Abstract
Chronic alcohol abuse is a systemic disorder and a risk factor for acute respiratory distress syndrome (ARDS) and chronic obstructive pulmonary disease (COPD). A significant amount of ingested alcohol reaches airway passages in the lungs and can be metabolized via oxidative and non-oxidative pathways. About 90% of the ingested alcohol is metabolized via hepatic alcohol dehydrogenase (ADH)-catalyzed oxidative pathway. Alcohol can also be metabolized by cytochrome P450 2E1 (CYP2E1), particularly during chronic alcohol abuse. Both the oxidative pathways, however, are associated with oxidative stress due to the formation of acetaldehyde and/or reactive oxygen species (ROS). Alcohol ingestion is also known to cause endoplasmic reticulum (ER) stress, which can be mediated by oxidative and/or non-oxidative metabolites of ethanol. An acute as well as chronic alcohol ingestions impair protective antioxidants, oxidize reduced glutathione (GSH, cellular antioxidant against ROS and oxidative stress), and suppress innate and adaptive immunity in the lungs. Oxidative stress and suppressed immunity in the lungs of chronic alcohol abusers collectively are considered to be major risk factors for infection and development of pneumonia, and such diseases as ARDS and COPD. Prior human and experimental studies attempted to identify common mechanisms by which alcohol abuse directly causes toxicity to alveolar epithelium and respiratory tract, particularly lungs. In this review, the metabolic basis of lung injury, oxidative and ER stress and immunosuppression in experimental models and alcoholic patients, as well as potential immunomodulatory therapeutic strategies for improving host defenses against alcohol-induced pulmonary infections are discussed.
1. Introduction
Chronic alcohol abuse or alcoholism cost ~$223 billion to US economy and 79,000 deaths each year (Bouchery et al., 2011). The worldwide death toll is estimated to be ~30 fold greater than that in the US (CDC, 2004; NIAAA, 2000). Approximately 10–20 million people meet the criteria of alcoholic dependence in the United States and >500 million worldwide (Grant et al., 2004; Lieber, 1995). Alcohol over consumption damages almost every organ in the body and predisposes the host to a wide range of infectious diseases such as pneumonia, acute respiratory distress syndrome (ARDS) and chronic obstructive pulmonary disease (COPD) (Liang et al., 2012; Pabst et al., 2011; Zhang et al., 2002a). Therefore, chronic alcohol abuse is a major health issue worldwide.
Although ingested alcohol is mainly metabolized in the liver, a sizable amount of the dose reaches the airway passages by the bronchial circulation and is metabolized via oxidative and/or non-oxidative pathways (Manautou et al., 1992; Manautou and Carlson, 1991). Some of this alcohol may be excreted unchanged in exhaled breath. Alcohol consumption compromises systemic immunity, thereby increasing the susceptibility of the host to pulmonary infections characterized by severe symptoms, and less favorable outcomes such as ARDS and COPD ((Liang et al., 2012; Moss et al., 1996; Pabst et al., 2011). Both, ARDS and COPD in chronic alcohol abusers result in hospitalization, extensive treatment cost and significant mortalities. Therefore, alcohol abuse is a systemic disorder with specific effects on the respiratory system associated with increased incidence of infections in the lung (Gamble et al., 2006; Shellito, 1998; Vander Top et al., 2005). In this review, we summarize current understanding of ethanol metabolism in the lungs and its consequential oxidative stress and ER stress, suppression of innate and adaptive immunity of the lungs. Finally, we review therapeutic strategies used to mitigate immunosuppression and oxidative stress.
2. Metabolism of alcohol in the lungs
The majority of ingested ethanol is metabolized in the liver by cytosolic alcohol dehydrogenase (ADH) to acetaldehyde, which is further oxidized by mitochondrial aldehyde dehydrogenase (ALDH) to acetate (Lieber, 2004). Mammalian lungs can metabolize ingested ethanol by ADH followed by ALDH at rates dependent on its concentration (Bernstein, 1982; Jones, 1995; Qin and Meng, 2006; Vasiliou and Marselos, 1989; Yin et al., 1992). Ethanol can also be metabolized by microsomal cytochrome P450 2E1 (CYP2E1) and peroxisomal catalase to acetaldehyde in both the liver and in lungs (Bernstein et al., 1990; Jones, 1995; Rikans and Gonzalez, 1990; Yin et al., 1992). CYP2E1 is particularly induced during chronic alcohol abuse and is shown to be responsible for production of reactive oxygen species (Lieber, 2004). However, catalase may not be an important enzyme for ethanol oxidative metabolism due to its inhibition by ethanol (Das and Mukherjee, 2010). Mammalian lung parenchyma consist of large squamous alveolar type I epithelial cells (8% of the cells, but one of the largest cells and cover ~97% of alveolar space area), alveolar type II cells (16% of the total alveolar cells, half that of the type I pneumocyte), capillary endothelial cells (30% of the lung cells) and variable number of alveolar macrophages. Cells in the interstitial space comprised of 37% of the total cells (Matalon, 1991). Whether all cell types in the lungs metabolize ethanol is very poorly studied. Bronchial and bronchiolar epithelium, Clara cells, type II pneumocytes, and alveolar macrophages from human lung have been shown to express CYP enzymes (Hukkanen J et al., 2002). Therefore, it is likely that most of resident cells express ethanol oxidizing activity and capable of oxidizing ethanol, but specific information on the metabolism of ethanol in various cell types in the lung is largely lacking.
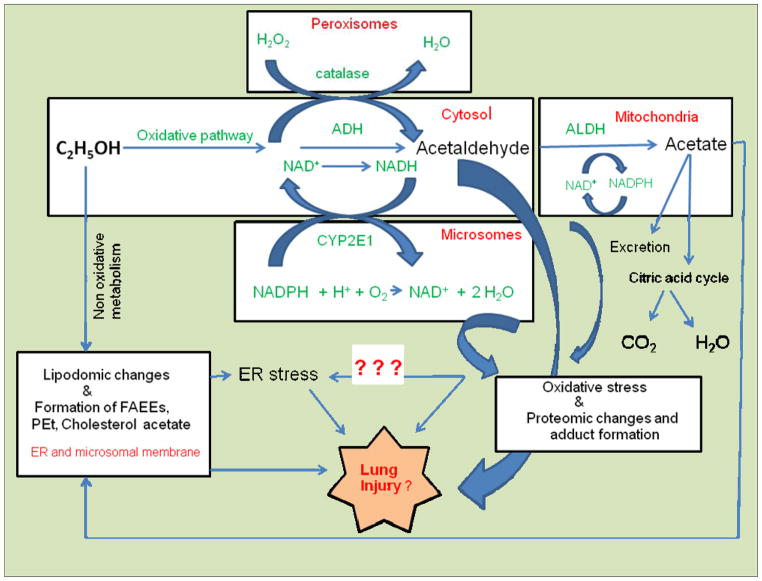
An alternative metabolism of ethanol is driven by fatty acid ester ethyl ester (FAEE) synthase, phospholipase D, sulfatase and glucuronidase, called as nonoxidative pathway, are also ubiquitous in the mammalian lungs (Aradottir et al., 2006; Lieber, 2004; Manautou and Carlson, 1991; Sharma et al., 1991; Zakhari, 2006). Ethyl sulfate and ethyl glucuronide are water soluble and thus rapidly excreted. On the other hand, phosphatidylethanol (PEt) and FAEEs, the products of nonoxidative metabolism of ethanol catalyzed by phospholipase D and FAEE synthase, respectively, are lipophilic and have been shown to accumulate in target organs including lungs (Aradottir et al., 2006; Aradottir et al., 2002; Bernstein et al., 1990; Kaphalia et al., 2004; Laposata and Lange, 1986; Manautou and Carlson, 1991). Although toxicity of nonoxidative metabolites of ethanol in mammalian lungs has not been investigated, mammalian lungs are well equipped for both oxidative and non-oxidative metabolism of ethanol and potential target of injury by a wide range of ethanol metabolites formed in the tissue (Fig. 1).
Fig. 1.
Alcohol metabolism and putative mechanism of alcoholic lung injury. The canonical pathway for ethanol metabolism is shown in the green.
