Abstract
Evidence derived from a vast array of laboratory studies and epidemiological investigations have implicated diets rich in fruits and vegetables with a reduced risk of certain cancers. However, these approaches cannot demonstrate causal relationships and there is a paucity of randomized, controlled trials due to the difficulties involved with executing studies of food and behavioral change. Rather than pursuing the definitive intervention trials that are necessary, the thrust of research in recent decades has been driven by a reductionist approach focusing upon the identification of bioactive components in fruits and vegetables with the subsequent development of single agents using a pharmacologic approach. At this point in time, there are no chemopreventive strategies that are standard of care in medical practice that have resulted from this approach. This review describes an alternative approach focusing upon development of tomato-based food products for human clinical trials targeting cancer prevention and as an adjunct to therapy. Tomatoes are a source of bioactive phytochemicals and are widely consumed. The phytochemical pattern of tomato products can be manipulated to optimize anticancer activity through genetics, horticultural techniques, and food processing. The opportunity to develop a highly consistent tomato-based food product rich in anticancer phytochemicals for clinical trials targeting specific cancers, particularly the prostate, necessitates the interactive transdisciplinary research efforts of horticulturalists, food technologists, cancer biologists, and clinical translational investigators.
Keywords: Tomato, Food science, Cancer, Horticulture, Prevention, Clinical trials
1 Introduction
1.1 Historical perspectives on foods and cancer prevention
In the middle of the last century, the establishment of cancer registries around the world provided data suggesting that cancer risks were highly variable around the globe leading scientists to develop hypotheses that could explain these observations [1-3]. In parallel, cancer epidemiology emerged as a rapidly growing discipline and it was soon realized that migrant populations often developed cancer risk profiles of the host nation in conjunction with adaption of new cultural and lifestyle patterns, strongly implicating environmental influences as opposed to inherited genetics as the primary driver of cancer risk [4, 5]. The emergence of nutritional epidemiology generated a vast array of hypotheses regarding dietary patterns and cancer risk, with laboratory scientists also demonstrating potent effects of nutrients, energy intake, and energy source on carcinogenesis [6]. One theme that emerged and has been adopted for public health recommendations is the potential for diets rich in fruits and vegetables to inhibit various types of cancer. The landmark report of Block et al. in 1992 compiled the accumulated epidemiologic evidence associating fruit and vegetable intake with risk of cancers from an array of organs, concluding that diets rich in these components reduced the risk of many malignancies [7]. The 1997 report of the AICR/WCRF, which was updated in 2007, was the most thorough review of diet and cancer risk ever undertaken, and also concluded that a plant-based diet was the foundation for a cancer prevention dietary pattern [8, 9]. Government and philanthropic agencies developed public health programs to educate populations and invested in research regarding enhancing implementation of cancer prevention programs [10, 11]. Yet, we are now a decade into the twenty first century and we have no clear evidence that these recommendations are effective in reducing the cancer burden. Randomized trials of changing to plant-based dietary pattern or the testing of specific plant-based interventions are rarely supported by funding agencies.
One approach to test the anticancer properties of a specific fruit or vegetable is to focus upon the development of a food product that can easily be incorporated into the diet, following the established and accepted paradigm of pharmaceutical agents. A food product that is fully characterized in terms of phytochemical and nutrient content, provides a precise daily dose of phytochemicals, is tasteful to enhance compliance and is easily incorporated into a participants dietary pattern are necessary prerequisites. Furthermore, stability of bioactives during processing and storage will require careful consideration.
Key decisions in the development of a food-based cancer prevention strategy include which type of cancer to target and what fruit or vegetable to employ. The scientific evidence from several sources including epidemiology, analytical chemistry, and cancer biology should drive these decisions. Ideally, the documentation of bioactive components in the food of interest that demonstrate anticancer activity using a combination of preclinical in vitro and in vivo approaches and characterization of biomarkers of exposure and of efficacy that can be applied to human translational studies, are necessary for moving forward with definitive prevention trials. Initial human studies (phase I and II) focus upon defining optimal dosages that provide excellent compliance, with safety, and impact biomarkers of activity [12]. Definitive phase III trials must also consider the population to target, which is likely driven by funding available. Large-scale studies of primary prevention will be rare in the current funding climate. Thus, a focus upon high-risk populations will be most desirable and the frequency of cancer outcome will be higher than in a general population thus allowing smaller studies of shorter duration. Efforts will focus upon those with a family history or defined genetic predisposition to the cancer of interest, presence of premalignant conditions, prior exposure to cancer causing agents, or survivors of a cancer that have high risk of a second primary in the same organ.
In this review, we will focus our attention on developing novel tomato (Solanum lycopersicum) products for cancer prevention studies, with a particular emphasis upon prostate cancer. We provide an overview of these efforts, their justification, and directions for future efforts.
1.2 Tomato domestication and horticulture
The present-day tomato does not have a long history of human consumption. Considerable genetic evidence suggests that the cultivated tomato has its origins as a wild small green fruit in the foothills of the Andes in the vicinity of present day Peru [13]. One species, a yellow variety the size of current cherry tomatoes, was domesticated, cultivated, and consumed by the Aztecs of Central America, which they called xitomatl, starting around 700 AD. Early in the 15th century, tomato seeds were first introduced to Europe and other parts of the globe by the Spanish, likely from the Central American cultivars after colonization [14]. Records identifying tomato use in Europe were documented in 1544 in Italy [13] but they were considered toxic and therefore predominantly used for ornamental purposes while gradually integrating into European cuisine over the next two centuries [14]. Throughout the 1800s, toxicity concerns were dispelled and consumption increased in many areas of the world. Tomato production soared by the 1920s with the application of safe mass canning that built upon the development of mechanized peeling, juice extraction, and sterilization techniques [15]. With increasing demand and knowledge derived from horticultural genetics, more desirable features were selected for varietal improvement to improve appearance, size, and quality of fruit. Over recent decades, selection of strains to enhance disease resistance, improve mechanical harvesting, facilitate processing to various products, and impact ripening and transportation has promoted the economic value of tomatoes. Current consumer interest in potential health benefits, tomato quality and organic farming, with continued anxiety over genetic manipulation, has created many avenues for the further development of tomatoes with diverse characteristics to target various stakeholders, including those on both the production and consumption sides.
1.3 Tomato production and consumption
Tomatoes are a popular food item and a generally acceptable addition to the diet of those at risk for cancer. The global annual production of tomatoes is nearly 130 Mt (1 Mt=106 t) and the USA currently ranks second in production (Fig. 1) [16]. By weight, the tomato ranks third in global production of fruits and vegetables behind potatoes and sweet potatoes. In the USA, the tomato ranks fifth in crop production behind potatoes, lettuce, onions, and watermelon [17]. The annual per capita consumption in the USA averages approximately 20 lb of fresh tomatoes and 70 lb of processed tomatoes [17] divided among sauces (35%), followed by paste (18%), canned whole tomato products (17%), and catsup and juice (each about 15%).
Fig. 1.
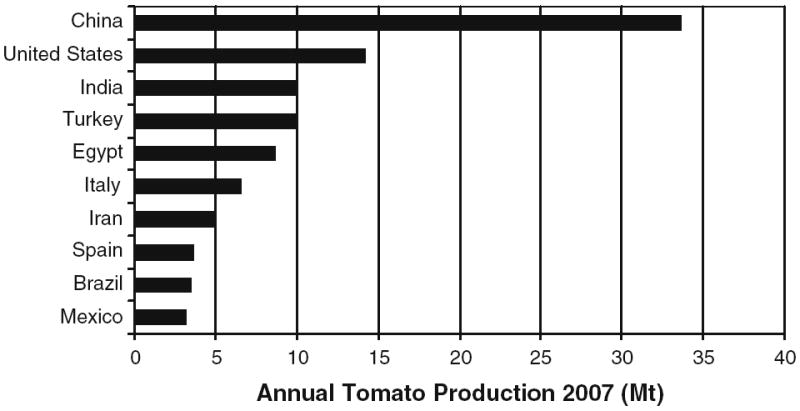
The 2007 tomato production data of the leading ten countries as compiled by the United Nations, Food and Agriculture Organization, FAOSTAT
2 Bioactive phytochemicals in tomatoes
The emergence of epidemiological studies suggesting health benefits of tomatoes immediately led to speculation regarding the potential bioactive components that may mediate the putative benefits, with a major focus upon carotenoids. Lycopene, the carotenoid providing the red color to tomatoes, was an easy target as the United States Department of Agriculture (USDA) had extensive food analysis data defining carotenoid content of foods due to the importance of carotenoids (β-carotene and other provitamin A carotenoids) in providing vitamin A in the diet. The rapid commercialization of lycopene supplements and tomato extracts marketed to consumers soon followed. However, with time, several lines of evidence indicated that a reductionist approach, focusing only upon lycopene, may be too simplistic. Indeed, our studies of experimental prostate cancer in rodent models indicated that tomato powder was more effective than lycopene in the inhibition of carcinogenesis [18, 19]. These observations indicating that lycopene may contribute to anticancer activity, but not account for all of the anticancer benefits of tomatoes, have led to the evaluation of other phytochemicals and the consideration of whole-food approaches for human translational studies of cancer prevention.
Tomatoes are cholesterol-free and low in fat and calories, thus often incorporated into a healthy dietary pattern [9]. Tomatoes are typically 5–10% dry matter, nearly half of which is reducing sugars and about 10% organic acids, primarily citrate and malate. In addition, multiple components are present which may impact health, including nutrients and an array of non-nutrient phytochemicals (Fig. 2) [20]. For example, tomatoes contribute significant amounts of potassium, folate, ascorbic acid, vitamin A (β-carotene), and tocopherols while providing about 2% of weight as fiber and 1% of weight as protein. Many non-nutrient phytochemicals are also present. Non-provitamin A carotenoids such as lycopene, phytoene, and phytofluene are the focus of significant attention [21]. An array of polyphenols are also present, primarily as flavonoids, with the USDA nutrient bank reporting that flavanones (e.g., naringenin), flavones (e.g., apigenin and luteolin), and flavonols (e.g., kaempferol, myricetin, quercetin) are the major components. The major effort thus far has focused upon the carotenoids and polyphenols as the active anticancer components.
Fig. 2.
Several of the phytochemicals found in tomatoes and tomato products
In the following sections, we consider the epidemiological and experimental data regarding tomatoes and their phytochemicals in prostate carcinogenesis. We will then provide a more detailed evaluation of various phytochemicals that may mediate anticancer activity. We then suggest opportunities for future human studies to evaluate the hypothesis that tomato products may serve as a vehicle for prostate cancer prevention.
3 Tomatoes and prostate cancer
3.1 Epidemiology of tomato intake, lycopene exposure, and prostate cancer
The hypothesis that diets rich in tomatoes may have a role in prostate cancer prevention was driven primarily by epidemiological investigations. The fact that the USDA also had a database for carotenoid content of foods allowed epidemiologists to link estimated intakes of tomato products with the average carotenoid content to obtain an estimate of lycopene exposure. Thus, the focus of attention was immediately upon lycopene, as opposed to other phytochemicals, and was further facilitated by the ability to measure serum lycopene concentrations as a biomarker of exposure by HPLC for additional epidemiologic investigations. This review is not encyclopedic, but rather selects illustrative studies and provides an overview of our conceptual evolution regarding the relationships between tomatoes and their components in prostate carcinogenesis.
