Targeting antigen to ‘steady state’ DCs causes deletion of endogenous islet-specific T cells.
Keywords: Autoimmunity, diabetes, NOD mice
Abstract
CD8+ T cells specific for islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) have been implicated in type 1 diabetes in both humans and non-obese diabetic (NOD) mice, in which T cells specific for IGRP206–214 are highly prevalent. We sought to manipulate these pathogenic T cells by exploiting the ability of steady-state dendritic cells (DCs) to present antigens in a tolerogenic manner. The endocytic receptor DEC-205 was utilized to deliver an IGRP206–214 mimotope to DCs in NOD mice, and the impact of this delivery on a polyclonal population of endogenous islet-reactive cognate T cells was determined. Assessment of islet-infiltrating CD8+ T cells showed a decrease in the percentage, and the absolute number, of endogenous IGRP206–214-specific T cells when the mimotope was delivered to DCs, compared with delivery of a specificity control. Employing an adoptive transfer system, deletion of CD8+ T cells as a result of DEC-205-mediated antigen targeting was found to occur independently of programmed death-1 (PD-1) and its ligand (PD-L1), both often implicated in the regulation of peripheral T-cell tolerance. Given its promise for the manipulation of self-reactive polyclonal T cells demonstrated here, the distinctive characteristics of this antigen delivery system will be important to appreciate as its potential as an intervention for autoimmune diseases continues to be investigated.
Introduction
In the absence of infection, or when antigens are experimentally delivered without an adjuvant, steady-state dendritic cells (DCs) present antigens in a tolerogenic manner that leads to deletion (1–3) or unresponsiveness (2, 4, 5) of cognate T cells or manipulates them to become regulatory (6–8). In the periphery, a major means of inducing tolerance to self-antigens is their presentation by steady-state DCs, which are an important tool for antigen-specific immunomodulatory therapeutic interventions in autoimmune diseases like type 1 diabetes (9). A variety of molecules for receptor-mediated endocytosis of antigens are employed by DCs, of which DEC-205 (CD205) (10) has a special ability to uptake and subsequently present antigens via both MHC class I (cross-presentation) (1) and class II (11, 12). DEC-205, expressed at high levels on certain DC subsets (13–15), has been used to target antigens specifically to DCs in mice (1–6, 8). Such targeting leads to greater efficiency in antigen presentation by both of the MHC classes (1). Selective delivery of a foreign antigen to DCs in the steady-state in vivo leads to deletion of transferred cognate CD8+ T cells and the establishment of tolerance in non-autoimmunity-prone C57BL/6 mice (1).
Type 1 diabetes is an autoimmune disease characterized by T-cell-mediated destruction of the pancreatic islet beta cells. In the non-obese diabetic (NOD) mouse model of the disease, as well as in patients, CD8+ T cells are important targets for therapeutic interventions (16–21). To harness the tolerogenic properties of DCs in the development of an intervention for type 1 diabetes, we previously demonstrated that antigen targeting to DEC-205+ DCs led to deletion of adoptively transferred TCR-transgenic autoreactive CD8+ T cells and the establishment of tolerance to the antigen in autoimmunity-prone NOD mice (3). However, the ability of DEC-205-mediated antigen targeting to manipulate cognate endogenous CD8+ T-cell populations, required for clinical translation of this strategy, remained to be investigated.
To that end, we sought to target the endogenous population of autoreactive CD8+ T cells in NOD mice specific for amino acids 206–214 of islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP206–214) presented by H-2Kd (22). Apart from being a prevalent population in the islets of NOD mice (22–24), monitoring the number of these CD8+ T cells in the blood can be used to predict disease onset (23). Moreover, islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) epitopes have also been found to be targeted by CD8+ T cells in type 1 diabetes patients (25), and establishment of CD8+ T-cell tolerance to IGRP in NOD mice expressing HLA-A2, but no murine class I MHC molecules, had a diabetes-protective effect (18). Given the importance of IGRP-specific CD8+ T cells in disease development, we produced anti-DEC-205 linked to NRP-V7, a superagonist mimotope of IGRP206–214 (26), to manipulate IGRP-reactive CD8+ T cells in NOD mice. We found that deletion of endogenous IGRP206–214-specific CD8+ T cells from pancreatic islets could be achieved by treatment with anti-DEC-205/NRP-V7. This finding suggests the efficacy of antigen-linked anti-DEC-205 in manipulating disease-relevant endogenous CD8+ T-cell populations specific for self-antigens even in the setting of an ongoing autoimmune process.
Despite a number of studies demonstrating induction of tolerance by DEC-205-mediated antigen delivery in the absence of an adjuvant (1–5), the molecular pathways responsible for the deletion of cognate CD8+ T cells have not yet been identified. Investigation of these pathways might suggest ways to improve the performance of natural tolerance induction processes that operate even in autoimmunity- prone individuals such as NOD mice. Furthermore, an understanding of the participating pathways might suggest adjunct agents to improve the therapeutic efficacy of this treatment and avoid untoward side-effects once the therapies are evaluated in humans. Given the involvement of programmed death-1 (PD-1; CD279) and its ligand (PD-L1; B7-H1; CD274) in the regulation of peripheral T-cell tolerance (27), we hypothesized that the PD-1 pathway mediates the T-cell deletion observed in response to delivery of antigen to steady-state DCs. We tested this notion using blocking antibodies and our previously described T-cell adoptive transfer model (3). This work revealed that blockade of PD-1 or PD-L1 could not inhibit the deletion of transferred T cells by DEC-205-mediated targeting of their antigen to steady-state DCs. Our present study stands in apparent disparity with previous reports implicating the PD-1 pathway in DC-mediated tolerance induction in the periphery (28, 29). This is likely due to the unique scenario presented by our study, in which antigen is delivered in a systemic manner via the endocytic receptor DEC-205 to a particular subset of DCs. Given its promise for the manipulation of autoreactive polyclonal T-cell populations demonstrated here, the distinctive characteristics of this system will be important to appreciate as its potential as an intervention for autoimmune diseases continues to be investigated.
Methods
Mice
8.3-NOD mice (30) are transgenic for the TCR of the NOD-derived H-2Kd-restricted T-cell clone NY8.3 (31), which recognizes IGRP206–214 (22) and the mimotope peptide NRP-V7 (KYNKANVFL) (26). NOD-AI4αβTg (32) and NOD.Rag1 null .AI4αβTg mice (33) express the TCR from the NOD islet-derived H-2Db-restricted T-cell clone AI4 (32), which recognizes the mimotope peptide YAIENYLEL (MimA2) (34). NOD.NON-Thy1 a mice have been described (35). NOD-AI4αβTg.NON-Thy1 a mice were generated by crossing NOD-AI4αβTg mice with NOD.NON-Thy1 a mice and then crossing the progeny to obtain mice carrying the AI4αβ transgenes and homozygous for Thy1 a. All mice were housed under specific pathogen-free conditions at the Albert Einstein College of Medicine following protocols approved by the Institutional Animal Care and Use Committee.
