terminus has two initiation sites. Within the myosin-like domain, there are leucine zipper motifs within two coiled-coil regions (aa 74-110 and 118-186).11,12 The leucine zipper and the olfactomedin domains are well conserved across species. At the secondary level, the N-terminal region is primarily α-helical and the C-terminal consists mostly of β-strands.13 In glaucoma patients, most of the myocilin mutations are mapped to the third exon of the gene within the C-terminal olfactomedin domain.
The expression of myocilin is mainly in the eye, but it has also been observed in a number of other tissues in the body including the skeletal muscle and brain. In the eye, myocilin is seen in many tissues such as the sclera, ciliary body and the optic nerve head14 although its expression is the highest in the TM.
The human optineurin gene has a total of 16 exons; the first 3 are non-coding and the remaining 13 exons code for a 577-amino acid protein that contains multiple coiled-coil domains and a C-terminal zinc finger.15,16 The optineurin protein from different species has high amino acid homology.16 The amino acid 50 glutamic acid residue is conserved in mouse, rat, chicken and cow. Optineurin is also ubiquitously expressed in non-ocular tissues such as the heart and brain and in ocular tissues including the retina, trabecular meshwork, and non-pigmented ciliary epithelium. In the retina, RGCs are immunolabeled with high intensity.15,16
Protein characteristics
Myocilin was initially identified as a protein secreted into the media of TM cultures after induction with glucocorticoids such as dexamethasone (DEX).12 One unique feature is that the myocilin expression is highly upregulated by DEX in TM cells but not in other cell types such as corneal fibroblasts.11,17 Containing a signal peptide sequence at the N-terminus, myocilin is, at least in part, secreted via the traditional secretory pathway. Myocilin has additionally been shown to associate with exosome-like vesicles and may also use this alternative mechanism to be released from the cell.11
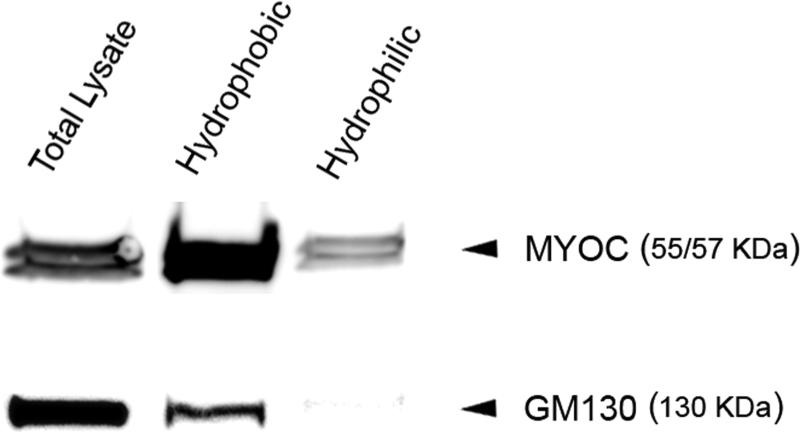
Myocilin has been shown to be a glycoprotein, N-glycosylated at amino acid residues 57-59 (Asn-Glu-Ser).11 On Western blots, it is seen as 53-57-kilodalton (kDa) doublet bands although some antibodies also yield a 66-kDa protein band. When subjected to membrane protein extraction, myocilin in TM cell lysates distributed largely in the hydrophobic fraction in association with membranes (Fig. 1). The myocilin protein has in addition been reported to be cleaved into a 20 kDa N-terminal fragment and a 35 kDa C-terminal fragment both in vitro and in vivo. The proteolytic processing of myocilin is suggested to have a role in regulating its molecular interactions.18
Figure 1. Western blot analysis for myocilin in hydrophilic and hydrophobic fractions of human TM cell lysates.
Total proteins in TM lysates were subjected to membrane protein extraction followed by Western blotting. The hydrophobic fraction contained membrane proteins and the hydrophilic fraction contained predominantly non-membrane cytosolic proteins. Myocilin (MYOC) in total cell lysates was detected largely in the hydrophobic fraction although a small portion was also seen in the cytosolic, hydrophilic fraction. GM130, a Golgi marker used as membrane protein positive control, was detected exclusively in the hydrophobic fraction.
