Abstract
PET and MRI provide complementary information in the study of the human brain. Simultaneous PET/MR data acquisition allows the spatial and temporal correlation of the measured signals, opening up opportunities impossible to realize using stand-alone instruments. This paper reviews the methodological improvements and potential neurological and psychiatric applications of this novel technology. We first present methods for improving the performance and information content of each modality by using the information provided by the other technique. On the PET side, we discuss methods that use the simultaneously acquired MR data to improve the PET data quantification. On the MR side, we present how improved PET quantification could be used to validate a number of MR techniques. Finally, we describe promising research, translational and clinical applications that could benefit from these advanced tools.
Keywords: simultaneous PET/MRI, multimodal imaging, neurology
Magnetic resonance (MR) and positron emission tomography (PET) provide complementary anatomical, physiological, metabolic and functional information about the brain. Pooling information obtained with each modality has long been performed through a parallel analysis of the sequentially acquired data and, more commonly today, by using software co-registration techniques. However, underlying such studies is the assumption that no significant changes in the physiological or cognitive conditions have occurred between the two examinations; while a good assumption for some studies, this may not be not the case more generally, particularly when probing a subject’s mental state, which may change on time frames from minutes to even seconds, in some disease conditions such as acute ischemic stroke or migraine where physiological and metabolic changes can occur on the order of minutes, or in cases where therapeutic interventions may cause rapid changes in baseline physiology, such as neuro-oncologically directed treatments with anti-angiogenic agents.
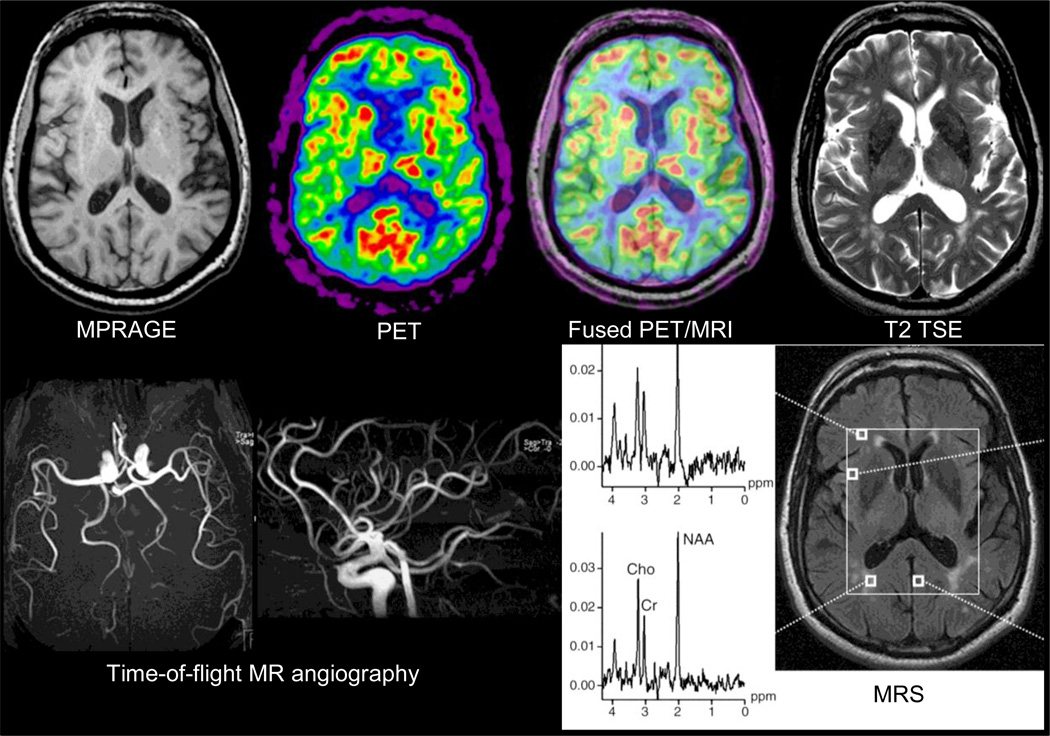
One means to address such potential pitfalls is through the simultaneous collection of MRI and PET data. The feasibility of simultaneous PET and MR data acquisition for human studies was first demonstrated in 2007 and proof-of-principle brain data (Figure 1) were collected using a prototype MR-compatible PET insert – called BrainPET – positioned inside a commercially available 3-Tesla MR Trio system (Siemens Healthcare Inc.) (1). As of 2010, a fully integrated PET/MR scanner is also available for human whole-body imaging (Siemens Biograph mMR) (2).
Figure 1.
First simultaneous PET/MR study in a 66-year-old healthy volunteer. The MR sequences run included T2 turbo spin echo, EPI, time-of-flight MR-angiography and MRS. The PET image displayed was reconstructed from the twenty-minute emission data recorded at steady state after injection of 370 MBq of FDG. Data acquired on the BrainPET prototype, Siemens, Knoxville, TN. Reproduced with permission from (1).
Simultaneous PET/MR allows for both the spatial and temporal correlation of the signals, opening up opportunities impossible to realize using sequentially acquired data. The features of this new technology may be particularly appealing to applications in neuroscience and translational neurologic/psychiatric research, considering that MR represents the first-line diagnostic imaging modality for numerous indications and that a great number of specific PET tracers are available today to assess functional and molecular processes in the brain. In studying physiological brain function, simultaneous acquisition may allow improved in vivo assessment and cross-correlation of a number of neuropsychological events: changes in hemodynamics including quantitative assessment of cerebral blood flow (CBF), volume (CBV) and oxygenation, neurovascular coupling, inflammation and microglial activation, ischemia, necrosis, apoptosis, etc. The understanding of pathophysiological mechanisms underlying disease states and the clinical evaluation of various disorders of the central nervous system over time could also be improved: quantitative measurements of pathological processes in brain cancer before and after treatment; neurodegenerative diseases; acute and chronic stroke, including neuroplasticity associated with stroke recovery, etc. In addition, other disorders associated with changes in mental status, including depression, dementia, schizophrenia, obsessive-compulsive disorders, etc., could be investigated in new ways, combining anatomical, functional and metabolic measurements during identical examination conditions.
In this manuscript, we discuss the methodological opportunities opened up by simultaneous PET/MR data acquisition and describe how this novel imaging modality could benefit neurological and psychiatric applications.
METHODOLOGICAL AND SCIENTIFIC ADVANTAGES
Simultaneous acquisition immediately brings to mind the possibility of improving the performance and information content of one instrument using the information obtained from the other modality. This street does not need to be one-way – the perspective to combine the strengths of both techniques for mutual improvement appears tempting. The accuracy of the PET estimates could be improved by including the MR information as the structural framework underlying the distribution of the PET signal. Reciprocally, the strength of PET to provide absolute quantitative information might help validate several MR techniques in vivo. Next, we give examples of methodological improvements made possible via simultaneous acquisition.
MR-based PET Motion Correction
Subject head motion is difficult to avoid, leading to blurring of PET images that could even offset the benefit of using high-resolution scanners. Various techniques have been proposed for restraining the subject’s head (with limited success) or for correcting for head movements (3–11). However, most of these methods require a relatively unobstructed view of the optical sensors from outside the scanner, which is not typically the case on an integrated PET/MR scanner because of the radiofrequency coils.
In an integrated scanner, the MR data acquired simultaneously with the PET data can be used to obtain high temporal resolution rigid-body motion estimates, most often in the background of the actual MR data acquisition. One such method consists of repeatedly acquiring anatomical data and co-registering the individual MR volumes. When performing fast imaging with EPI or spiral sequences, such methods provide temporal updating of spatial information in times typically from 2–6 seconds. These methods are particularly attractive because they allow the simultaneous acquisition of functional MRI (fMRI) and PET data, which is of interest for several research applications. For more conventional acquisition methods (e.g. high-resolution anatomical imaging), motion estimates with very high temporal resolution (e.g. every 20 ms) can be obtained using embedded navigator pulses (12). These could potentially be used to correct the PET data in very short frames which could be particularly important for performing motion correction in the early phases of a dynamic PET study when frames as short as one second are often used to sample the radiotracer arterial input function (AIF).
