The contrasting and controversial roles of T-cell subsets in atherosclerosis.
Keywords: atherosclerosis, inflammation, T cell
Abstract
Atherosclerosis is a chronic inflammatory disease of the artery wall. Atherosclerotic lesions contain monocytes, macrophages, smooth muscle cells and T lymphocytes. Here, we review the role of T-lymphocyte subsets in atherosclerosis. Among CD4+ T cells, Th1 cells are pro-atherogenic, Treg cells are athero-protective and the role of Th2 and Th17 cells remains unclear. The role of follicular helper T cells in atherosclerosis remains unknown, as is the role of CD8+ T cells. NKT cells bind glycolipid antigens and exert a pro-atherogenic role. The antigen specificity of T-cell responses in atherosclerosis is poorly understood. In order to enable antigen-specific prevention or therapy, a better understanding of these mechanisms is needed.
Introduction
Immunologic and inflammatory mechanisms of atherosclerosis
Atherosclerosis and the resulting cardiovascular diseases are the leading causes of both death and disability in North America (1). The cause of atherosclerosis has traditionally been viewed as a chronic response to arterial injury. In the last two decades, the role of local arterial tissue inflammation has been increasingly studied and hypothesized to be a major underlying mechanism in the development of atherosclerosis (2–6). Subsequent investigations into the mechanisms of atherosclerosis have suggested that both innate and adaptive immunity play a significant role in the development and progression of atherosclerosis (2, 6–8).
The innate response initiates with the activation of monocytes/macrophages in the vessel wall, followed by more specific adaptive responses mediated by T and B cells (8, 9). In early lesions, macrophages are found in abundance. Stimulated by macrophage colony-stimulating factor and various other cytokines, these cells up-regulate their pattern recognition receptors and mediate lipoprotein internalization, leading to their foam-cell appearance (10). Macrophages are the source of the inflammatory cytokine TNF, which fuels the inflammatory cycles by recruiting more T cells, B cells (11) and macrophages into the lesion site.
Subsequent lymphocyte recruitment promotes chemokine secretion and localized inflammation (2, 12, 13). Continuous influx of mononuclear cells and inefficient clearance of dead cells (efferocytosis) eventually gives rise to the formation of atherosclerotic plaques with a central necrotic lipid core and a fibrous cap (14, 15). In mature lesions, immune responses mediated by T cells and B cells may become the dominant forces in enhancing inflammation. In fact, the presence of auto-antibodies against oxidized low-density lipoprotein (oxLDL) in the lesions of atherosclerotic humans and animal models provided initial evidence of the involvement of adaptive immunity in the development of atherosclerosis (16). Subsequently, in both human and murine atherosclerotic plaques, all T-cell subsets (CD4+, CD8+, TCRγδ+ and NKT cells) have been found (2, 5, 13, 17).
Candidate autoantigens that are thought to activate T cells in atherosclerosis
T cells have been the focus of intense research over the last 15 years because of their potential role in the development and progression of atherosclerosis. Several publications in the mid-1990s and early 2000s showed a preponderance of CD4+ T cells when compared with CD8+ T cells (13, 18, 19) and polyclonal expansion of T cells isolated from patients was accompanied by production of (predominantly) IFN-γ (18, 19). Subsequent studies have shown that the T cells found within the atherosclerotic plaques are far more activated than their peripheral blood counterparts, within both the CD4+- and CD8+-cell compartments (20).
The antigens leading to T-cell activation are not known with certainty, but clearly involve epitopes of oxLDL (18), and possibly heat-shock protein 60/65 (HSP60/65) (21–23). Currently, the leading autoantigen candidate is oxLDL. T cells within the atherosclerotic plaque have been shown to respond in an antigen-specific manner to oxLDL (18). Auto-antibodies to HSP60 have been found to correlate with increasing risk of coronary artery disease, and in a mouse model of atherosclerosis immunization of pro-atherosclerotic mice with HSP65 resulted in worsening aortic plaques compared with controls (21–23).
Development of T cells and their subsets
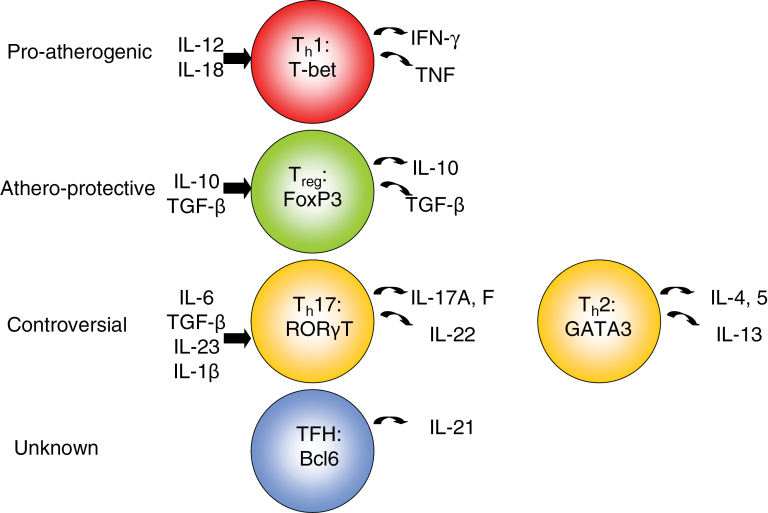
Naive CD4+ T cells have the ability to differentiate into various subtypes of helper T cells (Fig. 1). These include Th1-, Th2-, Th17- and Treg-cell lineages. Helper T-cell polarization into the various lineages is influenced by the cytokine environment. Specifically, the presence of IL-12 results in STAT4 (signal transducer and activator of transcription 4) induction and subsequent activation of the transcription factor, T box expressed in T cells (T-bet). T-bet activation not only results in IFN-γ production but also inhibits IL-4 expression (thus inhibiting Th2 lineage commitment). IL-18 also skews toward a Th1 phenotype. Th2 commitment occurs in the presence of IL-4. IL-4 increases expression of GATA-binding protein 3 (GATA3), which through a positive feedback loop potentiates IL-4 expression while inhibiting IFN-γ expression. Th17 differentiation occurs under the influence of TGF-β with either IL-6 or IL-21, IL-1β and IL-23 and the transcription factors STAT3, retinoic acid-related orphan receptor γT (RORγT) and RORα (24, 25).