Myocilin interacts with itself at sites of the leucine zipper/coiled coil domain to form dimers and possibly multimers.11,19,20 It also interacts with a number of proteins including flotillin-1(a structural protein of lipid rafts), optimedin, extracellular proteins such as fibronectin and fibrillin-1, as well as matricellular proteins hevin and SPARC.11,18
Optineurin, by contrast, is not secreted.21 It is neither N- nor O-glycosylated but is serine- and tyrosine-phosphorylated.22 Optineurin is shown to be, exclusively, a hydrophilic, cytosolic protein.22 It possesses an ubiquitin binding domain, is ubiquitinated, and is processed through the ubiquitin-proteasome pathway.22,23 By native blue gel electrophoresis, optineurin is found to be capable of forming 420 kDa homo-oligomers, which, based on the 67 kDa monomer size, is estimated to be hexamers.22 Furthermore, optineurin interacts and associates with Rab8, myosin VI, and transferrin receptor,22,24 either singly or in combination, to form supermolecular complexes with sizes larger than 400 kDa.22
Optineurin has in addition been shown to interact with transcription factor IIIA,25 huntingtin,26 metabotropic glutamate receptor,27 and TANK-binding kinase 1 (TBK1).28 It shares 53% amino acid homology with NEMO (NF-κB essential modulator) and is identified as a NEMO-related protein.29 Optineurin expression is upregulated by tumor necrosis factor-α (TNFα)15 and interferon and its phosphorylation is induced upon phorbol 12-myristate 13-acetate stimulation.29
Localization
Myocilin protein is localized to both intra- and extra-cellular sites in TM cells. Immunofluorescence has shown that the intracellular form of myocilin is distributed diffusely in the cytoplasm including perinuclear regions.11,17 Subcellular fractionation and/or immunoelectron microscopy indicated that intracellular myocilin in TM cells is associated not only with endoplasmic reticulum, Golgi apparatus, vesicles, but also with mitochondria.11,30 Immunogold labeling documented the extracellular localization of myocilin.11,31,32 It is intriguing that myocilin is associated mainly with microfibrillar architecture in sheath-derived plaques where pathologic changes have been reported to occur in eyes of POAG patients.32
Optineurin also has a diffuse, cytoplasmic distribution but a population of the protein is associated with the Golgi apparatus.21 As optineurin is a cytosolic protein not associated with membranes, the optineurin-Golgi association is probably indirect via interactions of other Golgi-associated proteins such as Rab8.22
Consequences of Overexpression and Mutation and/or Possible Functional Roles
De-adhesive activity, Rho inactivation, mitochondrial association, and inhibition on neurite outgrowth – myocilin phenotype
The extracellular myocilin was demonstrated to be a very poor substrate for TM cells.33 It blocks the TM attachment to fibronectin, retards migration, causes a dramatic reduction in actin stress fibers and focal adhesions,33,34 and triggers TM cells to assume a stellate morphology with microspikes.34 Myocilin has been shown to interact with fibronectin via the heparin (Hep) II domain.35,36 This domain, along with the Arg-Gly-Asp site, is where fibronectin is linked to the actin cytoskeleton, transducing signals from the exterior to the interior of the cells. The fibronectin-TM cell interaction is possibly mediated via binding of myocilin to the Hep II domain.36 Purified recombinant myocilin protein has also been noted more recently to be a modulator of the Wnt signaling.37
Overexpressing wild type or full length myocilin intracellularly38 by transfection or by protein transduction39 in TM cells results in an alteration in actin architecture, similar to that seen with the extracellular form of myocilin. Key findings include a loss of actin stress fibers and vinculin-positive focal adhesions, and a reduction in cell adhesion to fibronectin, vitronectin and collagen types I and IV.40 The fibronectin deposition is diminished and the trypsinization time needed to round up or suspend TM cells from plates is significantly shorter for myocilin transfectants than that for mock controls, signifying a compromised cell-matrix adhesiveness.40 Myocilin therefore appears to possess a de-adhesive activity, capable of changing TM cells in culture from a state of strong adherence, containing robust focal adhesions and actin-containing stress fibers, to a state of compromised adhesiveness. The de-adhesion process, on a long-term chronic basis, may render TM cells vulnerable. Myocilin overexpressing cells have been shown to display an increased susceptibility to apoptotic challenge.38 It is speculated that the cell vulnerability, in conjunction with additional stress or insults, may be a key factor in the development of glaucomatous conditions.