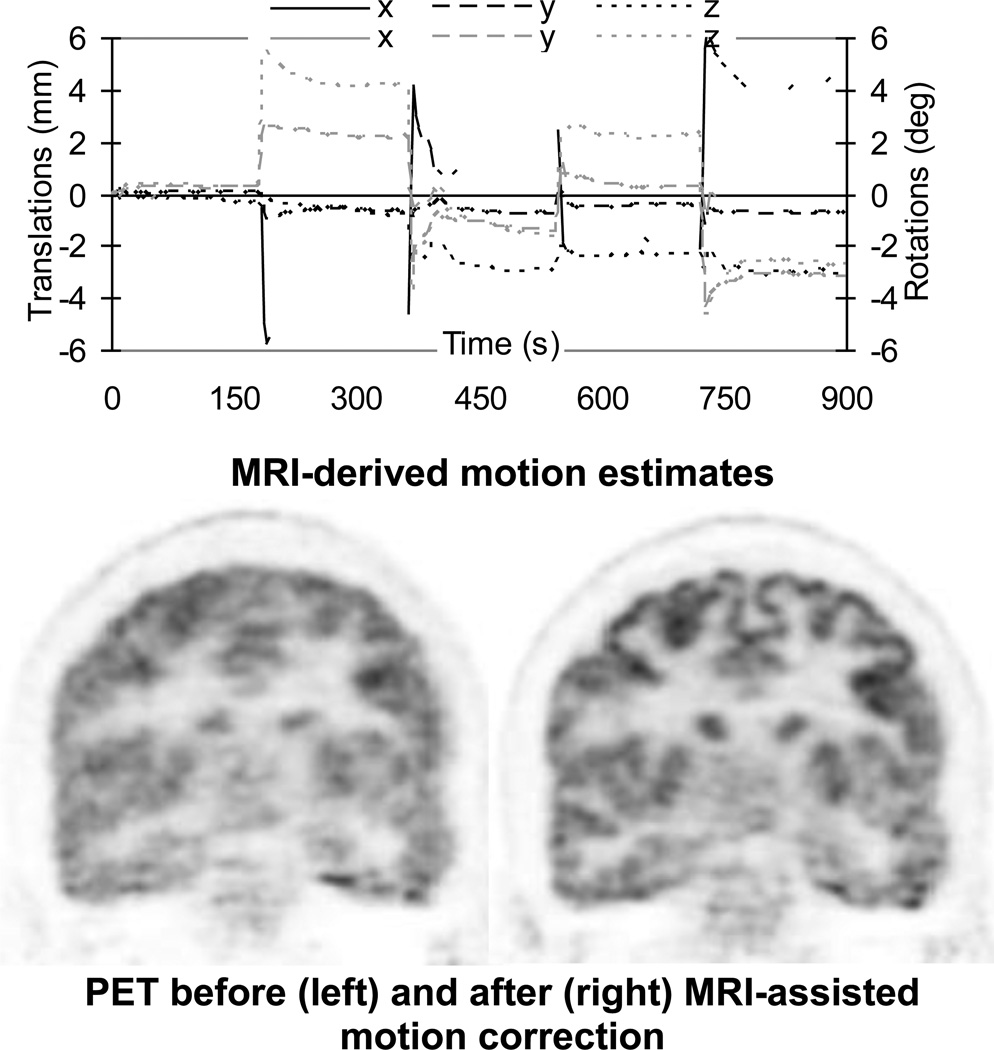
Proof-of-principle MR-assisted PET rigid-body motion correction studies have already been performed in healthy volunteers using the BrainPET scanner (Figure 2) (13). MR-based motion correction has the potential to improve PET as a quantitative method. First, the nominal spatial resolution of the current state-of-the-art scanners can be achieved. Second, the mismatch between the attenuation and emission volumes can be eliminated, assuming that the former was also derived from the simultaneously acquired MR data (14). Third, better estimates of the radiotracer AIF could be obtained using image-based approaches from the motion corrected data. Together these methods could increase the reliability and reproducibility of the PET data and this could potentially benefit a number of neurological applications that require precise quantification (e.g. neuroreceptor studies) or that involve uncooperative subjects (e.g. Alzheimer’s disease (AD), movement disorders or pediatric patients).
Figure 2.
MRI-assisted PET motion correction in a healthy volunteer using EPI-derived motion estimates. Upper row: plots of motion estimates – translations along (black) and rotations about (gray) the three orthogonal axes. Lower row: PET data reconstructed before (left) and after (right) motion correction. Substantial improvement in image quality can be observed after correction. Data acquired on the BrainPET prototype, Martinos Center, MGH.
Partial Volume Effects Correction
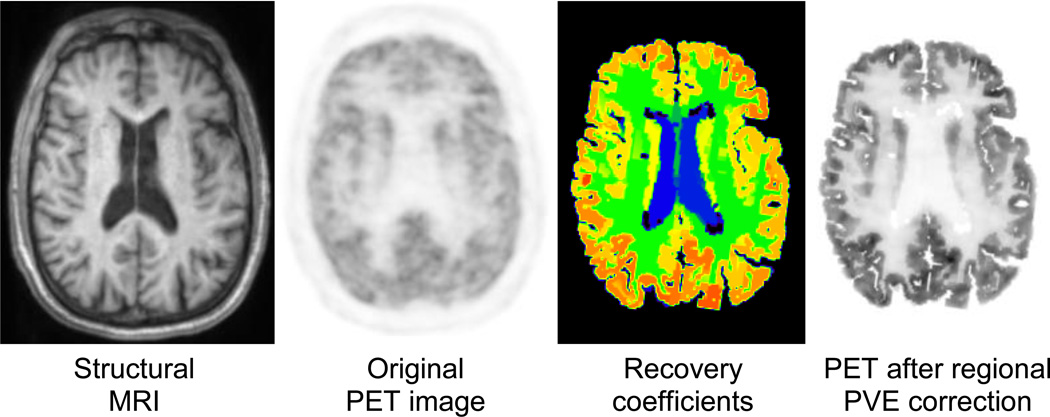
Partial volume effects (PVE) lead in PET to an under- or overestimation of tissue activity concentrations that depends on the activity distribution and the size and shape of the structures of interest (15). As a general rule, the signal is not accurately recovered in structures smaller than 2–3 times the full-width-at-half-maximum spatial resolution of the scanner. Because the spatial resolution of the current state-of-the-art clinical PET scanners is comparable to the cortical gray matter thickness (i.e. 2–3 mm), the activity concentration in these structures is clearly affected by PVE in virtually all neurological studies performed today (Figure 3).
Figure 3.
Influence of MR-based PVE correction on PET image contrast for a “normal” brain. From left to right, the MR image used to automatically segment ROIs of multiple brain structures, the original PET image, PVE correction factors for mean ROI values calculated via the geometric transfer matrix method using the segmented MR and original PET images as inputs, and the original PET image after application of the recovery coefficients. Data acquired on the BrainPET prototype, Martinos Center, MGH. Images courtesy of Spencer Bowen, PhD.
Several algorithms have been proposed for correcting for PVE, ranging from methods that apply the correction to the measured values derived from regions of interest (16–19), to those that attempt to apply the correction on a voxel-by-voxel basis (20) or to those using the anatomical information provided by MR as prior information to regularize the PET images in statistical reconstruction algorithms (21–23) and thus to model the PVE in the reconstruction.
Although these PVE correction methods have been used for numerous clinical studies, none of them has been widely accepted. This is because many factors affect the accuracy of PVE correction: segmentation of structures of interest from the anatomical MR data, spatial co-registration of the MR and PET volumes, characterization of the scanner’s point spread function and the assumptions made during the correction (23–25). The spatial co-registration problems are eliminated in the case of an integrated PET/MR system, where simultaneous acquisition guarantees spatial correlation. Furthermore, head motion, which is an additional source of image blurring, can be minimized using MR-based motion correction.
Image-based Radiotracer Arterial Input Function Estimation
Accurate PET quantification requires an input function (i.e. plasma time-activity curve of the tracer delivery to the tissue) to the compartment models used for estimating parameters of interest related to normal and pathological changes in tissue function or metabolism. The “gold standard” method for determining the AIF involves radial artery catheterization, which limits its usefulness in routine clinical PET studies. Alternatively, less invasive methods such as arterialized venous blood sampling or non-invasive techniques (i.e. image-based, population-based) have been proposed (see (26) for an excellent review).