Fig. 1.
The effects of T-cell subsets on atherosclerosis. Pro-atherogenic: Th1 (red). The presence of IL-12 and IL-18 causes Th1 skewing. IL-12 results in activation of the downstream transcription factor, T-bet. T-bet activation results in pro-inflammatory cytokine production, including IFN-γ and TNF. Athero-protective: Treg (green). IL-10 and TGF-β are required for Treg commitment. nTregs, and some iTregs, express the transcription factor FoxP3 and can produce IL-10 and/or TGF-β. Controversial: Th17 (yellow, left) and Th2 (yellow, right). Th17 differentiation requires a combination of several cytokines, including IL-6, TGF- β, IL-23 and IL-1β. These cells express the transcription factor RORγT, and produce IL-17A/F and IL-22. Naive CD4+ T cells commit to a Th2 lineage under the influence of IL-4. In a positive feedback loop, IL-4 increases expression of the transcription factor GATA3, which produces additional IL-4 and inhibits IFN-γ production. Unknown: Follicular helper T cells (blue). These are CD4+ T cells found in the B-cell follicles of secondary lymphoid organs. They constitutively express CXCR5 on their cell surface. They secrete IL-21 and express the transcription factor Bcl6.
Treg commitment is driven by the presence of TGF-β and IL-10 (26, 27), but recently, it has been shown to be influenced by TLR-activated CD11c+CD103+ dendritic cells (DCs) that appear to have a role in Treg differentiation (28). Each helper T-cell subtype has a unique cytokine expression profile and separate roles in immunity. Th1 cells are considered pro-inflammatory through their secretion of IFN-γ. Th2 cells play a crucial role in the development of atopic/allergic diseases via secretion of IL-4, IL-5 and IL-13. Th17 cells secrete IL-17A/F and IL-22, and have been implicated in various autoimmune diseases.
The role of mouse models in defining the role of T cells in atherosclerotic disease
There has been increasing interest to define the role of various T-cell subsets in the development of atherosclerosis. The interest is 2-fold. First, defining the roles of various subsets of CD4+ helper T cells will provide a greater understanding of which (if any) T cells are friend rather than foe in the context of atherosclerosis. Second, because maturation of the different T-cell subsets can be manipulated by the local cytokine milieu, understanding their different roles will likely be of great assistance in developing novel therapeutic measures to limit plaque progression and subsequent rupture.
Although experiments performed in mouse models are not exact representations of the disease processes in humans (29), mouse studies have proven to be valuable in the characterization of T-cell responses in atherosclerosis. Both knockout (KO) and over-expression experiments of various T-cell lineages and their cytokines have given researchers much insight into the role of various T-cell subsets in the development and progression of atherosclerotic disease.
In nearly all of these experiments, murine lines prone to developing atherosclerosis are used. These include both the LDL receptor (LDLR) KO and apolipoprotein E (ApoE) KO strains. LDLR−/− mice develop atherosclerotic lesions within the aorta only after several weeks of being fed a high-fat and high-cholesterol diet [western diet (WD)]. ApoE−/− mice, in contrast, develop spontaneous plaque lesions in the proximal aorta as early as 3 months of age, which can be further accelerated by WD feeding. Although a normal mouse serum cholesterol level is 80–100mg dl−1, LDLR–/– mice have baseline elevated serum cholesterol levels of 200–400mg dl−1 that rise even further upon WD feeding to >2000mg dl−1. Similarly, ApoE–/– mice can have total cholesterol levels ~10 times higher than normal mice. This review will focus almost exclusively on mouse studies that have helped to elucidate the role of T cells in atherosclerosis.
Th1 cells
Th1 cells exceed the number of Th2 cells in atherosclerotic plaques (30, 31). Differentiation into a Th1 phenotype requires the transcription factor T-bet, and Th1-skewing is accomplished by the cytokines IL-12 and IL-18 produced by activated macrophages (32). As stated earlier, IFN-γ is the prototypic cytokine secreted by Th1 cells.
IFN-γ has been shown to exacerbate plaque formation. When exogenous recombinant IFN-γ was injected intra-peritoneally into ApoE–/– mice for 30 days, researchers observed a 15% increase in lesion size compared with mice receiving PBS injections (33). This result was in accordance with loss-of-function studies using IFN-γR–/–/ApoE–/– double KO (dKO) mice, which resulted in a 60% decrease in lesion size when compared with their ApoE–/– single KO counterparts. Interestingly, these researchers also noted significantly less cellularity in the lesions of IFN-γR–/–/ApoE–/– dKO mice, along with an increase in lesional collagen content suggesting greater plaque stability.
Upstream of IFN-γ are the cytokines IL-12 and IL-18. Both are secreted by macrophages, and result in Th1 differentiation and IFN-γ production. Studies of these two cytokines have also shown that they exacerbate atherosclerosis, likely via increased production of IFN-γ. IL-12–/–/ApoE–/– mice had a 52% reduction in plaque area in the aortic root (34). Functional blockade of endogenous IL-12 via anti-IL-12 antibodies resulted in a significant decrease in atherogenesis (68%) compared with control mice, with an increase in collagen content (35). To investigate the role of IL-18, researchers have administered a plasmid encoding a soluble, recombinant IL-18-binding protein into ApoE–/– mice resulting in decreased bioavailable IL-18 and less atherosclerosis. These researchers also noted decreased lesional cellularity and increased collagen content (36). Subsequently, an IL-18–/–/ApoE–/– dKO mouse model showed that IL-18 deficiency reduced lesion size by ~35%, reduced I-Ab expression and increased the proportion of smooth muscle cells that were positive for smooth muscle-actin (compatible with a more stable lesion phenotype).