Blocking antibodies
The PD-1 blocking antibody 29F.1A12 (36) was prepared in-house. Its isotype control (rat IgG2a) and the PD-L1 blocking antibody 10F.9G2 (37) and its isotype control (rat IgG2b) were all obtained from Bio X Cell (West Lebanon, NH, USA).
Antibodies for flow cytometry
mAbs to murine Thy1.1 (OX-7), Thy1.2 (53–2.1), CD8α (53–6.7), CD11c (HL3), PD-L1 (MIH5), TCRβ (H57-597) and TCR Vα8 (B21.14) were purchased from BD Biosciences (Franklin Lakes, NJ, USA). mAbs to DEC-205 (205yekta), DCIR2 (33D1) and PD-L2 (clone 122) were purchased from eBioscience (San Diego, CA, USA).
Hybrid antibody production
The constructs encoding the heavy and light chains of anti-DEC-205/MimA2 (3) and anti-DEC-205/ovalbumin (38) have been described. The construct for the heavy chain of anti-DEC-205/NRP-V7 was prepared as described for that of anti-DEC-205/MimA2 (3), except that oligonucleotides encoding the NRP-V7 peptide were used. Hybrid antibodies were produced by transient transfection of 293T cells using calcium phosphate as described (3). All antibody batches were tested for the presence of endotoxin using the PYROGENT Plus Gel Clot LAL Assay (Lonza, Basel, Switzerland) and all were negative.
Binding of hybrid anti-DEC-205 antibodies to CHO/mDEC-205 cells
CHO cells stably transfected to express cell-surface murine DEC-205 (39) were generously provided by C. G. Park (The Rockefeller University). Wild-type CHO cells or CHO/mDEC-205 cells were incubated with either anti-DEC-205/NRP-V7 or anti-DEC-205/ovalbumin followed by FITC-labeled secondary antibody and analyzed by flow cytometry.
Isolation of DCs and in vitro T-cell proliferation assay
Splenic CD11c+ DCs were isolated from female NOD mice by low-density cell enrichment using 30% BSA (Sigma–Aldrich, St Louis, MO, USA), followed by magnetic separation using anti-mouse CD11c magnetic-activated cell sorting (MACS) microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were cultured overnight at 37°C with 5 μg ml−1 anti-DEC-205/MimA2 or anti-DEC-205/ovalbumin in the presence of 0.5 μg ml−1 LPS which was added 2h after the antibodies. On day 1, CD8+ AI4 T cells were purified from NOD-AI4αβTg mouse spleens by negative selection using MACS microbeads (Miltenyi Biotec). T cells (5×104) were added to graded doses of CD11c+ cells and cultured for 72h. Cells were labeled with BrdU (10 μM; Roche Applied Science, Indianapolis, IN, USA). The BrdU incorporation, indicative of cell proliferation, was measured by ELISA following the manufacturer’s protocol. Each batch of anti-DEC-205 antibody was tested in this way before use to verify its activity. Similar experiments using CD8+ T cells from 8.3-NOD mice were done to test the anti-DEC-205/NRP-V7 antibodies for the endogenous T-cell study.
Treatment of NOD mice with anti-DEC-205/NRP-V7 to study its effect on endogenous cognate CD8+ T cells
Female NOD mice obtained from The Jackson Laboratory were injected with equimolar amounts of either anti-DEC-205/NRP-V7 (10 μg) or anti-DEC-205/ovalbumin (15 μg) at 4 weeks and 8 weeks of age. At 12 weeks, the mice were killed; pancreatic islets were isolated and cultured for 3 days as described (40). Islet-associated T cells were stained with PE-labeled H-2Kd/NRP-V7 or H-2Kd/TUM (KYQAVTTTL) tetramers, followed by a labeled antibody to murine CD8α (53–6.7). The percent of NRP-V7-specific CD8+ T cells was determined by flow cytometry. Short-term culture of the islet infiltrates prior to analysis by flow cytometry was necessary to permit the expansion of NRP-V7-reactive cells, as they represent only a very small fraction of all the cells found in freshly isolated islets (16, 24). This precludes tetramer studies of infiltrates of individual mice directly ex vivo, particularly when there has been islet destruction and the islet yield is low. We have previously documented that short-term culture does not dramatically skew the islet CD8+ T-cell repertoire (16, 24, 40).
Expression of PD-1 ligands in splenic DC subsets
Splenic DC were isolated from NOD mice by collagenase D digestion followed by depletion of T, B and NK cells using magnetic beads (Miltenyi Biotec) and tested for expression of PD-L1 and PD-L2 by the CD11c+DEC-205+, CD11c+DEC-205− and CD11c+DCIR2+ DC subsets by flow cytometry.
In vivo deletion of transferred CD8+ T cells in presence of PD-1 or PD-L1 blocking antibodies
Splenic CD8+ AI4 T cells were purified from NOD.AI4αβTg or NOD.Rag1 null .AI4αβTg mice and injected i.v. into female NOD.NON-Thy1 a recipients (6–8 weeks of age). In some experiments (depending on availability), AI4 T cells were instead purified from the spleens of NOD-AI4αβTg.NON-Thy1 a mice and transferred to standard NOD recipients. Recipient mice were also injected i.p. with 200 μg of anti-PD-1 blocking antibody 29F.1A12 (36) or its isotype control. The following day, all mice were injected i.p. with 10 μg of anti-DEC-205/MimA2. The blocking antibody or the isotype control treatment was carried on thrice more on days 3, 6 and 9. Pancreatic lymph nodes and spleens were harvested 12 days later. Transferred cells were identified by positive staining for the appropriate Thy1 molecule and CD8. Samples were evaluated by multicolor flow cytometry using an LSRII (BD Biosciences) with subsequent analysis of data in FlowJo (Tree Star, Ashland, OR, USA). Also on day 12, pancreatic islets were isolated and cultured as described (40). Two days later, the cells were harvested and stained for the appropriate Thy1 molecule and CD8 and monitored by multicolor flow cytometry. In some cases, the pancreas was harvested as described (41) and used for flow cytometry to detect transferred CD8+ T cells. For some experiments, H-2Db/MimA2 tetramers were used to detect the transferred CD8+ T cells using H-2Db/lymphocytic choriomeningitis virus (LCMV) (KAVYNFATC) tetramers as the irrelevant control.
Similar strategies were used to assess the effect of PD-L1 blocking on DEC-205-mediated antigen targeting. Briefly, CD8+ AI4 T cells purified as just described were transferred to female recipients (6–8 weeks of age) which were also treated with 200 μg of anti-PD-L1 blocking antibody 10F.9G2 (37) or its isotype. The following day, recipient mice were injected i.p. with anti-DEC-205/MimA2. The blocking antibody or the isotype control treatment was repeated as before. At day 12 post-transfer, spleens and pancreatic lymph nodes were isolated to trace the fate of the transferred T cells.