The landmark publication by Giovannucci et al. [22] is illustrative of the important prospective cohort studies that support the tomato–prostate cancer hypothesis. The report is derived from the prospective Health Professional’s Follow-up Study, a cohort of over 50,000 American men that has been monitored since 1984. This effort is based upon a food-frequency questionnaire evaluation of usual dietary intake and the prospective evaluation of prostate cancer diagnosis which is verified by pathologic records. By 1992, 812 new cases of prostate cancer were documented. Among the dozens of vegetables and fruits or related products examined, the authors reported a significantly lower prostate cancer risk for tomato sauce (P=0.001), tomatoes (P=0.03), and pizza (P=0.05). Using the USDA database for carotenoid content of foods, the authors examined the relationship of carotenoids to risk. They observed no relationship between β-carotene, α-carotene, lutein, and β-crypotoxanthin and prostate cancer; only lycopene intake was associated with a lower risk (age- and energy-adjusted RR=0.79; 95% CI=0.64–0.99 for high versus low quintile of intake; P=0.04). The combined intake of tomatoes, tomato sauce, tomato juice, and pizza (accounting for 82% of lycopene intake) was inversely associated with risk of prostate cancer (multivariate RR=0.65; 95% CI=0.44–0.95, for consumption frequency greater than 10 versus less than 1.5 servings per week; P for trend=0.01) and locally advanced or metastatic prostate cancers (multivariate RR=0.47; 95% CI= 0.22–1.00; P for trend=0.03). This effort, with a large case sample and multiple dietary evaluations over time, seems to give credibility to a specific benefit for tomatoes and/or lycopene and has stimulated subsequent research. Using similar approaches, with dietary assessment tools, several prospective and case–control studies have been published with variable results and speculative conclusions [22-35]. Soon thereafter, epidemiologists also employed the assessment of serum lycopene as a biomarker of tomato exposure in nested case–control studies relative to risk of diagnosis or death. For example, the Physician’s Health Study is the largest plasma-based study to date [24]. Gann et al. showed that plasma lycopene level was significantly lower in all prostate cancer cases and in aggressive phenotype. Similarly, other studies, but not all, generally confirmed the inverse associations between elevated blood lycopene level and diminished prostate cancer risk [29, 36, 37].
Indeed, the variability in reported outcomes of epidemiologic studies should not be a surprise when we consider the magnitude and challenges of the endeavor. Prostate cancer is a heterogeneous disease with many subtypes that show a wide range of behavior, from very indolent to rapidly progressive. Indeed, we should not expect that a single dietary component, such as tomatoes, will impact all prostate cancers similarly. In addition, the diagnosis of prostate cancer in the era of prostate specific antigen (PSA)-based screening has changed the dynamics of prostate cancer epidemiology in the last two decades, as we now have shifted to early detection with greater cure rates and lower mortality, while also increasing our detection of indolent cases. Prostate cancer incidence currently is not the same as prostate cancer incidence prior to 1990. The accurate assessment of dietary intake is always a concern, although the current food-frequency questionnaires have been continually improved, error both in the quantity of specific foods consumed and the frequency adds to the imprecision of assessing relationships between tomato products and prostate cancer risk. The estimation of lycopene intake from the food frequency questionnaire is even more problematic, as the variability of lycopene content in foods can be quite significant based upon the source of tomatoes, ripeness, processing, and cooking. We also have very little mechanistic knowledge as to when in life tomato products may impact risk, yet epidemiologic studies are primarily confined to years immediately preceding diagnosis (prospective studies) or at the time of diagnosis (case–control studies). Although dietary patterns in adults are often fairly stable over time in many nations, prostate cancer in humans has a long process of carcinogenesis. The premalignant lesion, high-grade prostatic intraepithelial neoplasia, is detected in 10–20% of men between the ages of 20 and 30, suggesting that the critical time periods where diet may impact risk could be in the peri-pubertal and young adult age groups [38, 39]. Case–control studies are also plagued by reporting bias as cases typically will recall dietary patterns differently than when assessed prior to diagnosis [40, 41]. Thus, when we consider these issues in total, it is indeed remarkable that a trend toward a protective effect of tomatoes has emerged when the data is examined as a whole [9]. Although continued efforts to elucidate relationships between tomato exposure and prostate cancer risk are worthy to consider, the focus should be on prospective studies of sufficient statistical power and precision in the assessment of diet and cancer outcomes.
3.2 The lycopene and prostate cancer hypothesis: Biological plausibility
Nutritional epidemiology provided estimated exposure to lycopene and suggested correlations with reduced risk. Thus, the hypothesis that lycopene could mediate the anti-prostate cancer activity of tomato intake emerged rapidly and was particularly supported by the food and supplement industry. If a relationship truly exists, biological plausibility must be established by correlative scientific data. Indeed, it was key to document that lycopene was present in the blood and tissue of men. Our laboratory characterized carotenoid patterns in the human prostate from men undergoing prostatectomy for localized prostate cancer [42]. We reported that lycopene and β-carotene are the predominant carotenoids present, with means ± SE of 0.80 ±0.08 nmol/g and 0.54±0.09 nmol/g, respectively. The 9-cis β-carotene isomer, α-carotene, lutein, α-cryptoxanthin, zeaxanthin, and β-cryptoxanthin are consistently detectable in human prostate tissue. We further characterized tomato-based food products, serum, and prostate tissue for the presence of geometric lycopene isomers. All-trans lycopene accounted for 79–91% and cis-lycopene isomers for 9–21% of total lycopene in tomatoes, tomato paste, and tomato soup. Lycopene isomer patterns in the serum were 27–42% all-trans lycopene and 58–73% cis-isomers distributed among 12–13 peaks, depending upon their chromatographic resolution. In striking contrast with foods, all-trans lycopene accounts for only 12–21% and cis-isomers for 79–88% of total lycopene in benign or malignant prostate tissues. Thus, the human prostate contains lycopene and other dietary carotenoids, supporting the hypothesis that tomato-derived carotenoids may directly impact the prostate. Interestingly, we continue to speculate on the role of cis-isomers and their potential to impact biology.
3.3 Intervention studies of tomatoes or lycopene and prostate cancer
There are no long-term cancer prevention studies of tomatoes or lycopene for prostate cancer prevention. However, intervention studies have provided some provocative data. Serum lycopene concentrations can change quickly with alterations in the daily intake of tomato products. We [43] reported changes in plasma lycopene concentrations in healthy adults consuming standard daily servings of processed tomato products: tomato sauce (21 mg lycopene per serving), soup (12 mg lycopene per serving), or juice (17 mg lycopene per serving) for 4 weeks. Total plasma lycopene concentrations (mean ± SEM) decreased from 1.05±0.07 to 0.54±0.05 μM (49%, P<0.0001) during a 2-week washout period. Following intervention, plasma lycopene concentrations increased significantly for those consuming sauce, soup, and juice to 2.08 (192%, P<0.0001), 0.91 (122%, P<0.0001), and 0.99 (92%, P<0.0001) μM, respectively.
Other investigators examined the distribution of lycopene to the serum and prostate after food-based interventions. For example, Bowen et al. [23] provided pre-prostatectomy patients with 3 weeks of a tomato sauce-based pasta meal providing 30 mg of lycopene per day. After the dietary intervention, serum and prostate lycopene concentrations were statistically significantly increased, from 0.6 μM (95% confidence interval [CI]=0.5–0.7 μM) to 1.2 μM (95% CI= 1.0–1.4 μM; P<0.001) and from 0.28 μmol/g (95% CI=0.18–0.37 μmol/g) to 0.82 μmol/g (95% CI=0.57–1.11 μmol/g; P<0.001), respectively. Interestingly, leukocyte and prostate tissue oxidative DNA damage (8-OHdG as a biomarker) was reduced in tissues from the intervention group. Serum PSA levels decreased with dietary intervention, from 10.9 ng/mL (95% CI=8.7–13.2 ng/mL) to 8.7 ng/mL (95% CI=6.8–10.6 ng/mL) (P<0.001). Kucuk et al. [44] reported a similar pre-prostatectomy study employing a 3-week daily tomato oleoresin intake (30 mg lycopene/day) reporting that after intervention, subjects showed less aggressive pathological features of the cancer and lower plasma PSA concentrations.
We recently completed a study in men that failed curative therapy and have biochemical failure characterized by a progressively rising PSA, yet at the time were asymptomatic [45]. A combination of self-selected tomato products averaging 41 mg of lycopene/day and soy powder providing 40 g of soy protein/day was provided for 4 weeks. Serum lycopene increased from 0.72±0.09 μM to 1.21±0.10 μM (P<0.0001) and urinary isoflavone excretion increased from not detectable to 54.1±5.7 μM (P<0.05) with 8 weeks of diet intervention. Serum PSA decreased between weeks 0 and 8 for 14/41 men (34%). Mean serum vascular endothelial growth factor, a potent stimulus for cancer angiogenesis, was reduced from 87 to 51 ng/ml (P<0.05) over 8 weeks.
These examples illustrate that changing the daily intake of tomato products or lycopene oleoresin will significantly impact serum and prostate tissue lycopene concentrations over a relatively short period of a few weeks. In addition, the studies provide provocative correlative data suggesting the potential to impact PSA and other markers of activity, yet all studies thus far reported are very limited in statistical power and these findings must be considered with caution.
3.4 Tomatoes, lycopene, and experimental models of prostate carcinogenesis
Several studies have examined the impact of tomato components or lycopene on carcinogenesis or tumorigenesis for cancer derived from several tissues including mammary gland, colon, lung, liver, and urinary bladder [18, 46-58]. Prostate cancer has been examined in several different model systems. Imaida et al. [59] reported no significant effects of lycopene supplementation against rat prostate carcinogenesis. However, the small number of animals and low lycopene dosage that would not be expected to provide serum concentrations similar to humans, make this study inconclusive. Studies have investigated the combined effects of lycopene, vitamin E, and selenium [51, 60, 61], yet the rodent studies cannot differentiate the individual impact of lycopene. A study in a transplantable model of prostate tumorigenesis showed that tomato powder was more effective than lycopene for the inhibition of tumor growth [19]. In parallel, tomato powder diets produced a greater induction of tumor apoptosis and inhibition of proliferation compared with lycopene alone [19]. Boileau et al. [18] compared a control diet to those fed diets containing tomato powder or lycopene in a rat model with prostate cancer induced by testosterone and N-nitroso-N-methylurea. The control group experienced the greatest risk of prostate cancer, with tomato powder fed rats showing the lowest risk, with lycopene-fed rats experiencing an intermediate risk. In this study, plasma lycopene concentrations were similar in both tomato powder and lycopene-fed animals although the lycopene content in the tomato powder diet was approximately ten times lower than the lycopene beadlet diet. Thus illustrating, absorption of lycopene in the rats can be saturated. In addition, it is clear that absorption of lycopene by rats and mice is less efficient than in humans, a fact that should be considered in the design of studies. Clearly, measurement of blood carotenoids to document that physiological concentrations have been achieved is crucial to the interpretation of the experimental carcinogenesis studies.