One image-based approach is to derive the AIF from a region of interest placed across blood vessels after tracer administration. Correct definition of the region of interest over the vessel and correction for confounding effects such as spill-in from adjacent tissues or spill-out in the case of relatively small vessels can be challenging using only the PET images. In a combined scanner, the anatomical and physiological MR information could be used for this purpose. Using the co-registered MR anatomical images, the position and the size of the vessels of interest can be accurately determined (e.g. from the MR angiography data). With co-administration of both MR and PET tracers, MR could also provide additional information about the dynamics of bolus delivery to the tissue of interest and assess any local changes in blood flow, potentially reducing the problems of bolus delay and dispersion inherent in the global AIF estimate. For some radiotracers however, the AIF may still not be a suitable predictor of the input function of the parent radiolabeled compound and additional information regarding metabolites may be required.
Technique Cross-calibration and Validation
Cerebral Perfusion
H215O PET is considered the “gold standard” for measuring CBF, but a variety of MR methods have also emerged for the same purpose, including dynamic susceptibility contrast (DSC) imaging (27) and arterial spin labeling (ASL) (28). Perhaps not surprisingly, there are notable discrepancies between PET and MRI CBF measures (29), (as well as between the individual MR methods), but the degree to which this represents true technique variation versus differences in physiological state between distinct measurement periods, is unknown. Thus the use of simultaneous PET/MR imaging for an initial cross-validation of different techniques offers a unique means to begin to address confounds associated with this fundamental physiological measurement. Furthermore, beyond “perfusion”, both PET and MRI can be used independently to assess CBV, cerebral metabolic rate of oxygen (CMRO2), and oxygen extraction fraction (OEF). Given the overlapping abilities to perform these measurements with MR-based methods, a combined instrument could simultaneously perform multiple comparisons, cross-validating a number of physiological measurements.
Neuronal Activation
During periods of increased (or decreased) neural activation a variety of physiological states are known to change, including both hemodynamic and metabolic parameters. These changes, particularly in CBF and CBV, have been assessed for many years by PET to characterize regional brain activity – similar functional studies are now possible with MR (30, 31). One interesting observation from the early PET experience is that the increase in CBF was shown to be greater than the increase in the CMRO2 (32). The decrease in the concentration of paramagnetic deoxyhemoglobin in the venous blood as a result of the increase in venous blood oxygenation level is the basis of the increased blood oxygen level-dependent (BOLD) signal intensity (33) which is most typically used for human fMRI studies. The decrease in the cerebral OEF can also be measured using 15O2 PET studies. Combined PET/MR allows for the validation and detailed quantitative modeling of the relationship between OEF, CBV, CBF, and BOLD signals, and perhaps would reveal new aspects not obvious when the experiments are performed using separate instruments at different times. A simultaneous measurement of H215O PET activation and fMRI would also permit a more selective differentiation of local responses in the tissue (CBF PET) from those in the vascular system (BOLD or CBF MRI). Such data should permit a better understanding of the coupling between regional neuronal activity, changes in CBF and CBV, and oxygen metabolism.
Brain Baseline State
The concept of baseline state is an essential, though often overlooked, aspect of brain activation studies because the change in signal reported in response to a stimulus (measured either with PET or MR imaging techniques) is typically assessed with respect to an (unquantified) baseline condition. Furthermore, identifying a physiological baseline state is extremely relevant for assessing pathological changes in a number of conditions (e.g. AD, head trauma, stroke, neuropsychiatric disorders, etc.). PET and MR imaging techniques have been proposed for studying many aspects of this very complex state. For example, the CBF measurements mentioned above (e.g. H215O-PET and ASL MR) can be used to obtain precise measurement of the resting cerebral perfusion; measurements of the cerebral metabolic rate of glucose and CMRO2 can inform us about the basal metabolism; OEF can be used to provide a functional baseline (34, 35). Even with these advanced methods, brain activity “at rest” is far from being completely understood. An integrated PET/MR scanner offers the unique opportunity to simultaneously quantify and correlate the physiological, metabolic and functional baselines of the brain.
Brain Connectivity Measures
The brain remains the organ about which we know the least regarding the fundamental relationship between its structure and its function, in large part because methods to map the complex organizational relationships between its component parts in vivo have not existed. This is beginning to change. For example, diffusion MR imaging allows the identification of white matter orientation locally, permitting estimates of white matter connectivity between brain regions. In addition to information on structural connectivity"resting state” fMRI procedures allow the identification of the functional connectivity between different brain areas through the observation of the interregional correlation of physiological “noise” on time scales of seconds (36). What is missing in these measurements is of course the role of specific neurotransmitters. Adding information on transmitter release and receptor occupancy as well as on metabolism in connected areas of functional networks may be of special interest.
Such data are of more than just academic interest. It may be of great clinical relevance to study the state of functional connectivity together with functional PET markers. Recent studies were already able to demonstrate a regional interrelation between hypometabolism ([18F]FDG-PET) and disruption of functional connectivity in patients at risk for AD (37). As another example, the combination of information from diffusion tractography can provide important clinical information in settings of brain tumors by informing on the displacement and integrity of peritumoral fiber tracts, while its combination with PET ligand data on location of vital tumor tissue may be highly important for treatment planning, while identification of epileptic foci can be confirmed with confidence through the confluence of connectional and metabolic data. Ultimately, the diagnosis of developmental “connectopathies” such as autism and schizophrenia, and other behavioral disorders, may come within reach of this approach.
ADVANTAGES OF SIMULTANEOUS PET/MR IN CLINICAL AND RESEARCH APPLICATIONS
As described, simultaneous PET/MR is expected to be a more quantitatively accurate tool than the two methods alone, and a number of diseases may yield their secrets best to highly quantitative methods. This advantage alone could positively impact all clinical and research applications that require improved quantification.
One practical advantage that cannot be overstated is that because most patients receiving a PET examination for neuropsychiatric diseases typically receive an MR scan as well as part of their routine care, the option to perform the two examinations at the same time in the same scanner represents a significant increase in patient comfort. This “one stop shop” decreases the number of visits of the patient to the imaging departments, and it also reduces the overall imaging time.
Although technical challenges still exist, particularly on the PET side (e.g. MR-based attenuation correction is still an area of active research), the goal of the scanners’ manufacturers is to combine the two devices without compromising their individual performance. Indeed, all the standard and advanced MR methods for assessing brain anatomy, physiological parameters, water diffusion, metabolite concentrations, etc. are available in a combined scanner and can be profitably combined with PET methods (e.g. see Figure 1) using proven and novel radiotracers.
We describe next potential applications that could take advantage of the improved quantification or cross-calibrated measurements, without aiming to comprehensively review the usefulness of all MR and PET techniques for these applications. Instead, we provide examples of how one could use simultaneous PET/MR to further our knowledge in these fields. In each case, we first describe some of the limitations of the existing techniques, then discuss how simultaneous PET/MR could help from a methodological point of view and cautiously speculate on the potential clinical impact.
