Abstract
1) Aims
Dyssynergic reflexive external urethral sphincter (EUS) activity following spinal cord injury can prevent bladder voiding, resulting in significant medical complications. Irreversible sphincterotomies or neurotomies can prevent EUS activation and allow bladder voiding, but may cause incontinence or loss of sacral reflexes. We investigated whether kilohertz frequency (KF) electrical conduction block of the sacral roots could prevent EUS activation and allow bladder voiding.
2) Methods
The S2 sacral nerve roots were stimulated bilaterally to generate bladder pressure in 6 cats. One S1 nerve root was stimulated proximally (20 Hz biphasic pulse trains) to evoke EUS pressure, simulating worst-case dyssynergic EUS reflexes. KF waveforms (12.5 kHz biphasic square wave) applied to an electrode implanted distally on the S1 nerve root blocked nerve conduction, preventing the increase in EUS pressure and allowing voiding.
3) Results
Applying KF waveforms increased bladder voiding in single, limited-duration trials from 3 ± 6% to 59 ± 12%. Voiding could be increased to 82 ± 9% of the initial bladder volume by repeating or increasing the duration of the trials.
4) Conclusions
Sacral nerve block can prevent EUS activation and allow complete bladder voiding, potentially eliminating the need for a neurotomy. Eliminating neurotomy requirements could increase patient acceptance of bladder voiding neuroprostheses, increasing patient quality of life and reducing the cost of patient care.
Keywords: Electrical Stimulation, Spinal Cord Injury, Rehabilitation, Urinary Bladder, Nerve Block
Introduction
Detrusor sphincter dyssynergia (DSD), the simultaneous uncoordinated contraction of the bladder and the external urethral sphincter (EUS), can obstruct bladder emptying following neurological disease or injury (Blaivas et al., 1981). DSD can lead to urinary tract infections, autonomic dysreflexia, vesico-ureteric reflux and hydronephrosis (Hackler, 1977). Such complications remain a principle cause of hospitalization for people with spinal cord injury (Cardenas et al., 2004).
Surgical neurotomies can allow bladder voiding by disrupting the spinal reflexes causing DSD and preventing EUS contractions. The Brindley bladder system, which requires an afferent rhizotomy, achieves complete, cost-effective bladder voiding using sacral root stimulation (Creasey and Dahlberg, 2001) and has been implanted in over 3000 patients (Finetech Medical LTD, 2010). Pudendal (PN) neurotomies have also been shown to improve bladder voiding (Engel and Schirmer, 1974). However, surgical neurotomies are irreversible, and any residual sensation and sacral reflexes controlling defecation, erection and ejaculation may be lost. These faculties are very important to quality of life (Anderson, 2004) and patients would prefer a device that does not require a neurotomy (Sanders et al., 2010).
Electrical nerve block provides a non-destructive alternative to a surgical neurotomy. Immediate, reversible electrical pudendal nerve block using kilohertz frequency stimulation (KF block) has been demonstrated in animals (Bhadra and Kilgore, 2005; Gaunt and Prochazka, 2008; Kilgore and Bhadra, 2004). Complete bladder voiding has been demonstrated using bilateral electrical pudendal nerve block to prevent EUS activation (Boger et al., 2008; Tai et al., 2007) without an afferent rhizotomy or PN neurotomy.
Electrical block of the sacral roots may provide an alternative to electrical pudendal nerve block. Muscles of the pelvic floor not innervated through the pudendal nerve may contribute to urethral outlet resistance (Juenemann et al., 1988). Sacral KF stimulation could block these muscles, potentially allowing voiding in patients for whom PN block is insufficient. Existing sacral neuroprostheses for bladder voiding could be modified to utilize sacral nerve block, speeding clinical translation.
The purpose of this study was to determine 1) the voiding efficiency achievable with sacral KF stimulation during dyssynergia and 2) whether sacral KF stimulation could allow bladder voiding during simulated DSD equivalent to unobstructed bladder voiding. Sacral KF block may increase patient acceptance of bladder voiding neuroprostheses by eliminating the requirement for surgical rhizotomies, thereby reducing patient morbidity and cost of care.
Materials and Methods
This study was conducted on six male cats. The Institutional Animal Care and Use Committee of Case Western Reserve University approved all animal care and experimental procedures. Data were collected under a combination of intravenous α-chloralose anesthesia (65 mg kg−1, Sigma, MO) and Isoflurane (0.25 – 3%). Subcutaneous buprenorphine (0.01 mg kg−1, Henry Schein, NY) was injected at induction and approximately every 12 hours thereafter. An anesthetic monitoring system (SurgiVet V9200 Advisor Monitor, Smiths Medical PM Inc, WI) was used to observe body temperature, ECG, blood oxygen saturation, blood pressure, and expired pCO2. Blood pressure, heart rate, and withdrawal and blink reflexes were monitored to maintain the appropriate depth of anesthesia. Respiration was maintained by a pressure-regulated respirator (ADS 1000, Engler Engineering Corporation, FL) and adjusted in response to expired pCO2 measurements. Temperature was maintained between 37 and 39 °C with a heating blanket. Saline (0.9%) with 8.4 mg mL−1 sodium bicarbonate and 5% dextrose was administered (3–20 ml hr−1, IV). A foam stand was used to support the legs and elevate the pelvis.