When treated with lysophosphatic acid, a Rho stimulator, the fibronectin deposition in myocilin transfectants was enhanced, suggesting a role of small GTPase Rho family in the myocilin phenotype.40 Subsequent pull down assays verify that indeed the level of GTP-bound or active RhoA in myocilin transfectants is lower than that in controls. Further assays, in consistence with the notion that the regulation of Rho function represents a major target for protein kinase A (PKA) in cytoskeletal modulation, disclose that along with RhoA inactivation, the adenosine 3’,5’-cyclic monophosphate (cAMP) level and the PKA activity are enhanced upon myocilin transfection. Inhibition of PKA by inhibitor H-89 in myocilin-transfected cells prevents, at least partially, the stress fiber dissolution. Time lapse video microscopy corroborates that both the H-89 treatment and co-transfection with constitutively active RhoA delay the rounding up or prolong the trypsinization time of myocilin-GFP-expressing cells.40
The association of myocilin with mitochondria was examined by mitochondrial import assays.41 Cell free-translated myocilin was incubated with isolated mitochondria and the assays revealed that myocilin is robustly imported into the mitochondria isolated from TM cells, but only minimally into those from corneal fibroblasts and mouse liver. The myocilin imported into TM mitochondria is targeted mostly to the membranes. When upregulated or overexpressed, myocilin induces a drop of the mitochondrial membrane potential, indicative of mitochondrial depolarization or dysfunction.41
The effect of myocilin on neurite outgrowth was investigated.42 Neuronal PC12 and RGC5 cells were transfected to express myocilin-GFP and allowed to differentiate. While the mock-transfected control cells produced long neurites, the length of neurites in wild type myocilin-transfected cells was reduced to nearly half of that of controls. The number of neurites was likewise decreased. Myocilin mutant P370L and Q368X constructs also elicited inhibitory effects on neurite outgrowth, comparable to that seen with the wild type myocilin.42
Optineurin transfection, on the other hand, has no effect on the actin structure, focal adhesion formation, or trypsin sensitivity of the cells.40 The PKA activity was also unaltered (Fig. 2).
Figure 2. PKA activity in optineurin-expressing cells.
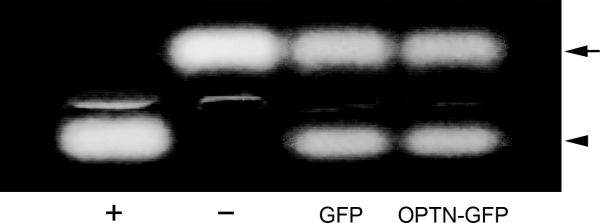
Human TM cells were transfected with pEGFP-N1 (GFP), or pOptineurin-GFP (OPTN-GFP) for 24 hours. Equal amounts of protein lysates were subjected to PKA assays. Positive (+) and negative (-) controls were included. The non-phosphorylated (upper band, arrow) and the phosphorylated (lower band, arrowhead) substrates were resolved on agarose gels. The PKA activity in optineurin transfectants, judged by the level of the phosphorylated substrate and determined by densitometric analyses, was similar to that in the GFP control.