3. Toxicity of ethanol metabolites
Both, ADH- and CYP2E1-catalyzed oxidation of ethanol generate a reactive metabolite acetaldehyde, which readily forms adducts with proteins and causes oxidative stress (Das and Mukherjee, 2010; Jones, 1995; Zakhari, 2006). Oxidative metabolism of ethanol also increases the ratio of NADH to NAD resulting in a dysregulation of lipid metabolism (Day and Yeaman, 1994). Genetic polymorphisms and altered levels of ADH, ALDH and CYP2E1 proteins influence the consumption of and susceptibility to ethanol and is possibly involve organ-specific injuries (Yin, 1994). Once formed, acetaldehyde is rapidly absorbed through the lungs (NIAAA, 2000). Biological consequences of acetaldehyde exposure include reduced phagocytotic index of lung macrophages and degeneration of the nasal olfactory epithelium (Appelman et al., 1986; Wyatt et al., 2012). Malondialdehyde (MDA, lipid peroxidation products) and acetaldehyde (MAA)-adducted proteins formed in the lungs of mice after co-exposure of cigarette smoke and alcohol are shown to stimulate bronchial epithelial cell interleukin-8 (IL-8) production via the activation of proteins kinase C epsilon. Proinflammatory responses of MAA-adducted proteins in vitro indicates that lung surfactant proteins are biologically relevant targets for MDA and acetaldehyde adduction (Wyatt et al., 2012)
FAEEs are shown to be formed in the lungs of rats and rabbits (Manautou and Carlson, 1991). These esters are reported to be cytotoxic to cells in culture and cause pancreatic toxicity in rats administered FAEEs (Werner et al., 1997; Wu et al., 2006). However, to the best of our knowledge, the pulmonary toxicity of FAEEs in vitro or in vivo has not been investigated. Another prominent nonoxidative metabolite of ethanol reportedly present in the lungs of alcoholics is PEt (Alling et al., 2005). Therefore, an evaluation of lung toxicity of FAEEs and PEt is warranted.
4. Lungs as target of alcohol-induced oxidative stress
Oxidative stress is thought to be a central feature of alcohol over-consumption-associated tissue injury and mechanism of alcoholic lung disease and several other diseases (Halliwell, 1996; Heffner and Repine, 1989). Therefore, understanding the role of oxidative stress in the pathophysiology of alcoholic lung disease is important for devising therapeutic approaches to reverse the disease progression.
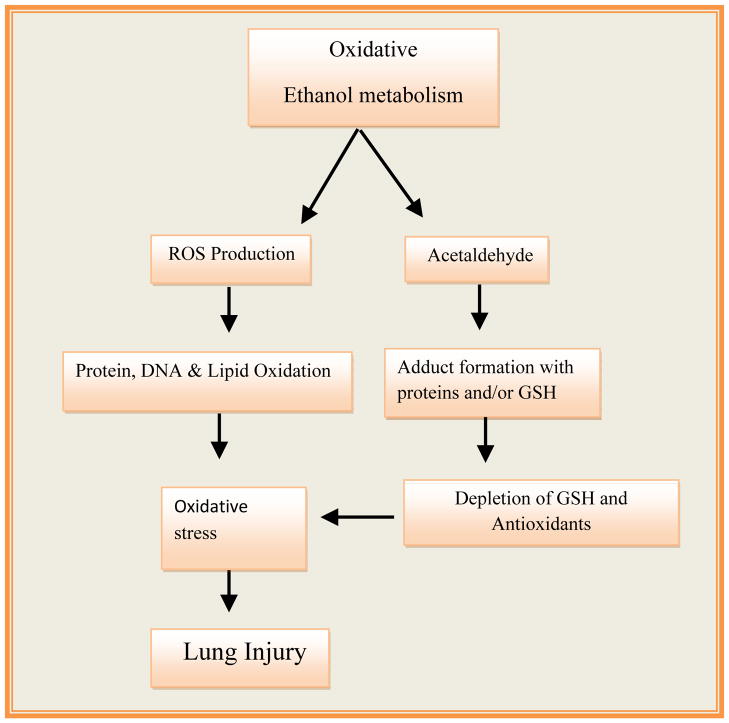
Among the organ systems, mammalian lungs are a potential target of oxidative stress resulting from chronic alcohol abuse due to their direct exposure to toxic environmental pollutants, smoke, and other particulate matter present in the ambient air. Alcohol is a well recognized systemic toxin and its oxidative metabolism to acetaldehyde generates a series of ROS and free radicals, which decreases the system’s ability to detoxify the ROS generated reactive intermediates or their products (Cederbaum, 2010). ROS-induced reactions develop an imbalance between oxidant production and system’s ability to detoxify the ROS generated reactive intermediates and products (Fig. 2). Reduced glutathione (GSH) and a number of antioxidant enzymes are known to reduce the systemic oxidative stress (Rahman and MacNee, 1996). Oxidation of alcohol to acetaldehyde catalyzed by ADH and/or CYP2E1 (Fig. 1) generates ROS such as superoxide anion (O2•−), the hydroxyl (•OH), and 1-hydroxyethyl (C2H5O•) radicals (Cederbaum, 2010). Such radicals can damage cellular proteins, lipids and DNA through oxidation reactions most importantly via peroxidation of unsaturated fatty acids. A significant amount of ethanol metabolized oxidatively in the lungs can be excreted in exhaled breath by volatilization across the alveolar-capillary membrane interface. However, local alcohol metabolism within the lung may be sufficient to exert significant oxidative stress in view of an increased ratio of oxidized to reduced GSH in lung lavage fluid, as has been reported in alcohol-fed animals and alcohol abusers (Holguin et al., 1998; Moss et al., 2000). An altered redox state associated with increased production of ROS may damage lungs through ethanol-associated oxidation of cellular proteins, lipids and DNA. ROS and their products with cellular proteins, lipids and DNA can also act as cellular messengers in redox signaling pathways and may have far reaching adverse systemic consequences.
Fig. 2.
Oxidative metabolism of ethanol and related oxidative stress in alcoholic lung Injury.
5. Oxidative stress and ARDS and COPD
Epidemiological studies reveal that oxidative stress is an important risk factor in the etiology of ARDS and COPD (MacNee, 2001; Moss et al., 1996). Often oxidative stress is linked with the oxidation of proteins, lipids and DNA to form oxidized proteins, lipid aldehydes and peroxides such as malondialdehyde (MDA), hexanals and 4-hydroxynenonal (4-HNE), and 8-hydroxy-deoxy-guanosine, which disrupt normal signaling events required for homeostasis and cause cell injury. Therefore, establishing a cause and effect interrelationship between toxicity and oxidative stress by assessing oxidized proteins, lipids or DNA could provide a better understanding of lung injury. However, ROS-derived reactions with endogenous biomolecules are often complex and multifaceted, resulting in the generation of secondary and tertiary reactive products and causing dimerization and polymerization between and among the radicals and adducts. These reactions are driven by significant change in redox status such as increased NADH/NAD ratio, up-regulation of NADH oxidase, and depletion of reduced GSH via its de novo synthesis and/or increased oxidation and a compromised antioxidant system (Aytacoglu et al., 2006; Polikandriotis et al., 2006; Yeh et al., 2007; Yeligar et al., 2012). Such conditions often compromise immunity and cause increased susceptibility to infection in lungs (Moss et al., 1996).
6. Antioxidants
Although the interrelationship between oxidative stress and antioxidants is not fully explored in alcoholic lung injury, reduced GSH appears to be a determining factor in human and experimental models of alcoholic lung disease (Guidot and Roman, 2002). To maintain normal physiological redox status, GSH, which is synthesized primarily in the liver, is circulated to all other organs including the lungs. Chronic alcohol ingestion depletes reduced GSH within the alveolar space by as much as 80–90%, and, consequently impairs alveolar epithelial surfactant production and barrier integrity, decreases alveolar macrophage function, and increases lung susceptibility to oxidant-mediated injury. Alcohol administration also increases glutathione turnover, a process independent of glutathione oxidation, glutathione S-transferase (GST) and glutathione peroxidase (GPX) activities. Precisely how alcohol decreases GSH levels in the lung are not well understood.
7. Alcohol abuse and endoplasmic reticulum (ER) stress in lungs
As discussed earlier alcohol abuse is a significant risk factor for ARDS and COPD. Studies in to the etiology of lung diseases like COPD and idiopathic lung fibrosis indicate a role for ER stress and unfolded protein response (UPR) pathways in their pathogenesis (Greene and McElvaney, 2010; Malhotra and Kaufman, 2007). However, scant literature exists regarding ER stress in lungs during chronic alcohol abuse.
In addition to its key role in the synthesis of proteins and in xenobiotic metabolism, another important role of ER is to correctly fold proteins and modify their tertiary and quaternary structures or direct misfolded proteins to ER associated degradation (ERAD) (Ji, 2012). Accumulation of misfolded proteins in the lumen of ER membrane causes ER stress, which activates the UPR to correctly fold unfolded proteins or remove the damaged cells by apoptosis. The UPR corrects ER stress through attenuating general protein synthesis and translation, by increasing protein folding capacity, and by expediting the degradative process of misfolded proteins (Ji et al., 2011).