Sphincter and bladder pressures and voided volumes were recorded onto a computer through a data acquisition card (NI PCI-6221, National Instruments Corporation, TX, sampling rate 99 Hz) using a customized interface (Labview 8.0, National Instruments Corporation, TX). Signal conditioning and routing was provided by strain gauge signal-conditioning modules and configurable feed-through modules (NI SCC-SG24 and SCC-FT01, National Instruments Corporation, TX) contained in an NI terminal block (NI SC-2345, National Instruments Corporation, TX). Data were also recorded on a strip chart (TA-11, Gould Inc., AZ). Volume voided was measured using a high-resolution single point load cell (s215, 0.9 kg full scale, Strain Measurement Devices, CT or ESP 0.6 kg full scale, Load Cell Central, PA). Sphincter pressure was measured using a 4F catheter-mounted dual sensor microtransducer (C7C4F, MMI-Gaeltech, NJ) placed at the level of the urethral sphincter (Baoqing et al., 1999). The position and orientation of the catheter were adjusted to maximize transduction of the evoked urethral pressure response to sacral root stimulation (Bhadra et al., 2006a; Boger et al., 2008).
Bladder pressure was measured using a suprapubic, 6F dual-lumen catheter (DLC – 6D, Life Tech, TX) connected to an external pressure transducer (Deltran IV, Utah Medical Devices, UT). The bladder volume could be adjusted using the second lumen of the 6F dual-lumen catheter. Saline (30 ml total) was infused into the bladder at a rate of 1 – 4 ml minute−1 (Genie YA-12, Kent Scientific, CT). Initial bladder volume for each trial depended on the physiological urine generation rate, which varied between animals. Baseline bladder pressures preceding trials were less than 20 cmH2O and initial bladder volumes (34 ± 7 ml) were comparable among trials conducted in the same animal.
Following a lumbosacral laminectomy (L6 – S2), four extradural cuff electrodes were implanted on the spinal sacral roots. A proximal bipolar electrode and a distal tripolar electrode were implanted on the right S1 root (Figure 1: EUS Stim and EUS KF). Two tripolar electrodes were implanted bilaterally on the S2 sacral roots (Figure 1: Bladder Stim). A multichannel, isolated voltage-controlled stimulator was used to generate stimulation waveforms (DS8000 stimulator and DLS 100 isolators, World Precision Instruments Inc, FL).
Figure 1.
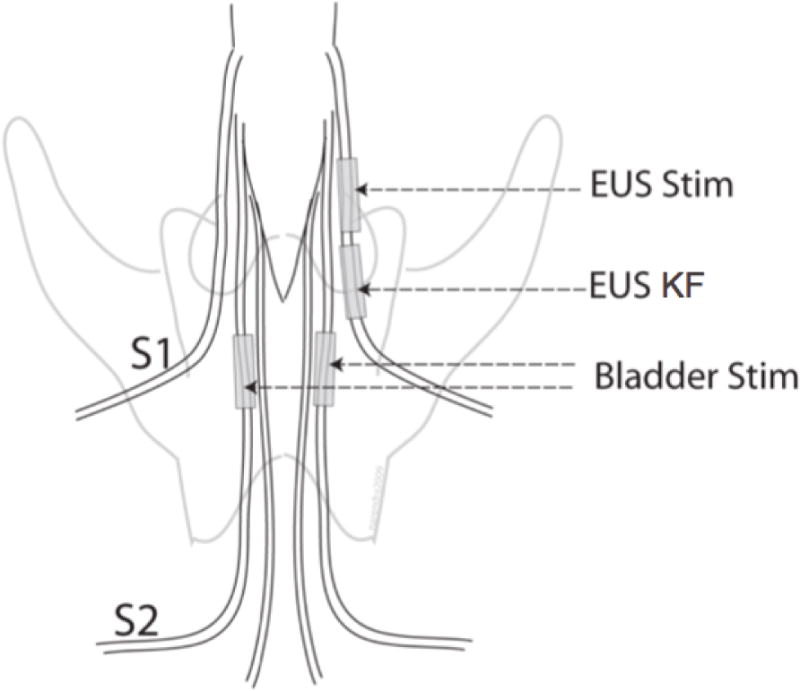
Electrode locations. Continuous proximal S1 spinal root stimulation evoked EUS pressure, mimicking dyssynergia (EUS Stim: 20 Hz, 100 μs pulse width, balanced biphasic, first phase cathodic). Kilohertz frequency waveforms applied to the distal S1 spinal root blocked nerve impulses evoked by the EUS Stim (EUS KF: 12.5 kHz, biphasic square wave). Intermittent bilateral S2 spinal root stimulation generated bladder pressure (Bladder Stim: 20 Hz, 100 μs pulse width, balanced biphasic, first phase cathodic, 2 s on / 4 s off).
