Summary
Macrophages are best known for their protective search and destroy functions against invading micro-organisms. These processes are commonly known as chemotaxis and phagocytosis. Both of these processes require actin cytoskeletal remodeling to produce distinct F-actin rich membrane structures called lamellipodia and phagocytic cups. This review will focus on the mechanisms by which macrophages regulate actin polymerization through initial receptor signaling and subsequent Arp2/3 activation by nucleation promoting factors like the WASP/WAVE family, followed by remodeling of actin networks to produce these very distinct structures.
Keywords: chemotaxis, FcγR, GPCR, CSF-1, WASP/WAVE, Arp2/3, integrin
General introduction
Elie Metchnikoff was the first to morphologically distinguish macrophages from other white blood cells, and his pioneering work laid the foundation for our understanding and recognition of these immune cells as indispensable to our innate and adaptive immunity (1). Macrophages are best known for their protective functions that include seeking out and eliminating infectious agents, repairing tissue damage, and mediating inflammatory response (2). In addition, macrophages play important roles in homeostatic tissue maintenance. Macrophages are resident cells in tissues including lung (alveolar), liver (Kuppfer cells), brain (microglia), and bone (osteoclast), originating from either primitive myeloid progenitors, which arise in the yolk sac, or recruited peripheral monocyte precursors that differentiate into mature macrophages in situ (3). Macrophages possess a highly specialized set of abilities that allow them to carry out these important functions.
Macrophages are highly motile cells able to move in a wide range of environments. Monocyte/macrophages are capable of endothelial transmigration (diapedesis) that consist of paracellular or transcellular transmigration across the endothelial cells of blood vessels followed by breaking through the associated basement membrane (4). Monocyte/macrophages are also able to migrate along 2-dimensional (2D) surfaces such as blood vessel walls, as in patrolling monocytes (5), as well as in complex 3-dimensional (3D) extracellular environments. Furthermore, macrophages have the ability to alternate between amoeboid or mesenchymal-like migratory modes in 3D environments (6).
Another major feature of macrophages is their ability to internalize particles larger than 0.5 µm by a process known as phagocytosis (hence their name – big eaters). As opposed to other ways to internalize external content, such as endocytosis or macro and micropinocytosis, phagocytosis targets big solid particles and needs to be triggered by direct ligand-receptor contacts between the particle and the phagocyte. The ability of phagocytes to engulf and ingest external pathogens, dead cells, or many other types of particles is a fundamental process in homeostasis and the immune defense of the organism (7).
The nucleation-promoting factors: key to F-actin formation
Both migration and phagocytosis involve two processes: first the sensing of external cues (chemoattractants or phagocytic targets), and second the controlled generation of mechanical forces which will lead to conspicuous deformation of the cell body. These changes in cell morphology are dependent on Arp2/3 triggered actin polymerization (8, 9). Indeed, the Arp2/3 complex is critical for many cellular functions in addition to phagocytosis and migration including endocytosis, endosomal fusion and formation of adhesions and junctions (reviewed in 10). Consistent with its importance in cell function, Arp2/3 is normally kept in an inactivate state until needed for the formation of F-actin and it relies on nucleation-promoting factors (NPFs) to favor the initiation of new filaments. Many of the NPFs for Arp2/3 belong to the well-known Wiskott-Aldrich syndrome protein (WASP) family. This family consists of WASP (restricted to hematopoietic cells), neural WASP (N-WASP), WASP family Verproline-homologous protein (WAVE) 1, 2, and 3, and other more exotic members WASH, WHAMM, and JMY. WHAMM has been shown to be important for endoplasmic reticulum (ER) to Golgi transport (11), and WASH is involved in vesicle trafficking (12). While JMY has been implicated in cell motility and actin polymerization in an Arp2/3-independent manner (13), the other members stimulate de novo actin polymerization by binding to and activating the Arp2/3 complex via their conserved VCA domain (verprolin homology, central hydrophobic, and acid regions) (10).
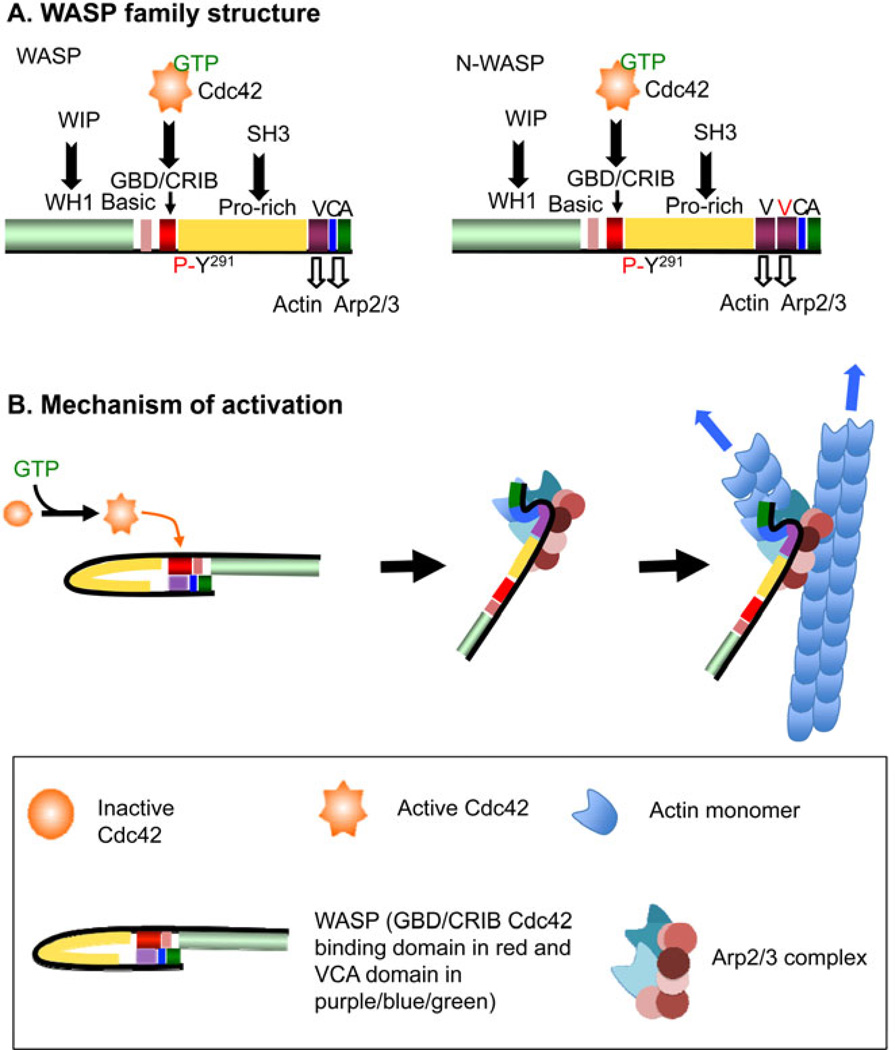
Only WASP, N-WASP, and WAVE2 have been implicated in the macrophage functions that are discussed here. More information of the other members of the WASP family can be found elsewhere (14). Adjacent to the VCA domain, a proline-rich region represents a binding site for SH3 domain-containing proteins and is also shared by these WASP family proteins. The N-termini are more divergent with WASP and N-WASP bearing a WASP homology domain (WH1), followed by a short basic region and a GTPase-binding domain (GBD) capable of binding GTP-loaded Cdc42 (Fig. 1A). WIP (WASP-interacting protein) interacts with the WH1 domain of N-WASP and WASP and performs critical regulatory functions and is primarily required for the stability of WASP protein levels in vivo (15). Additional structural differences between WASP and N-WASP may be responsible for the different functions of these proteins in vivo. In particular, N-WASP has an additional verprolin homology domain that enhances actin polymerization in vitro compared to WASP (16). Like Arp2/3, the NPFs are also kept in an inactive state until needed. Both WASP and N-WASP are autoinhibited through the sequestration of the VCA domain by its binding to the GBD. Activation of WASP family members occurs through a conformational change that frees the VCA domain to bind to the Arp2/3 complex and can occur through multiple mechanisms such as binding of Cdc42 or SH3 domain containing proteins (Fig. 1B) subsequent oligomerization of the VCA domain can also increase actin polymerization (reviewed in 17). Additional signals can also activate WASP and tyrosine phosphorylation on Tyr291 residue of WASP results in activation of Arp2/3-mediated actin polymerization in vitro (18). Cdc42-dependent WASP conformational opening is prerequisite for the tyrosine phosphorylation of WASP and related effects (19, 20), demonstrating a key role for Cdc42 in WASP function.
Fig. 1. Mechanism of activation of WASP/N-WASP.
(A) Domain structure of WASP and N-WASP (N-WASP differs from WASP by containing an additional V domain). (B) Mechanism of activation of WASP by relaxation of the autoinhibited conformation allowing for actin monomer and Arp2/3 binding to the VCA domain. Note that other pathways can induce or potentiate activation of WASP.