The recent work of Mossine et al. [62] provides an interesting twist to the concept that freeze-dried tomato powder can inhibit prostate carcinogenesis. They investigated whether ketosamines, a class of carbohydrate derivatives present in dehydrated tomato products, combined with lycopene may impact tumorigenesis of the highly metastatic rat prostate adenocarcinoma MAT-LyLu. The FruHis (a ketosamine)/lycopene combination significantly inhibited in vivo tumor formation by MAT-LyLu cells. Another experiment with diets supplemented with tomato paste, tomato powder, or tomato paste plus FruHis, were fed to rats treated with N-nitroso-N-methylurea and testosterone. The proportion of rats with macroscopic prostate cancer at necropsy in the control, tomato paste, tomato powder, and tomato paste/FruHis groups were 63%, 39%, 43%, and 18%, respectively. Thus FruHis or other ketosamines may be non-carotenoid components of tomato products that could inhibit prostate carcinogenesis.
Overall, the studies in rodent models are supportive of a protective effect of tomato products on prostate carcinogenesis and tumorigenesis, with a less potent, but detectable impact of pure lycopene. Thus, lycopene is likely one component, but not the only component of tomatoes that impact prostate cancer risk.
3.5 Evidence from cell culture
In vitro studies provide a convenient and controlled approach for elucidating the cellular and molecular mechanisms potentially mediated by phytochemicals. Nonetheless, the challenge is that numerous tomato carotenoids such as β-carotene and lycopene are particularly sensitive to degradation in cell culture systems [63-67]. Thus, it is a challenge to elucidate the roles of the carotenoids in vitro although the polyphenols are comparatively easy to examine in cell culture. Accordingly, it is critical that investigators appreciate and document the stability of agents under investigation. Data derived from cell culture studies is discussed in sections below focusing upon mechanisms of action.
4 Carotenoids in tomatoes
4.1 Carotenoid content of tomatoes and processed tomato products
Tomatoes are a rich source of carotenoids, particularly lycopene that provides the familiar red color of tomato. Although up to 20 different carotenoids have been detected in tomatoes, lycopene will typically account for 70–90% of carotenoids present, approximately 3–5 mg/100g of raw fruit, which is defined by genetics, environmental factors, and state of ripening [68, 69]. Specific yellow varieties may contain only 0.5 mg lycopene/100g whereas the deep-red varieties can contain up to 10–15 mg/100g. Lycopene is about 3–5 times higher in concentration in the tomato skin compared to the pulp [69]. The presence of lycopene in the insoluble fiber portion of the tomatoes may impact absorption and in vivo biodistribution. The remaining carotenoids include the metabolic precursors of lycopene, phytoene and phytofluene, along with β-carotene and neurosporene, and much smaller amounts of α-carotene, γ-carotene, and leutin/zeaxanthin [70].
The impact, if any, of a low-dose exposure to the carotenoid array found in tomatoes for cancer prevention, as opposed to pharmacologic approaches with lycopene alone, is not well understood. It remains a viable hypothesis, but not proven, that the pattern of carotenoids may underlie the greater benefits of tomato products compared to lycopene. The lessons of employing pure β-carotene for lung cancer chemoprevention in smokers should not be forgotten, yet we must not allow this experience to summarily disregard the potential of carotenoids to have beneficial impacts under different circumstances [71, 72].
4.2 Lycopene chemistry, isomerization, and degradation
The unique acyclic structure of lycopene with an array of conjugated double bonds and hydrophobicity certainly impacts its biologic properties. Lycopene typically exists in the all-trans configuration in tomatoes, the most thermodynamically stable form. However, seven of the bonds can isomerize and form mono- or poly-cis isomers upon exposure to heat, light, or certain chemical reactions. Thus, lycopene isomerization can occur during processing or storage [69, 70]. Interestingly, cis-isomers account for over 50% of the total lycopene in human serum and over 80% in tissues such as prostate [42, 43, 73, 74]. The cis-isomers are considered to be more polar and less prone to crystallization, but how they form in vivo and their impact on host biology is poorly understood.
Lycopene degradation occurs with light, heat, oxygen, and acids with metallic ions of copper and iron catalyzing oxidation [69]. The potential of these non-enzymatic reactions to impact lycopene destruction in vivo is uncertain, but is critical when considering laboratory investigations of carotenoids in cell culture and in animal models. Carotenoids are not inherently stable in vitro and degradation occurs quickly under standard conditions of cell culture [47, 75, 76]. Thus, consideration of how degradation products may impact the biology understandably is crucial and investigators can enhance the value of their scientific contributions through inclusion of analytic data in their publications. Rodent studies also require careful consideration regarding lycopene stability. Lycopene, either as a pure agent or as part of tomato components, can be incorporated into semi-purified diets for studies of carcinogenesis or tumorigenesis. Again, careful documentation of concentrations of carotenoids in the ingredients, the formulated diet, and stability under conditions of feeding are essential components of sound scientific technique. Ambient lighting during formulation and the potential of heating and drying processes during pelleting contribute to significant degradation is also a key consideration.
4.3 Lycopene metabolism
The enzymatic metabolism of lycopene and other carotenoids is only beginning to be understood. The recent characterization of the enzymes carotenoid mono-oxygenase 1 (central cleavage) and 2 (eccentric cleavage) as mediators of carotenoid cleavage provides a basis for greater understanding of metabolism, particularly when coupled with modern analytic technology [77]. Lycopene, like β-carotene, when metabolized by carotenoid mono-oxygenase 2 will generate apo-lycopenals. We have observed [78] several apo-lycopenals in tomato-derived food products, but also the plasma of individuals who had consumed tomato juice for 8 weeks. Apo-6′-, apo-8′-, apo-10′-, apo-12′-, and apo-14′-lycopenals were detected and quantified in plasma. The sum of apo-lycopenals was 1.9 nmol/L plasma. The presence of apo-lycopenals in plasma may derive from the absorption of apo-lycopenals directly from food and/or human metabolism. Hormonal status also impacts lycopene metabolism and tissue distribution, yet this remains poorly understood. For example, we observed that castration (depriving androgen) results in doubling of hepatic lycopene, despite a 20% lower lycopene consumption in castrated rats [79]. The role of lycopene metabolites is under investigation in regards to lung cancer, but not yet examined in prostate cancer. For example, an apo-10′-lycopenoic acid-fed diet significantly reduced the number of lung tumors in a chemical-induced carcinogenesis animal model [80]. Although the mechanisms are still speculative, lycopenoids, the metabolic products of lycopene, may possess more or less bioactive functions than lycopene itself [81]. The use of new murine models with targeted defects in carotenoid mono-oxygenase 1 and 2 will also provide novel tools for understanding lycopene metabolism and the impact of lycopene metabolites on biological outcomes [81, 82]. The use of radio-labeled or stable isotope technology will allow investigators to define lycopene metabolism more precisely than in the past [83, 84].
4.4 Lycopene absorption and bioavailability
Carotenoid absorption is highly variable, yet we are elucidating many factors that impact the process. Isomerization of lycopene impacts absorption efficiency. Cis-isomers of lycopene are produced during processing and cooking of tomato products, in addition, some isomerization may occur in the gastrointestinal tract, especially in the environment of the stomach. Studies with lymph-cannulated ferrets demonstrated that a lycopene dose that contained <10% cis-lycopene, lead to higher concentrations of cis-isomers in the small intestinal mucosal cells (58%), mesenteric lymph (77%), serum (52%), and tissues (47–58%), primarily the 5-cis-isomer [75]. We have observed that cis-lycopene-rich tomato sauce has higher bioavailability than trans-rich tomato sauce in healthy adult subjects [85]. Perhaps, all-trans-lycopene, a long linear molecule, may be less soluble in bile acid micelles. In contrast, cis-isomers of lycopene may move more efficiently across plasma membranes and preferentially incorporate into chylomicrons [86].
The interaction between the carotenoids in the ingested food influences the absorption of individual carotenoids. Studies of humans consuming food with multiple carotenoids may increase or decrease the individual carotenoids in plasma, compared with those consuming purified carotenoids [87, 88] and the mechanisms remain to be defined. The food matrix impacts absorption as well. For example, lycopene from tomato oleoresin or tomato juice (processed tomatoes) was better absorbed compared to lycopene from raw tomatoes [89]. It is well known that carotenoid–protein complexes are denatured by the cooking of vegetables and may impact bioavailability from the food matrix [90].
The absorption of a hydrophobic and lipophilic molecule such as lycopene is impacted by dietary lipids. Fielding et al. [91] showed addition of olive oil to diced tomatoes during cooking greatly increases the absorption of lycopene. We reported that salsa with the natural lipid source of avocado greatly enhanced carotenoid absorption from meals [92]. Similarly, the absorption of carotenoids from salad with low-fat salad dressing was impaired compared with the absorption of carotenoids from salad with regular full-fat dressing [93]. However, Ahuja et al. [94] reported no difference in serum lycopene concentrations when provided with 15% of energy from fat or 38% of energy from fat, suggesting that the relationship is not linear.
Age may be another factor impacting lycopene absorption [95]. The bioavailability of lycopene was less in those 60–75 years of age compared to those 20–35. Interestingly, there was no major difference in the bioavailability of β-carotene, α-carotene, and lutein.
5 Lycopene, tomatoes, and prostate cancers: mechanisms
Although epidemiology is suggestive and rodent models are supportive of the anti-prostate cancer effects of tomatoes and lycopene, the underlying mechanisms remain very speculative and the predominant hypotheses are illustrated in the following sections.
5.1 Antioxidant properties
Prolonged oxidative damage caused by reactive oxygen species has long been hypothesized as a possible mechanism attributed to the occurrence of many cancers [63, 96, 97]. In vitro, lycopene can neutralize free radicals and quench singlet oxygen more efficiently than β-carotene and α-tocopherol [63, 98, 99]. Thus, lycopene is hypothesized to protect critical biomolecules such as DNA, protein, and lipids from free radical damage. On the contrary, in vivo evidence for this activity is difficult to document. Tomato extracts decreased xenobiotic induced oxidative stress and toxicity in the rat liver [100]. Lycopene supplementation dramatically decreased iron-induced oxidative stress in rats [101]. Consumption of tomato products decreased DNA damage, LDL oxidation, production of lipid peroxide, and oxidative stress in lymphocytes [33, 102-106]. Two studies also demonstrated no beneficial effects from lycopene supplements despite higher lycopene levels compared with tomato product supplementation. This raises the possibility that lycopene together with various micro-nutrients and phytochemicals in tomato products may provide a better defense against oxidative stress.