Data processing was done in Matlab (Mathworks, Inc., MA). Statistical analyses were performed using Minitab (Minitab Inc, PA).
Electrical Stimulation: Description and Purpose
Intermittent bilateral S2 sacral root stimulation (20 Hz, 100 μs pulse width, balanced biphasic, first phase cathodic, Figure 1: Bladder Stim) was used to evoke bladder pressure. A 33% duty cycle (2 s on / 4 s off) allowed voiding between stimulation intervals. Bladder stimulation amplitudes (7 ± 3 Vpp; range 3 – 8 Vpp) were adjusted as necessary to remain supramaximal. Bladder stimulus duration was 60 or 90 seconds (except a single trial of 73 seconds duration).
Continuous stimulation (20 Hz, 100 μs pulse width, balanced biphasic, first phase cathodic, Figure 1: EUS Stim) of the S1 sacral root through the proximal bipolar cuff electrode was provided to evoke consistent external urethral sphincter pressure, mimicking dyssynergic continence reflexes absent in this acute, anesthetized preparation. The stimulation amplitude (3 ± 1 Vpp; range 2 – 4 Vpp) was chosen to minimize voiding during bladder stimulation and was adjusted as necessary.
Kilohertz frequency waveforms applied to the distal tripolar S1 cuff electrode (12.5 kHz, biphasic square wave, Figure 1: EUS KF) blocked transmission of nerve impulses evoked by the proximal EUS Stim. The stimulation frequency was chosen to reduce the EUS KF onset response based on previous results (Bhadra et al., 2006a). During the experiment the stimulation amplitude was adjusted to allow voiding while remaining below 20 Vpp (15 ± 3 Vpp; range of 12 – 20 Vpp with a single trial conducted at 24 Vpp).
Bladder Voiding Trials: Description and Purpose
Max Voiding trials, Min Voiding trials, and KF trials investigated the effect of sacral root block on the voided bladder volume, the bladder pressure, and the urine flow rate (Figure 2). KF Max trials quantified the maximum voiding achievable using sacral root block in the preparation (Figure 3).
Figure 2.
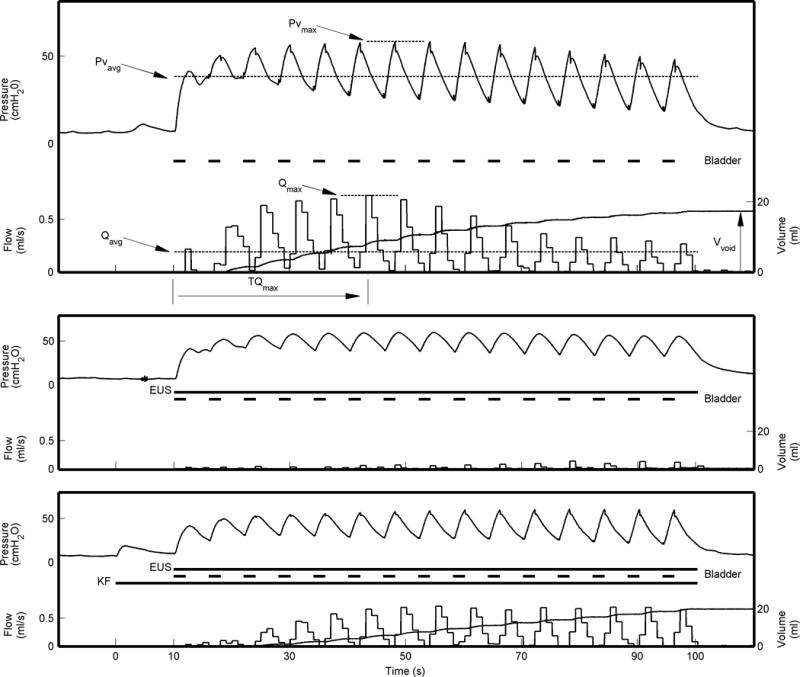
Stimulus timings and physiological measurements for Max Voiding trials (Top), Min Voiding trials (Middle) and Kilohertz Frequency (KF) trials (Bottom). Stimulus Timings: The horizontal lines below each bladder pressure trace show the duration of the bladder, EUS, and KF stimuli. Bladder) Intermittent bilateral S2 Bladder Stim generated bladder pressure. EUS) Continuous proximal S1 EUS Stim was applied in Min Voiding and Kilohertz Frequency trials. EUS stim directly evoked urethral pressure in the Min Voiding trials, limiting bladder voiding. KF) Distal S1 EUS KF waveforms were applied in KF trials, preventing proximally evoked action potentials from reaching the urethral sphincter and allowing bladder voiding. Physiological Measurements: The maximum and average evoked bladder pressure (Pvmax and Pvavg) and volume voided (Vvoid) were measured. The maximum and average flow rate (Qmax and Qavg) and the elapsed time from the start of Bladder Stim until the maximum flow rate was achieved (TQmax) were calculated from the volume voided. For clarity, physiological measurements are only shown for the Max Voiding trial.