Statistical analysis
P-values were calculated by Mann–Whitney test using GraphPad Prism Version 5.04.
Results
Anti-DEC-205/NRP-V7 induces deletion of CD8+ T cells specific for IGRP206–214 in the islets of NOD mice
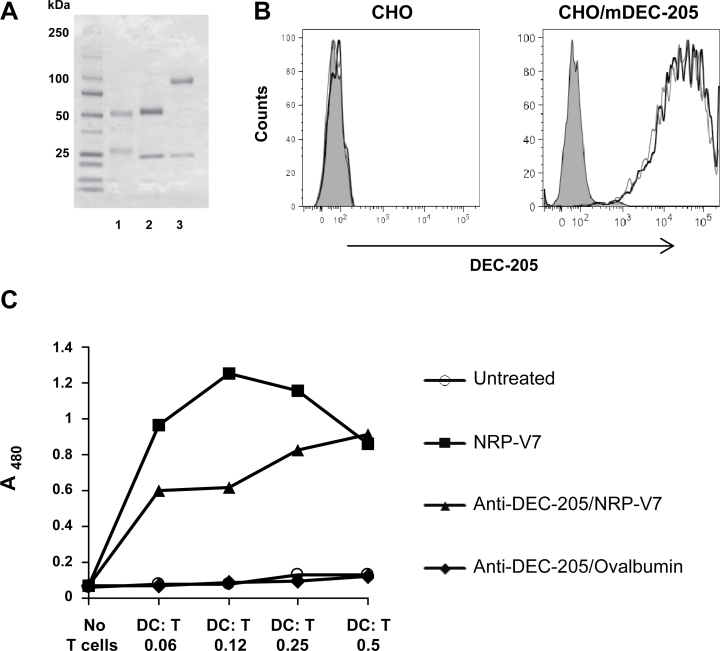
In our earlier work, transferred TCR-transgenic AI4 CD8+ T cells were found to be deleted when the superagonist mimotope peptide MimA2 was targeted to DCs via an anti-DEC-205 antibody (3). Having established the effect of DEC-205-mediated antigen targeting on transferred cognate CD8+ T cells, we now wanted to determine its outcome on a polyclonal population of endogenous islet-reactive T cells. Because AI4-like T cells are somewhat rare in NOD mice (24), and given the importance of IGRP-specific T cells in disease development (22–24), to more easily examine the influence of DEC-205-mediated antigen targeting on endogenous autoreactive T cells, we prepared a hybrid anti-DEC-205 antibody linked to NRP-V7 (Fig. 1A), a superagonist mimotope peptide for IGRP206–214 (26). Both anti-DEC-205/NRP-V7 and anti-DEC-205/ovalbumin (containing the entire ovalbumin protein), which would serve as the specificity control in the subsequent experiments, were analyzed by SDS-PAGE under reducing conditions (Fig. 1A). The molecular weights of the light chains and the antigen-linked heavy chains were as expected (light chains, 25 kD; anti-DEC-205/NRP-V7 heavy chain, 51 kD; anti-DEC-205/ovalbumin heavy chain, 93 kD). Both antibodies demonstrated specific binding to the DEC-205 receptor on CHO/mDEC-205 cells, as evidenced by lack of binding to CHO cells not expressing DEC-205 (Fig. 1B). DCs treated in vitro with anti-DEC-205/NRP-V7 selectively stimulated cognate 8.3 CD8+ T cells to proliferate, whereas those treated with anti-DEC-205/ovalbumin did not have any effect (Fig. 1C). Therefore, NRP-V7 delivered via the hybrid anti-DEC-205 antibody could be cross-presented to CD8+ T cells by DCs.
Fig. 1.
Characterization of anti-DEC-205/NRP-V7 which can specifically stimulate reactive CD8+ T cells in vitro. The hybrid antibodies anti-DEC-205/ovalbumin and anti-DEC-205/NRP-V7 were produced by transient transfection of 293 T cells with calcium phosphate. (A) SDS-PAGE analysis showing bovine γ-globulin standard (lane 1), anti-DEC-205/NRP-V7 (lane 2) and anti-DEC-205/ovalbumin (lane 3) after affinity purification on a Protein G column. Molecular weight standards are in the left lane. The molecular weights of the light chains and the antigen-linked heavy chains were as expected (light chains, 25 kD; anti-DEC-205/NRP-V7 heavy chain, 51 kD; anti-DEC-205/ovalbumin heavy chain, 93 kD). (B) Binding of anti-DEC-205/NRP-V7 or anti-DEC-205/ovalbumin to CHO cells (left panel) or CHO cells expressing DEC-205 (right panel). Cells were stained with 0.5 μg ml−1 anti-DEC-205/NRP-V7 (thick black line) or anti-DEC-205/ovalbumin (thin gray line) followed by FITC-labeled secondary antibody or secondary antibody alone (goat anti-mouse IgG; filled histogram) and analyzed by flow cytometry. (C) Splenic CD11c+ DCs were isolated from NOD mice and incubated overnight in the presence of LPS with 5 μg ml−1 anti-DEC-205/NRP-V7 or anti-DEC-205/ovalbumin or with 10−6 M NRP-V7 peptide. CD8+ T cells from the spleens of 8.3-NOD mice were purified and 2×104 T cells were incubated at the indicated ratios with the DCs. After 3 days of co-culture, proliferation was monitored by BrdU incorporation.