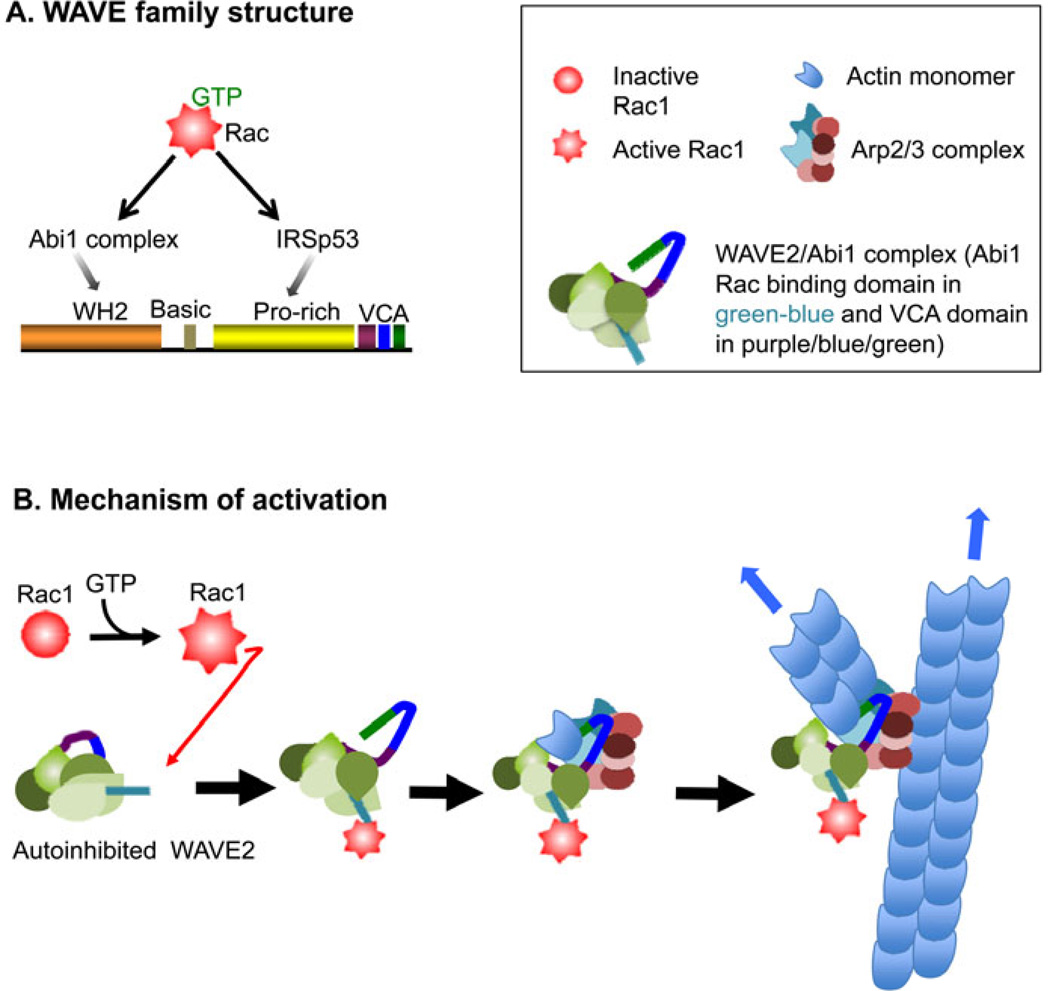
While there are three members of the WAVE family, monocytes express both WAVE1 and WAVE2, and only WAVE2, the ubiquitously expressed family member, is present in mature macrophages (21). WAVE proteins possess their own N-terminal WAVE homology domain (WH2) immediately followed by a basic stretch that binds to PI(3,4,5)P3, a proline-rich region known to interact with SH3 domain containing proteins, and a C-terminal VCA domain found in all WASP/WAVE proteins (Fig. 2A). The WH2 domain of WAVE binds to a complex of proteins called the Abi1 complex that is important in WAVE protein stabilization. Importantly, while WAVE proteins do not have a comparable region to the GBD, it is a downstream effector of Rac. The interaction of Rac with WAVE proteins has been proposed to occur through either the Abi1 complex or with IRSp53 (reviewed in 22–24). Recently, the mechanism by which WAVE is activated has been identified through structural analysis, where the binding of Rac and phospholipids recruits WAVE2 to the membrane and frees the VCA domain for Arp2/3 binding (25)(Fig. 2B). In addition, there is evidence for the importance of phosphorylation of WAVE and the Abi1 complex in the regulation of WAVE activity (reviewed in 26). All this suggests that the regulation of WAVE proteins is as complex, if not more so, than the WASP family members. Overall, the WASP/WAVE NPFs can be seen as signaling hubs that integrate various upstream cues to elicit appropriate actin responses.
Fig. 2. Mechanism of activation of WAVE.
(A) Domain structure of WAVE2. (B) Mechanism of WAVE2 activation downstream of Rac1. Rac1 does not bind directly to WAVE complex but depends instead on interactions with Abi1 and IRSp53. WAVE complex in turn activates the ARP2/3 complex, which nucleates branched F-actin.
Despite the functional importance of actin response in migration and phagocytosis, many gaps exist in our understanding of its dynamics leading to the generation of distinct F-actin rich membrane structures. This review presents our current knowledge.
Phagocytosis
Phagocytosis requires a conspicuous change of shape of the cell: it needs to deform to engulf particles that may represent a substantial fraction of its cellular size. It is now beyond doubt that the actin cytoskeleton, as a major actor of cellular morphology, is the primary force driving phagocytosis. However, more than a hundred years after the first description of this phenomenon, many gray areas remain.
One of the difficulties encountered when studying phagocytosis is the tremendous diversity of receptors and ligands that can trigger it, each resulting in different downstream pathways. In macrophages research has mostly focused on two phagocytic receptors: the Fcγreceptor (FcγR) and the complement receptor 3 (CR3) (also known as integrin αMβ2, Mac1 and CD11b/CD18). The former helps recognize particles opsonized by antibodies and the latter binds particles coated with the complement molecule C3b.
Given that most of the available data has been obtained with the FcγR-triggered phagocytosis, we use it as a standard model. Similarities and differences with CR3-triggered phagocytosis will be discussed in a second part. This review focuses only on actin and actin-related roles in phagocytosis.
FcγR phagocytosis
In pioneer studies several decades ago, it was observed that macrophages in the presence of IgG-opsonized beads would progressively extend membrane processes to wrap around the bead until engulfment was complete. However, when only one hemisphere of the beads was coated the macrophages stopped at the boundary between the opsonin and the IgG-free halves (27). The term ‘zipper model’ has thus been coined to describe the idea that the IgG-coated surface was acting as a railway to guide the extending membrane of the macrophages around the particle. This model has evolved over the years. It appeared that FcγRs are not passive sticky molecules and their lateral mobility and clustering is necessary for phagocytosis to occur (28). Also, it has recently been demonstrated that the IgG coating is not sufficient, as certain shapes are not suitable for engulfment (29). However, the central idea remains that there is a succession of local signaling hotspots as phagocytosis progresses.
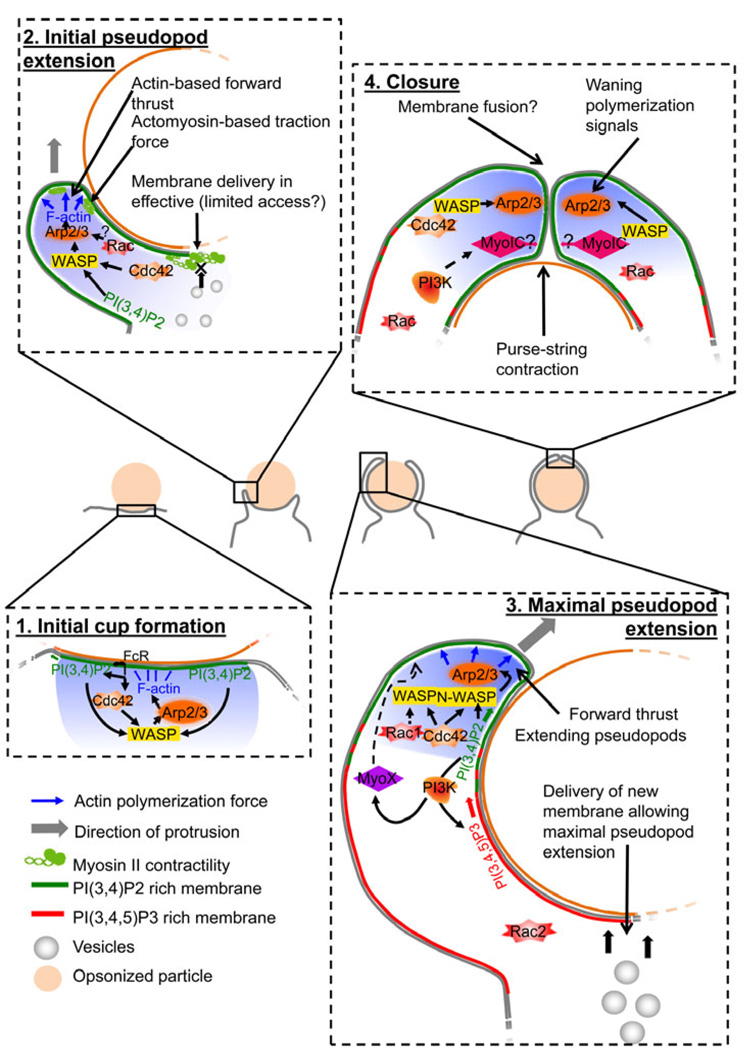
FcγR-triggered phagocytosis occurs as a continuous, progressive transition. The process has generally been subdivided into steps that are mostly based on morphological observations. Although no clear-cut molecular markers of each step have been defined, some molecules can be associated with or play a predominant role in the different stages of progression leading to internalization allowing the division of the phagocytic process into four steps (Fig. 3). The first very transient step is when the early stages of signaling downstream of FcγR occur. Some core elements of phagocytosis, such as the molecular pathways leading to actin polymerization, are recruited and activated at the site of particle binding. At this stage, no clear protrusions are morphologically detectable, but F-actin is assembled beneath the particle. The second step is the extension of an initial pseudopod protruding along the targeted particle generating a detectable phagocytic cup. The third step involves the continuous extension of the pseudopod, where the phagocytic cup wraps around to the most distal parts of the particle. The reason for considering this as a separate step is that it necessitates the utilization of different molecular events to overcome mechanical constraints involved in the engulfment of large particles (see below). Eventually, during the fourth step, the rims join on the distal side of the particle during the closure step. The rim of the phagocytic cup fuses, sealing the phagocytic cup into a completely internalized phagosome. Important events take place subsequently, such as phagosome retraction and maturation, that are very important in our understanding of pathogens killing or evasion (30).