Figure 3.
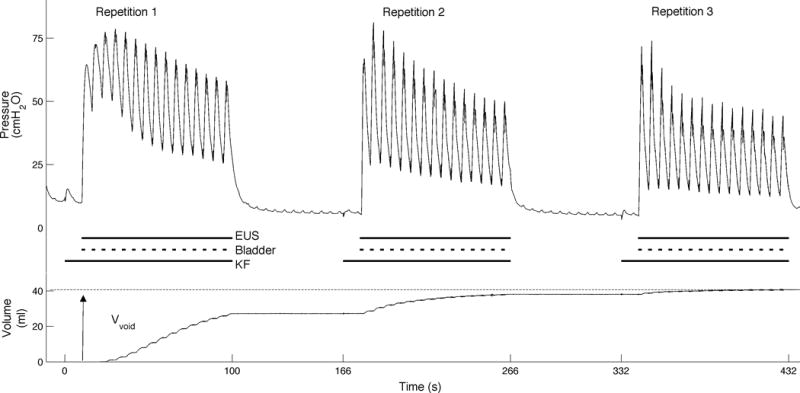
Kilohertz Frequency Max (KF Max) trials were intended to maximize the bladder voiding achieved using sacral block. KF Max trials included either three repeated stimulation intervals separated by approximately 60 seconds (as shown, n = 9) or a single stimulation interval of extended duration (not shown, n=2). Stimulus Timings: The horizontal lines below the bladder pressure trace show the duration of the bladder, EUS, and kilohertz frequency stimuli. Bladder) Intermittent bilateral S2 Bladder Stim evoked bladder pressure. EUS) Continuous proximal S1 EUS Stim mimicked maximally dyssynergic reflexes. KF) Distal S1 EUS KF stimulation prevented external urethral sphincter activation. Bladder Stim and EUS Stim were applied simultaneously and began 10 seconds after EUS KF stimulation. Extracted Measurements: The overall voided volume (Vvoid) at the end of three trials was recorded. In KF Max trials including three stimulation intervals, the first stimulation interval was also analyzed separately as a KF trial (see Figure 2 for KF trial description).
Max Voiding trials simulated unobstructed post-stimulus voiding and included only Bladder Stim (for definition of stimulation types see Figure 1). Min Voiding trials simulated complete bladder sphincter dyssynergia. Bladder pressure was evoked with Bladder Stim and urethral pressure was evoked with EUS Stim. KF trials included Bladder Stim, EUS Stim, and KF Stim. Comparable voiding in KF trials and Max Voiding trials would suggest effective nerve block, while comparable voiding in KF trials and Min Voiding trials would suggest poor nerve block.
Max Voiding trials and Min Voiding trials were also used to identify problems and ensure consistency of the experimental preparation. Significant voiding during Min Voiding trials indicated sphincter damage or fatigue, as the fully activated urethral sphincter was no longer capable of preventing voiding. Insignificant voiding during Max Voiding trials indicated either insufficient bladder pressure or elevated urethral resistance.
In Max Voiding trials, Min Voiding trials, and KF trials the total volume voided, the maximum vesicular pressure, and the average vesicular pressure were recorded (Figure 2: Vvoid, Pvmax, Pvavg). The flow rate was derived every second from the change in volume voided. The maximum flow rate, the average flow rate, and the elapsed time from the start of the trial until the maximum flow rate was reached were calculated (Figure 2: Qmax, Qavg, TQmax). After trials with significant voiding the bladder was drained and the residual volume, Vresidual, was measured. The bladder was not drained following trials with minimal voiding. Instead, Vresidual was estimated as the sum of subsequent voiding and the next directly measured residual bladder volume. The voiding efficiency, Veff, was defined as Vvoid / (Vresidual + Vvoid).
An analysis of variance with post hoc comparisons of means by trial type was used to investigate the dependence of the variables recorded in Max Voiding trials, Min Voiding trials, and KF trials on trial type and animal. No interaction between trial type and animal was assumed. Voiding during EUS control trials was rare, so only KF and Max Voiding trials were included in the analysis of TQmax.