Brain Tumors
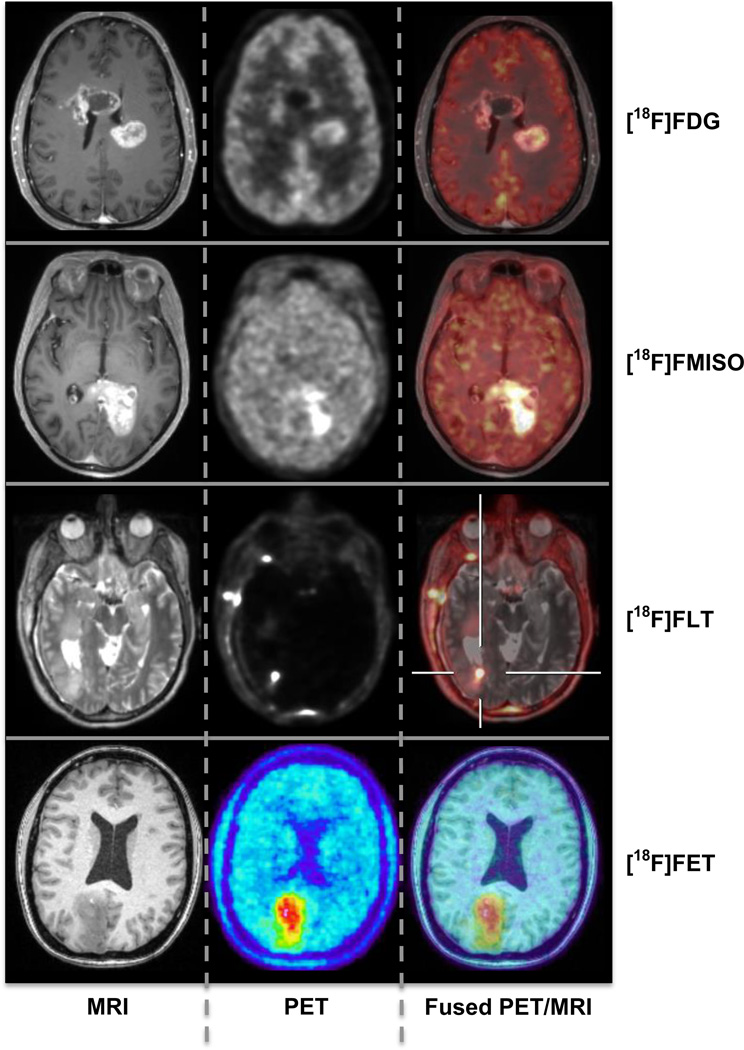
MRI is firmly established as a diagnostic and assessment method of choice for brain tumor patients and it has found increasing use as a cancer imaging biomarker (38–41). A number of quantitative MR methods (e.g. dynamic contrast enhanced (DCE) MRI, DSC MRI, MR spectroscopy (MRS), diffusion MRI) have been used to improve cancer imaging. However, these MRI techniques have also limitations. DCE MRI, for example, provides a marker of enhancement – but enhancement depends on CBF and CBV, the permeability of the capillary bed, and the surface area of the capillary bed (42) – and sorting out which of these is changing after a given treatment can be challenging (43). Furthermore, contrast enhancement in the brain is dependent on the integrity/disruption of the blood-brain barrier. PET tracers for studying amino acid transport (e.g. [11C]methionine, MET, [18F]fluoroethyltyrosine, FET), cellular proliferation (e.g. [18F]fluorothymidine, FLT), and tissue hypoxia (e.g. [18F]fluoromisonidazole, FMISO) (Figure 4) have been demonstrated to have the potential to circumvent several of the existing limitations of MRI for brain tumor diagnosis. However, the PET estimates are also influenced by permeability or other delivery related factors that could be better understood using MRI techniques. For example, separating the FLT transport and metabolic trapping components (44, 45) and comparing these with the permeability changes assessed with MRI might provide additional insights beyond either alone. As another example, the importance of assessing tumor perfusion and hypoxia independently was demonstrated using a dynamic model for analyzing dynamic FMISO PET data (46) – MR offers the ability to directly quantify brain and tumor perfusion for inclusion in PET kinetic modeling. All these studies would greatly benefit from the improved PET data quantification obtained using the simultaneously acquired MR information as described in the previous sections.
Figure 4.
Simultaneous PET/MR studies in patients with brain tumors. From left to right: axial MR, PET and fused images are shown for different tracers: FDG, FMISO, FLT (data acquired on the BrainPET prototype, Martinos Center, MGH) and FET (data acquired on the BrainPET prototype, Forschungszentrum Juelich, Germany; images courtesy of Hans Herzog, PhD and Karl-Josef Langen, MD).
Glioblastoma, the most aggressive and uniformly fatal brain tumor, is a disease in desperate need of successful therapies and quantitative imaging biomarkers are required for evaluating the effects and understanding the mechanisms of action of novel therapeutic agents (e.g. anti-angiogenic agents (47, 48)). Simultaneous MR measurements of microvascular proliferation, permeability, and PET tracer uptake could help quantify precisely how tumor proliferation, tumor vascular properties, and anti-tumor effects occur and interact, thus enabling more precise understanding of tumor biology, evolution, and therapeutic response on an individual basis.
In combination, PET and MR data should allow improved diagnostic accuracy and could be used for surgery and radiation therapy planning (49). It has been demonstrated that the combination of both modalities results in changes of the target volume used for radiation therapy, which may have significant effects on patient outcome (50). An integrated imaging device becomes especially important when brain areas of critical function must be identified in the vicinity of tumors for planning of radiation therapy or surgical intervention (51).
Dementia / Neurodegeneration
In the work-up of patients with cognitive impairment or suspected dementia, morphological procedures (i.e. CT or MRI) are used to exclude non-neurodegenerative and potentially treatable causes for the symptomatic appearance. Recently, novel MR techniques have emerged that go beyond this. These include quantitative MRI morphology (52–56), diffusion tensor imaging (57, 58), fMRI (59, 60) and methods that allow the assessment of changes in brain resting state functional connectivity networks such as the default mode network (61, 62). However, these tools are not yet proven to provide high diagnostic sensitivity and specificity. PET findings, on the other hand, allow more reliable diagnosis and differential diagnosis of dementing disorders as well as prediction in pre-dementia stages. These include specific patterns of regional glucose metabolism indicating neuronal dysfunction (Figure 5) (63) and changes in neurotransmitter systems (e.g. cholinergic, serotonergic, dopaminergic) (63–66). Recently, new tracers for molecular imaging of ß-amyloid plaques (67, 68) have been introduced. Many modern therapy approaches are directed towards the prevention/removal of this pathology and, thus, the expectations towards these PET tracers with regard to early diagnosis, patient selection and therapy monitoring are very high.
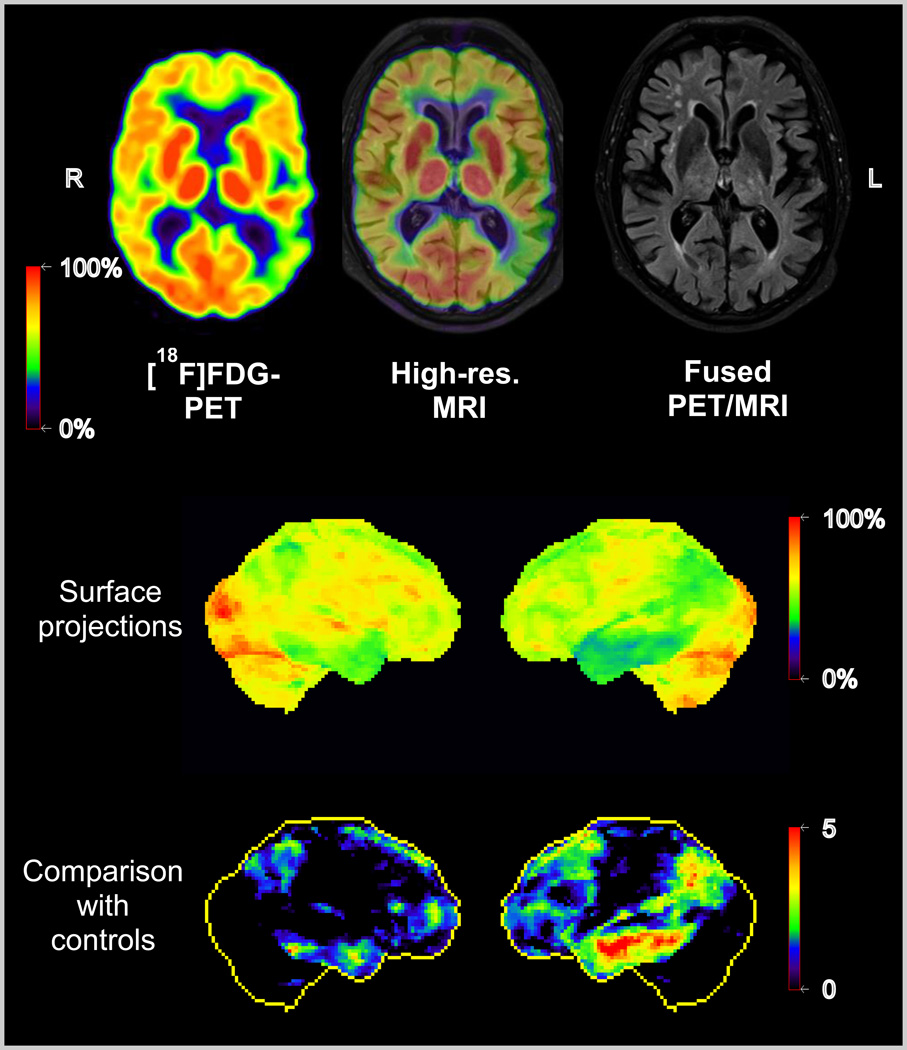
Figure 5.
Simultaneous PET/MR study in an AD patient. Upper row: axial FDG-PET, high resolution MRI and fusion image. Areas with reduced metabolism (green) representing impaired neuronal function are visible in the left temporo-parietal cortex. Lower rows: Surface projections of cerebral metabolism and of Z-scores images (comparison with controls). Data acquired on the Biograph mMR scanner, Munich.