5.2 Proliferation and apoptosis
Antiproliferative effects of lycopene have been reported in several cancer cell lines including those derived from prostate cancers and prostate epithelial cells [55, 80, 97, 107-122]. For example, a lower dose of lycopene inhibited the growth of LNCaP prostate cancer cells while a higher dose blocked cells in G2/M phase and induced apoptosis [112]. Several in vivo studies supported the antiproliferative and proapoptotic capabilities of lycopene or tomato components, such as in the in vivo transplantable tumor models [19]. A unique approach has recently been employed with LNCaP prostate cancer cell lines exposed to sera from volunteers who consumed tomato paste or purified lycopene [123]. Expression array analysis of cells exposed to serum from patients after red tomato consumption demonstrated several changes in pathways involved in cell proliferation, apoptosis, and stress responses including cyclin D1, p53, Bax:Bcl-2, and IGFBP-3. Metabolic changes in cells are necessary for rapid cell turnover. Fatty acid synthase, for example, is increased during prostate carcinogenesis and down-regulated by lycopene [124].
5.3 Growth factor and steroid hormone action
Insulin-like growth factor (IGF-I) is postulated to be a contributor to prostate carcinogenesis [125-129]. Recent epidemiological studies suggested that higher dietary intake of lycopene is correlated with a lower circulating IGF-I and higher levels of IGFBPs [122, 130, 131]. Lycopene at pharmacologic concentrations inhibited prostate cancer cell IGF-I receptor expression and Akt phosphorylation [114]. Lycopene has been reported to inhibit dihydrotestosterone and IGF-I signaling by suppressing autocrine IGF-I networks and by attenuating the stimulus of IGF-I on serine phosphorylation of Akt/GSK3β [117]. Interestingly, a large study has linked lower levels of IGF-I with increased tomato intake in the diet [132]. Yet, other studies have not seen this relationship [133]. Thus, the existing in vivo data is inconsistent. Our group identified that numerous tomato polyphenols (e.g., quercetin) inhibit IGF-I signaling [119]. One working hypothesis is that bioactive tomato compounds may interact and downregulate androgen signaling networks and has been the subject of several studies [54, 79, 134, 135] but a clear conclusion cannot be drawn at this time. In a rat model, lycopene was also found to reduce several genes related to androgen metabolism including 5α-reductase type 2, 17β-HSD, and CYP7B1 [135]. Perhaps the most interesting finding is that lycopene down-regulated 5-α-reductase type 2, an enzyme responsible for converting testosterone to the active ligand for the androgen receptor [135]. Drugs, such as finasteride and dutasteride which also target this enzyme are both known to have chemopreventive activity in humans.
5.4 Epigenetics
The complex epigenetic changes that are known to occur in carcinogenesis are only beginning to be understood in the prostate. Investigators have explored the ability of lycopene to impact DNA methylation [136]. The authors found that lycopene partially demethylated the promoter of the GSTP1 tumor suppressor gene in breast cancer cells. Remarkably, GSTP1 has also been shown as a biomarker in human prostate cancer and is frequently methylated [137]. In addition, the RAR β2 gene, a member of the nuclear receptor superfamily, was also demethylated by lycopene in normal breast cells [136]. At this time, a role for lycopene or other tomato phytochemicals in epigenetic regulation within the prostate and its impact on carcinogenesis is speculative.
5.5 Carcinogen-metabolizing enzymes
The phase II enzymes generally increase the hydrophilicity of carcinogens and further enhance their detoxification and excretion [138]. Accumulating evidence links the beneficial effects of lycopene to the induction of phase II detoxification enzymes [139]. Expression of phase II enzymes are regulated by the antioxidant response element (ARE) and the transcription factor Nrf2 which is involved in regulating the expression of many antioxidant and detoxifying enzymes [140-142]. Lycopene and other carotenoids induce phase II enzymes such as NQO1 and GCS in mammary and liver cancer cells and the mechanism is regulated by the ARE system. More interestingly, Nrf2, which is typically found in the cytoplasm, translocated to the nucleus after carotenoid treatments. Furthermore, an ethanolic extract of lycopene containing unidentified hydrophilic derivatives activated ARE-driven reporter gene [140]. Taken together, this suggests that lycopene oxidative metabolites may play a role, at least in part, for the induction of phase II enzymes through ARE system. An in vitro study has shown that apo-10′-lycopenoic acid elevated phase II enzyme expression in human bronchial epithelial cells [143]. Furthermore, apo-10′-lycopenoic acid, the carotenoid-metabolizing cleavage product, demonstrated a dose- and time-dependent elevation in nuclear Nrf2 protein accumulation. Whether lycopene, other carotenoids, or their metabolites have more potent effects on detoxification enzymes merits additional investigation. However, the role of this process in prostate carcinogenesis is unclear, as chemical carcinogens involved in human prostate carcinogenesis have not been fully characterized.
5.6 Gap junction communication
One of the first hypotheses to emerge regarding how lycopene may impact cancer risk focused upon enhancing normal intracellular communication, with gap junctions as a biomarker for this pro-differentiation activity. Gap junctions are protein channels in cell membranes that allow nutrients, ions, and small molecules to pass between adjacent cells [144-146]. Each gap junction is derived from a total of 12 connexin proteins. Among the connexin family, connexin 43 (Cx43) is the most widely expressed and induced by retenoids, carotenoids, or their metabolites. Remarkably, evidence now suggests loss of gap junction communication is a common characteristic of carcinogenesis [122, 147]. It was indicated that both pro-vitamin A and non-provitamin A carotenoids suppressed carcinogen-induced neoplastic transformation and increased Cx43 gene expression [148, 149]. It is intriguing that the central cleavage product of lycopene, acyclo-retinoic acid demonstrated the potential to increase gap junction communication [150]. In addition, in rat liver epithelial cells, lycopene oxidation products revealed a stimulatory effect on gap junction communication [144]. In prostate cancer cells treated with serum from rats fed red and yellow tomatoes, a significantly increased Cx43 expression was seen while serum from those fed lycopene beadlets with the identical lycopene content as red tomatoes did not exert such effects [151]. The findings demonstrate the importance of tomato components other than lycopene in gap junction communication. Interestingly, another in vivo study gavaged rats with lycopene and found the lower dose (5 mg/Kg/day) enhanced gap junction communication while the higher dose (50 mg/Kg/day) inhibited this process [152]. Overall, the cell culture work is suggestive of an impact of lycopene, its metabolites, or other tomato-derived components on impacting gap junction communication. However, verification of this finding in vivo and the integration of these findings into a cohesive hypothesis remains an intriguing challenge.
5.7 Anti-inflammatory activity
Inflammation is a likely component of the initiation of prostate cancer, a process that may be enhanced by testosterone. We have yet to thoroughly evaluate the anti-inflammatory impacts of tomato phytochemicals in the prostate. Tomato phenolic treatment of KB cells results in suppression of COX-2 expression [153]. In healthy volunteers, both tomato juice with or without fortified vitamin C reduced total cholesterol and C-reactive protein, a serum biomarker of inflammation [154]. The study by Riso et al. [155] also examined the impact of a tomato-based drink on markers of inflammation and found TNF-α was 34% lower after 26 days of intervention. A rodent study revealed that lycopene supplementation led to a down-regulation of the gene expression for numerous cytokines, chemokimes, and immunoglobulins such as IL-1β and MIP-2, a potent neutrophil chemotactic factor [135]. On the contrary, others found little impact from instituting a tomato-rich diet on inflammatory markers [156]. Although these examples illustrate the potential, much more research is necessary with the integration of in vitro, rodent, and human data to make firm conclusions in regards to an anti-inflammatory impact of tomato products and their components.
6 Causality
At this point in time, it is clear that we have not established causality, and this issue has been previously addressed [157]. The accumulated data is supportive of a hypothesis that tomato products, lycopene, or other tomato bioactives may have anti-prostate carcinogenesis activity. Thus, well designed and executed studies are clearly needed using a variety of experimental approaches. It is critical that those evaluating cancer biology also integrate their efforts with the analytic chemists in order to better interpret their findings. The chemical instability of lycopene and other tomato-derived carotenoids requires that investigators take special precautions to insure quality and informative data.
7 Tomato products for future human studies
The tomato is a popular and widely consumed product. The concept that we can manipulate the tomato and create novel tomato-based food products, also known as functional foods that are specifically designed to target prostate carcinogenesis is very attractive. As we learn which components of the tomato are most desirable for cancer prevention, we have several options that will allow the development of a more potent agent for intervention trials.
We can begin with tomato horticulture and examine the genetic factors that impact tomato phytochemical profiles. Through genetic manipulation or breeding strategies we can better optimize a desired phytochemical pattern while also maintaining critical growth characteristics and disease resistant features that may be desirable by the producer or processor [158, 159]. Enhancing solid content and fruit color are two important characteristics in improving fruit quality. Developing cultivars with enhanced lycopene concentrations has already become an important area of research. Introducing lycopene β-cyclase into tomato plastid genome to increase conversion of lycopene into β-carotene caused a 50% increase in total carotenoids. As long ago as 1935, ethylene was suggested as the plant hormone responsible for fruit ripening as well as inhibition of vegetative tissues. Recently, it was concluded by transcriptome metabolite analysis that ethylene is involved in several carotenoid biosynthesis processes affecting their qualitative and quantitative deposition. Thus, by increasing expression of ethylene production in tomatoes we can also alter carotenoid patterns [160].
Environmental conditions, such as air temperature, solar radiation, water and ripeness are also to be considered in optimizing a desired phytochemical pattern [161]. For example, a favorable temperature to enhance lycopene content of specific cultivars is 22–25°C while those at higher temperatures have a dramatically lower content [161]. Solar radiation influences synthesis of carotenoids or other phytochemicals and accumulation is interactive with the genotype of the tomato plant [162, 163]. Differences in soil water impact fruit quality including the color of tomato [164]. Stages of ripening influence the concentration of carotenoids in tomatoes [161]. The interactions between the horticultural specialists and the cancer biologists as they establish a desired phytochemical pattern will allow for the development of a tomato-based food product with a great potential to impact disease processes.
We believe that a processed food that is reproducible and consistent in phytochemical pattern will be the optimal approach for a long-term cancer prevention trail in men. For example, we currently feel that a tomato juice product is an ideal approach, due to convenience. Thus, consideration of the processing aspects of product development to insure a highly desirable product while maintaining the anticancer activity is a key issue. The tomato cultivar chosen must also have characteristics that allow efficient processing to produce juice. The processing of tomatoes and developing a product involves several treatments such as chopping, hot break extraction, sieving, evaporation, sterilization, and storage. Each step has the potential to impact phytochemical profiles in the commercial product [70]. For example, the fruit-breaking step and the removal of seed and skin significantly affects the levels of antioxidants and many other metabolites present in tomato paste [165]. Thermal processing may disrupt the matrix structure encapsulating lycopene and other phytochemicals such as polyphenols in tomatoes and release more bioactives [166]. The impact of packing material, time, and temperature of storage are also considerations for developing a product for clinical trials [167].
One of the strategies that we are pursuing is the combination of tomato products in juice matrix with other components that may have anti-prostate cancer properties. For example, we have previously shown excellent compliance with a diet rich in both tomato products and soy powder, with encouraging declines in PSA in men with active prostate cancer. Thus, we are developing and testing a novel tomato–soy juice that is now in a phase I/II clinical trial. Indeed, this approach can create a novel food product that is “personalized” to a specific disease process and can take full advantage of the scientific evidence that is available regarding different food sources and phytochemicals to create a more effective intervention.