Unlike myocilin, optineurin is not imported into the mitochondria.41 Neither overexpression nor mutation of optineurin results in any compromise of the mitochondrial membrane potential (data not shown). There was also no inhibitory effect on the neurite outgrowth.42
Golgi fragmentation, impaired transferrin uptake, NF-κB pathway and inflammation regulation, and cell death – optineurin phenotype
When transfected to express optineurin-GFP fusion protein, diffuse green fluorescence is seen in the cytoplasm of human retinal pigment epithelial (RPE),21 RGC5,22 PC12,42 and mouse neuroblastoma Neuro2a (Fig. 3) cells. Notably, bright granular or punctuate structures are also observed in perinuclear regions of all cell types. These structures, termed foci, are dynamic. Visualized by live cell imaging, they move around in both short and long ranges.22 Subsequent experimentation using nocadazole proved that the formation of optineurin foci is microtubule dependent.21,22 Such foci are likewise formed in cells after transfection to overexpress mutant E50K optineurin-GFP. The number and the size of the E50K-GFP foci are even greater than those of the wild type.21
Figure 3. Foci formation in optineurin-expressing Neuro2a neuronal cells.
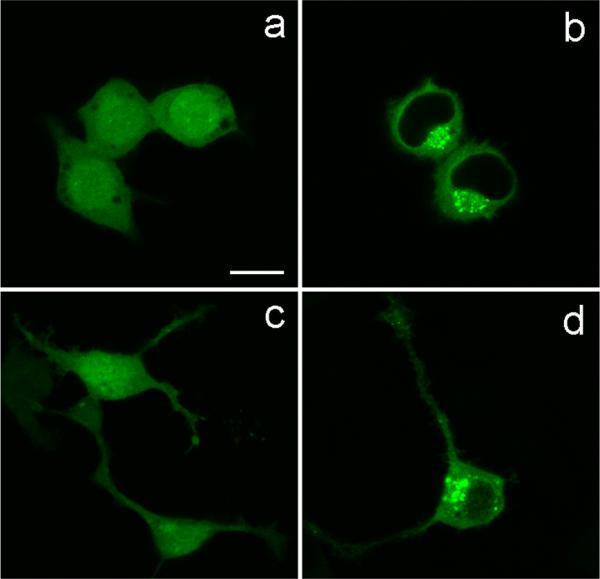
The cells were transfected for 20 hours with pEGFP-N1 (a and c, mock control) to express GFP, or pOptineurin-EGFP (b and d) to express wild type optineurin-GFP. When transfected with pEGFP-N1, green fluorescence was observed in the entire cells including the nucleus. When transfected with pOptineurin-EGFP, diffuse green fluorescence was seen in the cytoplasm of Neuro2a cells. Strikingly, bright granular or punctuate structures termed foci were inaddition noted in the perinuclear region. The foci were observed in cells without (c) and with (d) neuronal morphology showing neurite extensions. Scale bar, 10 μm.
Golgi fragmentation was noted upon optineurin overexpression when the cells were immunostained with Golgi marker GM130 or Golgin97. The degree of fragmentation is also more severe in E50K transfectants than the wild type.21,22
Optineurin, as discussed above, is known to interact with Rab8, myosin VI, huntingtin, and transferrin receptor, all are players in membrane trafficking. To determine the role of optineurin in protein trafficking, transferrin uptake, an indicator of receptor-mediated endocytosis process, was examined in RPE and RGC5 cells after transfection to overexpress wild type and E50K optineurin. Results obtained indicated that the transferrin uptake was significantly decreased in optineurin-expressing cells.43 The reduction was observed from the initial 2- and 5-min time points. Co-transfection with transferrin receptor, but not Rab8 or myosin VI, construct, rescued the optineurin inhibitory effect. The ectopic expression of optineurin caused transferrin receptor to colocalize with the foci. Surface biotinylation experiments further showed that the surface level of transferrin receptor was lowered, leading presumably to an impeded transferrin uptake.43 The optineurin phenotype is thus likely mediated by the interaction between optineurin and transferrin receptor molecules. The E50K mutation, compared to the wild type, yielded a more pronounced impairment in the transferrin uptake.43
Optineurin, sharing an amino acid homology with NEMO, is demonstrated to be a negative regulator of NF-κB.44,45 It is well known that transcription factor NF-κB is retained in an inactive form in the cytoplasm. Upon activation by TNFα, signaling intermediates such as RIP (receptor interacting protein) are recruited to the TNF receptor, resulting in activation of the IκB kinase (IKK) complex. The inhibitory proteins of the IκB family are then degraded and NF-κB is translocated to the nucleus to trigger gene regulation. NEMO is the catalytic unit known as IKKγ of the IKK complex. Optineurin competes with NEMO for binding to ubiquitinated RIP to inhibit the TNFα-induced NF-κB activation. There appears to be a negative feedback loop; the optineurin gene expression is elevated by treatment of TNFα via the NF-κB pathway, and the optineurin induced in turn inhibits the NF-κB activation, suggesting a physiological role for optineurin in dampening the TNFα signaling.44,45
Via interaction with TBK1, optineurin has been shown to inhibit virus triggered interferon-β induction.46 Similar to the NF-κB signaling, the ubiquitin binding domain of optineurin appears to be essential for regulation of the antiviral response.46 The role of optineurin may therefore be expanded from a modulator of TNFα signaling to a broad-spectrum negative regulator of inflammation.