Fig. 3. The four steps of FcγR-driven phagocytosis.
Note that some molecules have been omitted for clarity of the figure. For example, PI3K is present and active all around the cup; Rac2 is present and active at the base of the cup.
Initial cup formation (step 1)
Three classes of FcγRs have been recognized: FcγRI, FcγRII, and FcγRIII with several individual receptor isoforms (31). Most of these FcγR isoforms, including FcγRI, FcγRIIA, and FcγRIIIA, are able to mediate phagocytosis (32). Clustering of FcγRs with Fc domains of IgG triggers phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) that are located either in the cytoplasmic domain of FcγRIIA or in γ chains associated with FcγRI and FcγRIIIA and plays a key role in the transduction of phagocytic signaling cascades leading to particle engulfment (33).
One of the consequences of early FcγR signaling is a rapid formation of F-actin that is an absolute requisite for phagocytosis, since cells pretreated with polymerization inhibitory drugs are unable to even initiate a phagocytic cup (34, 35). Several NPFs are involved in initial F-actin generation at this stage. The importance of WASP as a key activator of actin polymerization is illustrated in Wiskott-Aldrich syndrome (WAS) patients. The plethora of immunological defects in WAS patients indicates the critical role of WASP for normal function across the hematopoietic cell lineage (36). Lorenzi and colleagues (37) established that macrophages from WAS patients lacked WASP and presented a marked impairment in actin polymerization at phagocytic cups resulting in reduced phagocytosis. Experiments in primary macrophages and macrophage cell lines using short interfering RNA (siRNA) to reduce endogenous levels of WASP confirm that WASP is recruited at the phagocytic cup and is strongly required for actin polymerization, cup initiation, and, logically, complete phagocytosis (38, 39). Another member of the WASP family, N-WASP, is also recruited at the phagocytic cup; its role however, might differ from WASP (see below). Consistent with the role of WASP, a study has reported a recruitment of Arp2/3 to the phagocytic cup (8). Also, artificial disruption of the binding between WASP and Arp2/3 by overexpression of the VCA domain of WASP leads to a loss of preferential recruitment of F-actin at the cup, decrease in cup formation and phagocytosis in macrophage cell lines (8). All this evidence together demonstrates that the engagement of the FcγR rapidly leads to an activation of WASP and a subsequent Arp2/3-mediated generation of a branched F-actin network.
One can wonder how the actin-regulating proteins such as WASP and Arp2/3 are recruited and activated downstream of FcγR. Most probably, several intertwined pathways are involved and many unknown elements remain. First, an hypothesis based on membrane dynamics can be suggested for the recruitment of WASP at the cup. Magenau and colleagues (40) have recently proposed that macrophages undergo a rapid actin-independent ceramide and lipid rafts enrichment of the membrane at the site of contact with IgG-coated beads. Importantly, inhibition of this membrane enrichment causes a marked defect of F-actin polymerization and phagocytosis. The authors suggest that the enrichment generates strain forces bending the membrane inward. Proteins containing BAR domains can recognize such a local deformation. Among them, formin-binding protein 17 (FBP17) has been reported to recruit WASP and its partner WIP to the nascent phagocytic cup (41). Reduction of FBP17 levels leads to a threefold decrease of phagocytic cup formation. Therefore, it is possible that the contact between IgG and FcγR triggers a very swift modification of the membrane with passive curving causing the initial recruitment of FBP17 and WASP-WIP, thus allowing the early formation of F-actin. Second, in addition to recruitment, WASP also needs to be activated. This can be achieved by several pathways. The most well studied upstream inducers of F-actin are the RhoGTPases, and it is therefore not surprising that they play a central role in phagocytic cup formation. Several have been linked to a downstream actin polymerization. WASP is a well-known target of Cdc42, which is rapidly activated downstream of the FcγR (42, 43), and it has been clearly demonstrated that the activity of Cdc42 has a pivotal role in WASP activation and induction of cup formation and phagocytosis (38, 44, 45).
However, WASP may not be completely responsible for all of the actin polymerization that occurs downstream of FcγRs. The guanine nucleotide exchange factor Vav that has activity to several RhoGTPases including Rac, Cdc42, and Rho (46) is also required for FcγR-mediated Rac activation and phagocytosis (47). Both Rac1 and Rac2 are activated shortly after FcγR triggering albeit with slightly different kinetics and localization patterns suggesting that they have different functions in phagocytosis (42, 43). Several groups observed that dominant negative mutants of Rac1, functional silencing of Rac1, or Rac1/2 double knockout bone marrow-derived macrophages impair both the accumulation of F-actin in cups and phagocytosis (44, 45, 48). Some controversy remains, as in a different study Rac1 was not required for phagocytosis in a siRNA screen using a macrophage cell line (49). This discrepancy might be explained by potential compensation by Rac2 which is also required for phagocytosis(50). We can conclude nonetheless that all three of these RhoGTPases contribute to actin polymerization to various extents during this initial step in the formation of the phagocytic cup.
The actual pathway linking Rac to actin polymerization is yet unclear. While Rac1 is well known to activate Arp2/3 through the WAVE complex, WAVE2 surprisingly is not required for FcγR phagocytosis in macrophages (21). It is possible that Rac1 indirectly promotes actin polymerization and cup formation through generation of another very important WASP activators: PI(3,4)P2 (51). PI(4,5)P2 is generated upon FcγR binding by PIPKIa (51), and the inhibition of PIPKIa decreases PI(4,5)P2 accumulation. This diminishes and slows down F-actin production, and eventually results in a defect of phagocytosis (52). Importantly, some groups have indeed described a role for Rac1 in activating PIPKI (phosphatidylinositol-4-phosphate 5-kinase I) (53) although this remains to be confirmed in macrophages.
The importance of the dynamics of F-actin in shaping the phagocytic cup is further reinforced by the observation that Ena/VASP is rapidly recruited to the cup. Ena/VASP is a ligand of the G-actin partner profilin and therefore helps recruit monomeric actin at the sites of intense polymerization. Elegant SEM shows that the disruption of Ena/VASP activity completely abolished F-actin accumulation and cup formation at the sites of FcγR triggering (54). Given that PI(4,5)P2 is also a docking site for the aforementioned BAR domain-containing FBP17, it becomes clear that different pathways involving RhoGTPases, some phosphoinositides and other proteins act synergistically to trigger an early recruitment and activation of WASP, Arp2/3 and F-actin accumulation thereby setting the stage for cup progression around the particle.
Initial pseudopod extension (Step 2)
Accumulation of F-actin at the zone of contact with the target results in the progression of the cup to enwrap the particle. The behavior of a growing phagocytic cup also depends on the size, the shape, and the stiffness of the particles. One clear observation is that as the polymerization of actin continues it generates the force required for the progression of the phagocytic cup. This could be due to continuous actin polymerization alone or by the involvement of myosin motor proteins.
Several papers have reported that the actin progresses as a ring moving from the base to the tip of the cup (42, 43). Many of the upstream regulators of F-actin formation described in the first step also play a role in this step as well. Of course, given the progression of the actin ring, this requires tight spatio-temporal control. Consistent with its prominent role in cup initiation, Cdc42 presents a pattern similar to the progression of the ring of actin: the fraction of active Cdc42 is concentrated at the rim of the extending cup (42, 43). It would be reasonable to assume that WASP activity follows the same kind of pattern. It is possible that not only the activation of Cdc42 is necessary for the production of F-actin but also that its restriction to a limited zone ensures a directed displacement of the area of polymerization by creating de facto a rim to base polarity in the cup. Supporting this concept is the observation that the overexpression of constitutively active Cdc42 leads to a loss in the constraint of F-actin to the extending rim, as well as a defect in the completion of phagocytosis (43). Both Rac1 and Rac2 show different localization patterns, being active all around the cup or more restricted to the base of the phagocytic cup, respectively. However, the consequences of this pattern on F-actin dynamics are not clear. Given that all of these RhoGTPases are activated at several times and locations during phagocytosis makes the study of these proteins in the later steps of phagocytosis complicated. Manipulation of both the spatial and temporal regulation of these proteins would be needed in order to determine the role of continuous actin polymerization and of the individual RhoGTPases in pseudopod extension during phagocytosis.
In addition to actin polymerization, the progression of the phagocytic cup is accompanied by myosin contractility, observable when deformable particles are used (42). Actomyosin contraction has been predicted in a mechanical model of neutrophil phagocytosis as a mechanism to avoid the particle being pushed away by the force of actin polymerization at the membrane-particle interface (55). This membrane proximal contraction might also be important to create a bracing force allowing a better coupling of actin fibers to the membrane and hence a better transmission of mechanical forces. This coupling has been described in the context of cell migration (56) as a molecular clutch between the cytoskeleton, the membrane, and beyond it the surface onto which the protrusion is progressing. Importantly it has been confirmed in macrophages that myosin II is responsible for the tight apposition of the cup around the particle (35) and for phagocytosis (57). When myosin II is inhibited an F-actin-rich cup still forms but remains loose and is unable to elongate (35). This indicates that actin is able to polymerize and to initiate pseudopod extension without a local binding of FcγR, and that long distance elongation requires an actomyosin-mediated adhesion onto the particle.