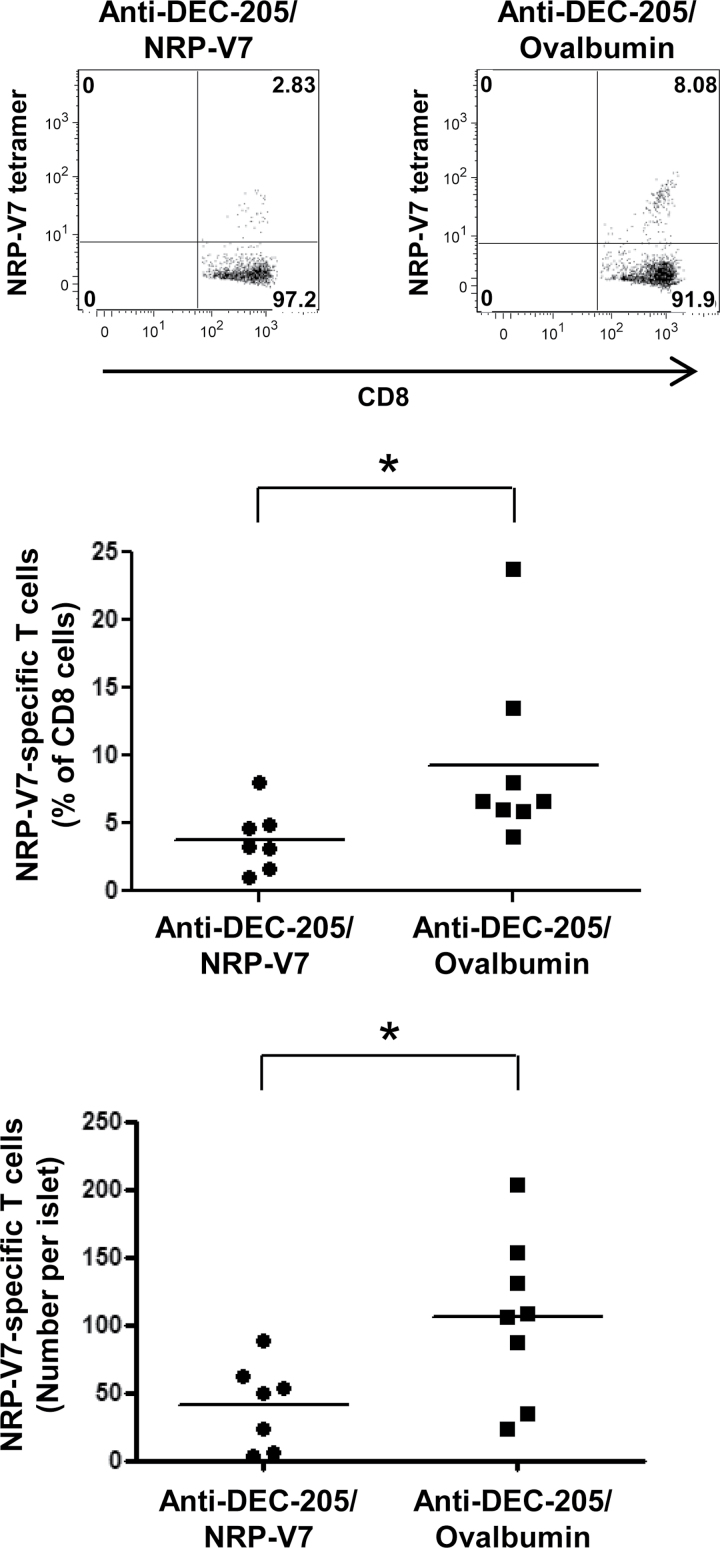
To determine whether anti-DEC-205/NRP-V7 antibody can cause deletion of cognate endogenous CD8+ T cells, we treated NOD mice with equimolar amounts of either anti-DEC-205/NRP-V7 or anti-DEC-205/ovalbumin. Assessment of islet-infiltrating CD8+ T cells showed a clear decrease in the percentage, as well as the absolute number, of endogenous IGRP206–214-specific T cells in mice treated with anti-DEC-205/NRP-V7 compared with those treated with the specificity control anti-DEC-205/ovalbumin (Fig. 2). These findings demonstrate the ability of DEC-205-mediated antigen targeting to manipulate a disease-relevant endogenous, autoreactive CD8+ T-cell population.
Fig. 2.
DEC-205-mediated antigen delivery in NOD mice leads to deletion of endogenous cognate CD8+ T cells. Female NOD mice were treated with either anti-DEC-205/NRP-V7 or anti-DEC-205/ovalbumin at 4 weeks and 8 weeks of age. At 12 weeks of age, pancreatic islets were isolated and cultured for 3 days in the presence of IL-2. Islet-infiltrating cells were stained with anti-CD8 antibody and PE-labeled H-2Kd/NRP-V7 tetramers to quantify the IGRP206–214-specific T cells by flow cytometry (gating on CD8+ cells). Representative plots are shown in the top panel. A statistically significant decrease in both the percentage of endogenous IGRP206–214-specific T cells in the islet infiltrate (P = 0.01; middle panel) and the absolute number of these cells per islet (P = 0.03; bottom panel) was observed in mice treated with anti-DEC-205/NRP-V7 compared with those treated with the specificity control anti-DEC-205/ovalbumin (n = 7 and 8, respectively). *P < 0.05.
Anti-PD-1 blocking antibody does not have any effect on the deletion of transferred CD8+ T cells mediated by anti-DEC-205/MimA2
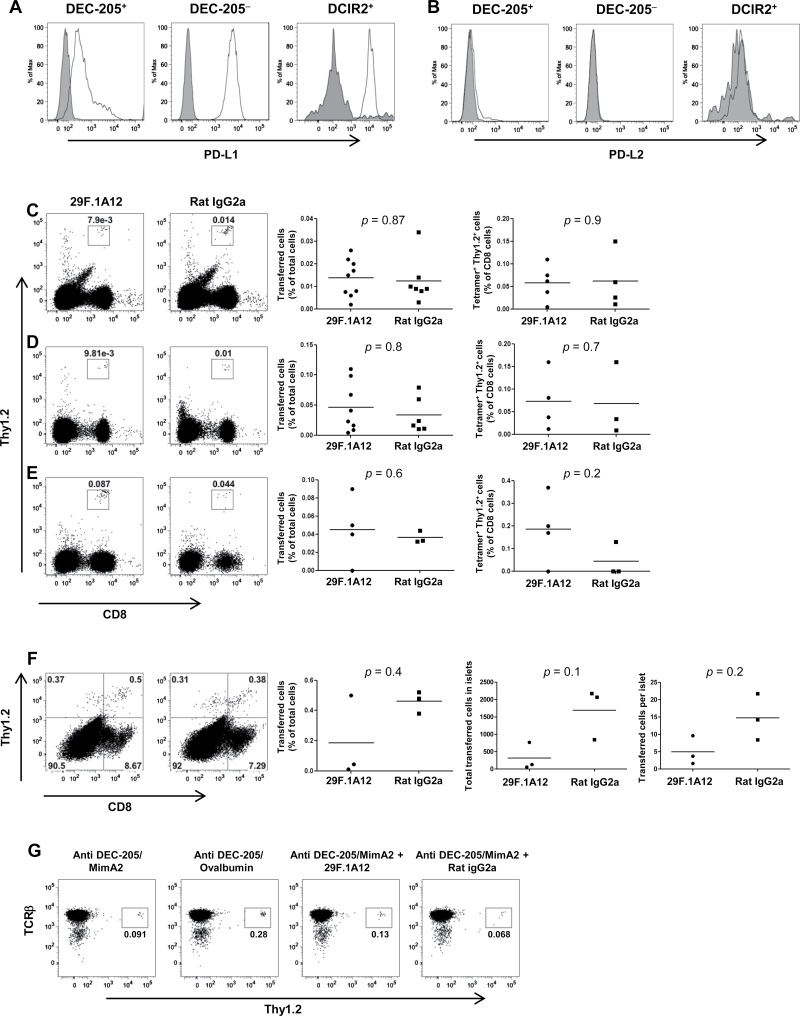
Knowing that DEC-205-mediated antigen targeting could lead to the deletion of cognate endogenous CD8+ T cells, we proceeded to look at the operative mechanism involved in the process. We hypothesized that the PD-1 pathway was a participant in this process, given its frequent implication in the regulation of peripheral T-cell tolerance (27). As further support for this hypothesis, splenic DC subsets from NOD mice were analyzed for expression of the PD-1 ligands. We found that the CD11c+DEC-205+, CD11c+DEC-205− and CD11c+DCIR2+ splenic DC subsets all expressed PD-L1 (Fig. 3A), but not PD-L2 (Fig. 3B), in the absence of immune stimulation, consistent with the findings of others (42, 43). Therefore, we proceeded to investigate the role of PD-1 and PD-L1 in the CD8+ T-cell deletion process induced by DEC-205-mediated antigen targeting.