A variety of pathological conditions (oxidative stress, hypoxia or altered calcium homeostasis, glucose starvation, and infections) or chemical exposure can result in ER stress. Glucose-regulated protein (GRP) 78 and other- prosurvival ER chaperones that control stress signaling pathways in the ER membrane are activated by ER stress. When cells undergo ER stress, the UPR is activated by three resident sensors: i) protein kinase-like ER kinase (PERK); ii), inositol-requiring enzyme (IRE)-1α; and iii) activating transcription factor 6 (ATF6) (Ji, 2012). Studies of chronic inflammatory lung diseases (bronchial asthma, COPD, and cystic fibrosis) as well as data from our laboratory show increasing evidence of ER stress in ethanol-induced lung injury (Greene and McElvaney, 2010; Kaphalia and Calhoun, 2012; kaphalia et al., 2013). Earlier, Shang et al. reported activation of the ER stress pathways in the lungs of lipopolysaccharide (LPS)-treated mice (Shang et al., 2011). ER stress has been reported in the lungs of patients with familial and sporadic idiopathic pulmonary fibrosis, and produces a dysfunctional epithelial cell phenotype, facilitating fibrotic remodeling (Lawson et al., 2011). Cigarette smoke (CS) can also cause ER stress in the lungs of patients with COPD (Kelsen et al., 2008). Lung epithelial cells treated with CS extract induce ER stress and apoptosis (Tagawa et al., 2008). Impaired or inhibited proteasomal activity disrupts ERAD and causes accumulation of misfolded proteins resulting in ER stress (Chauhan et al., 2008; Kardosh et al., 2008). Therefore, ER stress appears to be a common pathological condition in various lung diseases caused by exposure to CS and inhaled environmental pollutants. Further studies to elucidate the role of ER stress and UPR in pathogenesis of alcohol abuse-related lung injury could thus lead to a better understanding of the mechanism(s) of alcoholic lung disease.
Recently, we found that primary bronchial smooth muscles cells treated with bronchial lavage fluid from severe asthmatic patients cause ER stress (Kaphalia and Calhoun, 2012). In other study, we found an up-regulation of ATF6 and PERK and/or their downstream signaling in the lungs of hepatic ADH-deficient mice fed ethanol daily for 3 months (kaphalia et al., 2013). In these studies, we found activation of XBP1, protein disulfide isomerase (PDI) and C/EBP homologous protein (CHOP) suggesting a role of ER stress in ethanol-induced lung injury. It is likely that a prolonged ER stress also contribute to the etiologies of chronic diseases. Since very little is known about ER stress in the lungs of alcohol abusers, understanding the role of ER stress in alcoholic lung disease could be useful for developing preventive measures at molecular levels.
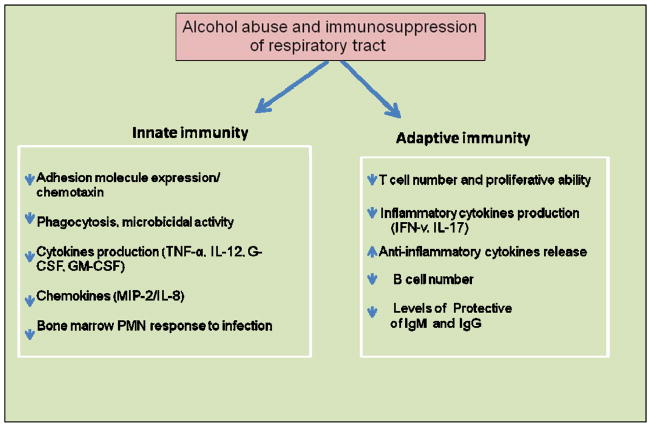
8. Alcohol abuse and immunosuppression of respiratory tract
The respiratory tract has a sophisticated immune defense mechanism that effectively protects lungs from chemical insults, and from bacterial or viral infections. However, excess alcohol consumption suppresses the immune defense network and predisposes the respiratory tract to a range of infections (Macgregor and Loubia, 1997). Reduced innate and adaptive immunity may be an important co-morbidity in alcoholic patients, as the mortality rate alone from pneumonia is more than double in alcoholics compared with non-alcoholics (Capps and Coleman, 1923)). Because of their direct exposure to the environment, host immunity expressed in the lungs is critically important to survival, and when impaired, the lungs become potential targets of pneumonia related to bacterial and viral infections.
8.1. Innate immunity
Major components of non-specific host defense include structural airway barriers that prevent entry of pathogens into the respiratory tract, and the mucociliary lining in the surface of airways, pathogen killing mechanisms such as the production of antimicrobial peptides, ROS, hypochlorous acid, and phagocytic defense by polymorphonuclear leukocytes (PMNs or neutrophils) and alveolar macrophages. Acute and chronic alcohol intoxication interferes with the innate response at structural and barrier levels causing leakiness between the physical barriers and mucosal organs, increased lung permeability due compromised tight junctions between the epithelial cells, and reduced cell mediated host defense mechanisms.
The cell mediated arm of innate immunity is primarily controlled by granulocytes (leukocytes or PMNs), monocytes/macrophages, dendritic cells and natural killer (NK) cells. Neutrophils are the most abundant type of PMNs in the lungs. Both chronic alcohol abuse and experimental acute and chronic alcohol ingestion inhibit pulmonary recruitment of PMNs and their functions, resulting in increased susceptibility to bacterial infections, and impaired bacterial clearance, in turn resulting in an increased incidence of lung diseases and related mortality (Zhang et al., 2002b). Alcohol-mediated effects range from the initial stages of primitive hematopoietic precursor commitment to impaired recruitment of PMNs, their adherence to endothelial cells and production of superoxide, elastase and nitric oxide synthase (Greenberg et al., 1999; Macgregor et al., 1988; Siggins et al., 2011; Stoltz et al., 1999; Vander Top et al., 2006). Alcohol ingestion interferes with differentiation and impairs phagocytic functions of alveolar macrophages (Bermudez and Young, 1991; Dannenberg, 1989; Laso et al., 2007; Lau et al., 2009; Siggins et al., 2009; Szabo et al., 2004).
The nature of immune responses and immune cell trafficking are controlled by cytokines and chemokines, respectively, although functional overlap exists. Alcohol predisposes the host to a variety of complications including an inability to produce important activating and chemotactic cytokines, and lung innate immunity by altering the expression of proinflammatory mediators. Alcohol-mediated suppression of pro-inflammatory cytokines (TNF and IL-1β) and chemokines (CINC and MIP-2) and induction of anti-inflammatory cytokines (IL-10) could be associated with an impaired host defense against infection, or an altered resolution of inflammation (Boe et al., 2003; Mandrekar et al., 1999; Standiford and Danforth, 1997; Stoltz et al., 2000; Zisman et al., 1998).
Granulocyte colony stimulating factor (G-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF), growth factors involved in the production or differentiation of granulocytes and granulopoietic response are also significantly reduced after ethanol exposure (Basu et al., 2002; Joshi et al., 2006; Joshi et al., 2005; Zhang et al., 2005). Given the key role of neutrophils in host defense against bacterial infections, this finding is of great potential clinical relevance in alcohol-related pneumonias.
Interferons (IFNs) secreted from host cells in response to the presence of pathogens, particularly viruses, trigger protective immune defense and interfere with viral replications. Alcohol ingestion in humans and rodents causes reduced secretion of IFNs and contributes to an increased risk of bacterial and viral infections (Starkenburg et al., 2001; Szabo et al., 2001). The lungs of alcohol-fed rodents infected with Klebsiella pneumoniae showed a decreased and delayed production of IFN-γ mRNA and related proteins resulting in reduced bacterial clearance from the lungs and reduced survival of the animals (Zisman et al., 1998).
Finally, the complement system is also affected by alcohol exposure. The complements system refers to a series of proteins circulating in the blood in an inactive form, but become sequentially activated in response to the recognition of molecular components of microorganisms. Once activated, complements cover the surface of the pathogen to be recognized for the phagocytosis. Chronic alcoholic patients express abnormally low levels of complements precursors in the circulation (Bhopale et al., 2011). Further, some studies have also reported reduced activation of the complements after chronic alcohol exposure (Bykov et al., 2007; Roychowdhury et al., 2009). Either of these abnormalities could impair complement-dependent host defense.
Therefore, a better understanding of the mechanisms by which ethanol and its metabolites regulate the expression and function of transcription factors and inflammatory mediators is needed. Many proofs of concept experiments could be evaluated using primary cell culture and animal models exposed to ethanol compared to its oxidative and non oxidative metabolites. Moreover, alcohol metabolites can also act as triggers for airway disease exacerbations especially in atopic asthmatics and in Asian populations who are known to have a reduced capacity to metabolize alcohol. Therefore, epidemiological studies in larger cohorts would improve understanding of the effects of chronic alcohol abuse and metabolites of ethanol on the complement system.