8 Conclusions
The possibility that tomato products have anti-prostate cancer properties remains a viable hypothesis. It is very clear that causality has not been established and that significant amounts of additional research are necessary to solidify such a relationship. Unfortunately, the marketing of lycopene supplements and various related products, both by manufacturers and the popular press has misinformed the public with regards to therapeutic outcomes and disease prevention. Nevertheless, this remains an exciting area for future research, combining the skills and knowledge of a diverse set of disciplines, including horticulture, food science and technology, experimental carcinogenesis, and clinical investigation.
Contributor Information
Hsueh-Li Tan, The Ohio State University Nutrition (OSUN) Graduate Program, The Ohio State University, Columbus, OH 43210, USA.
Jennifer M. Thomas-Ahner, Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA
Elizabeth M. Grainger, Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA
Lei Wan, The Ohio State University Nutrition (OSUN) Graduate Program, The Ohio State University, Columbus, OH 43210, USA.
David M. Francis, Department of Horticulture and Crop Sciences, Ohio Agricultural Research and Development Center, The Ohio State University, Wooster, OH 44691, USA
Steven J. Schwartz, Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA Department of Food Science and Technology, College of Food, Agriculture, and Environmental Science, The Ohio State University, Columbus, OH 43210, USA.
John W. Erdman, Jr., Department of Food Science and Human Nutrition and the Division of Nutritional Sciences, University of Illinois, Urbana, IL 61801, USA
Steven K. Clinton, Email: clinton.8@osu.edu, Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA; Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Ohio State University, A456 Starling Loving Hall, 320 West 10th Ave, Columbus, OH 43210, USA.
References
- 1.Parkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR, et al. Fifty years of cancer incidence: Ci5 i-ix. International Journal of Cancer. 2010 doi: 10.1002/ijc.25517. [DOI] [PubMed] [Google Scholar]
- 2.Guinee VF, Smart CR, Waterhouse J, Muir CS, Parkin DM, Zippin C, et al. Cancer data systems. Current Problems in Cancer. 1985;9(3):1–77. [PubMed] [Google Scholar]
- 3.Doll R, Peto R. The causes of cancer (Quantitative estimates of avoidable risks of cancer in the United States today) Oxford: Oxford University Press; 1981. [PubMed] [Google Scholar]
- 4.McCredie M. Cancer epidemiology in migrant populations. Recent Results in Cancer Research. 1998;154:298–305. doi: 10.1007/978-3-642-46870-4_21. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM. Studies of cancer in migrant populations. IARC Scientific Publications. 1993;123:1–10. [PubMed] [Google Scholar]
- 6.Willett W. Nutritional epidemiology (Monographs in epidemiology and biostatistics) Vol. 15. New York: Oxford University Press; 1990. [Google Scholar]
- 7.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutrition and Cancer. 1992;18(1):1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund. Food, nutrition and the prevention of cancer: A global perspective. Washington, D.C.: American Institute for Cancer Research; 1997. [DOI] [PubMed] [Google Scholar]
- 9.World Cancer Research Fund. Food, nutrition and the prevention of cancer: A global perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 10.National Academy of Science. Diet, nutrition, and cancer. Washington DC: National Academy Press; 1982. [Google Scholar]
- 11.Melnik TA, Rhoades SJ, Wales KR, Cowell C, Wolfe WS. Food consumption patterns of elementary school-children in New York City. Journal of the American Dietetic Association. 1998;98(2):159–164. doi: 10.1016/S0002-8223(98)00040-6. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald L, Foster BC, Akhtar H. Food and therapeutic product interactions—A therapeutic perspective. Journal of Pharmacy & Pharmaceutical Sciences. 2009;12(3):367–377. doi: 10.18433/j30p4c. [DOI] [PubMed] [Google Scholar]
- 13.Peralta IE, Spooner DM. History, origin and early cultivation of tomato (Solanaceae) In: Razdan MK, Mattoo AK, editors. Genetic improvement of solanaceous crops. Vol. 2. 2007. pp. 1–27. [Google Scholar]
- 14.Smith AF, Peralta IE, Spooner DM. Early history, culture, and cookery. Vol. 5. University of Illinois Press; 2001. [Google Scholar]
- 15.Gould W. Tomato production, processing and quality evaluation. Westport: Aviation Publishers Company, Limited; 1974. [Google Scholar]
- 16.Faostat. [June–July];2010 Available at http://faostat.fao.org/
- 17.United States Department of Agriculture. [2010];Economic research service. 2010 Available at http://www.ers.usda.gov/briefing/vegetables/tomatoes.htm.
- 18.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, Clinton SK. Prostate carcinogenesis in n-methyl-n-nitrosourea (nmu)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. Journal of the National Cancer Institute. 2003;95(21):1578–1586. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 19.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW., Jr Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Research. 2007;67(2):836–843. doi: 10.1158/0008-5472.CAN-06-3462. [DOI] [PubMed] [Google Scholar]
- 20.Beecher GR. Nutrient content of tomatoes and tomato products. Proceedings of the Society for Experimental Biology and Medicine. 1998;218(2):98–100. doi: 10.3181/00379727-218-44282a. [DOI] [PubMed] [Google Scholar]
- 21.Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutrition Reviews. 1998;56(2 Pt 1):35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. Journal of the National Cancer Institute. 1995;87(23):1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 23.Bowen P, Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, et al. Tomato sauce supplementation and prostate cancer: lycopene accumulation and modulation of biomarkers of carcinogenesis. Experimental Biology and Medicine. 2002;227(10):886–893. doi: 10.1177/153537020222701008. [DOI] [PubMed] [Google Scholar]
- 24.Gann PH, Ma J, Giovannucci E, Willett W, Sacks FM, Hennekens CH, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Research. 1999;59:1225–1230. [PubMed] [Google Scholar]
- 25.Grant WB. An ecologic study of dietary links to prostate cancer. Alternative Medicine Review. 1999;4(3):162–169. [PubMed] [Google Scholar]
- 26.Hodge AM, English DR, McCredie MR, Severi G, Boyle P, Hopper JL, et al. Foods, nutrients and prostate cancer. Cancer Causes & Control. 2004;15(1):11–20. doi: 10.1023/B:CACO.0000016568.25127.10. [DOI] [PubMed] [Google Scholar]
- 27.Hsing AW, Comstock GW, Abbey H, Polk BF. Serologic precursors of cancer-retinol, carotenoids, and tocopherol and risk of prostate-cancer. Journal of the National Cancer Institute. 1990;82(11):941–946. doi: 10.1093/jnci/82.11.941. [DOI] [PubMed] [Google Scholar]
- 28.Lagiou A, Trichopoulos D, Tzonou A, Lagiou P, Mucci L. Are there age-dependent effects of diet on prostate cancer risk? Sozial- und Praventivmedizin. 2001;46(5):329–334. doi: 10.1007/BF01321084. [DOI] [PubMed] [Google Scholar]
- 29.Lu QY, Hung JC, Heber D, Go VL, Reuter VE, Cordon-Cardo C, et al. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiology, Biomarkers & Prevention. 2001;10(7):749–756. [PubMed] [Google Scholar]
- 30.McCann SE, Ambrosone CB, Moysich KB, Brasure J, Marshall JR, Freudenheim JL, et al. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in Western New York. Nutrition and Cancer. 2005;53(1):33–41. doi: 10.1207/s15327914nc5301_4. [DOI] [PubMed] [Google Scholar]
- 31.Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. 1989;64(3):598–604. doi: 10.1002/1097-0142(19890801)64:3<598::aid-cncr2820640306>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Norrish AE, Jackson RT, Sharpe SJ, Skeaff CM. Prostate cancer and dietary carotenoids. American Journal of Epidemiology. 2000;151(2):119–123. doi: 10.1093/oxfordjournals.aje.a010176. [DOI] [PubMed] [Google Scholar]
- 33.Rao AV, Fleshner N, Agarwal S. Serum and tissue lycopene and biomarkers of oxidation in prostate cancer patients: A case–control study. Nutrition and Cancer. 1999;33(2):159–164. doi: 10.1207/S15327914NC330207. [DOI] [PubMed] [Google Scholar]
- 34.Tzonou A, Singorello LB, Lagiou P, Wuu J, Trichopoulos D, Trichopoulou A. Diet and cancer of the prostate: A case–control study in Greece. International Journal of Cancer. 1999;80:704–708. doi: 10.1002/(sici)1097-0215(19990301)80:5<704::aid-ijc13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Vogt TM, Mayne ST, Graubard BI, Swanson CA, Sowell AL, Schoenberg JB, et al. Serum lycopene, other serum carotenoids, and risk of prostate cancer in US blacks and whites. American Journal of Epidemiology. 2002;155(11):1023–1032. doi: 10.1093/aje/155.11.1023. [DOI] [PubMed] [Google Scholar]
- 36.Wu K, Erdman JW, Jr, Schwartz SJ, Platz EA, Leitzmann M, Clinton SK, et al. Plasma and dietary carotenoids, and the risk of prostate cancer: A nested case–control study. Cancer Epidemiology, Biomarkers & Prevention. 2004;13(2):260–269. doi: 10.1158/1055-9965.epi-03-0012. [DOI] [PubMed] [Google Scholar]
- 37.Nomura AM, Stemmermann GN, Lee J, Craft NE. Serum micronutrients and prostate cancer in Japanese Americans in Hawaii. Cancer Epidemiology, Biomarkers & Prevention. 1997;6(7):487–491. [PubMed] [Google Scholar]
- 38.Sakr W, Grignon D, Haas G, Schomer K, Heilburn L, Cassin B, et al. Epidemiology of high-grade prostatic intraepithelial neoplasia. Pathology, Research and Practice. 1995;191:838–841. doi: 10.1016/s0344-0338(11)80965-9. [DOI] [PubMed] [Google Scholar]
- 39.Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. Journal d’Urologie. 1993;150(2 Pt 1):379–385. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 40.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. Journal of the National Cancer Institute. 1999;91(4):317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 41.Giovannucci E. Tomato products, lycopene, and prostate cancer: A review of the epidemiological literature. The Journal of Nutrition. 2005;135(8):2030S–2031S. doi: 10.1093/jn/135.8.2030S. [DOI] [PubMed] [Google Scholar]
- 42.Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, et al. Cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiology, Biomarkers & Prevention. 1996;5(10):823–833. [PubMed] [Google Scholar]