Consistent with these cytokine-based studies, T-bet deficiency also results in reduction of atherosclerosis by ~30%, with a concurrent increase in Th2 cytokine production (IL-4, IL-5 and IL-10) and an increase in E06 IgM natural antibody (37) (an athero-protective antibody shown to be up-regulated in the presence of IL-5) (38). Overall, the IFN-γ–IL-12–IL-18 axis of cytokines is strongly inflammatory and promotes and accelerates lesion development.
Th2 cells
IL-4 produced by Th2 cells counteracts the production of IFN-γ (39). The role of the Th2 pathway in the development of atherosclerosis, however, remains controversial. IL-4, IL-5 and IL-13 are stereotypic Th2 cytokines (IL-10 is another Th2 cytokine, but for the purposes of this review, we will discuss it in Treg cells). Because the Th2 phenotype opposes differentiation of pro-atherogenic IFN-γ-producing Th1 cells, it would seem that Th2 cells ought to be athero-protective. In human studies, this does appear to be the case, as a recent publication found that high numbers of Th2 cells found in the peripheral blood were independently associated with a decrease in mean common carotid intima-media thickness (a clinical marker of atherosclerosis), and in women was associated with a reduced risk of acute myocardial infarction (40). However, in murine studies of the Th2 cytokines IL-4 and IL-5, this association is blurred.
Besides opposing Th1 effects and lineage commitment, IL-4 and IL-5 also exert their influence on other cells, thus making their specific role in atherosclerosis difficult to elucidate. For example, mouse studies have shown that IL-4 production leads to up-regulation in the expression of CD36 (41, 42) and class A scavenger receptor on macrophages (43), vascular cell adhesion molecule-1 (VCAM-1) (44, 45), matrix metalloproteinase 1 (46) and monocyte chemoattractant protein 1 (47), which would all be theorized to lead to increased atherosclerosis. Although one publication demonstrated a significant reduction in atherosclerotic lesion size in the aortic arch and thoracic aortic when IL-4–/– bone marrow was adoptively transferred into irradiated LDLR–/– mice (48), subsequent studies using either IL-4–/–/ApoE–/– dKO or IL-4–/–/LDLR–/– dKO have shown no difference in atherosclerosis compared with IL-4 wild-type control mice (49).
Conversely, IL-5 seems to have a protective role against atherosclerosis. Engraftment of IL-5-deficient bone marrow into LDLR KO mice leads to enhanced lesion formation (38), possibly due to decreased production of natural IgM antibodies from B-1 cells, which in turn act as blocking antibodies against oxLDL (50). Indeed, it has been shown that although natural antibodies are poly-specific, they do have a preference for other immunoglobulins, self-antigens and common bacterial antigens. These natural antibodies have been shown to bind oxidized phospholipids and oxidized phospholipoproteins and inhibit macrophage uptake of oxLDL, which leads to an athero-protective effect (51).
Th17 cells
Th17 cells are induced in the presence of IL-6 and TGF-β, and secrete IL-17A, IL-17F and IL-22. The IL-17 family of cytokines consists of six members—IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F. In general, all of the IL-17 family members activate NF-κB, ERK1/2, CCAAT/enhancer-binding protein β (C/EBPβ) and C/EBPδ signaling in target cells to varying degrees (52), which results in pro-inflammatory cytokine production including TNF, IL-1β (53, 54), IFN-γ (55) and granulocyte colony-stimulating factor (56). In patients with unstable angina, levels of plasma IL-17A and the Th17-related cytokines IL-6 and IL-23 are elevated (57), and cultured human T cells isolated from atherosclerotic coronary arteries also produce a unique combination of IL-17A and IFN-γ after polyclonal stimulation compared with T cells extracted from non-diseased vessels (58, 59). The role of Th17 cells in atherosclerosis, though, remains controversial.
Various methods used for knocking down expression of IL-17A and its receptor IL-17RA yielded conflicting results. Using a rat anti-mouse-IL-17A antibody in ApoE KO mice fed 12 weeks of chow diet, one publication reported a 50% reduction in aortic root lesions and increased collagen content with decreased cellularity that suggests a more stable plaque phenotype (53). Although these findings were repeated in a subsequent study, this group found no evidence of reduced IL-17A signaling despite the use of rat anti-mouse-IL-17A antibodies to decrease atherosclerosis (60). Interestingly, when these authors used a mouse anti-mouse-IL-17A antibody, they found no improvement in atherosclerotic burden despite significant reduction in IL-17A production (60), suggesting that the athero-protective effects afforded by exogenous anti-IL-17A antibodies might not actually be from reduced levels of IL-17A but may instead be attributed to the species from which the antibody was derived (rat anti-mouse versus mouse anti-mouse) (52, 60).
Blockade of IL-17A was also done using an Fc-containing fusion protein that also included the IL-17RA extracellular domain (IL-17RA–Fc) expressed in vivo from an adenovirus construct (AdIL-17RA–Fc) in ApoE–/– mice fed WD for 15 weeks (58). Blockade of IL-17A using this method resulted in 54% smaller lesions throughout the aorta compared with controls.
Experiments with KO mice have also yielded inconsistent results. IL-17A–/–/LDLR–/– dKO mice demonstrate no difference in plaque burden within the aortic roots or descending aorta despite decreases in aortic macrophages, CD11b+CD11c+ cells and T-cell cellularity (55). These findings contrast with a recent publication where IL-17A–/– and IL-17RA–/– mice were bred into an ApoE–/– background and showed that IL-17A or IL-17RA deficiency led to a 35% or 25% reduction in atherosclerosis along the entire aorta, respectively, after 15 weeks of WD (61). In this study, the athero-protection was predominantly noted in the ascending portion of the aorta (roots and arch), but not in the descending thoraco-abdominal portion of the aorta. This study also demonstrated decreased cellularity and IFN-γ-producing Th1 cells in the aortic arch, findings not observed in the descending aorta. Interestingly, mRNA expression levels of IL-17A and IL-17RA correlated with the distribution of plaque protection, as IL-17A is preferentially expressed in the aortic arch, whereas IL-17RA is ubiquitously expressed along the entire aorta.