Cell death ensued following optineurin overexpression.42 The cell loss is at least in part due to increased apoptosis.42 As is seen with other optineurin phenotypes, the cell loss is also more pronounced in E50K-expressing cultures.
Since the E50K mutation generates much striking effects compared to the wild type optineurin, this mutation is apparently a gain-of-toxicity mutation. The defective trafficking, deregulated NF-κB signaling, along with fragmentation of the Golgi complex and induction of apoptosis, are speculated to be the underlying bases why E50K mutation renders the patients predisposed to the glaucoma pathology.
Overexpression of wild type myocilin results in a diffuse distribution but little foci are observed in TM or RGC5 cells. No fragmentation of the Golgi is detected and the apparatus remains intact regardless whether the cells were transfected with wild type or mutant (P370L and Q368X) myocilin (Fig. 4).
Figure 4. The Golgi integrity in cells expressing wild type and mutant myocilin.
Inducible, stable RGC5 cells were untreated (Non-Induced; a, b, c) or treated with doxycycline for 24 hours. Upon treatment, the cells were induced to express wild type myocilin-GFP (MYOCWT; d, e, f), P370L myocilin-GFP (MYOCP370L; g, h, i), Q368X myocilin-GFP (MYOCQ368X; j, k, l), or wild type optineurin-GFP (OPTN; m, n, o). Immunofluorescence staining for Golgi marker, GM130, was performed. The myocilin- or optineurin-GFP-expressing cells were marked by the GFP green fluorescence, the nuclei were stained with DAPI in blue and the Golgi staining in both induced (green) and non-induced (non-green) cells was seen in red. Fragmentation of the Golgi apparatus is seen in cells expressing optineurin-GFP (as a positive control, n) but not in those expressing wild type and P370L and Q368X mutant myocilin-GFP. Scale bar: 10 μm.
Unlike optineurin, wild type myocilin does not affect the transferrin uptake or its kinetics.43 Also contrasting optineurin, overexpression of wild type myocilin does not invoke apoptosis in TM cells.41 However, treatment of anti-Fas does induce a significantly higher level of apoptosis in myocilin-expressing cells as compared to mock or nontransfected controls.29 Myocilin overexpression appears to sensitize cells to apoptotic challenges.
Of note is that P370L and Q368X myocilin mutants do trigger cell death.42 These mutants are not secreted.47 They are retained in the cells and have been shown to form aggregates to cause endoplasmic reticulum stress.48
It is unknown whether myocilin plays a role in either NF-κB signaling or regulation or antiviral response.
Conclusions
Myocilin mutations are typically associated with high IOP cases and the optineurin ones are associated with NTG. As reviewed above, these two glaucoma genes are different, exhibiting dissimilar characteristics and eliciting differential effects on signal transduction, neurite outgrowth, and protein trafficking process. The contrast implicates that myocilin and optineurin have different functional roles and that they contribute to the development of neurodegenerative glaucoma via distinct mechanisms. Continued efforts directed to unraveling of such mechanisms are likely to be fruitful, providing further insights into the molecular bases involved in pathogenesis of glaucoma.