For each KF Max trial the voiding efficiency was determined from the total volume voided. Student’s t-test was used to compare Veff for Min Voiding and KF Max trials.
Sacral Conduction Block
The quality of the sacral conduction block was measured in a subset of experiments. The bladder was drained and the urethral pressure recorded. Trials included either the same electrical stimulation as in the KF trials described above or continuous proximal stimulation combined with distal block as in previous PN block studies (Bhadra et al., 2006a). The EUS pressure was lowpass-filtered (5 Hz cutoff frequency) and the minimum EUS pressure during KF stimulation (Pumin) and the elapsed time until the EUS pressure decreased to within 10 cmH2O of Pumin (TPumin) were recorded.
Results
Voiding during KF trials was achieved in three of six animals. Block could not be achieved in one animal. Sacral stimulation evoked minimal bladder pressure in one animal, prevented voiding although EUS block was complete. The remaining animal did not void during Max Voiding trials due to a urethral obstruction despite sufficient bladder pressure, even under deep anesthesia.
Bladder Voiding
Forty voiding trials were conducted in three animals (Table 1). KF stimulation greatly improved voiding efficiency (Figure 4). KF Max trials voided 82 ± 9% and KF trials voided 59 ± 12% of the initial bladder volume, significantly more than the 3 ± 6% of the initial bladder volume voided in Min Voiding trials.
Table I.
Forty trials were conducted in three animals. Nine KF trials in animals 2 and 3 were also the first repeat in nine KF Max trials. Two extended-duration KF Max trials were conducted in animal 1.
| Animal | Min Voiding | Max Voiding | KF Trials | KF Max Trials |
|---|---|---|---|---|
| 1 | 3 | 5 | 5 | 2 |
| 2 | 3 | 4 | 6 | 5 |
| 3 | 3 | 5 | 4 | 4 |
|
| ||||
| Total | 9 | 14 | 15 | 11 |
Figure 4.
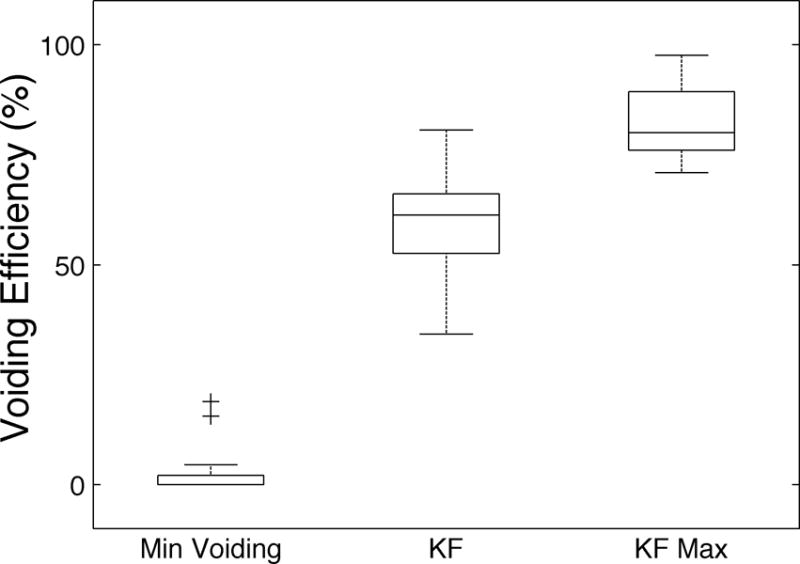
Minimal bladder voiding was achieved in Min Voiding trials, which served as negative controls. In KF trials, the addition of sacral kilohertz frequency waveforms permitted significant bladder voiding. In KF Max trials, voiding efficiencies comparable to those reported following pudendal neurotomies were achieved by repeating KF trials or extending the KF trial duration. Voiding efficiency was significantly greater in KF and KF Max trials than in Min Voiding trials (P < 0.01 for both). Most KF trials were the first of three repetitions constituting a KF Max trial; consequently, voiding efficiencies were greater in KF Max trials than KF trials.
Bladder voiding in KF trials was comparable to unobstructed bladder voiding in Max Voiding trials (Figure 5). Voiding efficiency and volume voided did not differ between KF trials and Max Voiding trials (Veff: p = 0.92, Vvoid: p = 0.98, n = 38). Bladder pressures and flow rates were also similar (Pvmax: p = 0.67, Pvavg: p = 0.22, Qmax: p = 0.43, Qavg: p = 1.0, n = 38). However, TQmax was greater in KF than in Max Voiding trials (33 ± 20 s versus 18 ± 10 s, p < 0.001, n = 29).
Figure 5.