Monocyte adherence is also affected by IL-17, as explanted aortas from ApoE KO mice showed enhanced adhesion capabilities (increased by 48%) when IL-17A was added to labeled monocytes from an ApoE KO mouse. This phenomenon was not observed under the same conditions when explanted aortas were from IL-17RA KO mice (61).
Two recent studies have cast doubt on the pro-atherosclerotic role of IL-17A (62, 63). Because Socs3 negatively regulates STAT3-dependent expression of IL-17A in T cells, these researchers used a Socs3–/–/LDLR–/– chimeric mouse strain to over-express IL-17A, which resulted in 50% smaller lesions within the aortic roots compared with Socs3+/+/LDLR–/– mice. Socs3 overproduction, which results in reduced IL-17 levels, actually accelerated atherosclerosis (62). Furthermore, treatment of LDLR KO mice with recombinant IL-17A in the same study resulted in reduced levels of VCAM-1, reduced vascular T-cell infiltration and decreased lesion sizes (62). Another study used IL-17A–/–/ApoE–/– dKO mice fed WD for 8 or 16 weeks that showed significantly more atherosclerosis (at 8 weeks, but not 16 weeks) compared with control ApoE–/– mice and had a more vulnerable plaque phenotype (63). Human studies may corroborate this finding as there are increased levels of STAT3 phosphorylation and IL-17 in patients with a more stable plaque phenotype (62). Thus, the role of Th17 cells and IL-17A is still an area of controversy and ongoing investigation.
Treg cells
Treg cells are characterized as negative regulators of immune effector cells. They have been shown to play a key role in preventing atherogenesis and have different functional phenotypes. Natural Treg cells (nTreg cells) are defined as a population of T cells expressing CD4, CD25 (IL-2R) and the transcription factor FoxP3. The nTreg cells mature within the thymus and have TCRs that have a high affinity for self-antigen (but that have escaped negative selection) and are therefore able to negatively regulate pro-inflammatory immune effects via the cytokines IL-10 and TGF-β. These T cells are unique in that they do not require antigen exposure in the periphery to gain their immunosuppressive activities.
Inducible Treg cells (iTreg cells) include type 1 regulatory T cells (Tr1 cells) and Th3 cells. The iTreg cells are derived from effector T-cell populations in the periphery only after exposure to antigen. They are also CD4+CD25+, but do not require FoxP3 expression to be functional (64). Tr1 cells are typically IL-10-secreting, whereas Th3 cells are TGF-β-producing. Both nTreg and iTreg cells have non-redundant roles in maintaining immunologic homeostasis through self-/non-self-recognition. They are able to prevent autoimmunity by competing with other T-cell subsets for complexes of antigen and MHC class II (MHCII) on antigen-presenting cells (APCs), by increasing the inhibitory receptor CTLA-4, by down-regulating co-stimulatory molecule expression (CD80/CD86) and through direct cytotoxic and/or inhibitory effects on other effector cells.
The initial observation that Treg cells are detected in much lower amounts in atherosclerotic plaques (1–5% of all T cells) than in other chronically inflamed tissues (~25% of all T cells in eczema or psoriasis) has led researchers to wonder if impairment of local tolerance against potential antigens in atherosclerotic lesions is a cause for inflammation and plaque formation (17, 65). A very recent paper (66) showed that in a prospective cohort in Sweden of 700 participants who were 68–73 years old, the number of circulating Treg cells in peripheral blood was inversely associated with the development of myocardial infarction (hazards ratio 1.9 for the lowest tertile of CD4+FoxP3+ T cells) and for coronary events in general (hazards ratio 2.6 in the lowest tertile). Interestingly, statin therapy (a mainstay in the treatment and prevention of cardiovascular disease) not only increased the number of CD4+CD25+FoxP3+ Treg cells in PBMCs cultured in vitro but also enhanced the suppressive function of Treg cells from acute coronary syndrome patients in vitro (67).
Adoptive transfer experiments of Tr1 cells administered to ApoE–/– mice showed a significant decrease in Th1 responses revealed by reduced IFN-γ production, increased IL-10 production and significant reduction in atherosclerotic lesion size when compared with control mice (68, 69). Treg cells also inhibited foam-cell formation in vitro and biased macrophage differentiation toward an M2 (anti-inflammatory) phenotype versus the M1 (pro-inflammatory) phenotype (70, 71). IL-10, secreted by Treg cells, is athero-protective and protects both from fatty streak formation and atherosclerotic plaque formation (13). The statin simvastatin increased the number of Treg cells in atherosclerotic plaques and the expression of mRNA for FoxP3, TGF-β and IL-10 when administered to ApoE–/– mice for 6 weeks (67).
The Treg cell’s link to innate immunity in atherosclerosis was demonstrated recently by Subramanian et al. (28), who showed that MyD88-dependent TLR signaling through CD11c+ DCs had an anti-atherosclerotic effect on irradiated Ldlr –/– mice reconstituted with bone marrow from Cd11cCre + Myd88 fl/fl mice. In these mice, CD11c+ cells lack the TLR adaptor MyD88, which decreases their ability to activate effector T cells and was expected to produce a decrease in atherosclerotic lesions, but instead resulted in ~50% increase in aortic root plaque area with concurrent decrease in percentage of Treg cells at the plaque site and IL-10 mRNA expression. Overall, the murine data suggest a protective role of Treg cells in atherosclerosis. Whether they are also protective in humans remains to be determined.
NKT cells
NKT cells are a unique subset of T cells that have both NK-specific (NK1.1 and Ly49) and T-cell-specific (CD4 and TCR) surface markers. These cells recognize antigen in the context of CD1d, not MHC, expressed on APCs. Their ligands are glycolipid antigens. They are found in both humans and mice, and are abundant in the liver and most lymphoid tissues. The invariant NKT (iNKT) cell has a limited TCR repertoire. Upon activation, these iNKT cells rapidly secrete large amounts of anti-inflammatory cytokines such as IL-4, IL-10 and IL-13, and pro-inflammatory cytokines such as IFN-γ (72). The iNKT cells have been suggested as possible autoregulatory cells that are capable of inducing tolerance by communicating with Treg cells (73). Although they may play a tolerogenic role in some diseases like type 1 diabetes in mice (74), it appears that they are likely to be pro-atherogenic.