8.2. Adaptive immunity
Cell mediated adaptive immunity is another important aspect of host defense which can be impaired by alcohol and its metabolites (Fig. 3). A pathogen encounter results in dendritic cell processing and antigen presentation, which then activates and differentiates T-cells into different subtypes such as T-helper cells (Th1, 2 and 17; characterized by surface expression of CD4+) and cytotoxic T-cells, characterized by expression of CD8+ and contain powerful enzymes for inducing the death of infected cells. Finally, interplay between the B-cells and T-cells is required for optimal immune responses to counteract the invasion of most the pathogens.
Fig. 3.
Schematic illustration by which alcohol abuse increases the risk of pulmonary infection by impairing the innate and adaptive immunity.
Acute and chronic alcohol ingestion can interfere with antigen presentation required to activate T- and B-cells and can also markedly affect the differentiation of dendritic cells (Ness et al., 2008). A significant reduction in absolute numbers of CD4+ T lymphocytes has been reported in chronic alcoholics (Saad and Jerrells, 1991). Generally, lymphocyte proliferative responses to specific antibodies against T-cell receptors are blunted by alcohol (Domiati-Saad and Jerrells, 1993). In alcoholics, a diminished capacity of T lymphocytes to produce IFN-γ, an important cytokine that stimulates cell-mediated immunity has been reported (Chadha et al., 1991). In addition, alcohol consumption can also suppress the recruitment of CD4+ and CD8+ T lymphocytes in response to P. carinii infection in the lungs (Shellito and Olariu, 1998). T cells isolated from chronic alcoholics and ethanol-intoxicated animals possess a decreased response to mitogen stimulation and an impaired hypersensitivity responses (Lundy et al., 1975; Spinozzi et al., 1991). Animal studies of pulmonary tuberculosis have shown decreased lung CD4+ and CD8+ T cells and diminished proliferation in ethanol-fed mice (Mason et al., 2004).
Pulmonary host defense against bacterial pathogens is dependent on intact type-1 T-cell immunity (Greenberger et al., 1996; Moore et al., 2002). Discovery of the T-cell cytokine IL-17 is important because it serves as a link between adaptive and innate immunity upregulating chemokines and cytokines to promote neutrophilic inflammation. Animals inoculated with K. pneumoniae exhibit induced expression of pulmonary IL-17 within 12 h, and animals deficient in the receptor for IL-17display an increased mortality from infection with this pathogen (Ye et al., 2001). Chronic alcohol intoxication inhibits the pulmonary IL-17 response to K. pneumoniae infection, and shows a dose-dependent inhibition of IL-17 by ethanol after in vitro stimulation of T cells (Shellito et al., 2001). Therefore, induction of IL-17 cytokine can improve survival of alcohol-treated animals (Ye et al., 2001).
Despite the decreases in B cell numbers, alcoholics with liver disease have increased levels of circulating nonprotective IgA, IgM, and IgG. In contrast, bronchoalveolar lavage (BAL) fluid in patients with alcoholic liver disease exhibits reduced levels of total IgG and IgG1 (Spinozzi et al., 1992). This defect closely correlates with the development of bacterial pneumonia. Replacement of immunoglobulin therapy partially restores BAL Ig levels and decreases the rate of subsequent pulmonary infection, further supporting the importance of airway antibodies in host defense.
9. Approaches for treatment of alcoholic lung disease
Most therapeutic efforts directed towards alcoholic lung disease are based upon the use of direct antioxidants such as N-acetylcysteine (NAC) and GSH. As a precursor of cysteine needed for the synthesis of GSH, NAC acts as indirect antioxidant. Therefore, restoring GSH homeostasis in the lung by GSH replenishment or by enhancing its endogenous synthesis has therapeutic potential for treating chronic alcoholic lung disease. Such an approach can also restore antioxidant enzymes (e.g. glutathione reductase and superoxide dismutase).
In animals, dietary GSH supplementation is effective in maintaining GSH homeostasis, but it requires chronic GSH ingestion to prevent oxidative damage (Guidot and Brown, 2000; Guidot et al., 2000; Holguin et al., 1998). Dietary supplementation of GSH precursors can also restore both the mitochondrial and cytosolic GSH pool, as well as alveolar epithelial functions and can significantly decrease the risk of alcoholic lung injury (Guidot and Brown, 2000). Therefore, adequate GSH precursor supplementation, which might significantly decrease risk of alcoholic lung injury and possibly even pneumonia, should be a better therapeutic approach.
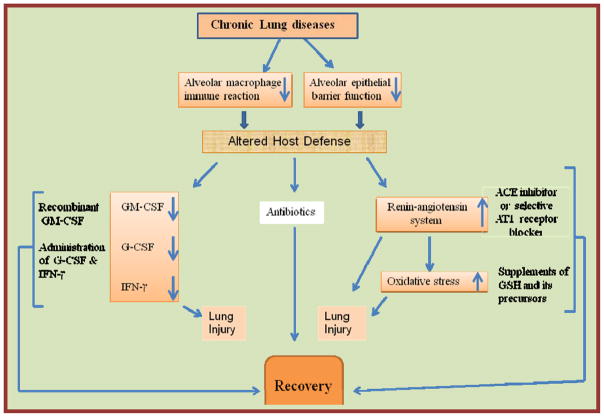
An association of the D/D genotype of the angiotensin converting enzyme (ACE) I/D polymorphism with phagocytic NADPH oxidase-mediated superoxide overproduction has been reported (Jose et al., 2009). The observation suggests that the mechanisms that control the angiotensin pathway are linked to the development of oxidative stress and potentially oxidative injury. Thus not surprisingly ACE II have been shown to significantly decrease the pulmonary complications due to alcohol abuse (Bechara et al., 2003). Therefore, ACE II inhibitors as well as AT1 receptor blockers could potentially be employed to prevent the alcoholic lung phenotype. (Fig. 4),
Fig. 4.
Lung injury targets in chronic alcoholic abuse and therapeutic approaches.
G-CSF enhances pulmonary clearance of bacteria from ethanol treated rats and improves their survival including the expression of adhesion molecules and phagocytosis (Stoltz et al., 1999; Zhang et al., 1999). Rats fed G-CSF (50 μg/kg) twice daily for 2 d can increase circulating PMN’s within the alveolar space by 5 – 7 folds (Zhang et al., 1999). In a clinical trial of 746 patients, subcutaneous injection of G-CSF (300 μg/day) for up to 10 d increased circulating PMNs 3-fold and exhibited less complications due to ARDS and disseminated intravascular coagulation (Nelson et al., 1998). Therefore, for alcoholic lung diseases, enhancing the chemotactic signals using G-CSF can enhance recruitment of circulating PMNs in to the alveolar space.
Expressions of the GM-CSF receptor, alveolar epithelial barrier function and fluid transport are significantly reduced in rats fed ethanol (Joshi et al., 2005; Mandujano et al., 1995). These expressions can be restored by intrapulmonary administration of recombinant GM-CSF, which improves functional activities of alveolar macrophages in alcohol-fed rats and restores alveolar epithelial barrier function damaged by alcohol ingestion (Pelaez et al., 2004).
GM-CSF therapy can reduce the intensity of pulmonary P. carinii infection associated with enhanced alveolar macrophage TNF production in CD4 depleted mice (Paine et al., 2000). Infusion of GM-CSF at a dose of 125 μg/m2 for 72 h causes up-regulation of CD11b expression on circulating PMNs and a more frequent resolution of infection in patients (Rosenbloom et al., 2005). In a clinical trial of 1200 neutropenic patients with pneumonia, a daily administration of GM-CSF (mean dose of 5 μg/kg daily) for a mean period of 13 d restored blood leukocyte counts (Dierdorf et al., 1997). Hematopoietic recovery was also observed in 74% of the patients with a good clinical and/or radiologic improvement. Therefore, GM-CSF therapy can be beneficial for alcoholic patients. However, higher GM-CSF daily doses (i.e., >15 μg/kg) are associated with serious side effects such as generalized inflammation and tissue injury (Arning et al., 1991).
Macrophages stimulated by IFN-γ are capable of killing over 3 dozen different pathogens (Murray, 1994). IFN-γ administered along with antibiotics produces synergistic effects in the treatment of certain pulmonary infections. Intratracheal instillation of IFN-γ or its aerosol inhalation activates alveolar macrophages that enhance the lung microbicidal activities (Beck et al., 1991; Jaffe et al., 1991). Local or systemic administration of IFN-γ alleviates alcohol-induced suppression of MIP-2 and CINC production in the lung following intrapulmonary LPS challenge. Interestingly, patients with multidrug resistant tuberculosis treated with IFN-γ rapidly clear the infection and eradicate the mycobacteria (Condos et al., 1997; Gallin et al., 1995).