- 43.Allen CM, Schwartz SJ, Craft NE, Giovannucci EL, De Groff VL, Clinton SK. Changes in plasma and oral mucosal lycopene isomer concentrations in healthy adults consuming standard servings of processed tomato products. Nutrition and Cancer. 2003;47(1):48–56. doi: 10.1207/s15327914nc4701_6. [DOI] [PubMed] [Google Scholar]
- 44.Kucuk O, Sarkar FH, Djuric Z, Sakr W, Pollak MN, Khachik F, et al. Effects of lycopene supplementation in patients with localized prostate cancer. Experimental Biology and Medicine. 2002;227(10):881–885. doi: 10.1177/153537020222701007. [DOI] [PubMed] [Google Scholar]
- 45.Grainger EM, Schwartz SJ, Wang S, Unlu NZ, Boileau TW, Ferketich AK, et al. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutrition and Cancer. 2008;60(2):145–154. doi: 10.1080/01635580701621338. [DOI] [PubMed] [Google Scholar]
- 46.Astorg P. Dietary n-6 and n-3 polyunsaturated fatty acids and prostate cancer risk: A review of epidemiological and experimental evidence. Cancer Causes & Control. 2004;15(4):367–386. doi: 10.1023/B:CACO.0000027498.94238.a3. [DOI] [PubMed] [Google Scholar]
- 47.Campbell JK, Engelmann NJ, Lila MA, Erdman JW. Phytoene, phytofluene, and lycopene from tomato powder differentially accumulate in tissues of male fisher 344 rats. Nutrition Research. 2007;27(12):794–801. doi: 10.1016/j.nutres.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen LA, Zhao Z, Pittman B, Khachik F. Effect of dietary lycopene on n-methylnitrosourea-induced mammary tumorigenesis. Nutrition and Cancer. 1999;34(2):153–159. doi: 10.1207/S15327914NC3402_5. [DOI] [PubMed] [Google Scholar]
- 49.Guttenplan JB, Chen M, Kosinska W, Thompson S, Zhao Z, Cohen LA. Effects of a lycopene-rich diet on spontaneous and benzo[a]pyrene-induced mutagenesis in prostate, colon and lungs of the lacz mouse. Cancer Letters. 2001;164(1):1–6. doi: 10.1016/s0304-3835(00)00705-9. [DOI] [PubMed] [Google Scholar]
- 50.Kim DJ, Takasuka N, Kim JM, Sekine K, Ota T, Asamoto M, et al. Chemoprevention by lycopene of mouse lung neoplasia after combined initiation treatment with den, mnu and dmh. Cancer Letters. 1997;120(1):15–22. doi: 10.1016/s0304-3835(97)00281-4. [DOI] [PubMed] [Google Scholar]
- 51.Limpens J, Schroder FH, de Ridder CM, Bolder CA, Wildhagen MF, Obermuller-Jevic UC, et al. Combined lycopene and vitamin e treatment suppresses the growth of pc-346c human prostate cancer cells in nude mice. The Journal of Nutrition. 2006;136(5):1287–1293. doi: 10.1093/jn/136.5.1287. [DOI] [PubMed] [Google Scholar]
- 52.Narisawa T, Fukaura Y, Hasebe M, Ito M, Aizawa R, Murakoshi M, et al. Inhibitory effects of natural carotenoids, alpha-carotene, beta-carotene, lycopene and lutein, on colonic aberrant crypt foci formation in rats. Cancer Letters. 1996;107(1):137–142. doi: 10.1016/0304-3835(96)04354-6. [DOI] [PubMed] [Google Scholar]
- 53.Narisawa T, Fukaura Y, Hasebe M, Nomura S, Oshima S, Sakamoto H, et al. Prevention of n-methylnitrosourea-induced colon carcinogenesis in f344 rats by lycopene and tomato juice rich in lycopene. Japanese Journal of Cancer Research. 1998;89(10):1003–1008. doi: 10.1111/j.1349-7006.1998.tb00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siler U, Barella L, Spitzer V, Schnorr J, Lein M, Goralczyk R, et al. Lycopene and vitamin e interfere with autocrine/paracrine loops in the dunning prostate cancer model. The FASEB Journal. 2004;18(9):1019–1021. doi: 10.1096/fj.03-1116fje. [DOI] [PubMed] [Google Scholar]
- 55.Tang L, Jin T, Zeng X, Wang JS. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in balb/c nude mice. The Journal of Nutrition. 2005;135(2):287–290. doi: 10.1093/jn/135.2.287. [DOI] [PubMed] [Google Scholar]
- 56.Nagasawa H, Mitamura T, Sakamoto S, Yamamoto K. Effects of lycopene on spontaneous mammary tumour development in shn virgin mice. Anticancer Research. 1995;15(4):1173–1178. [PubMed] [Google Scholar]
- 57.Sharoni Y, Giron E, Rise M, Levy J. Effects of lycopene-enriched tomato oleoresin on 7, 12-dimethyl-benz[a] anthracene-induced rat mammary tumors. Cancer Detection and Prevention. 1997;21(2):118–123. [PubMed] [Google Scholar]
- 58.Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. International Journal of Cancer. 2010;126(8):1788–1796. doi: 10.1002/ijc.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imaida K, Tamano S, Kato K, Ikeda Y, Asamoto M, Takahashi S, et al. Lack of chemopreventive effects of lycopene and curcumin on experimental rat prostate carcinogenesis. Carcinogenesis. 2001;22(3):467–472. doi: 10.1093/carcin/22.3.467. [DOI] [PubMed] [Google Scholar]
- 60.Venkateswaran V, Fleshner NE, Klotz LH. Synergistic effect of vitamin e and selenium in human prostate cancer cell lines. Prostate Cancer and Prostatic Diseases. 2004;7(1):54–56. doi: 10.1038/sj.pcan.4500707. [DOI] [PubMed] [Google Scholar]
- 61.Venkateswaran V, Klotz LH, Ramani M, Sugar LM, Jacob LE, Nam RK, et al. A combination of micronutrients is beneficial in reducing the incidence of prostate cancer and increasing survival in the lady transgenic model. Cancer Prevention Research Journal (Philadelphia, PA) 2009;2(5):473–483. doi: 10.1158/1940-6207.CAPR-08-0124. [DOI] [PubMed] [Google Scholar]
- 62.Mossine VV, Chopra P, Mawhinney TP. Interaction of tomato lycopene and ketosamine against rat prostate tumorigenesis. Cancer Research. 2008;68(11):4384–4391. doi: 10.1158/0008-5472.CAN-08-0108. [DOI] [PubMed] [Google Scholar]
- 63.Mein JR, Lian F, Wang XD. Biological activity of lycopene metabolites: Implications for cancer prevention. Nutrition Reviews. 2008;66(12):667–683. doi: 10.1111/j.1753-4887.2008.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nara E, Hayashi H, Kotake M, Miyashita K, Nagao A. Acyclic carotenoids and their oxidation mixtures inhibit the growth of hl-60 human promyelocytic leukemia cells. Nutrition and Cancer. 2001;39(2):273–283. doi: 10.1207/S15327914nc392_18. [DOI] [PubMed] [Google Scholar]
- 65.Williams AW, Boileau TW, Clinton SK, Erdman JW., Jr Beta-carotene stability and uptake by prostate cancer cells are dependent on delivery vehicle. Nutrition and Cancer. 2000;36(2):185–190. doi: 10.1207/S15327914NC3602_7. [DOI] [PubMed] [Google Scholar]
- 66.Williams AW, Boileau TW, Zhou JR, Clinton SK, Erdman JW., Jr Beta-carotene modulates human prostate cancer cell growth and may undergo intracellular metabolism to retinol. The Journal of Nutrition. 2000;130(4):728–732. doi: 10.1093/jn/130.4.728. [DOI] [PubMed] [Google Scholar]
- 67.Kim SJ, Nara E, Kobayashi H, Terao J, Nagao A. Formation of cleavage products by autoxidation of lycopene. Lipids. 2001;36(2):191–199. doi: 10.1007/s11745-001-0706-8. [DOI] [PubMed] [Google Scholar]
- 68.Agricultural Research Service. [2010];USDA database for the flavenoid content of selected foods. 2007 Release 2.1 Available at www.ars.usda.gov/nutrientdata.
- 69.Shi J, Le Maguer M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Critical Reviews in Biotechnology. 2000;20(4):293–334. doi: 10.1080/07388550091144212. [DOI] [PubMed] [Google Scholar]
- 70.Abushita AA, Daood HG, Biacs PA. Change in carotenoids and antioxidant vitamins in tomato as a function of varietal and technological factors. Journal of Agricultural and Food Chemistry. 2000;48(6):2075–2081. doi: 10.1021/jf990715p. [DOI] [PubMed] [Google Scholar]
- 71.Mayne ST, Walter M, Cartmel B, Goodwin WJ, Jr, Blumberg J. Supplemental beta-carotene, smoking, and urinary f2-isoprostane excretion in patients with prior early stage head and neck cancer. Nutrition and Cancer. 2004;49(1):1–6. doi: 10.1207/s15327914nc4901_1. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. International Journal of Cancer. 2009;126(8):1788–1796. doi: 10.1002/ijc.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allen CM, Smith AM, Clinton SK, Schwartz SJ. Tomato consumption increases lycopene isomer concentration in breast milk and plasma of lactating women. Journal of the American Dietetic Association. 2002;102(9):1257–1262. doi: 10.1016/s0002-8223(02)90278-6. [DOI] [PubMed] [Google Scholar]
- 74.Hadley CW, Clinton SK, Schwartz SJ. The consumption of processed tomato products enhances plasma lycopene concentrations in association with a reduced lipoprotein sensitivity to oxidative damage. The Journal of Nutrition. 2003;133(3):727–732. doi: 10.1093/jn/133.3.727. [DOI] [PubMed] [Google Scholar]
- 75.Boileau AC, Merchen NR, Wasson K, Atkinson CA, Erdman JW., Jr Cis-lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph-cannulated ferrets. The Journal of Nutrition. 1999;129(6):1176–1181. doi: 10.1093/jn/129.6.1176. [DOI] [PubMed] [Google Scholar]
- 76.Boileau TW, Boileau AC, Erdman JW., Jr Bioavailability of all-trans and cis-isomers of lycopene. Experimental Biology and Medicine (Maywood) 2002;227(10):914–919. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- 77.Gajic M, Zaripheh S, Sun F, Erdman JW., Jr Apo-8′-lycopenal and apo-12′-lycopenal are metabolic products of lycopene in rat liver. The Journal of Nutrition. 2006;136(6):1552–1557. doi: 10.1093/jn/136.6.1552. [DOI] [PubMed] [Google Scholar]
- 78.Kopec RE, Riedl KM, Harrison EH, Curley RW, Jr, Hruszkewycz DP, Clinton SK, et al. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. Journal of Agricultural and Food Chemistry. 2010;58(6):3290–3296. doi: 10.1021/jf100415z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boileau TW, Clinton SK, Erdman JW., Jr Tissue lycopene concentrations and isomer patterns are affected by androgen status and dietary lycopene concentration in male f344 rats. The Journal of Nutrition. 2000;130(6):1613–1618. doi: 10.1093/jn/130.6.1613. [DOI] [PubMed] [Google Scholar]
- 80.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the a/j mouse model in vivo. Carcinogenesis. 2007;28(7):1567–1574. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 81.Lindshield BL, Canene-Adams K, Erdman JW., Jr Lycopenoids: Are lycopene metabolites bioactive? Archives of Biochemistry and Biophysics. 2007;458(2):136–140. doi: 10.1016/j.abb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 82.Lindshield BL, King JL, Wyss A, Goralczyk R, Lu CH, Ford NA, et al. Lycopene biodistribution is altered in 15, 15′-carotenoid monooxygenase knockout mice. The Journal of Nutrition. 2008;138(12):2367–2371. doi: 10.3945/jn.108.099663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell JK, Rogers RB, Lila MA, Erdman JW., Jr Biosynthesis of 14c-phytoene from tomato cell suspension cultures (Lycopersicon esculentum) for utilization in prostate cancer cell culture studies. Journal of Agricultural and Food Chemistry. 2006;54(3):747–755. doi: 10.1021/jf0581269. [DOI] [PubMed] [Google Scholar]