Fig. 3.
Anti-PD-1 blocking antibody could not abrogate the deletion of cognate CD8+ T cells by DEC-205-mediated antigen targeting. DEC-205+, DEC-205− and DCIR2+ splenic DC subsets (CD11c+) from NOD mice were examined for expression of (A) PD-L1 or (B) PD-L2. The open histograms depict staining with the PD-L1 or PD-L2 antibodies, whereas the filled histograms depict binding of the correspondingisotype controls. (C–G) CD8+ T cells were purified from AI4 donors by negative selection using MACS microbeads (Miltenyi Biotec) and injected i.v. into female NOD.NON-Thy1 a recipients (6–8 weeks of age). The recipient mice were also injected i.p. with 200 μg of anti-PD-1 blocking antibody (29F.1A12) or its isotype control (rat IgG2a) soon after the cell transfer. The following day, the recipient mice were injected i.p. with 10 μg of anti-DEC-205/MimA2. The blocking antibody or the isotype control treatment was carried on thrice more on days 3, 6 and 9. (C) Spleens and (D) pancreatic lymph nodes were harvested 12 days later and single-cell suspensions were obtained from these organs. (E) Whole pancreas was collagenase digested to enumerate the transferred CD8+Thy1.2+ T cells in the pancreas. Cells were stained and analyzed for the percentage of transferred CD8+Thy1.2+ T cells by flow cytometry, gating on live cells. Also, in some experiments, cells were stained with an H-2Db/MimA2 tetramer to further characterize the transferred cells. No significant difference was found when 29F.1A12-treated mice were compared with those treated with rat IgG2a. (F) Pancreatic islets from both groups of mice were cultured for 2 days in the presence of IL-2, and the percentage or the total number of transferred cells in the islets or per islet was analyzed. No significant differences between the experimental and control groups were observed, thus demonstrating the lack of any effect of the PD-1 blocking antibody on the deletion of the transferred cells by DEC-205-mediated targeting. (G) To ensure that the DEC-205-mediated antigen targeting did not cause TCR down-regulation, the transferred cells were also stained with a TCRβ antibody and shown to have comparable TCR expression between mice treated with anti-DEC-205/MimA2 alone or anti-DEC-205/MimA2 with either 29F.1A12 or rat IgG2a or the specificity control anti-DEC-205/ovalbumin (gating on CD8+ cells). Data shown are representative of five independent experiments.
We utilized the AI4 transfer model to investigate the potential role of PD-1 in DEC-205 targeting, as it would be easier to track the fate of transferred cells than endogenous ones. CD8+ AI4 T cells were isolated from either NOD-AI4αβTg or NOD.Rag1 null .AI4αβTg mice (depending on availability). Donor mice that were Rag1-sufficient were subjected to extensive quality control steps to ensure that they highly expressed the transgenic specificity, as endogenous TCRs could potentially be expressed in these mice. Rag1-sufficient mice for which a PE-labeled H-2Db/MimA2 tetramer, and not a non-specific LCMV tetramer, bound to 90% or more of the CD8+ T cells in the blood (Supplementary Figure 1A is available at International Immunology Online) were recruited as donors. To further ensure the specificity of the CD8+ T cells in the AI4-transgenic mice, splenic CD8+ T cells were also stained with the H-2Db/MimA2 tetramer and with an antibody to the AI4 TCRα chain (Vα8) (Supplementary Figure 1B is available at International Immunology Online). Moreover, all batches of anti-DEC-205/MimA2 antibodies were checked for the ability to induce proliferation of the reactive CD8+ T cells in vitro using a BrdU incorporation assay (data not shown).
Recipient NOD.NON-Thy1 a mice treated with anti-DEC-205/MimA2 were also treated four times either with the PD-1 blocking antibody 29F.1A12 or, as a control, isotype-matched rat IgG2a. The transferred AI4 T cells were tracked, by staining with anti-CD8 and anti-Thy1.2 antibodies, in the spleen (Fig. 3C) and the pancreatic lymph nodes (Fig. 3D), as well as the pancreas (Fig. 3E) or the pancreatic islets (Fig. 3F). Most of the mice which were given the PD-1 blocking antibody (7 of 9) developed diabetes as reported (44), compared with none treated with the isotype, which proved that our treatment regimen with 29F.1A12 did cause blocking of PD-1 in the mice. If PD-1 was involved in the deletion of the transferred T cells by DEC-205-mediated antigen targeting, blocking of PD-1 would lead to an increase in the percentage of the CD8+Thy1.2+ cells. However, no significant difference in the percentage of CD8+Thy1.2+ cells could be detected in mice treated with the PD-1 blocking antibody in comparison with those treated with the isotype control in any of the locales that we investigated. When staining with the MimA2 tetramer or the non-specific LCMV tetramer staining was included in order to look more specifically at the transferred CD8+ T cells, the data similarly failed to reveal any difference between the two groups. The pancreatic islets, which are the most relevant site in this diabetes model, were also analyzed for the total number of transferred cells and the number of transferred cells per islet (Fig. 3F). Once again there was no significant difference in the number of transferred T cells between the groups treated with the blocking antibody or the isotype control. To further ensure that there was no TCR down-regulation, which could cause less tetramer binding to the cells, the transferred cells were also stained with an anti-TCR antibody. This analysis showed similar TCR expression in mice treated with anti-DEC-205/ovalbumin or anti-DEC-205/MimA2 alone or with either the PD-1 blocking antibody or the isotype control (Fig. 3G).