The signals triggering actomyosin contraction downstream of FcγRs are not clear. RhoA would be a good candidate for myosin II activation and the specific inhibition of Rho with C3 transferase was originally shown to prevent FcγR-mediated phagocytosis (58). While there was no effect of Rho inhibition in another study (45), in a more recent study Rho was shown to be necessary for efficient phagocytosis in primary macrophages, but dispensable for actin polymerization (48). Overall, these studies suggest that Rho activity is required for FcγR phagocytosis but not for actin polymerization.
It is apparent that while actin polymerization is required for the progression of the phagocytic cup, other factors are also necessary (59). When the cell is presented with an IgG-coated surface instead of a discrete particle to ingest, where extension of the basal side of the cell is comparable with a unique big and flat phagocytic cup, the cell extends and spreads on the surface. Using this technique, it has been shown that WASP, albeit important for the global amount of F-actin in macrophages, was only partially required for cell spreading, while the WASP family member N-WASP was required for spreading (38). This suggests that N-WASP has a specific role in membrane extension and may be important for the growth of pseudopods during the third step of phagocytosis.
Maximal pseudopod extension (Step 3)
Pseudopodial extension is naturally accompanied by an evolution of mechanical stress parameters, and the cell membrane and the underlying cortical actin is deformable to a limited extent. Indeed, the increase of membrane area during protrusion is opposed by an intrinsic physical feature of the cell called cortical tension (60). An increase in the cellular cortical tension can be observed during neutrophil phagocytosis (61). If the extent of pseudopodial extension is large, for example when a particle being engulfed is above a certain size, the polymerization force generated by actin dynamics would not be sufficient to override the opposing increased cortical tension. The pseudopod’s extension would then been stalled and phagocytosis would remain incomplete. For the pseudopod’s extension to be maximal, a number of specific actors are required. Several mechanisms may contribute to maximal pseudopodial extension.
One possible mechanism would be by simply relaxing (i.e. decreasing) the cortical tension. Such a relaxation of the cortical tension may be achieved by delivery of new membrane and several groups observed this event to occur at the base of the cup (59, 62). Arf6 has been implicated in the delivery of VAMP3 vesicles to the phagocytic cup (63, 64) for phagocytosis. Cargo delivery therefore requires free access to the forming phagosome, and may require the depolymerization of actin in this area. Consistent with the role of actin depolymerization in vesicle fusion, when the level of F-actin is artificially maintained high around the cup vesicle trafficking is abrogated and while the phagocytic cup forms it only partly elongates but remains unable to fully enclose the particle (62, 65).
Consistent with a need for actin depolymerization to allow for vesicle fusion, it has been observed in many studies that the production of F-actin during phagocytosis is transient. More precisely, the depolymerization of F-actin starts at the base of the cup, thereby creating a ring of F-actin progression at the tip of the extending phagocytic cup. Even in experimental settings where F-actin surrounds most of the cup without appearing as a bona fide ring, the base of the cup rapidly loses its F-actin (65). At least two mechanisms have been suggested to explain the drop of F-actin at the base. First, the same phenotype of stalled cup formation has been observed in cells with pharmacological inhibition of PI 3-kinase (PI3K) (35, 59, 66). This leads to an important idea in the field: although being present and active throughout phagocytosis, PI3K is not required for step 1 (initial cup formation) or for step 2 (initial pseudopod elongation) but is important to step 3 and transition to step 4 (closure). The mechanical importance of PI3K activity is well illustrated by the fact that the dependency of cells on PI3K activity is detectable when using large beads (5 µm of diameter) and not with small beads (2 µm). Engulfment of smaller beads requires less membrane extension and therefore mechanisms leading to cortical tension relaxation become dispensable. One functional link between PI3K activity and actin depolymerization has been established by Beemiller and colleagues (43). Using pharmacological inhibitors of PI3K, they demonstrated that PI3K was responsible for the extinction of Cdc42 activity, thereby halting WASP-mediated actin polymerization (43).
The second mechanism stems from the observation that the needed depolymerization at the base of the phagocytic cup extensively depends on the synchronized loss of PI(4,5)P2 that is a key factor controlling the amount of F-actin in the cup (51, 62, 65). At least three synergistic pathways can cause this loss of PI(4,5)P2. First, PLC hydrolyzes PI(4,5)P2 into DAG and IP3. Consistently, inhibition of PLC leads to an increase in PI(4,5)P2 and actin-rich stalled phagocytic cups (51, 65). Second, PI(4,5)P2 is also hydrolyzed into PI4P by the phosphatase OCRL1. OCRL1 is delivered to the base of the phagocytic cup and its reduction leads to an increase the amount of F-actin and decreases the efficiency of phagocytosis (62). However, since OCRL1 is targeted to the cup by vesicle trafficking, its recruitment would require an initial depolymerization of F-actin and therefore may constitute a positive feedback loop of F-actin loss. Third, PI(4,5)P2 is consumed by PI3K that transforms it into PI(3,4,5)P3.
PI3K actually seems to be a crucial player in the maximal extension of pseudopods. Indeed, PI3K activity controls other elements of the cytoskeleton necessary to the maximal elongation of pseudopods. For instance, myosin X is recruited upon PI3K activity, and disruption of its activity impairs the spreading on macrophages on IgG-coated surfaces (67). Importantly, the requirement for myosin X is dependent of the size of the target particles and the incomplete phagocytosis upon myosin X inhibition is rescued when cells are presented with smaller sized particles. This is consistent with a regulation of myosin X recruitment by PI3K. It also suggests that this myosin partakes in the regulation of the balance between polymerization force and cortical tension, probably through an action on actin filaments. However the mechanical details are currently unknown.
Phagosome closure (Step 4)
The last step of phagocytosis is the closure of the phagocytic cup with the target particle being sealed inside. The closure of the cup can be experimentally detected when particles become completely inaccessible to immunostaining without permeabilization (59). It is actually uneasy to set a clear limit between step 3 (pseudopod elongation) and step 4 (closure). Some authors refer to all the events past the moment where PI3K becomes necessary in step 3 as ‘closure’, whereas others adopt a narrower definition and only consider the last moments when the rims of the cup fuse together.
In spite of many studies, little is known about phagocytic cup closure in FcγR-phagocytosis and the role of actin in it. If we adopt a broad definition of what the closure is, we can say that the most noticeable feature of this step is a growing requirement for actomyosin contractility. Several myosins are recruited to the phagocytic cup (68). Among them, myosin IC appears to accumulate in a PI3K-dependent manner at the tip of the phagocytic cup (68, 69) and therefore could be a candidate to induce a purse string actomyosin contraction closing the cup. However, the lack of specific inhibitors and biosensors of atypical myosins significantly hinders the study of contractility during closure. Finally the closure step brings up the questions of membrane fusion and signal arrest, but these are yet to be investigated.
CR3 phagocytosis
Complement-triggered phagocytosis is another widespread phagocytic pathway involved in innate immunity. Upon opsonization by the soluble complement molecule C3bi, particles can be recognized by CR3 and ingested. The CR3-triggered phagocytosis is probably the second most studied pathway after FcγR. Both pathways share many common elements, however certain differences remain.
A first obvious difference is in the nature of the receptor: CR3, also known as αMβ2, is an integrin. β2 integrins rest in a bent low affinity conformation until intracellular, or inside-out, signals switch them to extended intermediate affinity conformation. They then naturally oscillate to an open high affinity conformation and are locked in that state upon ligand binding. Stabilization of the high affinity conformation by ligand results in signaling from the integrin to downstream pathways, a process termed ‘outside-in’. The specific mechanism of integrin activation suggests an important difference between CR3 and FcγR phagocytosis: the need for an independent inside-out activation of αMβ2 by external cues implies that the cells need to be put in an ‘activated’ state before phagocytosis begins, which could have important consequences on the dynamics of the cytoskeleton.
Initial studies reported conspicuous differences in the process of phagocytosis. Whereas the FcγR triggered the formation of a well characterized phagocytic cup protruding outward, CR3 activation was described as causing the particle to sink into the cytoplasm (34). Both types of phagocytosis required actin polymerization but the CR3-triggered sink was conceptually closer to endocytosis whereas the FcγR-triggered cup looked more similar to the macropinocytic process. Such a morphological difference suggested dissimilarities in the molecular pathways. These observations suggested that although many cytoskeleton-related elements were shared, their patterns of distribution were different (70).
More recent observations, however, indicate that a classic phagocytic cup may occur during CR3-mediated phagocytosis (71). The nature and morphology of these structures is debated. Indeed, pseudopods have been described either as forming a tight ‘classic’ cup (48) or slightly less protruding extensions (49). Another study reports even looser F-actin-rich protrusions called ruffles (72). In this work, ruffles are generated by external cues such as phorbol myristate acetate (PMA) or lipopolysaccharide (LPS) independently of CR3 binding. CR3 accumulates in the ruffles that therefore maximize the capture of particles prior to phagocytosis. Both the sinking and ruffling models could however be combined. It is conceptually possible that the inside out signal leading to activation of CR3 also triggers ruffling aiding in the capturing particles. Once the particles are bound, the ruffles might collapse and a depression might then appear as the particle sinks.