However, PET estimates in small and anatomically complex structures (such as those first affected in the progression of neurodegenerative disorders) are biased by confounding effects (e.g. patient motion, atrophy-induced PVE, etc.). PVE can actually be opposing, depending on the type of tracer used: e.g. regional atrophy could lead to overestimation of cortical hypometabolism as measured by [18F]FDG but to underestimation of amyloid tracer uptake, as measured by [11C]PiB. Simultaneous PET/MRI offers the unique option to carefully address these issues through very accurate MR-based motion and PVE correction. Furthermore, the exact relationship between PET and MRI findings can be investigated. This is of special interest as both strong overlap (69) and considerable discrepancy between MRI structural changes and PET metabolic changes or amyloid deposits (70) have been demonstrated in previous studies in the development of AD (71, 72). As atrophy is thought to represent a more downstream event in the course of neurodegeneration, the assessment of functional/molecular abnormalities together with the detection of the onset and extent of pathology may allow a more precise staging of disease. In addition, simultaneous acquisition of resting state fMRI, ASL MR and PET may allow for a direct comparison between these functional parameters and for a cross-evaluation of their diagnostic value.
Deep brain stimulation (e.g. repetitive transcranial magnetic stimulation or direct current stimulation of selected cortical areas) has recently been suggested for the treatment of AD. Multiparametric functional imaging could improve our understanding of the effect of stimulation therapy on functional networks (fMRI) and neuroreceptor/transmitter systems (PET).
Finally, the opportunity to obtain complementary PET and MRI data simultaneously in a single imaging session is also likely to represent a major convenience, an issue particularly important in these often elderly and fragile patients.
Stroke / Cerebrovascular Disorders
Based on the pioneering work performed with both PET and MRI in ischemic stroke, the concept of a potentially salvageable penumbra has emerged and is the most important (and today the only effective) basis for treatment of ischemic stroke. Therefore an exact determination of potentially salvageable tissue is mandatory. Although PET methods are still considered a gold standard for non-invasively identifying the relevant regions of hemodynamic and metabolic compromise in ischemic stroke (73), PET is not routinely integrated into the first-line diagnostic evaluation of patients with suspected cerebrovascular issues or stroke. This is because the PET studies that would be the most useful in the acute phase of ischemic stroke (i.e. those involving O-15 or C-11 labeled tracers) are also the most challenging logistically. In the acute setting, a fast diagnosis is needed as “time is brain”, particularly regarding differentiation between ischemic and hemorrhagic strokes, which require very different therapeutic approaches. Diagnosis is usually obtained by CT or increasingly also by means of MRI (74, 75). The MR perfusion-diffusion weighted (PW-DW) mismatch is believed to reflect tissue that is potentially salvageable (similar to the PET penumbra). However, PWI does not correctly estimate the PET penumbra as it only demonstrates hypoperfusion in the vascular bed. A direct comparison of simultaneously determined PWI hypoperfusion and low flow-preserved oxygen supplied tissue (increased OEF) is necessary to really discriminate tissue which can benefit from recanalization therapies or for excluding patients that are not likely to benefit from thrombolytic therapy.
Simultaneous measurements would elucidate the ongoing debate concerning the relationship between the perfusion-diffusion mismatch and the PET penumbra (Figure 6) (76–79). PET/MRI techniques cross-calibration/validation studies are likely to lead to the adoption of new PWI thresholds (80) that will allow a better selection of those patients that might still benefit from therapeutic interventions beyond the accepted 4.5-hour time window. As previously stated, “the time has come to calibrate simpler and widely applicable functional imaging procedures – especially diffusion- and perfusion-weighted MRI – on PET in order to make these modalities a reliable tool in the study of acute ischemic stroke.” (76)
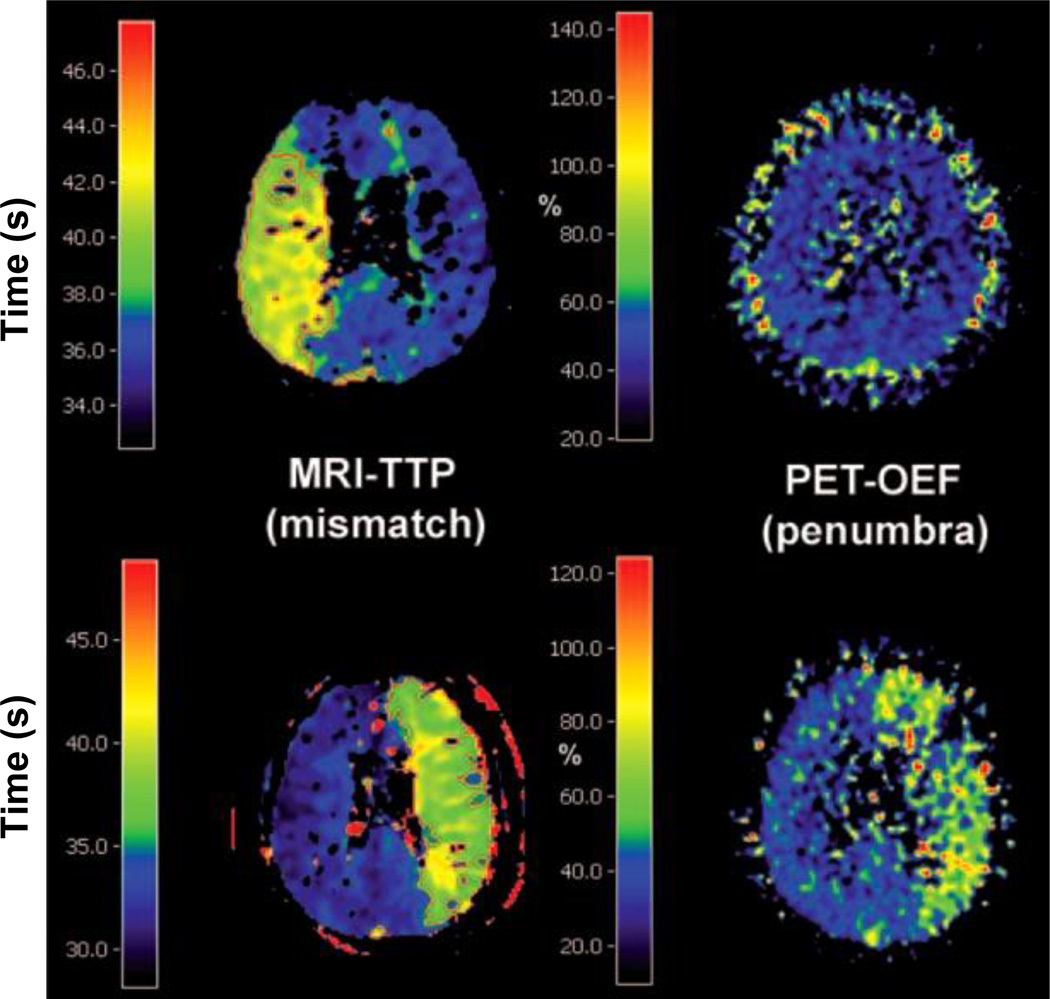
Figure 6.
Comparison of MRI time-to-peak (TTP) and PET OEF images in two patients measured in the chronic phase of stroke illustrating the mismatch-penumbra debate. Disagreement between the two techniques is observed in the first (upper row) and agreement in the second case (lower row). Figure reproduced from (80).
In the follow-up of ischemic stroke it is important to demonstrate the relationship between progression of lesion (assessed using 15O-PET and/or microglial activation using TPSO-PET) to changes in PW and DW images. Since these changes occur in small areas around the primary lesion and are transient, a simultaneous determination is necessary. In this context, activation studies (fMRI) would help to better understand the progression (or the recovery) of functional impairment in the surrounding of the ischemic lesion and within the functional network.
Epilepsy
Ictal-interictal SPECT is most often used for detecting the epileptogenic focus but this method relies on the presence of seizure activity. An imaging modality that allows the interictal detection of the lesion would be preferred and combining the PET and MRI data might facilitate this task. It is known that regional interictal hypometabolic lesions detected in FDG-PET are highly probable to represent epileptogenic foci, even in MRI-negative cases or to select the responsible focus out of several structural abnormalities present on the MRI-findings (81). In MRI-negative patients with temporal lobe epilepsy, [11C]flumazenil PET has been shown to be useful to detect the epileptic foci due to the reduced binding of this ligand to the inhibitory GABA-receptors (82). Nevertheless, high-resolution MRI brain scans are required for focus localization and precise coregistration of the PET and MRI volumes is very important for surgery planning. The combination of PET and fMRI may also allow detecting eloquent brain areas and the topographical association to the epileptogenic material.