Antibody blocking of PD-L1 shows no effect on the deletion of transferred CD8+ T cells mediated by anti-DEC-205/MimA2
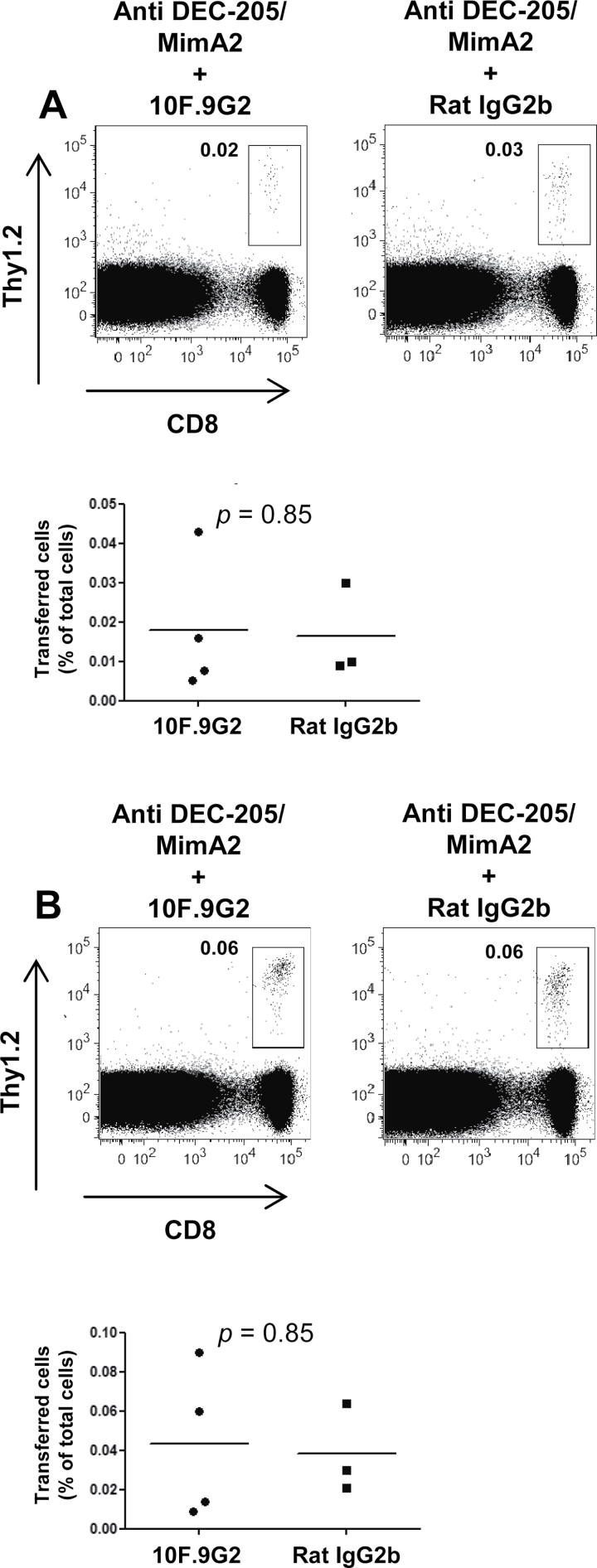
Among the two ligands of PD-1, PD-L1 is expressed on a variety of cells including pancreatic islet cells (42, 43), whereas PD-L2 is only expressed on activated DCs and macrophages (43). Islets lacking PD-L1 are rapidly destroyed when they are transplanted into diabetic mice, which shows that tissue expression of PD-L1 is important in peripheral tolerance mechanisms that protect target organs from pathogenic autoreactive T cells (45). Moreover, another costimulatory molecule, B7-1, a ligand for CD28 and CTLA-4, has also been identified as a second receptor for PD-L1 in both mice and humans (37, 46). This interaction has been shown to bidirectionally inhibit T-cell proliferation and cytokine production in vivo (47). Therefore, even though PD-1 was found to not be involved in the deletion process, it was important to investigate the role of PD-L1. For this purpose, we utilized the anti-PD-L1 antibody 10F.9G2, which blocks both PD-L1:PD-1 and PD-L1:B7-1 interactions (37). Consequently, any effect this blocking antibody might have on the outcome of DEC-205-mediated antigen targeting would reveal the role of PD-L1 regardless of its binding partner. AI4 T cells were transferred to recipient mice treated either with the anti-PD-L1 blocking antibody 10F.9G2 or its isotype control. All mice received anti-DEC-205/MimA2. If PD-L1 was involved in the deletion process, blocking of the molecule would cause an increase in the percentage of CD8+Thy1.2+ cells. Although diabetes was observed in 25% of the mice treated with anti-PD-L1 and not in the controls, blocking of PD-L1 did not have any effect on the deletion of cognate CD8+ T cells by DEC-205-mediated antigen targeting as measured in the spleen (Fig. 4A) or the pancreatic lymph nodes (Fig. 4B).
Fig. 4.
PD-L1 is not involved in the deletion of reactive CD8+ T cells induced by DEC-205-mediated antigen targeting. CD8+ AI4 T cells purified from spleens of NOD-AI4αβTg mice were injected i.v. into female NOD.NON-Thy1 a recipients (6–8 weeks of age). The recipient mice were also injected i.p. with 200 μg of anti-PD-L1 blocking antibody or its isotype control soon after the cell transfer. The following day, the recipient mice were injected i.p. with 10 μg of anti-DEC-205/MimA2. The blocking antibody or the isotype control treatment wascarried on thrice more on days 3, 6 and 9. (A) Spleens and (B) pancreatic lymph nodes were harvested 12 days later and analyzed by flow cytometry, gating on live cells. There was no significant difference in the percentage of transferred cells in the spleen or the pancreatic lymph nodes showing that PD-L1 is not required for the deletion by DEC-205-mediated antigen targeting. Data shown are representative of three independent experiments.
Discussion
The ability of DCs to present antigens in a manner subsequently resulting in tolerance to those antigens has been harnessed in a number of studies (1–5). Previously, we have shown that DEC-205-mediated antigen targeting to steady-state DCs leads to the deletion of cognate TCR-transgenic AI4 T cells in an adoptive transfer model, even in the face of ongoing autoimmunity (3). In order to investigate the effect of antigen targeting via DEC-205 on endogenous T-cell populations, we targeted the more prevalent, and therefore more readily traceable, CD8+ T-cell population that is specific for IGRP206–214 in the context of H-2Kd. IGRP206–214-specific CD8+ T cells constitute one of the most prevalent populations in islet infiltrates and are among the earliest T cells to infiltrate the islets (22). We found that NOD mice treated with anti-DEC-205/NRP-V7 had a significantly lower percentage of the endogenous IGRP-specific T cells in pancreatic islets, as well as a lower absolute number, in comparison with those treated with the specificity control. The ability to manipulate a naturally occurring and prevalent autoreactive T-cell population could be an important breakthrough in the development of new therapeutic strategies for type 1 diabetes.