Human studies have shown that iNKT cells are found in human carotid artery plaques (75) as well as in human atherosclerotic tissue from abdominal aortic aneurysms (76). Studies of ApoE–/–/CD1d–/– mice showed reduced atherosclerosis (77–79) and stimulation of ApoE–/– mice with α-galactosylceramide (a glycolipid that activates NKT cells via CD1d) worsened atherosclerosis (77, 78). Although most studies suggest a pro-atherosclerotic role for iNKT cells, at least one study suggests that they are athero-protective (80). In this study, the researchers activated iNKT cells exogenously with α-galactosylceramide and injections of these activated iNKT cells protected LDLR–/– (but not ApoE–/–) mice from carotid atherosclerosis, a site that was uniquely investigated in this particular iNKT cell study. These results indicate that further investigation is necessary to determine the role of iNKT cells in atherosclerosis (81).
Other T cells
Follicular helper T cells are a subset of CD4+ T cells that are required for germinal center formation and antibody production by B cells (82). Their role in atherosclerosis has not been investigated yet. Similarly, the role of CD8+ T cells in atherosclerosis is largely unexplored at this time.
Conclusions
Data from mouse models of atherosclerosis suggest a role for various T-cell subsets in the development and progression of atherosclerosis. CD4+ T cells in general are believed to exacerbate atherosclerotic disease, but when subsets of CD4+ T cells are examined individually, the different classes of helper T cells appear to exert very different influences on the atherosclerotic plaque.
Although the murine studies reviewed previously give us insight into the various roles of T-cell subsets in atherosclerosis, one critical element is still missing. The lack of understanding of what triggers T-cell activation (with the exception of nTreg-cell activation) has, so far, greatly hindered advances to eventually control the progression of atherosclerotic development. T cells are not activated by binding to the whole antigenic molecule. Instead, the antigenic molecules have to be broken down into small peptides by APCs and the TCR binds to a ligand composed of a small peptide of the antigenic molecule (an antigenic epitope) in association with the MHC proteins. Over the years, although several autoantigens have been identified as having properties of mounting atherosclerotic responses (21–23, 39, 83), the nature of the actual peptides (epitopes) that bind to the TCR of atherogenic T cells remains unexplored. Furthermore, the role of these antigen-specific T cells in atherosclerotic disease after peptide-specific activation has not been sufficiently queried.
Evidence for the existence of an autoantigen residing within the atherosclerotic plaque comes from live-cell imaging of explanted aortas from CD11cYFP/ApoE–/– mice after 12 weeks of WD. In this model, antigen-experienced ApoE–/– CD4+ T cells interact with yellow fluorescent protein (YFP) + CD11c-expressing APCs in the aortic wall (84). These T cells were effector-memory CD4+ T cells and had long interactions with APCs in the vessel wall. They also exhibited slowed migration speeds compared with T cells isolated from naive wild-type C57BL/6 mice. The functional significance of these interactions was demonstrated by increased T-cell proliferation and induction of pro-inflammatory cytokines (IFN-γ, TNF and IL-17).
One set of potentially relevant autoantigens are peptides derived from human ApoB-100, with P210 being the best known. P210 was first reported by Fredrikson et al. (85), who synthesized 302 overlapping 20-mer-peptides corresponding to the entire human ApoB-100 amino acid sequence and tested both native and oxidized forms of these peptides for binding by circulating antibodies from patients’ sera. Of the 302 peptides, 102 were found to bind antibodies in the sera. P210 is one of the 102 antibody-binding peptides, and the presence of both IgM and IgG auto-antibodies against P210 suggests that T-cell help (and therefore T-cell recognition of the autoantigen) was required during this process because of the observed class switching. Interestingly, repeated immunization of mice using P210 has shown an athero-protective effect (86, 87), which recently has been attributed to its ability to increase Treg cells in secondary lymphoid organs (88, 89).
It is interesting to note that P210 was initially identified as an auto-antibody-binding peptide, but it is now proposed to have Treg-induction properties (88, 89). For P210 to have direct effects on any CD4+ helper T cell, the peptide would have to first be bound to an MHCII molecule on an APC and be subsequently presented to a TCR capable of recognizing the peptide–MHC complex.
To determine whether P210 can indeed bind to MHCII, we sought to measure its capacity to bind its putative mouse (C57BL/6) MHCII restricting allele, I-Ab. Accordingly, we synthesized P210 and tested in a classical competitive inhibition assay utilizing purified MHC and high-affinity radiolabeled I-Ab ligands (90). Under the conditions utilized, where (label) < (MHC) and IC50 ≥ (MHC), the measured IC50 values are reasonable approximations of the true K d values (91, 92). We found that P210 did not bind I-Ab with biologically relevant affinity, with an IC50 of 34 000nM (Table 1). By comparison, well-known I-Ab-restricted T-cell epitopes, such as ovalbumin peptide323–339 (93) and myelin oligodendrocyte glycoprotein38–51 (94), have been shown to bind I-Ab with ~100-fold higher affinity.
Table 1.
H-2 I-Ab-binding affinity of P210 compared with known I-Ab-restricted T-cell epitopes
| Peptide | Sequence | Reference | H-2 I-Ab (IC50 nM) |
|---|---|---|---|
| P210 | KTTKQSFDLSVKAQYKKNKH | (85–87, 89) | 34 000 |
| OVA323–339 | ISQAVHAAHAEINEAGR | (93, 97) | 472 |
| MOG38–51 | GWYRSPFSRVVHLY | (94) | 354 |
| HBV core128–140 | TPPAYRPPNAPIL | (98) | 255 |
| AChR146–162 | PKYVKQNTLKLAT | (99) | 84 |
AChR, Torpedo californica acetylcholine receptor; HBV, hepatitis B virus core protein; MOG, Mus musculus myelin oligodendrocyte glycoprotein; OVA, Gallus gallus ovalbumin.