A major advantage of integrated PET/MR for epilepsy diagnosis is that the number of times sedation/anesthesia is required could be reduced, particularly in younger children who cannot remain motionless during the scans. Furthermore, the reduced radiation when compared with PET/CT could be particularly useful for assessing medically intractable epilepsy in children.
Neurobiology of Brain Activation
Simultaneous PET/MR may improve the understanding of the interaction between neurochemistry/transmitter-mediated processes and their functional, metabolic and hemodynamic consequences in healthy brain and various disorders.
For example, in Parkinson’s disease, PET and MR data could be used to interrogate simultaneously different phases of a complex pharmacological response such as the rate of dopamine synthesis, release, transport and receptor expression (PET) and neuronal activation (fMRI) after amphetamine stimulation (83).
Using an integrated scanner, the effect of deep brain stimulation of the nucleus accumbens (84) on metabolism and transmitter-receptor interaction in defined regions together with the connecting fiber tracts can be investigated. Similarly, the effects of repetitive transcranial magnetic stimulation or of direct current stimulation, which can be used to activate or inhibit selected areas of the cortex, on regional metabolism and on the involvement of transmitter-receptor systems with the connecting networks (85) can be studied.
A highly interesting scientific option is the simultaneous measurement of neurochemical changes (PET) and changes in regional brain activity (BOLD-fMRI). This includes effects of activation by specific tasks on transmitter release or receptor binding. For example, during learning of motor skills, effects of regional neuronal activation might be dynamically monitored by BOLD-fMRI and, simultaneously, could be detected by a PET study where a dopamine receptor ligand is displaced from the receptor site (86). In analogy, the response to pain with regard to neuronal activation and e.g. opiate receptor displacement could be assessed simultaneously. Findings from such simultaneous multimodal imaging could also be related with task performance, behavior, hormone levels, subjective perception etc. Because neurons represent the fastest acting cells in the human body, sequential imaging approaches may never be able to truly reproduce a situation and to synchronize the findings from the two modalities. Perfusion, metabolism, neuronal activity, receptor/transmitter status or functional connectivity may change within seconds. Furthermore, cerebral processes are affected by mood, hormonal status, vigilance or levels in medication, which can change within minutes. Finally, habituation may lead to differences in performance in repeated tasks (or their cerebral processing), as well as to changes in expectation or in the subjective evaluation of any repeated sensation. Thus, in a sequential approach, it will neither be possible to establish a temporal relation between PET and MRI findings nor it can be assured that the brain processes investigated are comparable. In contrast, simultaneous PET/MR imaging may open totally new perspectives in the analysis of neurochemical/electrophysiological interactions in the brain.
Translational Research
Tremendous progress is constantly being made in the molecular and cellular imaging field. Integrated PET/MR will be of great value for the transfer of these findings to humans and the development of innovative treatment strategies. Examples of applications that might benefit from simultaneous assessment of various parameters are monitoring therapeutic effects of targeted gene transfer, stem cell transplantation and cell replacement approaches.
Targeted genes can be transferred using various vectors for induction of foreign enzymes expression in selected cells. These foreign enzymes can render the induced cells susceptible to specific drugs, which then are converted into toxic compounds. This approach was applied for experimental treatment of glioblastoma by transferring the herpes simplex thymidine kinase gene, which made the cells susceptible for ganciclovir. The expression of the transferred gene as well as the effect on tumor metabolism and growth could be followed by PET and MR (87).
Simultaneous PET/MR can be used to show the viability and differentiation of transplanted cells and their effect on the neuronal network. Embryonic stem cells can be implanted into brain lesions, e.g. the destroyed striatum and the proliferation and differentiation of dopaminergic cells can be followed using combined measurements. The restored functional activity of the transplanted cells can be demonstrated by the response to amphetamine causing increase in relative CBV due to dopamine release (88).
Cell replacement approaches have been proposed for treatment of various neurological disorders (e.g. ischemic stroke). For this strategy, it is essential to monitor the location and follow the migration of grafted stem or progenitor cells. Various methods can be used for labeling these cells, making them detectable by MRI, e.g. iron oxide particles (89). The migration of these cells to the border zone of damaged brain tissue in the affected hemisphere can be followed by MRI. Monitoring viability and migration as well as integration of cells into functional networks by simultaneous PET/MR might become a qualifying step in strategies relying on transplantation of fetal grafts in various neurological diseases.
CONCLUSION
For years, simultaneous PET/MR was thought of as a potential “Holy Grail” of molecular imaging, and of molecular neuroimaging in particular. Integrated PET/MR imaging offers a number of promising options regarding the clinical evaluation of disorders of the brain. The high diagnostic value of MR and PET procedures for neuropsychiatric conditions may turn PET/MR into the hybrid imaging modality of choice for diagnostic questions directed towards pathologies inside the brain. It may help to improve the diagnostic value compared to each of the individual diagnostic procedures and it may optimize workflow and increase patient comfort.
From a scientific perspective, PET/MR should improve our understanding of healthy brain physiology and function and open new insights into the pathophysiological interrelation between different parameters involved in disorders of the central nervous system. The clever design and analysis of the data obtained with specific tracers will allow for the estimation of the change in receptor binding in response to a stimulus, a concept directly complementary to fMRI activation studies to investigate brain physiology. As we probe illnesses of the mind more thoroughly, the state of the mind and the state of the brain at any given instant become more relevant. In our quest for understanding how our mind works, the great opportunity (and challenge) opened up by simultaneous PET/MR is to use dynamic measures of brain physiology from MR and new generations of receptor specific tracers (with long plasma half-lives, rapid reversible displaceability) to perform receptor specific activation mapping using fMRI-like paradigms.
Although a range of existing studies, where data from both modalities is required, might benefit from this new imaging technique, we expect that the most exciting opportunities will emerge from new molecular neuroimaging applications, providing new and exciting scientific and medical benefits.
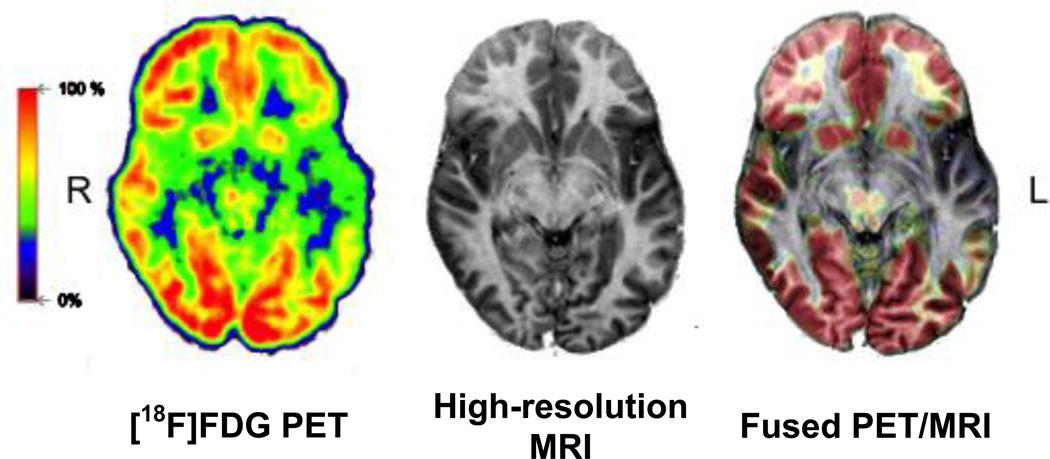
Figure 7.
Simultaneous PET/MR study in an epilepsy patient. From left to right: Axial FDG-PET (60–75 min post-injection), high resolution MRI-scan and fusion image. Distinct hypometabolism is visible in the polar region of the left temporal lobe, typically corresponding to the epileptogenic focus. Data acquired on the Biograph mMR scanner, Munich.