- 84.Zaripheh S, Erdman JW., Jr The biodistribution of a single oral dose of [14c]-lycopene in rats prefed either a control or lycopene-enriched diet. The Journal of Nutrition. 2005;135(9):2212–2218. doi: 10.1093/jn/135.9.2212. [DOI] [PubMed] [Google Scholar]
- 85.Unlu NZ, Bohn T, Francis DM, Nagaraja HN, Clinton SK, Schwartz SJ. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. The British Journal of Nutrition. 2007;98(1):140–146. doi: 10.1017/S0007114507685201. [DOI] [PubMed] [Google Scholar]
- 86.Erdman JW., Jr How do nutritional and hormonal status modify the bioavailability, uptake, and distribution of different isomers of lycopene? The Journal of Nutrition. 2005;135(8):2046S–2047S. doi: 10.1093/jn/135.8.2046S. [DOI] [PubMed] [Google Scholar]
- 87.Johnson EJ, Qin J, Krinsky NI, Russell RM. Ingestion by men of a combined dose of beta-carotene and lycopene does not affect the absorption of beta-carotene but improves that of lycopene. The Journal of Nutrition. 1997;127(9):1833–1837. doi: 10.1093/jn/127.9.1833. [DOI] [PubMed] [Google Scholar]
- 88.Micozzi MS, Brown ED, Edwards BK, Bieri JG, Taylor PR, Khachik F, et al. Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. The American Journal of Clinical Nutrition. 1992;55(6):1120–1125. doi: 10.1093/ajcn/55.6.1120. [DOI] [PubMed] [Google Scholar]
- 89.Bohm V, Bitsch R. Intestinal absorption of lycopene from different matrices and interactions to other carotenoids, the lipid status, and the antioxidant capacity of human plasma. European Journal of Nutrition. 1999;38(3):118–125. doi: 10.1007/s003940050052. [DOI] [PubMed] [Google Scholar]
- 90.Ryan L, O’Connell O, O’Sullivan L, Aherne SA, O’Brien NM. Micellarisation of carotenoids from raw and cooked vegetables. Plant Foods for Human Nutrition. 2008;63(3):127–133. doi: 10.1007/s11130-008-0081-0. [DOI] [PubMed] [Google Scholar]
- 91.Fielding JM, Rowley KG, Cooper P, OD K. Increases in plasma lycopene concentration after consumption of tomatoes cooked with olive oil. Asia Pacific Journal of Clinical Nutrition. 2005;14(2):131–136. [PubMed] [Google Scholar]
- 92.Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. The Journal of Nutrition. 2005;135(3):431–436. doi: 10.1093/jn/135.3.431. [DOI] [PubMed] [Google Scholar]
- 93.Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, et al. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. The American Journal of Clinical Nutrition. 2004;80(2):396–403. doi: 10.1093/ajcn/80.2.396. [DOI] [PubMed] [Google Scholar]
- 94.Ahuja KD, Ashton EL, Ball MJ. Effects of a high monounsaturated fat, tomato-rich diet on serum levels of lycopene. European Journal of Clinical Nutrition. 2003;57(7):832–841. doi: 10.1038/sj.ejcn.1601617. [DOI] [PubMed] [Google Scholar]
- 95.Cardinault N, Tyssandier V, Grolier P, Winklhofer-Roob BM, Ribalta J, Bouteloup-Demange C, et al. Comparison of the postprandial chylomicron carotenoid responses in young and older subjects. European Journal of Nutrition. 2003;42(6):315–323. doi: 10.1007/s00394-003-0426-2. [DOI] [PubMed] [Google Scholar]
- 96.Agarwal S, Rao AV. Tomato lycopene and its role in human health and chronic diseases. Cmaj. 2000;163(6):739–744. [PMC free article] [PubMed] [Google Scholar]
- 97.Ripple MO, Henry WF, Rago RP, Wilding G. Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. Journal of the National Cancer Institute. 1997;89(1):40–48. doi: 10.1093/jnci/89.1.40. [DOI] [PubMed] [Google Scholar]
- 98.Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Archives of Biochemistry and Biophysics. 1989;274(2):532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 99.Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA. Antioxidant activities of carotenes and xanthophylls. FEBS Letters. 1996;384(3):240–242. doi: 10.1016/0014-5793(96)00323-7. [DOI] [PubMed] [Google Scholar]
- 100.Jamshidzadeh A, Baghban M, Azarpira N, Bardbori AM, Niknahad H. Effects of tomato extract on oxidative stress induced toxicity in different organs of rats. Food and Chemical Toxicology. 2008;46(12):3612–3615. doi: 10.1016/j.fct.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 101.Matos HR, Marques SA, Gomes OF, Silva AA, Heimann JC, Di Mascio P, et al. Lycopene and beta-carotene protect in vivo iron-induced oxidative stress damage in rat prostate. Brazilian Journal of Medical and Biological Research. 2006;39(2):203–210. doi: 10.1590/s0100-879x2006000200006. [DOI] [PubMed] [Google Scholar]
- 102.Astley SB, Hughes DA, Wright AJ, Elliott RM, Southon S. DNA damage and susceptibility to oxidative damage in lymphocytes: Effects of carotenoids in vitro and in vivo. The British Journal of Nutrition. 2004;91(1):53–61. doi: 10.1079/bjn20031028. [DOI] [PubMed] [Google Scholar]
- 103.Misra R, Mangi S, Joshi S, Mittal S, Gupta SK, Pandey RM. Lycored as an alternative to hormone replacement therapy in lowering serum lipids and oxidative stress markers: A randomized controlled clinical trial. The Journal of Obstetrics and Gynaecology Research. 2006;32(3):299–304. doi: 10.1111/j.1447-0756.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 104.Porrini M, Riso P. Lymphocyte lycopene concentration and DNA protection from oxidative damage is increased in women after a short period of tomato consumption. The Journal of Nutrition. 2000;130(2):189–192. doi: 10.1093/jn/130.2.189. [DOI] [PubMed] [Google Scholar]
- 105.Rao A, Agarwal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutrition Research. 1999;19(2):305–323. [Google Scholar]
- 106.Riso P, Pinder A, Santangelo A, Porrini M. Does tomato consumption effectively increase the resistance of lymphocyte DNA to oxidative damage? The American Journal of Clinical Nutrition. 1999;69(4):712–718. doi: 10.1093/ajcn/69.4.712. [DOI] [PubMed] [Google Scholar]
- 107.Chalabi N, Delort L, Le Corre L, Satih S, Bignon YJ, Bernard-Gallon D. Gene signature of breast cancer cell lines treated with lycopene. Pharmacogenomics. 2006;7(5):663–672. doi: 10.2217/14622416.7.5.663. [DOI] [PubMed] [Google Scholar]
- 108.Chalabi N, Delort L, Satih S, Dechelotte P, Bignon YJ, Bernard-Gallon DJ. Immunohistochemical expression of raralpha, rarbeta, and cx43 in breast tumor cell lines after treatment with lycopene and correlation with rt-qpcr. The Journal of Histochemistry and Cytochemistry. 2007;55(9):877–883. doi: 10.1369/jhc.7A7185.2007. [DOI] [PubMed] [Google Scholar]
- 109.Tang FY, Cho HJ, Pai MH, Chen YH. Concomitant supplementation of lycopene and eicosapentaenoic acid inhibits the proliferation of human colon cancer cells. The Journal of Nutritional Biochemistry. 2009;20(6):426–434. doi: 10.1016/j.jnutbio.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 110.Obermuller-Jevic UC, Olano-Martin E, Corbacho AM, Eiserich JP, van der Vliet A, Valacchi G, et al. Lycopene inhibits the growth of normal human prostate epithelial cells in vitro. The Journal of Nutrition. 2003;133(11):3356–3360. doi: 10.1093/jn/133.11.3356. [DOI] [PubMed] [Google Scholar]
- 111.Haase I, Evans R, Pofahl R, Watt FM. Regulation of keratinocyte shape, migration and wound epithelialization by igf-1- and egf-dependent signalling pathways. Journal of Cell Science. 2003;116(Pt 15):3227–3238. doi: 10.1242/jcs.00610. [DOI] [PubMed] [Google Scholar]
- 112.Hwang ES, Bowen PE. Cell cycle arrest and induction of apoptosis by lycopene in lncap human prostate cancer cells. Journal of Medicinal Food. 2004;7(3):284–289. doi: 10.1089/jmf.2004.7.284. [DOI] [PubMed] [Google Scholar]
- 113.Ivanov NI, Cowell SP, Brown P, Rennie PS, Guns ES, Cox ME. Lycopene differentially induces quiescence and apoptosis in androgen-responsive and -independent prostate cancer cell lines. Clinical Nutrition. 2007;26(2):252–263. doi: 10.1016/j.clnu.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 114.Kanagaraj P, Vijayababu MR, Ravisankar B, Anbalagan J, Aruldhas MM, Arunakaran J. Effect of lycopene on insulin-like growth factor-i, igf binding protein-3 and igf type-i receptor in prostate cancer cells. Journal of Cancer Research and Clinical Oncology. 2007;133(6):351–359. doi: 10.1007/s00432-006-0177-6. [DOI] [PubMed] [Google Scholar]
- 115.Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor i receptor, phosphatidylinositol 3-kinase, and akt. Molecular and Cellular Biology. 1997;17(3):1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, et al. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutrition and Cancer. 1995;24(3):257–266. doi: 10.1080/01635589509514415. [DOI] [PubMed] [Google Scholar]
- 117.Liu X, Allen JD, Arnold JT, Blackman MR. Lycopene inhibits igf-i signal transduction and growth in normal prostate epithelial cells by decreasing dht-modulated igf-i production in co-cultured reactive stromal cells. Carcinogenesis. 2008;29(4):816–823. doi: 10.1093/carcin/bgn011. [DOI] [PubMed] [Google Scholar]
- 118.Peternac D, Klima I, Cecchini MG, Schwaninger R, Studer UE, Thalmann GN. Agents used for chemoprevention of prostate cancer may influence psa secretion independently of cell growth in the lncap model of human prostate cancer progression. The Prostate. 2008;68(12):1307–1318. doi: 10.1002/pros.20795. [DOI] [PubMed] [Google Scholar]
- 119.Wang S, DeGroff VL, Clinton SK. Tomato and soy polyphenols reduce insulin-like growth factor-i-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. The Journal of Nutrition. 2003;133(7):2367–2376. doi: 10.1093/jn/133.7.2367. [DOI] [PubMed] [Google Scholar]
- 120.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. Journal of the National Cancer Institute. 2000;92(18):1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 121.Klippel A, Kavanaugh WM, Pot D, Williams LT. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase akt through its pleckstrin homology domain. Molecular and Cellular Biology. 1997;17(1):338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sharoni Y, Danilenko M, Walfisch S, Amir H, Nahum A, Ben-Dor A, et al. Role of gene regulation in the anticancer activity of carotenoids. Pure and Applied Chemistry. 2002;74(8):1469–1477. [Google Scholar]
- 123.Talvas J, Caris-Veyrat C, Guy L, Rambeau M, Lyan B, Minet-Quinard R, et al. Differential effects of lycopene consumed in tomato paste and lycopene in the form of a purified extract on target genes of cancer prostatic cells. The American Journal of Clinical Nutrition. 2010;91(6):1716–1724. doi: 10.3945/ajcn.2009.28666. [DOI] [PubMed] [Google Scholar]