These findings suggest that the mechanism by which P210 produces athero-protection is unlikely to be through direct interactions with Treg cells, unless the peptide is in some way post-translationally modified in some as yet undetected manner. Our data do not rule out the possibility that P210 induces Treg functionality through interactions with other cell types such as DCs (95) or CD8+ T cells (96), which in turn may provide the stimuli for Treg production. Nevertheless, it is clear that, to advance our understanding of how each subset of T cells contributes to the initiation and progression of atherosclerosis, it is necessary to study each cell type in isolation to define what constitutes an atherogenic T cell.
Funding
National Institutes of Health (T32 AI 07469 to K.T.).
References
- 1. Kavey R. E., Daniels S. R., Lauer R. M., Atkins D. L., Hayman L. L., Taubert K. American Heart Association 2003. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation 107:1562. [DOI] [PubMed] [Google Scholar]
- 2. Hansson G. K., Hermansson A. 2011. The immune system in atherosclerosis. Nat. Immunol. 12:204. [DOI] [PubMed] [Google Scholar]
- 3. Wick G., Perschinka H., Millonig G. 2001. Atherosclerosis as an autoimmune disease: an update. Trends Immunol. 22:665. [DOI] [PubMed] [Google Scholar]
- 4. Silverstein R. L., Febbraio M. 2000. CD36 and atherosclerosis. Curr. Opin. Lipidol. 11:483. [DOI] [PubMed] [Google Scholar]
- 5. Libby P., Ridker P. M., Hansson G. K. 2011. Progress and challenges in translating the biology of atherosclerosis. Nature 473:317. [DOI] [PubMed] [Google Scholar]
- 6. Tse K., Ley K. 2012. Transforming growth factor-beta: transforming plaque to stability. Eur. Heart. J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ketelhuth D. F., Hansson G. K. 2011. Cellular immunity, low-density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thromb. Haemost. 106:779. [DOI] [PubMed] [Google Scholar]
- 8. Shimada K. 2009. Immune system and atherosclerotic disease: heterogeneity of leukocyte subsets participating in the pathogenesis of atherosclerosis. Circ. J. 73:994. [DOI] [PubMed] [Google Scholar]
- 9. Moore K. J., Tabas I. 2011. Macrophages in the pathogenesis of atherosclerosis. Cell 145:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shashkin P., Dragulev B., Ley K. 2005. Macrophage differentiation to foam cells. Curr. Pharm. Des. 11:3061. [DOI] [PubMed] [Google Scholar]
- 11. Zhou X., Hansson G. K. 1999. Detection of B cells and proinflammatory cytokines in atherosclerotic plaques of hypercholesterolaemic apolipoprotein E knockout mice. Scand. J. Immunol. 50:25. [DOI] [PubMed] [Google Scholar]
- 12. Zernecke A., Shagdarsuren E., Weber C. 2008. Chemokines in atherosclerosis: an update. Arterioscler. Thromb. Vasc. Biol. 28:1897. [DOI] [PubMed] [Google Scholar]
- 13. Andersson J., Libby P., Hansson G. K. 2010. Adaptive immunity and atherosclerosis. Clin. Immunol. 134:33. [DOI] [PubMed] [Google Scholar]
- 14. Thorp E., Subramanian M., Tabas I. 2011. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. Eur. J. Immunol. 41:2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thorp E., Tabas I. 2009. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J. Leukoc. Biol. 86:1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gounopoulos P., Merki E., Hansen L. F., Choi S. H., Tsimikas S. 2007. Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease. Minerva Cardioangiol. 55:821. [PubMed] [Google Scholar]
- 17. Lahoute C., Herbin O., Mallat Z., Tedgui A. 2011. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat. Rev. Cardiol. 8:348. [DOI] [PubMed] [Google Scholar]
- 18. Stemme S., Faber B., Holm J., Wiklund O., Witztum J. L., Hansson G. K. 1995. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl Acad. Sci. USA 92:3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Boer O. J., van der Wal A. C., Verhagen C. E., Becker A. E. 1999. Cytokine secretion profiles of cloned T cells from human aortic atherosclerotic plaques. J. Pathol. 188:174. [DOI] [PubMed] [Google Scholar]
- 20. Grivel J. C., Ivanova O., Pinegina N., et al. 2011. Activation of T lymphocytes in atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 31:2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Afek A., George J., Gilburd B., et al. 2000. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J. Autoimmun. 14:115. [DOI] [PubMed] [Google Scholar]
- 22. Zhu J., Quyyumi A. A., Rott D., et al. 2001. Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: evidence for an autoimmune component of atherogenesis. Circulation 103:1071. [DOI] [PubMed] [Google Scholar]
- 23. Kervinen H., Huittinen T., Vaarala O., et al. 2003. Antibodies to human heat shock protein 60, hypertension and dyslipidemia. A study of joint effects on coronary risk. Atherosclerosis 169:339. [DOI] [PubMed] [Google Scholar]
- 24. Dong C. 2006. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 6:329. [DOI] [PubMed] [Google Scholar]
- 25. Korn T., Bettelli E., Oukka M., Kuchroo V. K. 2009. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27:485. [DOI] [PubMed] [Google Scholar]
- 26. Lee Y. K., Mukasa R., Hatton R. D., Weaver C. T. 2009. Developmental plasticity of Th17 and Treg cells. Curr. Opin. Immunol. 21:274. [DOI] [PubMed] [Google Scholar]
- 27. Chaudhry A., Samstein R. M., Treuting P., et al. 2011. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Subramanian M., Thorp E., Hansson G. K., Tabas I. 2013. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J. Clin. Invest. 123:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seok J., Warren H. S., Cuenca A. G., et al. Inflammation and Host Response to Injury, Large Scale Collaborative Research Program 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl Acad. Sci. USA 110:3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laurat E., Poirier B., Tupin E., et al. 2001. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 104:197. [DOI] [PubMed] [Google Scholar]
- 31. Mallat Z., Taleb S., Ait-Oufella H., Tedgui A. 2009. The role of adaptive T cell immunity in atherosclerosis. J. Lipid Res. 50(Suppl.):S364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elhage R., Jawien J., Rudling M., et al. 2003. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc. Res. 59:234. [DOI] [PubMed] [Google Scholar]