REFERENCES
- 1.Schlemmer H-PW, Pichler BJ, Schmand M, et al. Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology. 2008;248:1028–1035. doi: 10.1148/radiol.2483071927. [DOI] [PubMed] [Google Scholar]
- 2.Drzezga A, Souvatzoglou M, Eiber M, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012 doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 3.Picard Y, Thompson CJ. Motion correction of PET images using multiple acquisition frames. IEEE Trans Med Imaging. 1997;16:137–144. doi: 10.1109/42.563659. [DOI] [PubMed] [Google Scholar]
- 4.Fulton RR, Meikle SR, Eberl S, Pfeiffer J, Constable CJ, Fulham MJ. Correction for head movements in positron emission tomography using an optical motion-tracking system. IEEE Trans Nucl Sci. 2002;49:116–123. [Google Scholar]
- 5.Bloomfield PM, Spinks TJ, Reed J, et al. The design and implementation of a motion correction scheme for neurological PET. Phys Med Biol. 2003;48:959–978. doi: 10.1088/0031-9155/48/8/301. [DOI] [PubMed] [Google Scholar]
- 6.Carson RE, Barker WC, Liow J-S, Johnson CA. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. Nuclear Science Symposium Conference Record, IEEE. 2003;5:3281–3285. [Google Scholar]
- 7.Buhler P, Just U, Will E, Kotzerke J, van den Hoff J. An accurate method for correction of head movement in PET. IEEE Trans Med Imaging. 2004;23:1176–1185. doi: 10.1109/TMI.2004.831214. [DOI] [PubMed] [Google Scholar]
- 8.Rahmim A, Bloomfield P, Houle S, et al. Motion compensation in histogram-mode and list-mode EM reconstructions: Beyond the event-driven approach. IEEE Trans Nucl Sci. 2004;51:2588–2596. [Google Scholar]
- 9.Herzog H, Tellmann L, Fulton R, et al. Motion artifact reduction on parametric PET images of neuroreceptor binding. J Nucl Med. 2005;46:1059–1065. [PubMed] [Google Scholar]
- 10.Raghunath N, Faber TL, Suryanarayanan S, Votaw JR. Motion correction of PET brain images through deconvolution: II Practical implementation and algorithm optimization. Phys Med Biol. 2009;54:813–829. doi: 10.1088/0031-9155/54/3/022. [DOI] [PubMed] [Google Scholar]
- 11.Olesen OV, Jørgensen MR, Paulsen RR, Højgaard L, Roed B, Larsen R. Structured light 3D tracking system for measuring motions in PET brain imaging. SPIE. 2010 Proceedings Vol. 7625. [Google Scholar]
- 12.van der Kouwe AJ, Benner T, Dale AM. Real-time rigid body motion correction and shimming using cloverleaf navigators. Magn Reson Med. 2006;56:1019–1032. doi: 10.1002/mrm.21038. [DOI] [PubMed] [Google Scholar]
- 13.Catana C, Benner T, van der Kouwe A, et al. MRI-assisted PET motion correction for neurologic studies in an integrated MR-PET scanner. J Nucl Med. 2011;52:154–161. doi: 10.2967/jnumed.110.079343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catana C, van der Kouwe A, Benner T, et al. Toward implementing an MRI-based PET attenuation-correction method for neurologic studies on the MR-PET brain prototype. J Nucl Med. 2010;51:1431–1438. doi: 10.2967/jnumed.109.069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman EJ, Huang SC, Phelps ME. Quantitation in positron emission computed-tomography. 1. Effect of object size. J Comput Assist Tomogr. 1979;3:299–308. doi: 10.1097/00004728-197906000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Meltzer CC, Leal JP, Mayberg HS, Wagner HN, Frost JJ. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J Comput Assist Tomogr. 1990;14:561–570. doi: 10.1097/00004728-199007000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Mullergartner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray-matter using positron emission tomography - MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–583. doi: 10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- 18.Labbé C, Koepp M, Ashburner J, et al. Absolute PET quantification with correction for partial volume effects within cerebral structures. In: Carson R, Daube-Witherspoon M, Herscovitch P, editors. Quantitative Functional Brain Imaging with Positron Emission Tomography. San Diego, CA: Academic Press; 1998. pp. 59–66. [Google Scholar]
- 19.Rousset OG, Ma YL, Evans AC. Correction for partial volume effects in PET: Principle and validation. J Nucl Med. 1998;39:904–911. [PubMed] [Google Scholar]
- 20.Kirov AS, Piao JZ, Schmidtlein CR. Partial volume effect correction in PET using regularized iterative deconvolution with variance control based on local topology. Phys Med Biol. 2008;53:2577–2591. doi: 10.1088/0031-9155/53/10/009. [DOI] [PubMed] [Google Scholar]
- 21.Leahy R, Yan X. Incorporation of anatomical MR data for improved functional imaging with PET. In: Hawkes D, Colchester A, editors. Information Processing in Medical Imaging. New-York: Wiley-Liss; 1991. pp. 105–120. [Google Scholar]
- 22.Wang CH, Chen JC, Kao CM, Liu RS. Incorporation of correlated MR images in MAP reconstruction of PET images. J Nucl Med. 2003;44:278. [Google Scholar]
- 23.Baete K, Nuyts J, Van Laere K, et al. Evaluation of anatomy based reconstruction for partial volume correction in brain FDG-PET. Neuroimage. 2004;23:305–317. doi: 10.1016/j.neuroimage.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 24.Rousset OG, Ma YL, Wong DF, Evans AC. Pixel- versus region-based partial volume correction in PET. In: Carson R, Daube-Witherspoon M, Herscovitch P, editors. Quantitative Functional Brain Imaging with Positron Emission Tomography. San Diego: Academic Press; 1998. pp. 67–75. [Google Scholar]
- 25.Strul D, Bendriem B. Robustness of anatomically guided pixel-by-pixel algorithms for partial volume effect correction in positron emission tomography. J Cereb Blood Flow Metab. 1999;19:547–559. doi: 10.1097/00004647-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Zanotti-Fregonara P, Chen K, Liow JS, Fujita M, Innis RB. Image-derived input function for brain PET studies: many challenges and few opportunities. J Cereb Blood Flow Metab. 2011;31:1986–1998. doi: 10.1038/jcbfm.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villringer A, Rosen BR, Belliveau JW, et al. Dynamic imaging with Lanthanide chelates in normal brain - contrast due to magnetic-susceptibility effects. Magn Reson Med. 1988;6:164–174. doi: 10.1002/mrm.1910060205. [DOI] [PubMed] [Google Scholar]
- 28.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic-resonance-imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donahue M, Lu H, Jones C, Pekar J, van Zijl P. An account of the discrepancy between MRI and PET cerebral blood flow measures. A high-field MRI investigation. NMR Biomed. 2006;19:1043–1054. doi: 10.1002/nbm.1075. [DOI] [PubMed] [Google Scholar]
- 30.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic MRI of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belliveau JW, Kennedy DN, McKinstry RC, et al. Functional mapping of the human visual-cortex by magnetic-resonance-imaging. Science. 1991;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- 32.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood-flow and oxidative-metabolism during somatosensory stimulation in human-subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic-resonance-imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolar DS. Magnetic Resonance Imaging of the Cerebral Metabolic Rate of Oxygen (CMRO2) Harvard University--MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology; 2010. [Google Scholar]
- 35.Fan AP, Benner T, Bolar DS, Rosen BR, Adalsteinsson E. Phase-based regional oxygen metabolism (PROM) using MRI. Magn Reson Med. 2012;67:669–678. doi: 10.1002/mrm.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 37.Drzezga A, Becker JA, Van Dijk KRA, et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain. 2011;134:1635–1646. doi: 10.1093/brain/awr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorensen AG. Magnetic resonance as a cancer imaging biomarker. J Clin Oncol. 2006;24:3274–3281. doi: 10.1200/JCO.2006.06.6597. [DOI] [PubMed] [Google Scholar]
- 39.Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–3298. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- 40.Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: A biomarker for treatment response in oncology. J Clin Oncol. 2007;25:4104–4109. doi: 10.1200/JCO.2007.11.9610. [DOI] [PubMed] [Google Scholar]
- 41.Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood-volume maps of gliomas - comparison with tumor grade and histologic-findings. Radiology. 1994;191:41–51. doi: 10.1148/radiology.191.1.8134596. [DOI] [PubMed] [Google Scholar]