- 124.Migita T, Ruiz S, Fornari A, Fiorentino M, Priolo C, Zadra G, et al. Fatty acid synthase: A metabolic enzyme and candidate oncogene in prostate cancer. Journal of the National Cancer Institute. 2009;101(7):519–532. doi: 10.1093/jnci/djp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, et al. Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science. 1998;279(5350):563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 126.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351(9113):1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 127.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. Journal of the National Cancer Institute. 1999;91(7):620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 128.Mantzoros CS, Tzonou A, Signorello LB, Stampfer M, Trichopoulos D, Adami HO. Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia. British Journal of Cancer. 1997;76(9):1115–1118. doi: 10.1038/bjc.1997.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: A case–control analysis. Journal of the National Cancer Institute. 1999;91(2):151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- 130.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor i and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiology, Biomarkers & Prevention. 2002;11(9):852–861. [PubMed] [Google Scholar]
- 131.Vrieling A, Voskuil DW, Bonfrer JM, Korse CM, van Doorn J, Cats A, et al. Lycopene supplementation elevates circulating insulin-like growth factor binding protein-1 and -2 concentrations in persons at greater risk of colorectal cancer. The American Journal of Clinical Nutrition. 2007;86(5):1456–1462. doi: 10.1093/ajcn/86.5.1456. [DOI] [PubMed] [Google Scholar]
- 132.Mucci LA, Tamimi R, Lagiou P, Trichopoulou A, Benetou V, Spanos E, et al. Are dietary influences on the risk of prostate cancer mediated through the insulin-like growth factor system? BJU International. 2001;87(9):814–820. doi: 10.1046/j.1464-410x.2001.02191.x. [DOI] [PubMed] [Google Scholar]
- 133.Schwarz S, Obermuller-Jevic UC, Hellmis E, Koch W, Jacobi G, Biesalski HK. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. The Journal of Nutrition. 2008;138(1):49–53. doi: 10.1093/jn/138.1.49. [DOI] [PubMed] [Google Scholar]
- 134.Campbell JK, Stroud CK, Nakamura MT, Lila MA, Erdman JW., Jr Serum testosterone is reduced following short-term phytofluene, lycopene, or tomato powder consumption in f344 rats. The Journal of Nutrition. 2006;136(11):2813–2819. doi: 10.1093/jn/136.11.2813. [DOI] [PubMed] [Google Scholar]
- 135.Herzog A, Siler U, Spitzer V, Seifert N, Denelavas A, Hunziker PB, et al. Lycopene reduced gene expression of steroid targets and inflammatory markers in normal rat prostate. The FASEB Journal. 2005;19(2):272–274. doi: 10.1096/fj.04-1905fje. [DOI] [PubMed] [Google Scholar]
- 136.King-Batoon A, Leszczynska JM, Klein CB. Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environmental and Molecular Mutagenesis. 2008;49(1):36–45. doi: 10.1002/em.20363. [DOI] [PubMed] [Google Scholar]
- 137.Phe V, Cussenot O, Roupret M. Methylated genes as potential biomarkers in prostate cancer. BJU International. 2010;105(10):1364–1370. doi: 10.1111/j.1464-410X.2009.09167.x. [DOI] [PubMed] [Google Scholar]
- 138.Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Archives of Pharmacal Research. 2005;28(3):249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 139.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12(1–4):5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 140.Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, et al. Carotenoids activate the antioxidant response element transcription system. Molecular Cancer Therapeutics. 2005;4(1):177–186. [PubMed] [Google Scholar]
- 141.Giudice A, Montella M. Activation of the nrf2-are signaling pathway: A promising strategy in cancer prevention. BioEssays. 2006;28(2):169–181. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- 142.Talalay P, Dinkova-Kostova AT, Holtzclaw WD. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Advances in Enzyme Regulation. 2003;43:121–134. doi: 10.1016/s0065-2571(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 143.Lian F, Wang XD. Enzymatic metabolites of lycopene induce nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. International Journal of Cancer. 2008;123(6):1262–1268. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aust O, Ale-Agha N, Zhang L, Wollersen H, Sies H, Stahl W. Lycopene oxidation product enhances gap junctional communication. Food and Chemical Toxicology. 2003;41(10):1399–1407. doi: 10.1016/s0278-6915(03)00148-0. [DOI] [PubMed] [Google Scholar]
- 145.Erdman JW, Jr, Ford NA, Lindshield BL. Are the health attributes of lycopene related to its antioxidant function? Archives of Biochemistry and Biophysics. 2009;483(2):229–235. doi: 10.1016/j.abb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Trosko JE, Chang CC, Upham B, Wilson M. Epigenetic toxicology as toxicant-induced changes in intracellular signalling leading to altered gap junctional intercellular communication. Toxicology Letters. 1998;102–103:71–78. doi: 10.1016/s0378-4274(98)00288-4. [DOI] [PubMed] [Google Scholar]
- 147.King TJ, Bertram JS. Connexins as targets for cancer chemoprevention and chemotherapy. Biochimica et Biophysica Acta. 2005;1719(1–2):146–160. doi: 10.1016/j.bbamem.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 148.Hossain MZ, Wilkens LR, Mehta PP, Loewenstein W, Bertram JS. Enhancement of gap junctional communication by retinoids correlates with their ability to inhibit neoplastic transformation. Carcinogenesis. 1989;10(9):1743–1748. doi: 10.1093/carcin/10.9.1743. [DOI] [PubMed] [Google Scholar]
- 149.Zhang LX, Cooney RV, Bertram JS. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in c3h/10t1/2 cells: relationship to their cancer chemopreventive action. Carcinogenesis. 1991;12(11):2109–2114. doi: 10.1093/carcin/12.11.2109. [DOI] [PubMed] [Google Scholar]
- 150.Stahl W, von Laar J, Martin HD, Emmerich T, Sies H. Stimulation of gap junctional communication: comparison of acyclo-retinoic acid and lycopene. Archives of Biochemistry and Biophysics. 2000;373(1):271–274. doi: 10.1006/abbi.1999.1510. [DOI] [PubMed] [Google Scholar]
- 151.Gitenay D, Lyan B, Talvas J, Mazur A, George S, Caris-Veyrat C, et al. Serum from rats fed red or yellow tomatoes induces connexin43 expression independently from lycopene in a prostate cancer cell line. Biochemical and Biophysical Research Communications. 2007;364(3):578–582. doi: 10.1016/j.bbrc.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 152.Krutovskikh V, Asamoto M, Takasuka N, Murakoshi M, Nishino H, Tsuda H. Differential dose-dependent effects of alpha-, beta-carotenes and lycopene on gap-junctional intercellular communication in rat liver in vivo. Japanese Journal of Cancer Research. 1997;88(12):1121–1124. doi: 10.1111/j.1349-7006.1997.tb00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shen YC, Chen SL, Zhuang SR, Wang CK. Contribution of tomato phenolics to suppression of cox-2 expression in kb cells. Journal of Food Science. 2008;73(1):C1–C10. doi: 10.1111/j.1750-3841.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 154.Jacob K, Periago MJ, Bohm V, Berruezo GR. Influence of lycopene and vitamin c from tomato juice on biomarkers of oxidative stress and inflammation. The British Journal of Nutrition. 2008;99(1):137–146. doi: 10.1017/S0007114507791894. [DOI] [PubMed] [Google Scholar]
- 155.Riso P, Visioli F, Grande S, Guarnieri S, Gardana C, Simonetti P, et al. Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. Journal of Agricultural and Food Chemistry. 2006;54(7):2563–2566. doi: 10.1021/jf053033c. [DOI] [PubMed] [Google Scholar]
- 156.Blum A, Monir M, Khazim K, Peleg A, Blum N. Tomato-rich (mediterranean) diet does not modify inflammatory markers. Clinical and Investigative Medicine. 2007;30(2):E70–E74. doi: 10.25011/cim.v30i2.982. [DOI] [PubMed] [Google Scholar]
- 157.Miller EC, Hadley CW, Schwartz SJ, Erdman JW, Jr, Boileau TW-M, Clinton SK. Lycopene, tomato products, and prostate cancer prevention. Have we established causality? Pure and Applied Chemistry. 2002;74(8):1435–1441. [Google Scholar]
- 158.Foolad MR. Genome mapping and molecular breeding of tomato. International Journal of Plant Genomics. 2007;2007:64358. doi: 10.1155/2007/64358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Apel W, Bock R. Enhancement of carotenoid biosynthesis in transplastomic tomatoes by induced lycopene-to-provitamin a conversion. Plant Physiology. 2009;151(1):59–66. doi: 10.1104/pp.109.140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, et al. Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. The Plant Cell. 2005;17(11):2954–2965. doi: 10.1105/tpc.105.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dumas Y, Dadomo M, Di Lucca G, Grolier P. Effects of environmental factors and agricultural techniques on antioxidantcontent of tomatoes. Journal of the Science of Food and Agriculture. 2003;83:369–382. [Google Scholar]
- 162.Giuntini D, Graziani G, Lercari B, Fogliano V, Soldatini GF, Ranieri A. Changes in carotenoid and ascorbic acid contents in fruits of different tomato genotypes related to the depletion of UV-b radiation. Journal of Agricultural and Food Chemistry. 2005;53(8):3174–3181. doi: 10.1021/jf0401726. [DOI] [PubMed] [Google Scholar]
- 163.Giuntini D, Lazzeri V, Calvenzani V, Dall’Asta C, Galaverna G, Tonelli C, et al. Flavonoid profiling and biosynthetic gene expression in flesh and peel of two tomato genotypes grown under UV-b-depleted conditions during ripening. Journal of Agricultural and Food Chemistry. 2008;56(14):5905–5915. doi: 10.1021/jf8003338. [DOI] [PubMed] [Google Scholar]
- 164.Colla G, Mitchell JP, Joyce BA, Huyck LM, Wallender WW, Temple SR, et al. Soil physical properties and tomato yield and quality in alternative cropping systems. Agronomy Journal. 2000;92:924–932. [Google Scholar]
- 165.Capanoglu E, Beekwilder J, Boyacioglu D, Hall R, de Vos R. Changes in antioxidant and metabolite profiles during production of tomato paste. Journal of Agricultural and Food Chemistry. 2008;56(3):964–973. doi: 10.1021/jf072990e. [DOI] [PubMed] [Google Scholar]
- 166.Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agricultural and Food Chemistry. 2002;50(10):3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 167.Garcia-Alonso FJ, Bravo S, Casas J, Perez-Conesa D, Jacob K, Periago MJ. Changes in antioxidant compounds during the shelf life of commercial tomato juices in different packaging materials. Journal of Agricultural and Food Chemistry. 2009;57(15):6815–6822. doi: 10.1021/jf900877c. [DOI] [PubMed] [Google Scholar]