Specific pathways: importance of RhoA and formins
Despite the Arp2/3-mediated actin polymerization in CR3-mediated phagocytosis (8), the NPFs regulating this process unclear. The participation of Rac1/2 and Cdc42 in actin dynamics appears to be a point of difference between the two modes of phagocytosis. As previously mentioned, Cdc42 is central in Fcγ-mediated phagocytosis and Rac1/2 is likely to act on F-actin production. By contrast, it is clear that Cdc42 and its downstream effector WASP are not required for CR3 phagocytosis (38, 45, authors’ unpublished observations). However, the involvement of Rac1/2 is unclear. Several reports state that Rac1/2 is not required for CR3 phagocytosis using cell lines (45, 49), while work with primary macrophages described a role for Rac1/2 in F-actin accumulation at the cup and phagocytosis (48). This discrepancy may be due to use of different cellular models and the possibility of compensation by related family members when one protein is absent.
Another important difference of CR3-mediated phagocytosis may be the involvement of RhoA. While its role in FcγR-triggered phagocytosis is controversial (45, 48, 49, 58), it is absolutely required in CR3-mediated phagocytosis (45, 48, 49). Beyond its expected role in actomyosin contractility through the ROCK-Myosin II pathway (57), the activity of RhoA seems to be an important regulator of actin dynamics in CR3-triggered phagocytosis. Indeed, reduction of RhoA levels in macrophage cell lines markedly reduces the accumulation of F-actin (49). RhoA may play a more unique role in actin responses downstream of CR3 by activating formins. Indeed, the formin mDia, a known effector of RhoA, is specifically recruited at sites of phagocytosis upon CR3 binding and not FcγR. Consistently, reduction of mDia causes a loss of F-actin at the phagocytic cups and an overall decrease in phagocytosis efficiency (73). RhoA can also enhance formin mediated actin polymerization by fostering the binding between G-actin and profilin (74). Given that formins generate unbranched F-actin filaments as opposed to Arp2/3, which nucleates branched F-actin meshworks, one can then wonder at the morphological and mechanical implications of the specific role of formins in CR3-triggered engulfment of particles, compared to the FcγR-dependent phagocytosis.
Another functional consequence of the use of formins in CR3 phagocytosis is the coordination between actin cytoskeleton and microtubules. Microtubules have long been associated with CR3 phagocytosis (70), and mDia is delivered to the site of phagocytosis by the microtubule-binding protein CLIP-170 (75). Consequently, disruption of CLIP-170/mDia interaction causes a defect in F-actin generation and phagocytosis. In a reverse effect, mDia also regulates microtubules dynamics in other cellular models (76). It appears then that the specific use of the RhoA-formin axis downstream of CR3 leads to a joint actin/microtubules dynamics important for phagocytosis.
Summary
A lot of data are now available about the actin response during phagocytosis. The polymerization force developed by the actin cytoskeleton is a central player in the change of shape of the cell. A ring a polymerizing actin pushes the membrane around the target particle, forming a cup. The role of actin goes beyond the protrusion and serves as a substrate for myosin contractility as well as a potential site for target vesicle trafficking. The idea that the phagocytic cup is a dynamically organized structure, with distinct patterns of molecules is now accepted. Many upstream regulators of actin are involved, particularly the RhoGTPases and the phosphoinositides and their associated enzymes (such as PI3K). Other elements of the cytoskeleton are also involved like several atypical myosin and the microtubules.
Several general questions remain only partly answered and should constitute points of focus in the field. In spite of informative pioneer studies, a unified understanding of the kinetics of the phagocytic cup is lacking. Higher resolution information of the activities of the major regulators, both in space and in time, would allow us to build a more detailed and comprehensive model of phagocytosis. Noticeable progress has been made in the development of sensitive biosensors in the past years and these tools would surely prove beneficial.
While it is generally admitted that signaling differs between FcγR- and CR3-driven processes, the morphological and biochemical discrepancies between these two modes of phagocytosis remain to be clearly established. A more systematic comparison between the two in the future studies would then be valuable. Beyond the differences, it would be crucial to determine whether these two different initial signaling processes have the exact same outcome or if there are subtle yet important immunological or homeostatic consequences of uptake by these two different systems.
Chemotaxis
Sites of infection or injury secrete an array of inflammatory cytokines that act as chemoattractants orchestrating the recruitment of monocytes/macrophages. Macrophages have the ability to sense and migrate along an external gradient of soluble factors, known as directed migration or chemotaxis (77). Macrophages use highly dynamic cytoskeletal remodeling to move through tissues of various topological and physical properties.
Motility cycle and leading-edge protrusions
Whereas tissues are three dimensional structures with highly variable properties, most of our understanding of cell motility comes from studies of cells in two dimensions where they move on uniform flat surfaces. Microscopy has revealed that during migration cells cycle through a set of actin-dependent events that include the extension of an actin-rich membrane protrusion, stabilization of the protrusion via integrin-mediated attachment to the underlying extracellular substrate, and the actomyosin-based contraction of the body and detachment at the rear (78). Chemotaxis adds an additional level of complexity beginning with the detection of an external gradient through signaling via specific cell surface receptors (77, 79), which remain equally distributed on the entire surface of cells during migration including macrophages (80). It has been suggested that Rho GTPases, together with PI3K, contribute to asymmetric PI(3,4,5)P3 distribution via a positive-feedback loop (81). Intracellular signaling downstream of these receptors induces the extension of an F-actin rich protrusion towards the chemoattractant source. Protrusions set key parameters of trajectory, including speed and direction of the cell (82). Efficient chemotaxis requires that cells migrate with directional persistence towards the chemoattractant source. It is believed that cells manifest persistence by continuously restricting lateral protrusion and favoring formation (via actin polymerization) and stabilization (via adhesions) of the actin-rich protrusion at the leading edge, toward the source of chemoattractant. This must rely on signaling networks at the leading edge to regulate actin polymerization and adhesions (83).
Several types of protrusions have been observed at the leading edge, depending on the cell and motility type (84).This review focuses on lamellipodia (also commonly called pseudopodia), as they are believed to be the major source of adhesion and force generation of the motile macrophage. These protrusions are thin, sheet-like membrane extensions. The lamellipodium of fibroblasts was first described in the landmark studies of Abercrombie as a thin region extending immediately from the plasma membrane, lacking organelles and only weakly attaching. Between the lamellipodia and the cell body is the lamella, which is thicker, containing organelles and strong adhesion sites. More importantly, these two regions have distinct and independent actin networks. The lamellipodium is structured by dendritically branched actin filaments with growth mediated by the Arp2/3 complex (85, 86). Arp2/3-mediated de novo actin polymerization immediately at the plasma membrane generates protrusive force (84, 87), and it is essential for persistent directional migration (88).
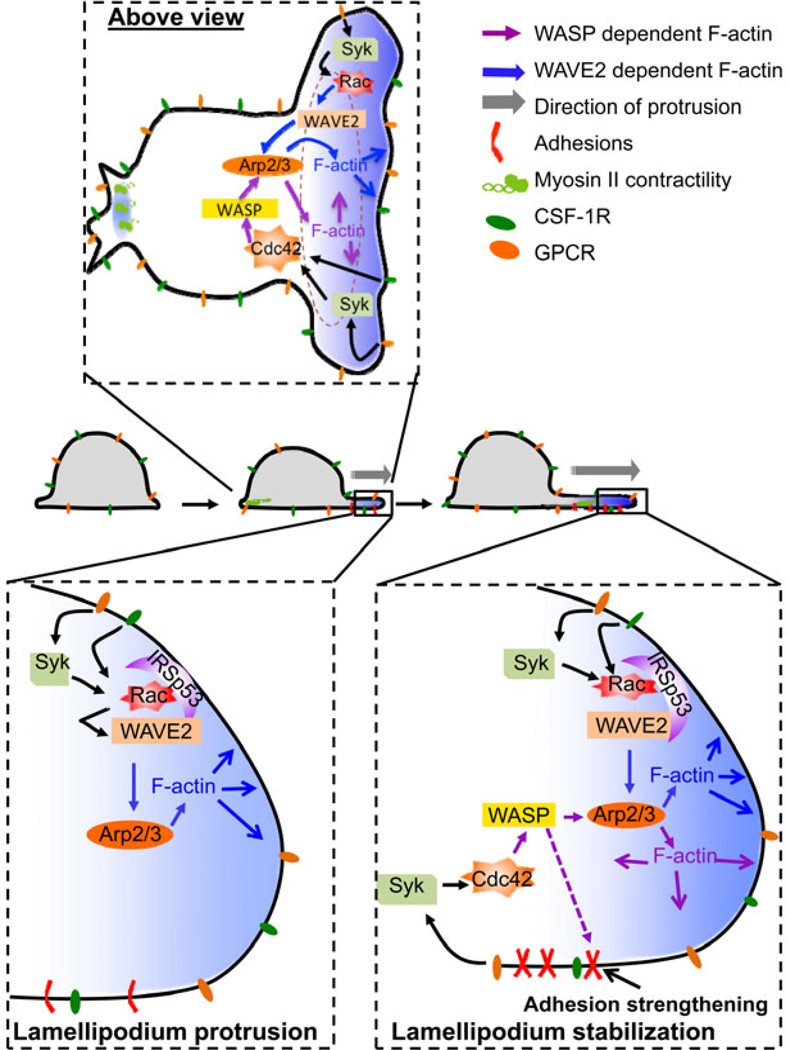
The extension of a leading lamellipodium during chemotaxis bears many similarities in structure to that of a phagocytic cup but with less precise spatial specificity. Both are defined in response to initial signaling induced by ligand binding followed by the extension of an actin driven protrusion. However, during chemotaxis the initial protrusion needs to be stabilized through substrate adhesion (Fig. 4)..
Fig. 4. Formation of chemotactic protrusions.