- 42.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 43.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 44.Muzi M, Spence AM, O'Sullivan F, et al. Kinetic analysis of 3'-deoxy-3'-F-18-fluorothymidine in patients with gliomas. J Nucl Med. 2006;47:1612–1621. [PubMed] [Google Scholar]
- 45.Schiepers C, Dahlbom M, Chen W, et al. Kinetics of 3'-Deoxy-3'-F-18-Fluorothymidine During Treatment Monitoring of Recurrent High-Grade Glioma. J Nucl Med. 2010;51:720–727. doi: 10.2967/jnumed.109.068361. [DOI] [PubMed] [Google Scholar]
- 46.Thorwarth D, Eschmann SM, Paulsen F, Alber M. A kinetic model for dynamic [F-18]-Fmiso PET data to analyse tumour hypoxia. Phys Med Biol. 2005;50:2209–2224. doi: 10.1088/0031-9155/50/10/002. [DOI] [PubMed] [Google Scholar]
- 47.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Bevacizumab plus Irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 48.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with Bevacizumab and Irinotecan. J Clin Oncol. 2008;26:271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heiss WD, Raab P, Lanfermann H. Multimodality assessment of brain tumors and tumor recurrence. J Nucl Med. 2011;52:1585–1600. doi: 10.2967/jnumed.110.084210. [DOI] [PubMed] [Google Scholar]
- 50.Grosu AL, Weber WA, Franz M, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:511–519. doi: 10.1016/j.ijrobp.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 51.Thiel A, Habedank B, Herholz K, et al. From the left to the right: How the brain compensates progressive loss of language function. Brain Lang. 2006;98:57–65. doi: 10.1016/j.bandl.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Ashburner J, Friston KJ. Voxel-based morphometry - The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 53.Karas GB, Burton EJ, Rombouts S, et al. A comprehensive study of gray matter loss in patients with Alzheimer's disease using optimized voxel-based morphometry. Neuroimage. 2003;18:895–907. doi: 10.1016/s1053-8119(03)00041-7. [DOI] [PubMed] [Google Scholar]
- 54.Chetelat G, Landeau B, Eustache F, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: A longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 55.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du AT, Schuff N, Kramer JH, et al. Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain. 2007;130:1159–1166. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bozzali M, Falini A, Franceschi M, et al. White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2002;72:742–746. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: a review. Curr Opin Neurol. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- 59.Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer's disease: Insights from functional MRI studies. Neuropsychologia. 2008;46:1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sperling RA, Dickerson BC, Pihlajamaki M, et al. Functional Alterations in Memory Networks in Early Alzheimer's Disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckner RL, Andrews-Hanna JR, Schacter DL. Year in Cognitive Neuroscience 2008. Vol 1124. Oxford: Blackwell Publishing; 2008. The brain's default network - Anatomy, function, and relevance to disease; pp. 1–38. [DOI] [PubMed] [Google Scholar]
- 62.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bohnen NI, Djang DSW, Herholz K, Anzai Y, Minoshima S. Effectiveness and safety of 18F-FDG PET in the evaluation of dementia: a review of the recent literature. J Nucl Med. 2012;53:59–71. doi: 10.2967/jnumed.111.096578. [DOI] [PubMed] [Google Scholar]
- 64.Pappata S, Salvatore E, Postiglione A. In vivo imaging of neurotransmission and brain receptors in dementia. J Neuroimaging. 2008;18:111–124. doi: 10.1111/j.1552-6569.2007.00194.x. [DOI] [PubMed] [Google Scholar]
- 65.Kendziorra K, Wolf H, Meyer PM, et al. Decreased cerebral alpha 4 beta 2* nicotinic acetylcholine receptor availability in patients with mild cognitive impairment and Alzheimer's disease assessed with positron emission tomography. Eur J Nucl Med Mol Imaging. 2011;38:515–525. doi: 10.1007/s00259-010-1644-5. [DOI] [PubMed] [Google Scholar]
- 66.Herholz K, Weisenbach S, Zundorf G, et al. In vivo study of acetylcholine esterase in basal forebrain, amygdala, and cortex in mild to moderate Alzheimer disease. Neuroimage. 2004;21:136–143. doi: 10.1016/j.neuroimage.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 67.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 68.Rowe CC, Villemagne VL. Brain amyloid imaging. J Nucl Med. 2011;52:1733–1740. doi: 10.2967/jnumed.110.076315. [DOI] [PubMed] [Google Scholar]
- 69.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drzezga A, Grimmer T, Henriksen G, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72:1487–1494. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- 71.Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 72.Mosconi L, Tsui WH, De Santi S, et al. Reduced hippocampal metabolism in MCI and AD - Automated FDG-PET image analysis. Neurology. 2005;64:1860–1867. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- 73.Heiss WD. Ischemic penumbra: Evidence from functional imaging in man. J Cereb Blood Flow Metab. 2000;20:1276–1293. doi: 10.1097/00004647-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 74.Lovblad KO, Laubach HJ, Baird AE, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. Am J Neuroradiol. 1998;19:1061–1066. [PMC free article] [PubMed] [Google Scholar]
- 75.Sorensen AG, Copen WA, Ostergaard L, et al. Hyperacute stroke: Simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology. 1999;210:519–527. doi: 10.1148/radiology.210.2.r99fe06519. [DOI] [PubMed] [Google Scholar]
- 76.Heiss WD. Best measure of ischemic penumbra: Positron emission tomography. Stroke. 2003;34:2534–2535. doi: 10.1161/01.STR.0000092396.70827.28. [DOI] [PubMed] [Google Scholar]
- 77.Warach S. Measurement of the ischemic penumbra with MRI: It's about time. Stroke. 2003;34:2533–2534. doi: 10.1161/01.STR.0000092395.19554.9A. [DOI] [PubMed] [Google Scholar]
- 78.Guadagno JV, Warburton EA, Jones PS, et al. How affected is oxygen metabolism in DWI lesions? A combined acute stroke PET-MR study. Neurology. 2006;67:824–829. doi: 10.1212/01.wnl.0000233984.66907.db. [DOI] [PubMed] [Google Scholar]
- 79.Takasawa M, Jones PS, Guadagno JV, et al. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative PET. Stroke. 2008;39:870–877. doi: 10.1161/STROKEAHA.107.500090. [DOI] [PubMed] [Google Scholar]
- 80.Sobesky J, Weber OZ, Lehnhardt FG, et al. Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke. 2004;35:2843–2847. doi: 10.1161/01.STR.0000147043.29399.f6. [DOI] [PubMed] [Google Scholar]
- 81.LoPinto-Khoury C, Sperling MR, Skidmore C, et al. Surgical outcome in PET-positive, MRI-negative patients with temporal lobe epilepsy. Epilepsia. 2012;53:342–348. doi: 10.1111/j.1528-1167.2011.03359.x. [DOI] [PubMed] [Google Scholar]
- 82.Hammers A, Koepp MJ, Hurlemann R, et al. Abnormalities of grey and white matter [11C]flumazenil binding in temporal lobe epilepsy with normal MRI. Brain. 2002;125:2257–2271. doi: 10.1093/brain/awf233. [DOI] [PubMed] [Google Scholar]
- 83.Chen YCI, Galpern WR, Brownell AL, et al. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: Correlation with PET microdialysis, and behavioral data. Magn Reson Med. 1997;38:389–398. doi: 10.1002/mrm.1910380306. [DOI] [PubMed] [Google Scholar]
- 84.van Kuyck K, Gabriels L, Cosyns P, et al. Behavioural and physiological effects of electrical stimulation in the nucleus accumbens: a review. Acta Neurochir Suppl. 2007;97:375–391. doi: 10.1007/978-3-211-33081-4_43. [DOI] [PubMed] [Google Scholar]
- 85.Thiel A, Schumacher B, Wienhard K, et al. Direct demonstration of transcallosal disinhibition in language networks. J CerebBlood Flow Metab. 2006;26:1122–1127. doi: 10.1038/sj.jcbfm.9600350. [DOI] [PubMed] [Google Scholar]
- 86.Badgaiyan RD, Fischman AJ, Alpert NM. Striatal dopamine release in sequential learning. Neuroimage. 2007;38:549–556. doi: 10.1016/j.neuroimage.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacobs A, Voges J, Reszka R, et al. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet. 2001;358:727–729. doi: 10.1016/s0140-6736(01)05904-9. [DOI] [PubMed] [Google Scholar]
- 88.Bjorklund LM, Sanchez-Pernaute R, Chung SM, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoehn M, Wiedermann D, Justicia C, et al. Cell tracking using magnetic resonance imaging. J Physiol. 2007;584:25–30. doi: 10.1113/jphysiol.2007.139451. [DOI] [PMC free article] [PubMed] [Google Scholar]