Whether any particular long term therapeutic strategy will be effective for either alcoholic lung disease, ARDS or COPD cannot be predicted with certainty. Combination therapies including antibiotics will be required to treat acute and chronic lung injuries. Though dietary supplementation with GSH precursors or selective inhibition of ACE II and/or AT receptors can limit lung injury in animal models, G-CSF appears to be most attractive candidate for treating the alcoholic lung disease as well as for ARDS and COPD.
10. Conclusions
Alcoholic lung disease is a significant public health concern and understanding its mechanism should enable us to develop effective therapeutic intervention and treatment. Immunosuppression due to acute or chronic alcohol abuse appears to be a significant risk factor in the initiation and progression of ARDS and COPD. Impaired alveolar epithelial barrier functions, mucocilliary clearance and GM-CSF signaling, and increased renin angiotensin activity are the major effects of acute and chronic alcohol abuse. A dramatic depletion of reduced glutathione in the lungs of ethanol fed animals is a hallmark of the prevailing oxidative stress. Therefore, oxidative metabolism of ethanol and its associated oxidative stress have been suggested as underlying mechanisms of alcoholic lung disease. ER stress and UPR signaling in COPD and lung fibrosis have also been reported. Therefore, ER stress and oxidative stress, both associated with ethanol abuse, could be critical factors for the suppression of innate and adaptive immunity in the lungs, as is reported in ARDS and COPD. In view of the metabolic basis of alcoholic lung diseases, studies investigating the role of nonoxidative metabolites of ethanol such as FAEEs and PEt are warranted. Although not discussed in this review, discovery driven approaches such as metabolomics and proteomics could be utilized to identify molecular pathways of disease, so as to develop biomarker candidates in alcoholic lung disease. Previous therapeutic studies have largely addressed replenishing reduced GSH levels or administering GM-CSF. However, treating immunosuppressive disease related to ethanol abuse becomes even more challenging because the targets are multiple and no biomarkers are yet identified. Therefore, understanding the mechanism of immunosuppresion by ethanol oxidative metabolites in conjunction with ER stress could open new avenues to identify therapeutic targets for alcoholic lung disease.
High lights.
Ethanol-induced immunosuppression and alcoholic lung disease
Metabolic basis of alcoholic lung disease
Ethanol-induced oxidative stress and endoplasmic stress in lungs
Alcoholic lung disease and innate and adaptive immunity
Therapeutic approaches for alcoholic lung disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alling C, Chick JD, Anton R, Mayfield RD, Salaspuro M, Helander A, Harris RA. Revealing Alcohol Abuse: To Ask or to Test? Alcoholism: Clinical and Experimental Research. 2005;29:1257–1263. doi: 10.1097/01.alc.0000171489.55856.a5. [DOI] [PubMed] [Google Scholar]
- Appelman LM, Woutersen RA, Feron VJ, Hooftman RN, Notten WRF. EFFECT OF VARIABLE VERSUS FIXED EXPOSURE LEVELS ON THE TOXICITY OF ACETALDEHYDE IN RATS. Journal of Applied Toxicology. 1986;6:331–336. doi: 10.1002/jat.2550060506. [DOI] [PubMed] [Google Scholar]
- Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C. Phosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol and Alcoholism. 2006;41:431–437. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- Aradottir S, Lundqvist C, Alling C. Phosphatidylethanol in rat organs after ethanol exposure. Alcoholism-Clinical and Experimental Research. 2002;26:514–518. [PubMed] [Google Scholar]
- Arning M, Kliche KO, Schneider W. GM-CSF THERAPY AND CAPILLARY-LEAK SYNDROME. Annals of Hematology. 1991;62:83–83. doi: 10.1007/BF01714907. [DOI] [PubMed] [Google Scholar]
- Aytacoglu BN, Calikoglu M, Tamer L, Coskun B, Sucu N, Kose N, Aktas S, Dikmengil M. Alcohol-induced lung damage and increased oxidative stress. Respiration. 2006;73:100–104. doi: 10.1159/000088680. [DOI] [PubMed] [Google Scholar]
- Basu S, Dunn A, Ward A. G-CSF: Function and modes of action (Review) International Journal of Molecular Medicine. 2002;10:3–10. [PubMed] [Google Scholar]
- Bechara RI, Brown LAS, Eaton DC, Roman J, Guidot DM. Chronic ethanol ingestion increases expression of the angiotensin II type 2 (AT(2)) receptor and enhances tumor necrosis factor-alpha- and angiotensin II-induced cytotoxicity via AT(2) signaling in rat alveolar epithelial cells. Alcoholism-Clinical and Experimental Research. 2003;27:1006–1014. doi: 10.1097/01.ALC.0000071932.56932.53. [DOI] [PubMed] [Google Scholar]
- Beck JM, Liggitt HD, Brunette EN, Fuchs HJ, Shellito JE, Debs RJ. REDUCTION IN INTENSITY OF PNEUMOCYSTIS-CARINII PNEUMONIA IN MICE BY AEROSOL ADMINISTRATION OF GAMMA-INTERFERON. Infection and Immunity. 1991;59:3859–3862. doi: 10.1128/iai.59.11.3859-3862.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez LE, Young LS. ETHANOL AUGMENTS INTRACELLULAR SURVIVAL OF MYCOBACTERIUM-AVIUM COMPLEX AND IMPAIRS MACROPHAGE RESPONSES TO CYTOKINES. Journal of Infectious Diseases. 1991;163:1286–1292. doi: 10.1093/infdis/163.6.1286. [DOI] [PubMed] [Google Scholar]
- Bernstein J. THE ROLE OF THE LUNG IN THE METABOLISM OF ETHANOL. Research Communications in Chemical Pathology and Pharmacology. 1982;38:43–56. [PubMed] [Google Scholar]
- Bernstein J, Basilio C, Martinez B. ETHANOL SULFATION BY THE PULMONARY ETHANOL METABOLIZING SYSTEM (PET) Research Communications in Chemical Pathology and Pharmacology. 1990;68:219–234. [PubMed] [Google Scholar]
- Bhopale K, Nauduri D, Soman K, Sood G, Okorodudu A, Ansari G, Kaphalia B. Differential Expression of plasma proteins in patients diagnosed with alcoholic liver disease or nonalcoholic steatohepatitis. Eur J Hepato-Gastroenterol. 2011;1:89–99. [Google Scholar]
- Boe DM, Nelson S, Zhang P, Quinton L, Bagby GJ. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcoholism-Clinical and Experimental Research. 2003;27:1838–1845. doi: 10.1097/01.ALC.0000095634.82310.53. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic Costs of Excessive Alcohol Consumption in the U.S., 2006. American Journal of Preventive Medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Bykov I, Junnikkala S, Pekna M, Lindros KO, Meri S. Effect of chronic ethanol consumption on the expression of complement components and acute-phase proteins in liver. Clinical Immunology. 2007;124:213–220. doi: 10.1016/j.clim.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Capps Coleman. Influence of alcohol on prognosis of pneumonia in cook county hospital: A statistical report. Journal of the American Medical Association. 1923;80:750–752. [Google Scholar]
- CDC. Alcohol-Attributable Deaths and Years of Potential Life Lost --- United States, 2001. MMWR. 2004;53:866–870. [PubMed] [Google Scholar]
- Cederbaum AI. Role of CYP2E1 in Ethanol-Induced Oxidant Stress, Fatty Liver and Hepatotoxicity. Digestive Diseases. 2010;28:802–811. doi: 10.1159/000324289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha KC, Stadler I, Albini B, Nakeeb SM, Thacore HR. EFFECT OF ALCOHOL ON SPLEEN-CELLS AND THEIR FUNCTIONS IN C57BL/6 MICE. Alcohol. 1991;8:481–485. doi: 10.1016/s0741-8329(91)90187-2. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Singh A, Brahmandam M, Podar K, Hideshirna T, Richardson P, Munshi N, Palladino MA, Anderson KC. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2008;111:1654–1664. doi: 10.1182/blood-2007-08-105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997;349:1513–1515. doi: 10.1016/S0140-6736(96)12273-X. [DOI] [PubMed] [Google Scholar]
- Dannenberg AM. IMMUNE-MECHANISMS IN THE PATHOGENESIS OF PULMONARY TUBERCULOSIS. Reviews of Infectious Diseases. 1989;11:S369–S378. doi: 10.1093/clinids/11.supplement_2.s369. [DOI] [PubMed] [Google Scholar]