- 33. Whitman S. C., Ravisankar P., Elam H., Daugherty A. 2000. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E-/- mice. Am. J. Pathol. 157:1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davenport P., Tipping P. G. 2003. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 163:1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hauer A. D., Uyttenhove C., de Vos P., et al. 2005. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation 112:1054. [DOI] [PubMed] [Google Scholar]
- 36. Mallat Z., Corbaz A., Scoazec A., et al. 2001. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ. Res. 89:E41. [DOI] [PubMed] [Google Scholar]
- 37. Buono C., Binder C. J., Stavrakis G., Witztum J. L., Glimcher L. H., Lichtman A. H. 2005. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl Acad. Sci. USA 102:1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Binder C. J., Hartvigsen K., Chang M. K., et al. 2004. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 114:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wurtz O., Bajénoff M., Guerder S. 2004. IL-4-mediated inhibition of IFN-gamma production by CD4+ T cells proceeds by several developmentally regulated mechanisms. Int. Immunol. 16:501. [DOI] [PubMed] [Google Scholar]
- 40. Engelbertsen D., Andersson L., Ljungcrantz I., et al. 2013. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler. Thromb. Vasc. Biol. 33:637. [DOI] [PubMed] [Google Scholar]
- 41. Feng J., Han J., Pearce S. F., et al. 2000. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. J. Lipid Res. 41:688. [PubMed] [Google Scholar]
- 42. Huang J. T., Welch J. S., Ricote M., et al. 1999. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature 400:378. [DOI] [PubMed] [Google Scholar]
- 43. Cornicelli J. A., Butteiger D., Rateri D. L., Welch K., Daugherty A. 2000. Interleukin-4 augments acetylated LDL-induced cholesterol esterification in macrophages. J. Lipid Res. 41:376. [PubMed] [Google Scholar]
- 44. Barks J. L., McQuillan J. J., Iademarco M. F. 1997. TNF-alpha and IL-4 synergistically increase vascular cell adhesion molecule-1 expression in cultured vascular smooth muscle cells. J. Immunol. 159:4532. [PubMed] [Google Scholar]
- 45. Lee Y. W., Kuhn H., Hennig B., Neish A. S., Toborek M. 2001. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J. Mol. Cell. Cardiol. 33:83. [DOI] [PubMed] [Google Scholar]
- 46. Sasaguri T., Arima N., Tanimoto A., Shimajiri S., Hamada T., Sasaguri Y. 1998. A role for interleukin 4 in production of matrix metalloproteinase 1 by human aortic smooth muscle cells. Atherosclerosis 138:247. [DOI] [PubMed] [Google Scholar]
- 47. Walch L., Massade L., Dufilho M., Brunet A., Rendu F. 2006. Pro-atherogenic effect of interleukin-4 in endothelial cells: modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis. 187:285. [DOI] [PubMed] [Google Scholar]
- 48. King V. L., Szilvassy S. J., Daugherty A. 2002. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor-/- mice. Arterioscler. Thromb. Vasc. Biol. 22:456. [DOI] [PubMed] [Google Scholar]
- 49. King V. L., Cassis L. A., Daugherty A. 2007. Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am. J. Pathol. 171:2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Binder C. J., Chou M. Y., Fogelstrand L., et al. 2008. Natural antibodies in murine atherosclerosis. Curr. Drug Targets 9:190. [DOI] [PubMed] [Google Scholar]
- 51. Hörkkö S., Bird D. A., Miller E., et al. 1999. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Invest. 103:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Butcher M., Galkina E. 2011. Current views on the functions of interleukin-17A-producing cells in atherosclerosis. Thromb. Haemost. 106:787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Erbel C., Chen L., Bea F., et al. 2009. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J. Immunol. 183:8167. [DOI] [PubMed] [Google Scholar]
- 54. Jovanovic D. V., Di Battista J. A., Martel-Pelletier J., et al. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 160:3513. [PubMed] [Google Scholar]
- 55. Madhur M. S., Funt S. A., Li L., et al. 2011. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 31:1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ley K., Smith E., Stark M. A. 2006. IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunol. Res. 34:229. [DOI] [PubMed] [Google Scholar]
- 57. Cheng X., Yu X., Ding Y. J., et al. 2008. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin. Immunol. 127:89. [DOI] [PubMed] [Google Scholar]
- 58. Smith E., Prasad K. M., Butcher M., et al. 2010. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 121:1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eid R. E., Rao D. A., Zhou J., et al. 2009. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 119:1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cheng X., Taleb S., Wang J., et al. 2011. Inhibition of IL-17A in atherosclerosis. Atherosclerosis 215:471. [DOI] [PubMed] [Google Scholar]
- 61. Butcher M. J., Gjurich B. N., Phillips T., Galkina E. V. 2012. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ. Res. 110:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Taleb S., Romain M., Ramkhelawon B., et al. 2009. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 206:2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Danzaki K., Matsui Y., Ikesue M., et al. 2012. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32:273. [DOI] [PubMed] [Google Scholar]
- 64. Ray A., Khare A., Krishnamoorthy N., Qi Z., Ray P. 2010. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 3:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. de Boer O. J., van der Meer J. J., Teeling P., van der Loos C. M., van der Wal A. C. 2007. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS One 2:e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wigren M., Björkbacka H., Andersson L., et al. 2012. Low levels of circulating CD4+FoxP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler. Thromb. Vasc. Biol. 32:2000. [DOI] [PubMed] [Google Scholar]