Our future studies will aim to develop DEC-205-mediated antigen targeting as an effective strategy for type 1 diabetes, initially in NOD mice. Since the prevalence of IGRP206–214-specific T cells can vary from mouse to mouse (24), it will be important to recruit only those that have a comparable presence of this population in the blood. Moreover, our target T-cell population, although found among the earliest infiltrates, increases with age and comes to predominate (16, 23). It has been demonstrated in earlier studies that elimination of IGRP206–214-specific CD8+ T cells at an early age (17), or tolerization to IGRP from birth (48) in NOD mice, did not have a marked effect on type 1 diabetes development. This is likely due to the development and expansion of other specificities that were able to occupy the ‘niche’ created by the absence of IGRP206–214-reactive T cells (17). Therefore, it may be necessary to intervene at a later stage of insulitis, when these cells have become more predominant. Interestingly, such late intervention was shown to be beneficial in a study where elimination of IGRP206–214-specific T cells by H-2Kd/NRP-V7 tetramers conjugated to the toxin saporin led to a reduction in diabetes incidence (21). Finally, the time between treatments will need to be optimized. In the work described here, mice were treated at 4 and 8 weeks of age. We chose this timing for our studies as we wanted to identify a regimen that would not be overly burdensome once translated to the clinic. However, as peptide presentation would be expected to occur only up to 2 weeks after administration of the antigen-linked anti-DEC-205 antibody (11), treatments spaced more closely will also be investigated in our future work.
Our studies demonstrate the lack of a requirement for PD-1 and PD-L1 in the deletional tolerance induced by DEC-205-mediated antigen delivery to DCs. These findings are in contrast to those obtained in certain other experimental tolerance induction systems. Specifically, in DIETER (DC-specific inducible expression of T-cell epitopes by recombination) mice, where CD11c+ cells can be induced to present viral CD8+ T-cell epitopes upon treatment with tamoxifen, the outcome of antigen presentation was T-cell tolerance that was T-cell-intrinsic and dependent on PD-1 expression (29). However, this system differs from ours in multiple respects. In our experiments, the antigen is delivered exogenously and is cross-presented exclusively by the CD8+ (DEC-205+) subset of DCs following uptake mediated by the endocytic receptor DEC-205. In contrast, in the DIETER mice, the viral epitopes are endogenous and are presented by all conventional DCs. These differences are likely among those that explain the differing requirement for the PD-1 pathway in the two systems. For example, the CD8+ and CD8− DC subsets are functionally distinct (8, 38), and microarray and proteomic analyses have both revealed DC heterogeneity (49, 50) that could potentially extend to expression of secreted or cell-surface molecules involved in T-cell deletion. Furthermore, although engagement of the DEC-205 receptor does not alter the DC maturation status (38), it could nonetheless alter steady-state DCs in ways that might influence the pathways utilized for the induction of T-cell tolerance. This area remains to be explored in detail.
PD-1 has also previously been shown to be necessary for the deletion of ovalbumin-specific CD8+ T cells transferred to mice expressing ovalbumin under the control of the rat insulin promoter (RIP-OVAhigh mice) (28). However, in this case, the antigen is presented locally in the pancreatic lymph nodes, whereas in our system, antigen administration using a fusion antibody to DEC-205 results in widespread presentation, with exposure of cognate T cells to their antigen in multiple sites, including spleen, pancreatic lymph nodes and peripheral lymph nodes (3). Such differences in antigen availability, sites of presentation and mode of uptake (i.e. whether the DEC-205 receptor is employed or not) may all influence the differential requirement for PD-1 for T-cell deletion.
In summary, we demonstrate in this study the potential of antigen-linked anti-DEC-205 administration to favorably manipulate self-reactive T cells in autoimmune diseases like type 1 diabetes. It is our hope that future studies with NOD mice and ‘humanized’ derivative strains will ultimately lead to a therapeutic intervention that will be clinically relevant. The recent development of mice transgenic for human DEC-205 and of antibodies to this molecule (39) should facilitate these efforts.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
National Institutes of Health [R01 DK094327 and R01 DK064315 to T.P.D., R01 DK046266 and U01 DK072473 (Beta Cell Biology Consortium) to D.V.S., P01 AI056299 to A.S., P60 DK020541 (Albert Einstein College of Medicine’s Diabetes Research Center)]; the Juvenile Diabetes Research Foundation to D.V.S. and T.P.D.; the American Diabetes Association to D.V.S. and T.P.D.; the Irma T. Hirschl/Monique Weill-Caulier Trust to T.P.D.; Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases to K.V.T.; Canadian Institutes of Health Research to P.S.; Canadian Diabetes Association to Julia McFarlane Diabetes Research Centre; National Institutes of Health Cancer Center (P30 CA013330 for flow cytometry facility at Albert Einstein College of Medicine). P.S. is scientific founder of Parvus Therapeutics, Inc. and a scientist of Alberta Innovates-Health Solutions. T.P.D. is the Diane Belfer, Cypres & Endelson Families Faculty Scholar in Diabetes Research.
Supplementary Material
Acknowledgements
We thank Harry Chapman for preparing CD8+ AI4 T cells from NOD.Rag1 null .AI4αβTg mice for some of the experiments and Yang Yang for preparing tetramers. We thank Carla Smith for mouse breeding and genotyping, and we thank Tadashi Hanafusa for generating the expression construct for the heavy chain of anti-DEC-205/NRP-V7.