Signaling pathways downstream of GPCR or CSF-1R lead to activation of WAVE2 and N-WASP/WASP. However, subsequent actin polymerization serves different purposes, WAVE2 being major in the generation of a forward thrust of the membrane, WASP being more important for stabilization of protrusions. To illustrate these two non-overlapping roles of F-actin, two different colors have been used: blue for the WAVE2-ARP2/3 axis and purple for the WASP-ARP2/3 axis. Note that some molecules have been omitted for clarity of the figure.
CSF-1 stimulated migration
Colony-stimulating factor-1 (CSF-1)-induced chemotaxis has been studied extensively in macrophages (reviewed in 89). CSF-1, also known as MCSF, is primarily a macrophage growth factor stimulating its survival, proliferation and terminal differentiation from peripheral monocyte to macrophage, and it also acts as a potent chemoattractant (90). CSF-1-mediated macrophage recruitment and chemotaxis play key roles not only in normal physiological processes, but is associated with the progression of several human inflammatory diseases such as atherosclerosis, arthritis, and metastatic progression of cancer (91–93). CSF-1 signaling is linked to actin cytoskeleton rearrangement via the actin NPFs of the WASP/WAVE family. CSF-1 signals through its receptor tyrosine kinase (CSF-1R) that leads to tyrosine phosphorylation important for the control of actin dynamics (reviewed in 94). The CSF-1R belongs to the class III receptor tyrosine kinase subfamily, which contains a split cytoplasmic kinase domain. CSF-1 binding induces receptor dimerization, kinase activation, and phosphorylation of seven out of twenty tyrosine residues in the cytoplasmic domain. Phosphorylation of these tyrosine residues on the CSF-1R creates binding sites for Src homology 2 (SH2) or phosphotyrosine binding domain (PTB) signaling proteins (95–97), such as SFKs and PI3K, that are known to mediate chemotaxis as well as various cellular responses (reviewed in 98).
PI3K and CSF-1 induced actin polymerization
Current evidence overwhelmingly suggests that PI3K is required for migration and actin polymerization in macrophages. Receptor activation, which may be spatially localized, leads to local accumulation of PI3K lipid products at the plasma membrane. This becomes a critical link to downstream Rho-GTPase-mediated NPF activation to regulate the actin cytoskeleton. The p85 regulatory subunit of PI3K associates with the CSF-1R following CSF-1 treatment and CSF-1 chemotaxis is reduced in p85-deficient macrophages (99, 100). Studies examining the role of SHIP (SH2 domain-containing inositol phosphatase), a negative regulator of PI3K, also supports the requirement of PI3K in macrophage chemotaxis (101). Among the several different PI3K catalytic subunits regulated by the p85 subunit of PI3K, p110δ appears to be the most critical isoform regulating CSF-1-induced actin remodeling and migration using either injection of isoform specific antibodies or inhibitors (102–104). The primary macrophages derived from a mouse containing a replacement of the endogenous isoform with a kinase-dead p110δ version confirmed reduced F-actin content and formation of F-actin-rich membrane protrusions in response CSF-1 and impaired chemotaxis (103). The importance of PI3K in CSF-1-mediated signaling is further substantiated in studies using CSF-1R deficient macrophages reconstituted with a CSF-1R that is deficient in a single tyrosine residue, Y721 (M−/−.Y712F) that mediates the direct interaction of the p85 regulatory subunit of PI3K with the CSF-1R (105). M−/−Y721F BMMs exhibited reduced CSF-1-induced PI(3,4,5)P3 production, impaired chemotaxis and diminished formation of membrane protrusions in response to global CSF-1 stimulation. Unlike FcγR signaling, PI3K plays an important role in actin polymerization downstream of CSF-1R signaling and actin polymerization in response to CSF-1 is completely abolished by treating cells with PI3K inhibitors (68).
It is clear that actin polymerization in response to CSF-1 is required for the extension of lamellipodial protrusions. Similar to what occurs during the progression of the phagocytic cup, RhoGTPases and the WASP/WAVE family of NPFs are major downstream effectors in the production of chemotactic lamellipodia. Cdc42 and Rac are required for CSF-1-elicited actin rich protrusions (44, 106) through the activation of NPFs like the WASP/WAVE family members. The importance of WASP in macrophage directed migration, specifically chemotaxis, was clearly established from studies that tested macrophages or monocytes derived from WAS patients. WAS patient-derived monocytes/macrophages displayed reduced chemotaxis to CSF-1, MCP-1 and fMLP and failure to polarize, however they were still motile (107, 108). Interestingly, consistent with a role for Cdc42 in WASP activation (19, 109), macrophages were also able to migrate without Cdc42 but do not migrate directionally (110). These results suggest that WASP was not required for the generation of lamellipodia in response to CSF-1 but was required for a different but necessary step in chemotaxis that will be described later.
Lamellipodial extension by WAVE2
The only other major WASP/WAVE family member expressed in macrophages is the ubiquitously expressed WAVE2. WAVE2 is well known to be required for the generation of protrusions in multiple cell types. Macrophages depend on WAVE2 for actin polymerization and the production of protrusions in response to CSF-1. Macrophages with reduced levels of WAVE2 fail to migrate in response to CSF-1. A study using constitutively active forms of Rac1 failed to rescue F-actin-rich membrane protrusions in macrophages with reduced WAVE2 expression leading to the conclusion that in macrophages Rac1 is a required link with WAVE2 (21). Since WAVE2 does not contain a GBD domain the interaction between Rac and WAVE2 is indirect and can occur through the binding of Rac with either the Abi1 complex or with IRSp53 (Fig. 2A). Studies in macrophages suggest that WAVE 2 stimulated protrusions are dependent on IRSp53 and blockage of the interaction between IRSp53 and WAVE2 inhibits CSF-1 stimulated protrusions and IRSp53 is required for chemotaxis to CSF-1 (111). This also suggests that while WAVE2 mediated actin polymerization is important in many cell types different upstream regulators may be required dependent on the cell studied.
WASP and lamellipodial stabilization
Whereas WASP is required for macrophage chemotaxis to CSF-1, it is not required for the production of F-actin-rich protrusions, leading to the question of additional roles of actin polymerization, and of WASP, in chemotaxis. Interestingly, although tyrosine phosphorylation is not required for WASP activation (19), it still plays an important role in chemotaxis in addition to phagocytosis, as expression of the phosphodeficient WASPY291F mutants in macrophages resulted in diminished chemotaxis (110).
Chemoattractant stimulation of motile cells often leads to biphasic actin responses in cells such as Dictyostylium amoebae, neutrophils, and carcinoma cells. The initial spike in actin polymerization correlates with directional sensing and is followed by secondary responses over the next several minutes that are associated with motility (112). Activation of the CSF-1R also induces biphasic actin polymerization (109) with an initial peak of F-actin polymerization that correlates with the early activation of WASP and is dependent on WASP activity (19, 109), suggesting that WASP was required for directional sensing. However, high resolution time lapse imaging demonstrated that despite delayed onset WASP-deficient macrophages were still able to respond directionally to the gradient source, but these directional protrusions exhibited a decreased persistence suggesting that WASP was required for later steps in the generation of chemotactic protrusions (109). Consistent with a role for WASP phosphorylation in chemotaxis, expression of a phosphodeficient form of WASP (WASPY291F) behaved similarly to WASP deficient cells, but with somewhat enhanced delay in the onset of the protrusion (109). A role for WASP in protrusion stabilization could be to favor the adhesion of the lamellipodium onto the substrate. Often lamellipodia that do not attach sufficiently are observed to fold back at the cell edge towards the cell body, a phenomenon often described as ruffling (113, 114). Most cell types studied as models of motility utilize large focal adhesions to attach to the underlying substratum but macrophages utilize different strategies. Monocyte-derived cells employ small phospho-paxillin-rich point complexes, focal complexes, and podosomes to adhere and migrate (115, 116) and the absence of either Pyk2 or Fak alters adhesion dynamics and decreases lamellipodial persistence (117). Podosomes are larger in size than focal complexes and contain an actin-rich core, formed through WASP mediated Arp2/3 activation and surrounded by a ring of adhesion proteins such as integrins, vinculin, and talin. In addition, podosomes are much more dynamic than focal adhesions (118). Although podosomes share similar components of focal adhesions, such as Pyk2, FAK, and SFKs, their distinct properties suggest these structures have specific regulation and functions in macrophage biology (reviewed in 119). Podosomes have been implicated in cell migration on planar surfaces and CSF-1 can induce the formation of macrophage podosomes in a PI3K-dependent manner (120). Studies of macrophages obtained from WAS patients, which lack podosomes, have impaired chemotaxis to CSF-1, MCP-1, and fMLP, suggesting that podosomes may be critical for directional migration (107, 108). A role for podosomes in the stabilization of lamellipodia is supported by the fact that podosomes form at the leading edge of cells during migration (121) and the requirement for WASP in lamellipodial persistence (109). In monocyte-derived dendritic cells, HS1 (hematopoietic lineage cell-specific protein 1), the hematopoietic homolog of cortactin, was also found to regulate podosome dynamics through interaction with WASP and to be required for directional persistence (122). Additionally, studies in neutrophils suggest that the upstream activator of WASP Cdc42 stabilizes directional protrusions, but the mechanism of how Cdc42 mediates these effects in neutrophils remains unknown (123). However, the WASP-podosome link remains speculative with no direct evidence connecting podosomes to chemotaxis.