- Das SK, Mukherjee S. Long-term ethanol consumption leads to lung tissue oxidative stress and injury. Oxidative Medicine and Cellular Longevity. 2010;3:414–420. doi: 10.4161/oxim.3.6.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CP, Yeaman SJ. THE BIOCHEMISTRY OF ALCOHOL-INDUCED FATTY LIVER. Biochimica Et Biophysica Acta-Lipids and Lipid Metabolism. 1994;1215:33–48. doi: 10.1016/0005-2760(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Dierdorf R, Kreuter U, Jones T. A Role for Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) in the Treatment of Neutropenic Patients with Pneumonia. Braz J Infect Dis. 1997;1:68–76. [PubMed] [Google Scholar]
- Domiati-Saad R, Jerrells TR. THE INFLUENCE OF AGE ON BLOOD-ALCOHOL LEVELS AND ETHANOL-ASSOCIATED IMMUNOSUPPRESSION IN A MURINE MODEL OF ETHANOL-CONSUMPTION. Alcoholism-Clinical and Experimental Research. 1993;17:382–388. doi: 10.1111/j.1530-0277.1993.tb00780.x. [DOI] [PubMed] [Google Scholar]
- Gallin JI, Farber JM, Holland SM, Nutman TB. INTERFERON-GAMMA IN THE MANAGEMENT OF INFECTIOUS-DISEASES. Annals of Internal Medicine. 1995;123:216–224. doi: 10.7326/0003-4819-123-3-199508010-00009. [DOI] [PubMed] [Google Scholar]
- Gamble L, Mason CM, Nelson S. The effects of alcohol on immunity and bacterial infection in the lung. Medecine Et Maladies Infectieuses. 2006;36:72–77. doi: 10.1016/j.medmal.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug and Alcohol Dependence. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Greenberg SS, Zhao XF, Hua L, Wang JF, Nelson S, Ouyang J. Ethanol inhibits lung clearance of Pseudomonas aeruginosa by a neutrophil and nitric oxide-dependent mechanism, in vivo. Alcoholism-Clinical and Experimental Research. 1999;23:735–744. [PubMed] [Google Scholar]
- Greenberger MJ, Kunkel SL, Strieter RM, Lukacs NW, Bramson J, Gauldie J, Graham FL, Hitt M, Danforth JM, Standiford TJ. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. Journal of Immunology. 1996;157:3006–3012. [PubMed] [Google Scholar]
- Greene CM, McElvaney NG. Z α-1 antitrypsin deficiency and the endoplasmic reticulum stress response. World J Gastrointest Pharmacol Ther. 2010;1:94–101. doi: 10.4292/wjgpt.v1.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidot DM, Brown LAS. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcoholism-Clinical and Experimental Research. 2000;24:1070–1076. [PubMed] [Google Scholar]
- Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LAS. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2000;279:L127–L135. doi: 10.1152/ajplung.2000.279.1.L127. [DOI] [PubMed] [Google Scholar]
- Guidot DM, Roman J. Chronic ethanol ingestion increases susceptibility to acute lung injury - Role of oxidative stress and tissue remodeling. Chest. 2002;122:309S–314S. doi: 10.1378/chest.122.6_suppl.309s. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Vitamin C: antioxidant or pro-oxidant in vivo? Free Radic Res. 1996;25:439–454. doi: 10.3109/10715769609149066. [DOI] [PubMed] [Google Scholar]
- Heffner JE, Repine JE. PULMONARY STRATEGIES OF ANTIOXIDANT DEFENSE. American Review of Respiratory Disease. 1989;140:531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- Holguin F, Moss IM, Brown LAS, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. Journal of Clinical Investigation. 1998;101:761–768. doi: 10.1172/JCI1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Pelkonen O, Hakkola JHR. Expression and regulation of xenobiotic-metabolizing cytochrome P450 (CYP) enzymes in human lung. Critical Reviews in Toxicology. 2002;32:391–411. doi: 10.1080/20024091064273. [DOI] [PubMed] [Google Scholar]
- Jaffe HA, Buhl R, Mastrangeli A, Holroyd KJ, Saltini C, Czerski D, Jaffe HS, Kramer S, Sherwin S, Crystal RG. ORGAN SPECIFIC CYTOKINE THERAPY - LOCAL ACTIVATION OF MONONUCLEAR PHAGOCYTES BY DELIVERY OF AN AEROSOL OF RECOMBINANT INTERFERON-GAMMA TO THE HUMAN LUNG. Journal of Clinical Investigation. 1991;88:297–302. doi: 10.1172/JCI115291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C. Mechanisms of Alcohol-Induced Endoplasmic Reticulum Stress and Organ Injuries. Biochemistry Research International. 2012;2012:12. doi: 10.1155/2012/216450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS. Liver-Specific Loss of Glucose-Regulated Protein 78 Perturbs the Unfolded Protein Response and Exacerbates a Spectrum of Liver Diseases in Mice. Hepatology. 2011;54:229–239. doi: 10.1002/hep.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW. MEASURING AND REPORTING THE CONCENTRATION OF ACETALDEHYDE IN HUMAN BREATH. Alcohol and Alcoholism. 1995;30:271–285. [PubMed] [Google Scholar]
- Jose GS, Fortuno A, Moreno MU, Robador PA, Bidegain J, Varo N, Beloqui O, Diez J, Zalba G. The angiotensin-converting enzyme insertion/deletion polymorphism is associated with phagocytic NADPH oxidase-dependent superoxide generation: potential implication in hypertension. Clinical Science. 2009;116:233–240. doi: 10.1042/CS20080057. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Applewhite L, Mitchell PO, Fernainy K, Roman J, Eaton DC, Guidot DM. GM-CSF receptor expression and signaling is decreased in lungs of ethanol-fed rats. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2006;291:L1150–L1158. doi: 10.1152/ajplung.00150.2006. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, Brown LAS, Guidot DM. Chronic Ethanol Ingestion in Rats Decreases Granulocyte-Macrophage Colony-Stimulating Factor Receptor Expression and Downstream Signaling in the Alveolar Macrophage. The Journal of Immunology. 2005;175:6837–6845. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- Kaphalia BS, Cai P, Khan MF, Okorodudu AO, Ansari GAS. Fatty acid ethyl esters: markers of alcohol abuse and alcoholism. Alcohol. 2004;34:151–158. doi: 10.1016/j.alcohol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Kaphalia L, Calhoun WJ. Bronchoalveolar Lavage (BAL) fluid from asthmatic patients and lipopolysaccharide activate the endoplasmic reticulum stress response in primary human bronchial smooth muscle cells. Am J Respir Crit Care Med. 2012:A4278–4285. [Google Scholar]
- kaphalia L, Nahal B, Bhupendra SK, William JC. B57 OPPORTUNITIES FOR PREVENTION OF COMMUNITY AND WORKPLACE DISEASE. American Thoracic Society; 2013. Endoplasmic Reticulum Stress And Unfolded Protein Response In The Lungs Of Deer Mice After Chronic Exposure To Ethanol; pp. A3143–A3143. [Google Scholar]
- Kardosh A, Golden EB, Pyrko P, Uddin J, Hofman FM, Chen TC, Louie SG, Petasis NA, Schonthal AH. Aggravated endoplasmic reticulum stress as a basis for enhanced glioblastoma cell killing by bortezomib in combination with celecoxib or its non-coxib analogue, 2,5-dimethyl-celecoxib. Cancer Research. 2008;68:843–851. doi: 10.1158/0008-5472.CAN-07-5555. [DOI] [PubMed] [Google Scholar]
- Kelsen SG, Duan XB, Ji R, Perez O, Liu C, Merali S. Cigarette smoke induces an unfolded protein response in the human lung - A proteomic approach. American Journal of Respiratory Cell and Molecular Biology. 2008;38:541–550. doi: 10.1165/rcmb.2007-0221OC. [DOI] [PubMed] [Google Scholar]