References
- 1. Bonifaz L., Bonnyay D., Mahnke K., Rivera M., Nussenzweig M. C., Steinman R. M. 2002. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196:1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hawiger D., Inaba K., Dorsett Y., et al. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo . J. Exp. Med. 194:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mukhopadhaya A., Hanafusa T., Jarchum I., et al. 2008. Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc. Natl. Acad. Sci. USA 105:6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hawiger D., Masilamani R. F., Bettelli E., Kuchroo V. K., Nussenzweig M. C. 2004. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo . Immunity 20:695. [DOI] [PubMed] [Google Scholar]
- 5. Stern J. N., Keskin D. B., Kato Z., et al. 2010. Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. Proc. Natl. Acad. Sci. USA 107:17280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kretschmer K., Apostolou I., Hawiger D., Khazaie K., Nussenzweig M. C., von Boehmer H. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219. [DOI] [PubMed] [Google Scholar]
- 7. Mahnke K., Qian Y., Knop J., Enk A. H. 2003. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood 101:4862. [DOI] [PubMed] [Google Scholar]
- 8. Yamazaki S., Dudziak D., Heidkamp G. F., et al. 2008. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 181:6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luo X., Herold K. C., Miller S. D. 2010. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity 32:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang W., Swiggard W. J., Heufler C., et al. 1995. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 375:151. [DOI] [PubMed] [Google Scholar]
- 11. Bonifaz L. C., Bonnyay D. P., Charalambous A., et al. 2004. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahnke K., Guo M., Lee S., et al. 2000. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J. Cell Biol. 151:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inaba K., Swiggard W. J., Inaba M., et al. 1995. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell. Immunol. 163:148. [DOI] [PubMed] [Google Scholar]
- 14. Vremec D., Pooley J., Hochrein H., Wu L., Shortman K. 2000. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164:2978. [DOI] [PubMed] [Google Scholar]
- 15. Henri S., Vremec D., Kamath A., et al. 2001. The dendritic cell populations of mouse lymph nodes. J. Immunol. 167:741. [DOI] [PubMed] [Google Scholar]
- 16. Amrani A., Verdaguer J., Serra P., Tafuro S., Tan R., Santamaria P. 2000. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 406:739. [DOI] [PubMed] [Google Scholar]
- 17. Han B., Serra P., Amrani A., et al. 2005. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat. Med. 11:645. [DOI] [PubMed] [Google Scholar]
- 18. Niens M., Grier A. E., Marron M., Kay T. W., Greiner D. L., Serreze D. V. 2011. Prevention of “Humanized” diabetogenic CD8 T-cell responses in HLA-transgenic NOD mice by a multipeptide coupled-cell approach. Diabetes 60:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scott G. S., Fishman S., Khai Siew L., et al. 2010. Immunotargeting of insulin reactive CD8 T cells to prevent diabetes. J. Autoimmun. 35:390. [DOI] [PubMed] [Google Scholar]
- 20. Tsai S., Shameli A., Yamanouchi J., et al. 2010. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 32:568. [DOI] [PubMed] [Google Scholar]
- 21. Vincent B. G., Young E. F., Buntzman A. S., et al. 2010. Toxin-coupled MHC class I tetramers can specifically ablate autoreactive CD8+ T cells and delay diabetes in nonobese diabetic mice. J. Immunol. 184:4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lieberman S. M., Evans A. M., Han B., et al. 2003. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc. Natl. Acad. Sci. USA 100:8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trudeau J. D., Kelly-Smith C., Verchere C. B., et al. 2003. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J. Clin. Invest. 111:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lieberman S. M., Takaki T., Han B., Santamaria P., Serreze D. V., DiLorenzo T. P. 2004. Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J. Immunol. 173:6727. [DOI] [PubMed] [Google Scholar]
- 25. Mallone R., Martinuzzi E., Blancou P., et al. 2007. CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes 56:613. [DOI] [PubMed] [Google Scholar]
- 26. Amrani A., Serra P., Yamanouchi J., et al. 2001. Expansion of the antigenic repertoire of a single T cell receptor upon T cell activation. J. Immunol. 167:655. [DOI] [PubMed] [Google Scholar]
- 27. Francisco L. M., Sage P. T., Sharpe A. H. 2010. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keir M. E., Freeman G. J., Sharpe A. H. 2007. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 179:5064. [DOI] [PubMed] [Google Scholar]
- 29. Probst H. C., McCoy K., Okazaki T., Honjo T., van den Broek M. 2005. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 6:280. [DOI] [PubMed] [Google Scholar]
- 30. Verdaguer J., Schmidt D., Amrani A., Anderson B., Averill N., Santamaria P. 1997. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J. Exp. Med. 186:1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santamaria P., Utsugi T., Park B. J., Averill N., Kawazu S., Yoon J. W. 1995. Beta-cell-cytotoxic CD8+ T cells from nonobese diabetic mice use highly homologous T cell receptor alpha-chain CDR3 sequences. J. Immunol. 154:2494. [PubMed] [Google Scholar]
- 32. Graser R. T., DiLorenzo T. P., Wang F., et al. 2000. Identification of a CD8 T cell that can independently mediate autoimmune diabetes development in the complete absence of CD4 T cell helper functions. J. Immunol. 164:3913. [DOI] [PubMed] [Google Scholar]
- 33. DiLorenzo T. P., Lieberman S. M., Takaki T., et al. 2002. During the early prediabetic period in NOD mice, the pathogenic CD8(+) T-cell population comprises multiple antigenic specificities. Clin. Immunol. 105:332. [DOI] [PubMed] [Google Scholar]
- 34. Takaki T., Lieberman S. M., Holl T. M., et al. 2004. Requirement for both H-2Db and H-2Kd for the induction of diabetes by the promiscuous CD8+ T cell clonotype AI4. J. Immunol. 173:2530. [DOI] [PubMed] [Google Scholar]
- 35. Prochazka M., Serreze D. V., Worthen S. M., Leiter E. H. 1989. Genetic control of diabetogenesis in NOD/Lt mice. Development and analysis of congenic stocks. Diabetes 38:1446. [DOI] [PubMed] [Google Scholar]
- 36. Barber D. L., Wherry E. J., Masopust D., et al. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682. [DOI] [PubMed] [Google Scholar]
- 37. Butte M. J., Keir M. E., Phamduy T. B., Sharpe A. H., Freeman G. J. 2007. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dudziak D., Kamphorst A. O., Heidkamp G. F., et al. 2007. Differential antigen processing by dendritic cell subsets in vivo . Science 315:107. [DOI] [PubMed] [Google Scholar]
- 39. Cheong C., Choi J. H., Vitale L., et al. 2010. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood 116:3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jarchum I., Takaki T., DiLorenzo T. P. 2008. Efficient culture of CD8(+) T cells from the islets of NOD mice and their use for the study of autoreactive specificities. J. Immunol. Methods 339:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Samanta D., Mukherjee G., Ramagopal U. A., et al. 2011. Structural and functional characterization of a single-chain peptide-MHC molecule that modulates both naive and activated CD8+ T cells. Proc. Natl. Acad. Sci. USA 108:13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liang S. C., Latchman Y. E., Buhlmann J. E., et al. 2003. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 33:2706. [DOI] [PubMed] [Google Scholar]
- 43. Yamazaki T., Akiba H., Iwai H., et al. 2002. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 169:5538. [DOI] [PubMed] [Google Scholar]
- 44. Ansari M. J., Salama A. D., Chitnis T., et al. 2003. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 198:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Keir M. E., Liang S. C., Guleria I., et al. 2006. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 203:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Butte M. J., Peña-Cruz V., Kim M. J., Freeman G. J., Sharpe A. H. 2008. Interaction of human PD-L1 and B7-1. Mol. Immunol. 45:3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paterson A. M., Brown K. E., Keir M. E., et al. 2011. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo . J. Immunol. 187:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krishnamurthy B., Dudek N. L., McKenzie M. D., et al. 2006. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J. Clin. Invest. 116:3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Edwards A. D., Chaussabel D., Tomlinson S., Schulz O., Sher A., Reis e Sousa C. 2003. Relationships among murine CD11c(high) dendritic cell subsets as revealed by baseline gene expression patterns. J. Immunol. 171:47. [DOI] [PubMed] [Google Scholar]
- 50. Segura E., Kapp E., Gupta N., et al. 2010. Differential expression of pathogen-recognition molecules between dendritic cell subsets revealed by plasma membrane proteomic analysis. Mol. Immunol. 47:1765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.