WAVE2 activation and recruitment to chemotactic protrusions is independent of WASP (109), strongly suggesting that WASP and WAVE2 have non-overlapping roles but work in coordinated fashion to generate an efficient chemotactic pseudopod. In this model, WASP is necessary for keeping the protrusion on course, while WAVE2 would supply branched actin network for the formation of the chemotactic pseudopod (Fig. 4). How the WASP-Arp2/3 generated actin network plays a different role than the WAVE-ARP2/3 one is a fascinating question yet to be investigated. It would be interesting to visualize the coordination of localized WASP and WAVE2 activities in stimulated protrusions.
Myosin-mediated contractility
Cells need a certain level of adhesiveness on the exterior and tension in the interior to generate traction force for migration (124, 125). Rho is a well-known mediator of adhesion and RhoA-mediated contractility is essential for macrophage migration, as C3 transferase treatment inhibits CSF-1-induced migration (80). However, different members of the Rho family may play different roles in migration. Macrophages express RhoA and RhoB and lack the other major isoform RhoC. RhoB controls cell adhesion through regulated integrin expression that may play an important role in stabilizing protrusions during motility. This is in line with observations that genetic deletion of RhoB adversely affects macrophage migration on select substrates. Furthermore, RhoA is important in tail retraction during macrophage chemotaxis (126) probably through regulation of myosin activity.
Similar to phagocytosis, myosin-mediated contractility is important for cell migration (127). Myosins exert their contractile forces onto actin filaments; hence myosin activity and actin dynamics are intimately connected. While there are detailed studies on the role of myosin in other cells types (128–130), our understanding of the role of myosin in macrophage migration and its role in the regulation of chemotactic protrusion is limited. Although the role of myosin during CSF-1 stimulated locomotive protrusions has not been directly examined, the regulatory light chain of myosin (MLRC) associates with the CSF-1R in response to CSF-1 (99). Also, studies on a regulator of myosin S100A4 suggests that myosin plays in important role in generating stable protrusions during macrophage chemotaxis (131). S100A4 is a Ca2+-dependent molecular switch that interacts with a wide range of downstream targets, one of them being nonmuscle myosin-IIA. Li et al. have shown that loss of S100A4 results in overassembly of myosin IIA and β-actin, resulting decreased persistence and size of protrusions leading to defects in chemotaxis. This has direct relevance in vivo, as S100A4−/− mice had significantly decreased macrophage recruitment to inflammatory challenge. The role of other myosin family members in macrophage chemotaxis has not been investigated. While many of the individual signaling components that regulate formation and stability of F-actin-rich lamellipodia have been identified using motility and chemotaxis assays, it is still an open question what their precise role in generating chemotactic pseudopods is. More precisely, the functional connection between behavior (migration), morphology (protrusions and contraction) and biochemistry (signaling pathways) studies remains to be established and is an important challenge in the times ahead. A more high resolution analysis of chemotactic protrusions would aid in our understanding of the precise roles of these proteins, including WASP, in macrophage chemotaxis. A greater challenge will be to determine how the activities of these different regulators are coordinated in space and time to lead to efficient chemotaxis.
G-protein-coupled receptor-stimulated protrusions
Chemokines also act through GPCRs to mediate their biological effects. Different model systems have been extensively used to characterize the signaling pathways that regulate directed migration via G-protein-coupled receptors (GPCR), including cAMP in Dictyostelium (132) and N-formyl-methionine-leucine-phenylalanine (fMLP) in neutrophils (82, 133). However, GPCR-mediated chemotaxis of macrophages is largely under studied, and as such, the mechanism by which GPCR activation and signaling is linked to actin cytoskeletal rearrangement and pseudopod extension is currently undefined. Recent studies indicate that tyrosine kinases, such as Src family kinases (SFKs), and PI3K also serve as effectors for several GPCRs (reviewed in 134, 135). This finding suggests that many of the proteins that play a role in CSF-1-mediated chemotaxis, discussed above, may also be important for chemotaxis via GPCRs.
Actin polymerization and protrusions downstream of GPCRs
It is well known that macrophages respond by rapid actin remodeling and robust formation of F-actin-rich membrane protrusions downstream of GPCR activation. CX3CL1 has been shown to induce rapid actin reorganization, production of F-actin-rich pseudopods and directed migration in macrophages that is contingent upon tyrosine phosphorylation by the non-receptor cytoplasmic Syk tyrosine kinase (136). Syk leads to Cdc42 and Rac1 activation in a PI3K-dependent manner, although with differential activation kinetics (137). Expression of dominant negative Rac1 and Cdc42 nearly completely abolishes fMLP-induced membrane ruffling, indicating the critical role of these RhoGTPases in GPCR-mediated actin rearrangement (44). MIP-1α also induces lamellipodia formation in a Rac1-dependent, but RhoA and Cdc42-independent manner (138). Similarly, the chemokine RANTES activates Rac1. Micropipette experiments which selectively deliver chemokines locally to one side of a cell revealed that Rac was required for macrophages to be able to polarize and extend leading-edge protrusions towards the chemoattractant gradient and to translocate in response to a directional source of RANTES (139). Interestingly, the activation of Rac1 required PI3K and causes the activation of PAK2. Although RANTES has been shown to cause actin cytoskeleton remodeling and lamellipodia formation, the direct role and mechanism of the PI3K-Rac1-PAK2 or other signaling axes on actin rearrangement has not been examined in detail (138). It is likely Rac1 and Cdc42 are equally involved in the generation of chemotactic lamellipodia in a context of directed migration to many GPCRs, although it is yet to be clearly proven.
Not surprisingly, the WASP family of NPFs is also required for chemokine induced migration downstream of the RhoGTPases (reviewed in 140). CX3CL1 induced activation of Cdc42 and Rac1 results in WASP and WAVE2 activation, respectively (137). Similar to CSF-1, WASP and WAVE2 appear to have different roles in CX3CL1-induced chemotaxis. WAVE2 activation was required for both the formation of F-actin-rich membrane protrusions as well as for chemotaxis. Interestingly, Cdc42-mediated WASP activity appeared to be essential for chemotaxis only, as diminished endogenous WASP levels did not impair membrane protrusion formation as dramatically as that of WAVE2. Importantly, tyrosine phosphorylation of WASP was also required in CX3CL1-induced chemotaxis. The role of WASP in CX3CL1 chemotaxis appears to be very similar to that of CSF-1 chemotaxis, suggesting that WASP may be required for the stabilization of protrusions. Studies in neutrophils provide some clues for how WASP may function. WASP-deficient neutrophils exhibited defective β2-integrin clustering associated with reduced adhesion which resulted in reduced migration towards fMLP as well as reduced transendothelial migration (141). As discussed above integrin-mediated adhesions play an important role in persistent migration by stabilizing protrusions, and lamellipodia that fail to attach to the extracellular substratum lead to inefficient chemotaxis in different cell types (83, 113). In addition, WASP may play multiple roles in chemotaxis. In a recent report, Kumar et al. (142) found that WASP, under the control of Cdc42, regulates neutrophil polarity by stabilizing microtubules via CD11b clustering in the uropod. Mechanisms that control polarity by defining the rear of the cell may also lead to the stabilization of the leading-edge, thereby promoting persistence in directional migration (83). Obviously, these observations would need to be confirmed in macrophages. In summary, although WASP appears to be generally required for GPCR-mediated chemotaxis in macrophages, it has only been directly characterized in the case of CX3CL1-mediated actin remodeling. In addition, while it is clear that WASP and WAVE2 play critical roles in CX3CL-1-induced chemotaxis, their precise roles in regulating the dynamics of GPCR-mediated pseudopod extension is yet to be examined.
Myosins are also important for GPCR-mediated macrophage chemotaxis. Wilson et al. (143) examined the role of myosin light chain phosphorylation in macrophage motility. Either inhibition myosin light chain kinase (MLCK) using a MLCK-specific antibody or overexpressed a constitutively active form of the kinase in macrophages reduced macrophage chemotaxis. More interestingly, they found that phosphorylation of Myosin II light chain had to be tightly controlled, as either increase or decrease in phosphorylation inhibited chemotaxis (143). This observation was further supported in a subsequent study where the importance of myosin II for macrophage chemotaxis is further evidenced by in vivo studies on inflammatory infiltration. Inhibition of either Rho kinase, that phosphorylates myosin light chain, or myosin II was associated with decreased macrophage recruitment in vivo in different disease models (144, 145). Although it is clear that myosin II is required for macrophage chemotaxis, its specific role in generating chemotactic protrusions has not been directly examined. Other myosin family members also play a role in chemotaxis. A study by Hanley et al shows that without Myosin IXb macrophages do not extend lamellipodia and have severely reduced migration speed, with an impaired the overall chemotactic ability and a deficiency in polarization (146). Interestingly, myosin IXb−/− macrophages display similar phenotype to cells microinjected with constitutively active RhoA (106). However, the interpretation of this observation is complicated by the fact that myosin IXb acts as a RhoGAP that modulates Rho activity.
The data on the roles of the downstream effectors mediating chemotaxis to GPRC ligands suggest that the pathways are very similar to those elicited by CSF-1. However, differences do exists between chemotaxis via tyrosine kinase and G-protein coupled receptors. Indeed, the requirement for Syk is dependent on the receptor class involved, as CSF-1-induced chemotaxis was still normal in macrophages after reduction of endogenous Syk expression (136). Syk did not directly phosphorylate WASP, although was still pre-requisite for tyrosine phosphorylation of WASP. Instead, as shown by pharmacological inhibitors, Src family kinases (SFKs) mediate tyrosine phosphorylation of WASP (137). Rather, Syk seems to be important for the activation of major upstream regulators of WASP and WAVE2: the Rho GTPases Rac1 and Cdc42 (137). Another interesting consideration of GPCR-mediated in comparison to receptor tyrosine kinase-mediated chemotaxis is the role of actin dynamics. Macrophages display bi-phasic F-actin peaks in response to CSF-1 that have separate and independent roles where the first peak appears to mediate persistence under the control of WASP. Interestingly, macrophages display a rapid single-phase F-actin peak in response to CXC3L1 (136) and an overall much faster generation of protrusions downstream of GPCR (personal observations), unlike the slower biphasic response to CSF-1. Overall, this suggests that macrophages, although employing similar (overlapping) signaling components, use different mechanisms to control the persistence of chemotactic protrusions in GPCR- compared to RTK-mediated chemotaxis; however, the exact mechanisms remain to be further investigated.