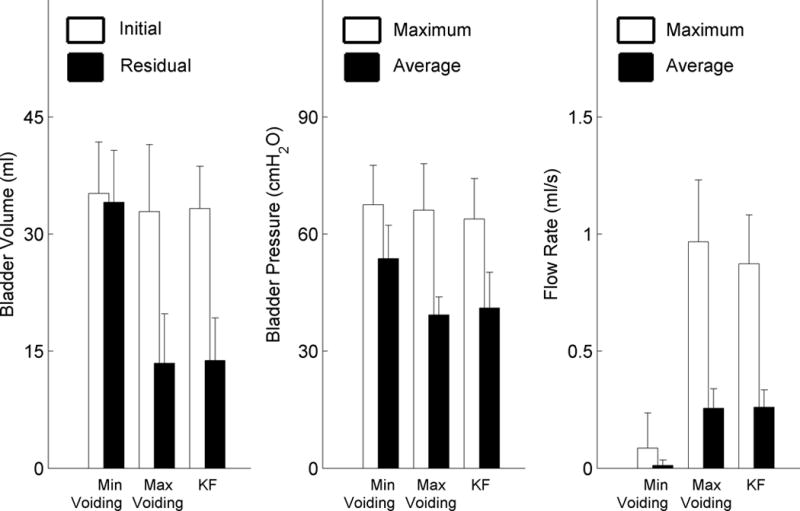
KF trials and Max Voiding trials exhibited similar urodynamic features. Left) Initial bladder volumes were similar for all trial types. Residual volumes were significantly greater for Min Voiding trials than KF or Max Voiding trials. Middle) Maximum evoked bladder pressures were similar all trial types, but average bladder pressures were significantly greater for Min Voiding trials than either KF or Max Voiding trials. Right) Maximum and average flow rates were similar for KF and Max Voiding trials and much greater than flow rates for Min Voiding trials. For all comparisons ∝ = 0.01.
Bladder voiding in KF trials was superior to bladder voiding in Min Voiding trials (Figure 5). Voiding efficiency and volume voided in KF trials was significantly greater than voiding efficiency and volume voided in Min Voiding trials. The residual bladder volume in KF trials was significantly less than the residual bladder volumes in Min Voiding trials. While maximum bladder pressures in KF trials and Min Voiding trials were comparable, average bladder pressures were significantly lower in KF trials than in Min Voiding trials. The maximum flow rate and average flow rate were greater in KF trials than in Min Voiding trials. TQmax was highly variable for Min Voiding trials (27 ± 30 s). Urine flow in Min Voiding trials occurred in either a single pulse at the initiation of bladder stimulation or at the very end of the trial.
Voiding efficiency and voided volume were consistent between animals (Vvoid: p = 0.16, Veff: p = 0.67, n = 38). However, residual bladder volume, maximum and average bladder pressure, maximum and average flow rate and the elapsed time until the maximum flow rate was reached differed significantly between animals (p < 0.01, n = 38).
Sacral Conduction Block
Conduction block quality was examined in five animals. Both the minimum EUS pressure during KF stimulation (Pumin: 22 ± 21 cmH2O, range 0 – 80 cmH2O) and the elapsed time until the EUS pressure decreased to within 10 cmH2O of Pumin (TPumin: 12 ± 14 s, range 3 – 43 s) varied between animals. Pumin was minimal in three animals (3 ± 3 cmH2O), of which two voided (one lacked evoked bladder pressure), but was high in the remaining two animals (18 and 80 cmH2O), which did not void.
Discussion
Effective bladder voiding was achieved using sacral nerve block to prevent EUS activation. Voiding efficiencies in KF Max trials (82 ± 9% of initial bladder volume) were comparable to voiding efficiencies achieved following a bilateral pudendal neurotomy (Boger et al., 2008; Boggs et al., 2006). Maximum flow, average flow, and bladder pressure were comparable in KF and Max Voiding trials, suggesting low outlet resistance during KF stimulation. While the maximum flow rate was reached later in KF trials than in Max Voiding trials, this delay did not reduce the total volume voided.
Sacral KF stimulation appears to have caused a temporary nerve conduction block. KF stimulus parameters were comparable to those achieving block in previous PN block preparations (Bhadra et al., 2006a; Boger et al., 2008). Min Voiding trials demonstrated that the urethral sphincter remained contractile throughout the experiment.
Sacral Stimulation for Voiding
The commercially available Finetech-Bridley Bladder Control System, which has been implanted in over 3000 patients (Finetech Medical, 2010), uses intermittent sacral stimulation to directly activate both the bladder and sphincter. Voiding occurs between stimulation intervals due to the difference in relaxation time between the detrusor and the EUS. However, to achieve effective bladder voiding, sacral sensory afferents must be transected to disable continence reflexes (Brindley, 1994).