- Laposata EA, Lange LG. PRESENCE OF NONOXIDATIVE ETHANOL-METABOLISM IN HUMAN ORGANS COMMONLY DAMAGED BY ETHANOL ABUSE. Science. 1986;231:497–499. doi: 10.1126/science.3941913. [DOI] [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcoholism-Clinical and Experimental Research. 2007;31:846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- Lau AH, Szabo G, Thomson AW. Antigen-presenting cells under the influence of alcohol. Trends in Immunology. 2009;30:13–22. doi: 10.1016/j.it.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XCC, Newcomb DC, Jones BR, Roldan J, Lane KB, Morrisey EE, Beers MF, Yull FE, Blackwell TS. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10562–10567. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Yeligar SM, Brown LAS. Chronic-Alcohol-Abuse-Induced Oxidative Stress in the Development of Acute Respiratory Distress Syndrome. The Scientific World Journal. 2012;2012:9. doi: 10.1100/2012/740308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS. SEMINARS IN MEDICINE OF THE BETH-ISRAEL-HOSPITAL, BOSTON - MEDICAL DISORDERS OF ALCOHOLISM. New England Journal of Medicine. 1995;333:1058–1065. doi: 10.1056/NEJM199510193331607. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Lundy J, Raaf J, Deakins S, Wanebo H, Jacobs D, Lee T, Jacobowitz D, Spear C, Oettgen H. The acute and chronic effects of alcohol on the human immune system. Surg Gynecol Obstet. 1975;141:212–218. [PubMed] [Google Scholar]
- Macgregor R, Loubia D. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- Macgregor RR, Safford M, Shalit M. EFFECT OF ETHANOL ON FUNCTIONS REQUIRED FOR THE DELIVERY OF NEUTROPHILS TO SITES OF INFLAMMATION. Journal of Infectious Diseases. 1988;157:682–689. doi: 10.1093/infdis/157.4.682. [DOI] [PubMed] [Google Scholar]
- MacNee W. Oxidative stress and lung inflammation in airways disease. European Journal of Pharmacology. 2001;429:195–207. doi: 10.1016/s0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Cycle or a Double-Edged Sword? Antioxidants & Redox Signaling. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Manautou J, Buss N, Carlson G. Oxidative and non-oxidative metabolism of ethanol by the rabbit lung. Toxicol letter. 1992;62:93–99. doi: 10.1016/0378-4274(92)90082-u. [DOI] [PubMed] [Google Scholar]
- Manautou JE, Carlson GP. Ethanol-induced fatty acid ethyl ester formation in vivo and in vitro in rat lung. Toxicology. 1991;70:303–312. doi: 10.1016/0300-483x(91)90005-l. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFκB activation by ethanol in human monocytes. International Immunology. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- Mandujano JF, Dsouza NB, Nelson S, Summer WR, Beckerman RC, Shellito JE. GRANULOCYTE-MACROPHAGE COLONY-STIMULATING FACTOR AND PNEUMOCYSTIS-CARINII PNEUMONIA IN MICE. American Journal of Respiratory and Critical Care Medicine. 1995;151:1233–1238. doi: 10.1164/ajrccm/151.4.1233. [DOI] [PubMed] [Google Scholar]
- Mason CM, Dobard E, Zhang P, Nelson S. Alcohol exacerbates murine pulmonary tuberculosis. Infection and Immunity. 2004;72:2556–2563. doi: 10.1128/IAI.72.5.2556-2563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon S. Mechanisms and regulation of ion transport in adult mammalian alveolar type II pneumocytes. American Journal of Physiology - Cell Physiology. 1991;261:C727–C738. doi: 10.1152/ajpcell.1991.261.5.C727. [DOI] [PubMed] [Google Scholar]
- Moore TA, Perry ML, Getsoian AG, Newstead MW, Standiford TJ. Divergent role of gamma interferon in a murine model of pulmonary versus systemic Klebsiella pneumoniae infection. Infection and Immunity. 2002;70:6310–6318. doi: 10.1128/IAI.70.11.6310-6318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. Jama-Journal of the American Medical Association. 1996;275:50–54. [PubMed] [Google Scholar]
- Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LAS. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. American Journal of Respiratory and Critical Care Medicine. 2000;161:414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- Murray HW. INTERFERON-GAMMA AND HOST ANTIMICROBIAL DEFENSE - CURRENT AND FUTURE CLINICAL-APPLICATIONS. American Journal of Medicine. 1994;97:459–467. doi: 10.1016/0002-9343(94)90326-3. [DOI] [PubMed] [Google Scholar]
- Nelson S, Belknap SM, Carlson RW, Dale D, DeBoisblanc B, Farkas S, Fotheringham N, Ho H, Marrie T, Movahhed H, Root R, Wilson J, Grp CAPS. A randomized controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalized patients with community-acquired pneumonia. Journal of Infectious Diseases. 1998;178:1075–1080. doi: 10.1086/515694. [DOI] [PubMed] [Google Scholar]
- Ness KJ, Fan J, Wilke WW, Coleman RA, Cook RT, Schlueter AJ. Chronic ethanol consumption decreases murine langerhans cell numbers and delays migration of langerhans cells as well as dermal dendritic cells. Alcoholism-Clinical and Experimental Research. 2008;32:657–668. doi: 10.1111/j.1530-0277.2007.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. 10th Special Report to the US Congress on Alcohol and Health. National Institute on Alcohol Abuse and Alcoholism; 2000. [Google Scholar]
- Pabst D, Kuehn J, Schuler-Luettmann S, Wiebe K, Lebiedz P. Acute Respiratory Distress Syndrome as a presenting manifestation in young patients infected with H1N1 influenza virus. European Journal of Internal Medicine. 2011;22:e119–e124. doi: 10.1016/j.ejim.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Paine R, Preston AM, Wilcoxen S, Jin H, Siu BB, Morris SB, Reed JA, Ross G, Whitsett JA, Beck JM. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. Journal of Immunology. 2000;164:2602–2609. doi: 10.4049/jimmunol.164.5.2602. [DOI] [PubMed] [Google Scholar]
- Pelaez A, Bechara RI, Joshi PC, Brown LAS, Guidot DM. Granulocyte/macrophage colony-stimulating factor treatment improves alveolar epithelial barrier function in alcoholic rat lung. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2004;286:L106–L111. doi: 10.1152/ajplung.00148.2003. [DOI] [PubMed] [Google Scholar]
- Polikandriotis JA, Rupnow HL, Elms SC, Clempus RE, Campbell DJ, Sutliff RL, Brown LAS, Guidot DM, Hart CM. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. American Journal of Respiratory Cell and Molecular Biology. 2006;34:314–319. doi: 10.1165/rcmb.2005-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin GH, Meng ZQ. The expressions of protooncogenes and CYP1A in lungs of rats exposed to sulfur dioxide and benzo(a)pyrene. Regulatory Toxicology and Pharmacology. 2006;45:36–43. doi: 10.1016/j.yrtph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radical Biology and Medicine. 1996;21:669–681. doi: 10.1016/0891-5849(96)00155-4. [DOI] [PubMed] [Google Scholar]
- Rikans LE, Gonzalez LP. ANTIOXIDANT PROTECTION SYSTEMS OF RAT LUNG AFTER CHRONIC ETHANOL INHALATION. Alcoholism-Clinical and Experimental Research. 1990;14:872–877. doi: 10.1111/j.1530-0277.1990.tb01830.x. [DOI] [PubMed] [Google Scholar]
- Rosenbloom AJ, Linden PK, Dorrance A, Penkosky N, Cohen-Melamed MH, Pinsky MR. Effect of granulocyte-monocyte colony-stimulating factor therapy on leukocyte function and clearance of serious infection in nonneutropenic patients. Chest. 2005;127:2139–2150. doi: 10.1378/chest.127.6.2139. [DOI] [PubMed] [Google Scholar]
- Roychowdhury S, McMullen MR, Pritchard MT, Hise AG, van Rooijen N, Medof ME, Stavitsky AB, Nagy LE. An Early Complement-Dependent and TLR-4-Independent Phase in the Pathogenesis of Ethanol-Induced Liver Injury in Mice. Hepatology. 2009;49:1326–1334. doi: 10.1002/hep.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad AJ, Jerrells TR. FLOW CYTOMETRIC AND IMMUNOHISTOCHEMICAL EVALUATION OF ETHANOL-INDUCED CHANGES IN SPLENIC AND THYMIC LYMPHOID-CELL POPULATIONS. Alcoholism-Clinical and Experimental Research. 1991;15:796–803. doi: 10.1111/j.1530-0277.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Shang Y, Wang F, Bai C, Huang Y, Zhao LJ, Yao XP, Li QA, Sun SH. Dexamethasone protects airway epithelial cell line NCI-H292 against lipopolysaccharide induced endoplasmic reticulum stress and apoptosis. Chinese Medical Journal. 2011;124:38–44. [PubMed] [Google Scholar]
- Sharma R, Gupta S, Singhal SS, Ahmad H, Haque A, Awasthi YC. INDEPENDENT SEGREGATION OF GLUTATHIONE-S-TRANSFERASE AND FATTY-ACID ETHYL-ESTER SYNTHASE FROM PANCREAS AND OTHER HUMAN TISSUES. Biochemical Journal. 1991;275:507–513. doi: 10.1042/bj2750507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellito JE. Alcohol and host defense against pulmonary infection with Pneumocystis carinii. Alcoholism-Clinical and Experimental Research. 1998;22:208S–211S. doi: 10.1111/j.1530-0277.1998.tb04003.x. [DOI] [PubMed] [Google Scholar]