Acknowledgments
The author thanks Ms. Hongyu Ying and Mr. Xiang Shen for expert technical assistance. The work was supported by grants EY005628 and EY018828 and core grant EY001792 from the National Eye Institute, Bethesda, Maryland, and a program of the American Health Assistance Foundation, Clarksburg, Maryland, for support of this research.
References
- 1.Bill A. The drainage of aqueous humor. Invest Ophthalmol Vis Sci. 1975;14:1–3. [PubMed] [Google Scholar]
- 2.Quigley HA. Glaucoma. Lancet. 2011;377:1367–77. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 3.Fan BJ, Wang DY, Lam DS, Pang CP. Gene mapping for primary open angle glaucoma. Clin Biochem. 2006;39:249–58. doi: 10.1016/j.clinbiochem.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Rao KN, Nagireddy S, Chakrabarti S. Complex genetic mechanisms in glaucoma: An overview. Indian J Ophthalmol. 2011;59:31–42. doi: 10.4103/0301-4738.73685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fingert JH. Primary open angle genes. Eye. 2011;25:587–95. doi: 10.1038/eye.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–70. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 7.Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–9. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 8.Gong G, Kosoko-Lasaki O, Haynatzki GR, Wilson MR. Genetic dissection of myocilin glaucoma. Hum Mol Genet. 2004;13:R91–102. doi: 10.1093/hmg/ddh074. [DOI] [PubMed] [Google Scholar]
- 9.Ayala-Lugo RM, Pawar H, Reed DM, Lichter PR, Moroi SE, Page M, et al. Variation in optineurin (OPTN) allele frequencies between and within populations. Mol Vis. 2007;13:151–63. [PMC free article] [PubMed] [Google Scholar]
- 10.Aung T, Rezaie T, Okada K, Viswanathan AC, Child AH, Brice G, et al. Clinical features and course of patients with glaucoma with the E50K mutation in the optineurin gene. Invest Ophthalmol Vis Sci. 2005;46:2816–22. doi: 10.1167/iovs.04-1133. [DOI] [PubMed] [Google Scholar]
- 11.Resch ZT, Fautsch MOP. Glaucoma-associated myocilin: A better understanding but much more to learn. Exp Eye Res. 2009;88:704–12. doi: 10.1016/j.exer.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–50. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- 13.Kanagavalli J, Krishnadas SR, Pandaranayaka E, Krishnaswamy S, Sundaresan P. Evaluation and understanding of myocilin mutations in Indian primary open angle glaucoma patients. Mol Vis. 2003;9:606–14. [PubMed] [Google Scholar]
- 14.Ezzat MK, Howell KG, Bahler CK, Beito TG, Loewen N, Poeschla EM, et al. Characterization of monoclonal antibodies against the glaucoma-associated protein myocilin. Exp Eye Res. 2008:376–84. doi: 10.1016/j.exer.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Kang J, Horwitz MS. Interaction of an adenovirus E3 14.7-kilodalton protein with a novel tumor necrosis factor α-inducible cellular protein containing leucine zipper domains. Mol Cell Biol. 1998;18:1601–10. doi: 10.1128/mcb.18.3.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rezaie T, Sarfarazi M. Molecular cloning, genomic structure, and protein characterization of mouse optineurin. Genomics. 2005;85:131–8. doi: 10.1016/j.ygeno.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21:395–428. doi: 10.1016/s1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 18.Aroca-Aguilar JD, Sánchez-Sánchez F, Ghosh S, Fernández-Navarro A, Coca-Prados M, Escribano J. Interaction of recombinant myocilin with the matricellular protein SPARC: functional implications. Invest Ophthalmol Vis Sci. 2011;52:179–89. doi: 10.1167/iovs.09-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wentz-Hunter K, Ueda J, Yue BYJT. Protein interactions with myocilin. Invest Ophthalmol Vis Sci. 2002;43:176–82. [PubMed] [Google Scholar]