Our approach would replace the dorsal rhizotomy required by the Brindley system with a temporary, non-destructive conduction block of EUS efferents. Elimination of the dorsal rhizotomy might promote patient acceptance of sacral conduction block for voiding. Though this study in acute cats demonstrated effective bladder voiding, further experimental validation using chronic spinal cats is warranted, as neuroanatomical changes following spinal cord injury could potentially reduce the effectiveness of the therapy.
Alternative methods for preventing EUS activation during sacral stimulation have been pursued. Sacral stimulation waveforms selectively activating only bladder efferents have been intraoperatively tested in patients (Rijkhoff et al., 1997). However, selective stimulation does not prevent activation of continence reflexes, and voiding experiments in animals have required a dorsal rhizotomy to achieve bladder voiding (Bhadra et al., 2006b). Mixed results have been achieved using sub-kilohertz sacral or PN stimulation: some studies have achieved voiding without an additional rhizotomy or neurotomy (Abdel-Gawad et al., 2001; Boyer et al., 2000; Thuroff et al., 1982), while other studies have not (Li et al., 1992; Li et al., 1995). Voiding using sub-kilohertz stimulation has been attributed to EUS fatigue or sacral nerve block. However, studies claiming nerve block did not include proximal stimulation (Abdel-Gawad et al., 2001; Boyer et al., 2000). Without proximal stimulation, conduction block cannot be distinguished from the neural fatigue observed by researchers using a similar 600 Hz waveform (Kilgore and Bhadra, 2004). In some studies, repeated fatiguing of the EUS resulted in sphincter hypertrophy (Bazeed et al., 1982), which could eventually inhibit voiding in a clinical implant.
Pudendal nerve conduction block using KF waveforms has successfully allowed voiding (Boger et al., 2008; Tai et al., 2007). Because the PN is the final motor pathway to the EUS, bilateral PN block can prevent direct EUS activation due to sacral stimulation or reflexive activation caused by dyssynergic continence reflexes. Sacral KF stimulation provides a complementary alternative to PN block for patients unable to void due to non-PN mediated continence mechanisms. Urethral pressure depends on both the EUS, innervated through the pudendal nerve, and the pelvic floor musculature, innervated directly from the S2 and S3 sacral nerve roots (Juenemann et al., 1988; Juenemann et al., 1987). Blocking reflexive EUS activation at the sacral level would therefore require multiple electrodes and effective nerve block of multiple sacral roots. Sacral KF block might be implemented more directly than PN block, as existing implanted Brindley devices might enable clinical testing of KF waveforms with human-approved electrodes.
Conclusion
KF sacral root stimulation allows effective bladder voiding by blocking EUS activation at the sacral root level. Bladder voiding during KF stimulation was equivalent to unobstructed bladder voiding. By eliminating requirements for a dorsal rhizotomy, sacral KF block neuroprostheses could increase patient acceptance of bladder voiding neuroprostheses, reducing patient morbidity and cost of care.
Acknowledgments
This work was supported by the Department of Veteran Affairs VA RR&D B6685R, B3675R; NIH DK077089, HD40298, AR07505, EB002091, EB004314; the State of Ohio BRTT03-10; and the Cleveland VA FES Center. The authors thank Jaime McCoin, Timothy Bruns, Tina Goetz, and Timothy Mariano for their experimental assistance.
References
- Abdel-Gawad M, Boyer S, Sawan M, Elhilali MM. Reduction of bladder outlet resistance by selective stimulation of the ventral sacral root using high frequency blockade: a chronic study in spinal cord transected dogs. J Urol. 2001;166(2):728–733. [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Baoqing W, Narendra B, Warren MG. Functional anatomy of the male feline urethra: morphological and physiological correlations. J Urol. 1999;161(2):654–659. [PubMed] [Google Scholar]
- Bazeed MA, Thuroff JW, Schmidt RA, Wiggin DM, Tanagho EA. Effect of chronic electrostimulation of the sacral roots on the striated urethral sphincter. J Urol. 1982;128(6):1357–1362. doi: 10.1016/s0022-5347(17)53507-7. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Bhadra N, Kilgore K, Gustafson KJ. High frequency electrical conduction block of the pudendal nerve. Journal of neural engineering. 2006a;3(2):180–187. doi: 10.1088/1741-2560/3/2/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra N, Grunewald V, Creasey GH, Mortimer JT. Selective activation of the sacral anterior roots for induction of bladder voiding. Neurourol Urodyn. 2006b;25(2):185–193. doi: 10.1002/nau.20184. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Kilgore KL. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve. 2005;32(6):782–790. doi: 10.1002/mus.20428. [DOI] [PubMed] [Google Scholar]