- Shellito JE, Olariu R. Alcohol Decreases T-Lymphocyte Migration into Lung Tissue in Response to Pneumocystis carinii and Depletes T-Lymphocyte Numbers in the Spleens of Mice. Alcoholism: Clinical and Experimental Research. 1998;22:658–663. doi: 10.1111/j.1530-0277.1998.tb04308.x. [DOI] [PubMed] [Google Scholar]
- Shellito JE, Zheng MQ, Ye P, Ruan S, Shean MK, Kolls J. Effect of Alcohol Consumption on Host Release of Interleukin-17 During Pulmonary Infection With Klebsiella pneumoniae. Alcoholism: Clinical and Experimental Research. 2001;25:872–881. [PubMed] [Google Scholar]
- Siggins RW, Bagby GJ, Molina P, Dufour J, Nelson S, Zhang P. Alcohol Exposure Impairs Myeloid Dendritic Cell Function in Rhesus Macaques. Alcoholism: Clinical and Experimental Research. 2009;33:1524–1531. doi: 10.1111/j.1530-0277.2009.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins RW, Melvan JN, Welsh DA, Bagby GJ, Nelson S, Zhang P. Alcohol Suppresses the Granulopoietic Response to Pulmonary Streptococcus pneumoniae Infection with Enhancement of STAT3 Signaling. The Journal of Immunology. 2011;186:4306–4313. doi: 10.4049/jimmunol.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinozzi F, Bertotto A, Rondoni F, Gerli R, Scalise F, Grignani F. T-LYMPHOCYTE ACTIVATION PATHWAYS IN ALCOHOLIC LIVER-DISEASE. Immunology. 1991;73:140–146. [PMC free article] [PubMed] [Google Scholar]
- Spinozzi F, Cimignoli E, Gerli R, Agea E, Bertotto A, Rondoni F, Grignani F. IGG SUBCLASS DEFICIENCY AND SINOPULMONARY BACTERIAL-INFECTIONS IN PATIENTS WITH ALCOHOLIC LIVER-DISEASE. Archives of Internal Medicine. 1992;152:99–104. [PubMed] [Google Scholar]
- Standiford TJ, Danforth JM. Ethanol feeding inhibits proinflammatory cytokine expression from murine alveolar macrophages ex vivo. Alcoholism-Clinical and Experimental Research. 1997;21:1212–1217. [PubMed] [Google Scholar]
- Starkenburg S, Munroe ME, Waltenbaugh C. Early alteration in leukocyte populations and Th1/Th2 function in ethanol-consuming mice. Alcoholism-Clinical and Experimental Research. 2001;25:1221–1230. [PubMed] [Google Scholar]
- Stoltz DA, Nelson S, Kolls JK, Zhang P, Bohm RP, Murphey-Corb M, Bagby GJ. In vitro ethanol suppresses alveolar macrophage TNF-alpha during simian immunodeficiency virus infection. American Journal of Respiratory and Critical Care Medicine. 2000;161:135–140. doi: 10.1164/ajrccm.161.1.9905016. [DOI] [PubMed] [Google Scholar]
- Stoltz DA, Zhang P, Nelson S, Bohm RP, Murphey-Corb M, Bagby GJ. Ethanol suppression of the functional state of polymorphonuclear leukocytes obtained from uninfected and simian immunodeficiency virus infected rhesus macaques. Alcoholism-Clinical and Experimental Research. 1999;23:878–884. [PubMed] [Google Scholar]
- Szabo G, Catalano D, White B, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcoholism-Clinical and Experimental Research. 2004;28:824–828. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Dolganiuc A, Catalano D, Kodys K. Reduced Alloreactive T-Cell Activation After Alcohol Intake is Due to Impaired Monocyte Accessory Cell Function and Correlates With Elevated IL-10, IL-13, and Decreased IFNγ Levels. Alcoholism: Clinical and Experimental Research. 2001;25:1766–1772. [PubMed] [Google Scholar]
- Tagawa Y, Hiramatsu N, Kasai A, Hayakawa K, Okamura M, Yao J, Kitamura M. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP) Free Radical Biology and Medicine. 2008;45:50–59. doi: 10.1016/j.freeradbiomed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Vander Top EA, Perry GA, Snitily MU, Gentry-Nielsen MJ. Smoke Exposure and Ethanol Ingestion Modulate Intrapulmonary Polymorphonuclear Leukocyte Killing, but Not Recruitment or Phagocytosis. Alcoholism: Clinical and Experimental Research. 2006;30:1599–1607. doi: 10.1111/j.1530-0277.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- Vander Top EA, Wyatt TA, Gentry-Nielsen MJ. Smoke Exposure Exacerbates an Ethanol-Induced Defect in Mucociliary Clearance of Streptococcus pneumoniae. Alcoholism: Clinical and Experimental Research. 2005;29:882–887. doi: 10.1097/01.alc.0000164364.35682.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliou V, Marselos M. Tissue Distribution of Inducible Aldehyde Dehydrogenase Activity in the Rat after Treatment with Phenobarbital or Methylcholanthrene. Pharmacology & Toxicology. 1989;64:39–42. doi: 10.1111/j.1600-0773.1989.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Werner J, Laposata M, FernandezDelCastillo C, Saghir M, Iozzo RV, Lewandrowski KB, Warshaw AL. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology. 1997;113:286–294. doi: 10.1016/s0016-5085(97)70106-9. [DOI] [PubMed] [Google Scholar]
- Wu H, Cai P, Clemens DL, Jerrells TR, Ansari GAS, Kaphalia BS. Metabolic basis of ethanol-induced cytotoxicity in recombinant HepG2 cells: Role of nonoxidative metabolism. Toxicology and Applied Pharmacology. 2006;216:238–247. doi: 10.1016/j.taap.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Kharbanda KK, McCaskill ML, Tuma DJ, Yanov D, DeVasure J, Sisson JH. Malondialdehyde–acetaldehyde-adducted protein inhalation causes lung injury. Alcohol. 2012;46:51–59. doi: 10.1016/j.alcohol.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of Interleukin 17 Receptor Signaling for Lung Cxc Chemokine and Granulocyte Colony-Stimulating Factor Expression, Neutrophil Recruitment, and Host Defense. The Journal of Experimental Medicine. 2001;194:519–528. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh MY, Burnham EL, Moss M, Brown LAS. Chronic alcoholism alters systemic and pulmonary glutathione redox status. American Journal of Respiratory and Critical Care Medicine. 2007;176:270–276. doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Harris FL, Hart CM, Brown LAS. Ethanol Induces Oxidative Stress in Alveolar Macrophages via Upregulation of NADPH Oxidases. The Journal of Immunology. 2012;188:3648–3657. doi: 10.4049/jimmunol.1101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin SJ, Liao CS, Chen CM, Fan FT, Lee SC. Genetic polymorphism and activities of human lung alcohol and aldehyde dehydrogenases: Implications for ethanol metabolism and cytotoxicity. Biochemical Genetics. 1992;30:203–215. doi: 10.1007/BF02399709. [DOI] [PubMed] [Google Scholar]
- Yin S. Alcohol dehydrogenase: enzymology and metabolism. Alcohol Alcohol Suppl. 1994;2:113–119. [PubMed] [Google Scholar]
- Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Boé DM, Zhong Q, Schwarzenberger P, Kolls JK, Summer WR, Nelson S. Acute Alcohol Intoxication Suppresses the CXC Chemokine Response During Endotoxemia. Alcoholism: Clinical and Experimental Research. 2002a;26:65–73. [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Happel KI, Summer WR, Nelson S. Pulmonary host defenses and alcohol. Frontiers in Bioscience. 2002b;7:D1314–D1330. doi: 10.2741/A842. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Stoltz DA, Summer WR, Nelson S. Granulocyte Colony-Stimulating Factor Modulates the Pulmonary Host Response to Endotoxin in the Absence and Presence of Acute Ethanol Intoxication. Journal of Infectious Diseases. 1999;179:1441–1448. doi: 10.1086/314763. [DOI] [PubMed] [Google Scholar]
- Zhang P, Quinton LJ, Gamble L, Bagby GJ, Summer WR, Nelson S. The Granulopoietic Cytokine Response and Enhancement of Granulopoiesis in Mice During Endotoxemia. Shock. 2005;23:344–352. doi: 10.1097/01.shk.0000158960.93832.de. [DOI] [PubMed] [Google Scholar]
- Zisman DA, Strieter RM, Kunkel SL, Tsai WC, Wilkowski JM, Bucknell KA, Standiford TJ. Ethanol Feeding Impairs Innate Immunity and Alters the Expression of Th1- and Th2-Phenotype Cytokines in Murine Klebsiella Pneumonia. Alcoholism: Clinical and Experimental Research. 1998;22:621–627. doi: 10.1111/j.1530-0277.1998.tb04303.x. [DOI] [PubMed] [Google Scholar]