- 20.Fautsch MP, Vrabel AM, Johnson DH. The identification of myocilin-associated proteins in the human trabecular meshwork. Exp Eye Res. 2006;82:1046–52. doi: 10.1016/j.exer.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Park BC, Shen X, Samaraweera M, Yue BYJY. Studies of optineurin, a glaucoma gene: Golgi fragmentation and cell death from overexpression of wild-type and mutant optineurin in two ocular cell types. Am J Pathol. 2006;169:1976–89. doi: 10.2353/ajpath.2006.060400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying H, Shen X, Park B, Yue BYJT. Posttranslational modifications, localization, and protein interactions of optineurin, the product of a glaucoma gene. PLoS One. 2010;5:e9168. doi: 10.1371/journal.pone.0009168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen X, Ying H, Qiu Y, Shyam R, Park J, Chi Z, et al. Cellular processing of optineurin in neuronal cells. J Biol Chem. 2011;286:3618–29. doi: 10.1074/jbc.M110.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chibalina MV, Roberts RC, Arden SD, Kendrick-Jones J, Buss F. Rab8-optineurin-myosin VI: analysis of interactions and functions in the secretory pathway. Methods Enzymol. 2008;438:11–24. doi: 10.1016/S0076-6879(07)38002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreland RJ, Dresser ME, Rodgers JS, Roe BA, Conaway JW, Conaway RC, et al. Identification of a transcription factor IIIA-interacting protein. Nucleic Acids Res. 2000;28:1986–93. doi: 10.1093/nar/28.9.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hattula K, Peranen J. FIP-2, a coiled-coil protein, links huntingtin to Rab8 and modulates cellular morphogenesis. Curr Biol. 2000;10:1603–6. doi: 10.1016/s0960-9822(00)00864-2. [DOI] [PubMed] [Google Scholar]
- 27.Anborgh PH, Godin C, Pampillo M, Dhami GK, Dale LB, Cregan SP, et al. Inhibition of metabotropic glutamate receptor signaling by the huntingtin-binding protein optineurin. J Biol Chem. 2005;280:34840–8. doi: 10.1074/jbc.M504508200. [DOI] [PubMed] [Google Scholar]
- 28.Morton S, Hesson L, Peggie M, Cohen P. Enhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucoma. FEBS Lett. 2008;582:997–1002. doi: 10.1016/j.febslet.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 29.Schwamborn K, Weil R, Courtois G, Whiteside ST, Israel A. Phorbol esters and cytokines regulate the expression of the NEMO-related protein, a molecule involved in a NF-κB-independent pathway. J Biol Chem. 2000;275:22780–9. doi: 10.1074/jbc.M001500200. [DOI] [PubMed] [Google Scholar]
- 30.Wentz-Hunter K, Ueda J, Shimizu N, Yue BYJT. Myocilin is associated with mitochondria in human trabecular meshwork cells. J Cell Physiol. 2002;190:46–53. doi: 10.1002/jcp.10032. [DOI] [PubMed] [Google Scholar]
- 31.Ueda J, Wentz- Hunter K, Cheng EL, Fukuchi T, Abe H, Yue BYJT. Ultrastructural localization of myocilin in human trabecular meshwork cells and tissues. J Histochem Cytochem. 2000;48:1321–9. doi: 10.1177/002215540004801003. [DOI] [PubMed] [Google Scholar]
- 32.Ueda J, Wentz-Hunter K, Yue BYJT. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci. 2002;43:1068–76. [PubMed] [Google Scholar]
- 33.Wentz-Hunter K, Kubota R, Shen X, Yue BYJT. Extracellular myocilin affects activity of human trabecular meshwork cells. J Cell Physiol. 2004;200:45–52. doi: 10.1002/jcp.10478. [DOI] [PubMed] [Google Scholar]