- Blaivas JG, Sinha HP, Zayed AA, Labib KB. Detrusor-external sphincter dyssynergia. J Urol. 1981;125(4):542–544. doi: 10.1016/s0022-5347(17)55099-5. [DOI] [PubMed] [Google Scholar]
- Boger A, Bhadra N, Gustafson KJ. Bladder voiding by combined high frequency electrical pudendal nerve block and sacral root stimulation. Neurourol Urodyn. 2008;27(5):435–439. doi: 10.1002/nau.20538. [DOI] [PubMed] [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. Journal of neural engineering. 2006;3(1):43–51. doi: 10.1088/1741-2560/3/1/005. [DOI] [PubMed] [Google Scholar]
- Boyer S, Sawan M, Abdel-Gawad M, Robin S, Elhilali MM. Implantable selective stimulator to improve bladder voiding: design and chronic experiments in dogs. IEEE Trans Rehabil Eng. 2000;8(4):464–470. doi: 10.1109/86.895949. [DOI] [PubMed] [Google Scholar]
- Brindley GS. The first 500 patients with sacral anterior root stimulator implants: general description. Paraplegia. 1994;32(12):795–805. doi: 10.1038/sc.1994.126. [DOI] [PubMed] [Google Scholar]
- Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Archives of physical medicine and rehabilitation. 2004;85(11):1757–1763. doi: 10.1016/j.apmr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Creasey GH, Dahlberg JE. Economic consequences of an implanted neuroprosthesis for bladder and bowel management. Archives of physical medicine and rehabilitation. 2001;82(11):1520–1525. doi: 10.1053/apmr.2001.25912. [DOI] [PubMed] [Google Scholar]
- Engel RM, Schirmer HK. Pudendal neurectomy in neurogenic bladder. J Urol. 1974;112(1):57–59. doi: 10.1016/s0022-5347(17)59641-x. [DOI] [PubMed] [Google Scholar]
- Finetech Medical LTD. Bladder Management Post Spinal Cord Injury 2010 [Google Scholar]
- Gaunt RA, Prochazka A. Transcutaneously Coupled, High-Frequency Electrical Stimulation of the Pudendal Nerve Blocks External Urethral Sphincter Contractions. Neurorehabil Neural Repair. 2008 doi: 10.1177/1545968308328723. [DOI] [PubMed] [Google Scholar]
- Hackler RH. A 25-year prospective mortality study in the spinal cord injured patient: comparison with the long-term living paraplegic. J Urol. 1977;117(4):486–488. doi: 10.1016/s0022-5347(17)58506-7. [DOI] [PubMed] [Google Scholar]
- Juenemann KP, Lue TF, Schmidt RA, Tanagho EA. Clinical significance of sacral and pudendal nerve anatomy. J Urol. 1988;139(1):74–80. doi: 10.1016/s0022-5347(17)42297-x. [DOI] [PubMed] [Google Scholar]
- Juenemann KP, Schmidt RA, Melchior H, Tanagho EA. Neuroanatomy and clinical significance of the external urethral sphincter. Urol Int. 1987;42(2):132–136. doi: 10.1159/000281871. [DOI] [PubMed] [Google Scholar]
- Kilgore KL, Bhadra N. Nerve conduction block utilising high-frequency alternating current. Med Biol Eng Comput. 2004;42(3):394–406. doi: 10.1007/BF02344716. [DOI] [PubMed] [Google Scholar]
- Li JS, Hassouna M, Sawan M, Duval F, Elhilali MM. Electrical stimulation induced sphincter fatigue during voiding. J Urol. 1992;148(3):949–952. doi: 10.1016/s0022-5347(17)36784-8. [DOI] [PubMed] [Google Scholar]
- Li JS, Hassouna M, Sawan M, Duval F, Elhilali MM. Long-term effect of sphincteric fatigue during bladder neurostimulation. J Urol. 1995;153(1):238–242. doi: 10.1097/00005392-199501000-00084. [DOI] [PubMed] [Google Scholar]
- Rijkhoff NJ, Wijkstra H, van Kerrebroeck PE, Debruyne FM. Selective detrusor activation by electrical sacral nerve root stimulation in spinal cord injury. J Urol. 1997;157(4):1504–1508. [PubMed] [Google Scholar]
- Sanders PMH, Ijzerman MJ, Roach MJ, Gustafson KJ. Patient preferences for next generation neural prostheses to restore bladder function. Spinal Cord. 2010 doi: 10.1038/sc.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Wang J, Wang X, Roppolo JR, de Groat WC. Voiding reflex in chronic spinal cord injured cats induced by stimulating and blocking pudendal nerves. Neurourol Urodyn. 2007;26(6):879–886. doi: 10.1002/nau.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuroff JW, Bazeed MA, Schmidt RA, Wiggin DM, Tanagho EA. Functional pattern of sacral root stimulation in dogs. I. Micturition. J Urol. 1982;127(5):1031–1033. doi: 10.1016/s0022-5347(17)54182-8. [DOI] [PubMed] [Google Scholar]