- 67. Meng X., Zhang K., Li J., et al. 2012. Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol. Med. 18:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mallat Z., Gojova A., Brun V., et al. 2003. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation 108:1232. [DOI] [PubMed] [Google Scholar]
- 69. Ait-Oufella H., Salomon B. L., Potteaux S., et al. 2006. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12:178. [DOI] [PubMed] [Google Scholar]
- 70. Lin J., Li M., Wang Z., He S., Ma X., Li D. 2010. The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation. J. Lipid Res. 51:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu G., Ma H., Qiu L., et al. 2011. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol. Cell Biol. 89:130. [DOI] [PubMed] [Google Scholar]
- 72. Kronenberg M., Gapin L. 2002. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2:557. [DOI] [PubMed] [Google Scholar]
- 73. Nowak M., Stein-Streilein J. 2007. Invariant NKT cells and tolerance. Int. Rev. Immunol. 26:95. [DOI] [PubMed] [Google Scholar]
- 74. Yang Y., Bao M., Yoon J. W. 2001. Intrinsic defects in the T-cell lineage results in natural killer T-cell deficiency and the development of diabetes in the nonobese diabetic mouse. Diabetes 50:2691. [DOI] [PubMed] [Google Scholar]
- 75. Bobryshev Y. V., Lord R. S. 2005. Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J. Histochem. Cytochem. 53:781. [DOI] [PubMed] [Google Scholar]
- 76. Chan W. L., Pejnovic N., Hamilton H., et al. 2005. Atherosclerotic abdominal aortic aneurysm and the interaction between autologous human plaque-derived vascular smooth muscle cells, type 1 NKT, and helper T cells. Circ. Res. 96:675. [DOI] [PubMed] [Google Scholar]
- 77. Tupin E., Nicoletti A., Elhage R., et al. 2004. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J. Exp. Med. 199:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Major A. S., Wilson M. T., McCaleb J. L., et al. 2004. Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 24:2351. [DOI] [PubMed] [Google Scholar]
- 79. Nakai Y., Iwabuchi K., Fujii S., et al. 2004. Natural killer T cells accelerate atherogenesis in mice. Blood. 104:2051. [DOI] [PubMed] [Google Scholar]
- 80. van Puijvelde G. H., van Wanrooij E. J., Hauer A. D., de Vos P., van Berkel T. J., Kuiper J. 2009. Effect of natural killer T cell activation on the initiation of atherosclerosis. Thromb. Haemost. 102:223. [DOI] [PubMed] [Google Scholar]
- 81. Braun N. A., Covarrubias R., Major A. S. 2010. Natural killer T cells and atherosclerosis: form and function meet pathogenesis. J. Innate Immun. 2:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29:621. [DOI] [PubMed] [Google Scholar]
- 83. Benagiano M., D’Elios M. M., Amedei A., et al. 2005. Human 60-kDa heat shock protein is a target autoantigen of T cells derived from atherosclerotic plaques. J. Immunol. 174:6509. [DOI] [PubMed] [Google Scholar]
- 84. Koltsova E. K., Garcia Z., Chodaczek G., et al. 2012. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J. Clin. Invest. 122:3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fredrikson G. N., Hedblad B., Berglund G., et al. 2003. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 23:872. [DOI] [PubMed] [Google Scholar]
- 86. Fredrikson G. N., Söderberg I., Lindholm M., et al. 2003. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler. Thromb. Vasc. Biol. 23:879. [DOI] [PubMed] [Google Scholar]
- 87. Fredrikson G. N., Björkbacka H., Söderberg I., Ljungcrantz I., Nilsson J. 2008. Treatment with apo B peptide vaccines inhibits atherosclerosis in human apo B-100 transgenic mice without inducing an increase in peptide-specific antibodies. J. Intern. Med. 264:563. [DOI] [PubMed] [Google Scholar]
- 88. Wigren M., Kolbus D., Dunér P., et al. 2011. Evidence for a role of regulatory T cells in mediating the atheroprotective effect of apolipoprotein B peptide vaccine. J. Intern. Med. 269:546. [DOI] [PubMed] [Google Scholar]
- 89. Herbin O., Ait-Oufella H., Yu W., et al. 2012. Regulatory T-cell response to apolipoprotein B100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 32:605. [DOI] [PubMed] [Google Scholar]
- 90. Sidney J., Southwood S., Moore C., et al. 2013. Measurement of MHC/peptide interactions by gel filtration or monoclonal antibody capture. Curr. Protoc. Immunol. 18.3.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cheng Y., Prusoff W. H. 1973. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22:3099. [DOI] [PubMed] [Google Scholar]
- 92. Gulukota K., Sidney J., Sette A., DeLisi C. 1997. Two complementary methods for predicting peptides binding major histocompatibility complex molecules. J. Mol. Biol. 267:1258. [DOI] [PubMed] [Google Scholar]
- 93. Alexander J., Sidney J., Southwood S., et al. 1994. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity 1:751. [DOI] [PubMed] [Google Scholar]
- 94. Petersen T. R., Bettelli E., Sidney J., Sette A., Kuchroo V., Bäckström B. T. 2004. Characterization of MHC- and TCR-binding residues of the myelin oligodendrocyte glycoprotein 38-51 peptide. Eur. J. Immunol. 34:165. [DOI] [PubMed] [Google Scholar]
- 95. Choi J. H., Cheong C., Dandamudi D. B., et al. 2011. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity 35:819. [DOI] [PubMed] [Google Scholar]
- 96. Chyu K. Y., Zhao X., Dimayuga P. C., et al. 2012. CD8+ T cells mediate the athero-protective effect of immunization with an ApoB-100 peptide. PLoS One 7:e30780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Barnden M. J., Allison J., Heath W. R., Carbone F. R. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76:34. [DOI] [PubMed] [Google Scholar]
- 98. Milich D. R., Hughes J. L., McLachlan A., Thornton G. B., Moriarty A. 1988. Hepatitis B synthetic immunogen comprised of nucleocapsid T-cell sites and an envelope B-cell epitope. Proc. Natl Acad. Sci. USA 85:1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wall K. A., Hu J. Y., Currier P., Southwood S., Sette A., Infante A. J. 1994. A disease-related epitope of Torpedo acetylcholine receptor. Residues involved in I-Ab binding, self-nonself discrimination, and TCR antagonism. J. Immunol. 152:4526. [PubMed] [Google Scholar]