- 34.Park BC, Shen X, Fautsch MP, Tibudan M, Johnson DH, Yue BYJT. Optimized bacterial expression of myocilin proteins and functional comparison of bacterial and eukaryotic myocilins. Mol Vis. 2006;12:832–40. [PMC free article] [PubMed] [Google Scholar]
- 35.Filla MS, Liu X, Nguyen TD, Polansky JR, Brandt CR, Kaufman PL, et al. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Invest Ophthalmol Vis Sci. 2002;43:151–61. [PubMed] [Google Scholar]
- 36.Peters DM, Herbert K, Biddick B, Peterson JP. Myocilin binding to Hep II domain of fibronectin inhibits cell spreading and incorporation of paxillin into focal adhesions. Exp Cell Res. 2005;303:218–28. doi: 10.1016/j.yexcr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Kwon H- S, Lee H- S, Ji Y, Rubin JS, Tomarev SI. Myocilin is a modulator of Wnt signaling. Mol Cell Biol. 2009;29:2139–54. doi: 10.1128/MCB.01274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wentz-Hunter K, Shen X, Okazaki K, Tanihara H, Yue BYJT. Overexpression of myocilin in cultured human trabecular meshwork cells. Exp Cell Res. 2004;297:39–48. doi: 10.1016/j.yexcr.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 39.Sakai H, Park BC, Shen X, Yue BYJT. Transduction of TAT-fusion proteins into the human and bovine trabecular meshwork. Invest Ophthalmol Vis Sci. 2006;47:4427–34. doi: 10.1167/iovs.06-0047. [DOI] [PubMed] [Google Scholar]
- 40.Shen X, Koga T, Park BC, SundarRaj N, Yue BYJT. Rho GTPase and cAMP/PKA signaling mediates myocilin induced alterations in cultured human trabecular meshwork cells. J Biol Chem. 2008;283:603–12. doi: 10.1074/jbc.M708250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai H, Shen X, Koga T, Park BC, Noskina Y, Tibudan M, et al. Mitochondrial association of myocilin in human trabecular meshwork cells. J Cell Physiol. 2007;213:775–84. doi: 10.1002/jcp.21147. [DOI] [PubMed] [Google Scholar]
- 42.Koga T, Shen X, Park J, Qiu Y, Park BC, Shyam R, et al. Effects of myocilin and optineurin, two glaucoma genes on neurite outgrowth. Am J Pathol. 2010;176:343–52. doi: 10.2353/ajpath.2010.090194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park BC, Ying H, Shen X, Park BC, Park JS, Qiu Y, Shyam R, et al. Impairment of protein trafficking upon overexpression and mutation of optineurin. PLoS One. 2010;5:e11547. doi: 10.1371/journal.pone.0011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu G, Wu CJ, Zhao Y, Ashwell JD. Optineurin negatively regulates TNFα- induced NF-κB activation by competing with NEMO for ubiquitinated RIP. Curr Biol. 2007;17:1438–43. doi: 10.1016/j.cub.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 45.Sudhakar C, Nagabhushana A, Jain N, Swarup G. NF-κB mediates tumor necrosis factor α-induced expression of optineurin, a negative regulator of NF-κB. PLoS ONE. 2009;4:e5114. doi: 10.1371/journal.pone.0005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mankouri J, Fragkoudis R, Richards KH, Wetherill LF, Harris M, Kohl A, et al. Optineurin negatively regulates the induction of IFNβ in response to RNA virus infection. PLoS Pathogens. 2010;6:e10000778. doi: 10.1371/journal.ppat.1000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Vollrath D. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum Mol Genet. 2004;13:1193–204. doi: 10.1093/hmg/ddh128. [DOI] [PubMed] [Google Scholar]
- 48.Yam GH, Gaplovska-Kysela K, Zuber C, Roth J. Aggregated myocilin induces Russell bodies and causes apoptosis: implications for the pathogenesis of myocilin-caused primary open-angle glaucoma. Am J Pathol. 2007;170:100–9. doi: 10.2353/ajpath.2007.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]