Conclusions
Leukocytes, in general, employ different strategies to regulate actin dynamics and adhesions and are more difficult to study than most other motile cells types because of their resistance to such tools as microinjection to target specific molecules or molecular biology approaches, until recently. Perhaps this is why the mechanics and signaling pathways of macrophage phagocytosis and motility have not been completely elucidated. Considering that immune functions are so fundamental to the development and survival of higher organisms, there should be far more advanced understanding of basic motility, phagocytosis, and actin-based antigen presentation (i.e. synapses not discussed in this review). These are all actin-based processes with parallel structures but each with its own peculiarities. A finer understanding could lead to targeted treatment for immune malfunction or to enhancement cellular responses. We hope that research will progress in this field so that a subsequent review not so many years from now would be able to paint a far more comprehensive picture of macrophage behaviors.
Acknowledgments
This work was supported by NIH grant GM071828 (DC). VM is a recipient of Cellular, Molecular Biology and Genetics training grant GM007491. We would like to thank Michael Cammer for critical reading of the manuscript.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Gordon S. Elie Metchnikoff: father of natural immunity. Eur J. Immunol. 2008;38:3257–3264. doi: 10.1002/eji.200838855. [DOI] [PubMed] [Google Scholar]
- 2.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 4.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat. Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 5.Auffray C, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 6.Vérollet C, Charriére GM, Labrousse A, Cougoule C, Cabec V Le, Maridonneau-Parini I. Extracellular proteolysis in macrophage migration: losing grip for a breakthrough. Eur. J. Immunol. 2011;41:2805–2813. doi: 10.1002/eji.201141538. [DOI] [PubMed] [Google Scholar]
- 7.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 8.May RC, Caron E, Hall A, Machesky LM. Involvement of the Arp2/3 complex in phagocytosis mediated by FcgammaR or CR3. Nat. Cell Biol. 2000;2:246–248. doi: 10.1038/35008673. [DOI] [PubMed] [Google Scholar]
- 9.Linder S, Hüfner K, Wintergerst U, Aepfelbacher M. Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J. Cell Sci. 2000;113(Pt 23):4165–4176. doi: 10.1242/jcs.113.23.4165. [DOI] [PubMed] [Google Scholar]
- 10.Rotty JD, Wu C, Bear JE. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat. Rev. Mol. Cell Biol. 2013;14:7–12. doi: 10.1038/nrm3492. [DOI] [PubMed] [Google Scholar]
- 11.Campellone KG, Webb NJ, Znameroski EA, Welch MD. WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell. 2008;134:148–161. doi: 10.1016/j.cell.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duleh SN, Welch MD. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskelet. Hoboken NJ. 2010;67:193–206. doi: 10.1002/cm.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuchero JB, Coutts AS, Quinlan ME, Thangue NBL, Mullins RD. p53-cofactor JMY is a multifunctional actin nucleation factor. Nat. Cell Biol. 2009;11:451–459. doi: 10.1038/ncb1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rottner K, Hänisch J, Campellone KG. WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol. 2010;20:650–661. doi: 10.1016/j.tcb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 15.García E, Jones GE, Machesky LM, Antón IM. WIP: WASP-interacting proteins at invadopodia and podosomes. Eur. J. Cell Biol. 2012;91:869–877. doi: 10.1016/j.ejcb.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Zalevsky J, Lempert L, Kranitz H, Mullins RD. Different WASP family proteins stimulate different Arp2/3 complex-dependent actin-nucleating activities. Curr. Biol. CB. 2001;11:1903–1913. doi: 10.1016/s0960-9822(01)00603-0. [DOI] [PubMed] [Google Scholar]
- 17.Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Annu. Rev. Biochem. 2010;79:707–735. doi: 10.1146/annurev.biochem.77.060407.135452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cory GOC, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation Wiskott-Aldrich Syndrome protein. J. Biol. Chem. 2002;277:45115–45121. doi: 10.1074/jbc.M203346200. [DOI] [PubMed] [Google Scholar]
- 19.Cammer M, Gevrey J-C, Lorenz M, Dovas A, Condeelis J, Cox D. The mechanism of CSF-1-induced Wiskott-Aldrich syndrome protein activation in vivo: a role for phosphatidylinositol 3-kinase and Cdc42. J. Biol. Chem. 2009;284:23302–23311. doi: 10.1074/jbc.M109.036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres E, Rosen MK. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol. Cell. 2003;11:1215–1227. doi: 10.1016/s1097-2765(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 21.Kheir WA, Gevrey J-C, Yamaguchi H, Isaac B, Cox D. A WAVE2-Abi1 complex mediates CSF-1-induced F-actin-rich membrane protrusions and migration in macrophages. J. Cell Sci. 2005;118:5369–5379. doi: 10.1242/jcs.02638. [DOI] [PubMed] [Google Scholar]
- 22.Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 2001;114:1801–1809. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- 23.Millard TH, Sharp SJ, Machesky LM. Signalling to actin assembly via the WASP (Wiskott-Aldrich syndrome protein)-family proteins and the Arp2/3 complex. Biochem. J. 2004;380:1–17. doi: 10.1042/BJ20040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stradal TEB, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 2004;14:303–311. doi: 10.1016/j.tcb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, et al. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468:533–538. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendoza MC. Phosphoregulation of the WAVE regulatory complex and signal integration. Semin. Cell Dev. Biol. 2013;24:272–279. doi: 10.1016/j.semcdb.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin FM, Jr, Griffin JA, Silverstein SC. Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J. Exp. Med. 1976;144:788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaumouillé V, Grinstein S. Receptor mobility, the cytoskeleton, and particle binding during phagocytosis. Curr. Opin. Cell Biol. 2011;23:22–29. doi: 10.1016/j.ceb.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith LM, May RC. Mechanisms of microbial escape from phagocyte killing. Biochem. Soc. Trans. 2013;41:475–490. doi: 10.1042/BST20130014. [DOI] [PubMed] [Google Scholar]
- 31.Ravetch JV, Bolland S. IgG Fc receptors. Annu. Rev. Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 32.García-García E, Rosales C. Signal transduction during Fc receptor-mediated phagocytosis. J. Leukoc. Biol. 2002;72:1092–1108. [PubMed] [Google Scholar]
- 33.Strzelecka A, Kwiatkowska K, Sobota A. Tyrosine phosphorylation and Fcgamma receptor-mediated phagocytosis. FEBS Lett. 1997;400:11–14. doi: 10.1016/s0014-5793(96)01359-2. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan G. Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand. J. Immunol. 1977;6:797–807. doi: 10.1111/j.1365-3083.1977.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 35.Araki N, Hatae T, Furukawa A, Swanson JA. Phosphoinositide-3-kinase-independent contractile activities associated with Fcgamma-receptor-mediated phagocytosis and macropinocytosis in macrophages. J. Cell Sci. 2003;116:247–257. doi: 10.1242/jcs.00235. [DOI] [PubMed] [Google Scholar]
- 36.Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nat. Rev. Immunol. 2010;10:182–192. doi: 10.1038/nri2724. [DOI] [PubMed] [Google Scholar]
- 37.Lorenzi R, Brickell PM, Katz DR, Kinnon C, Thrasher AJ. Wiskott-Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood. 2000;95:2943–2946. [PubMed] [Google Scholar]
- 38.Park H, Cox D. Cdc42 regulates Fc gamma receptor-mediated phagocytosis through the activation and phosphorylation of Wiskott-Aldrich syndrome protein (WASP) and neural-WASP. Mol. Biol. Cell. 2009;20:4500–4508. doi: 10.1091/mbc.E09-03-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuboi S. Requirement for a complex of Wiskott-Aldrich syndrome protein (WASP) with WASP interacting protein in podosome formation in macrophages. J. Immunol. Baltim. Md 1950. 2007;178:2987–2995. doi: 10.4049/jimmunol.178.5.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magenau A, et al. Phagocytosis of IgG-coated polystyrene beads by macrophages induces and requires high membrane order. Traffic Cph. Den. 2011;12:1730–1743. doi: 10.1111/j.1600-0854.2011.01272.x. [DOI] [PubMed] [Google Scholar]
- 41.Tsuboi S, et al. FBP17 Mediates a Common Molecular Step in the Formation of Podosomes and Phagocytic Cups in Macrophages. J. Biol. Chem. 2009;284:8548–8556. doi: 10.1074/jbc.M805638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoppe AD, Swanson JA. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol. Biol. Cell. 2004;15:3509–3519. doi: 10.1091/mbc.E03-11-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beemiller P, et al. A Cdc42 activation cycle coordinated by PI 3-kinase during Fc receptor-mediated phagocytosis. Mol. Biol. Cell. 2010;21:470–480. doi: 10.1091/mbc.E08-05-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 46.Abe K, et al. Vav2 is an activator of Cdc42, Rac1, and RhoA. J. Biol. Chem. 2000;275:10141–10149. doi: 10.1074/jbc.275.14.10141. [DOI] [PubMed] [Google Scholar]
- 47.Patel JC, Hall A, Caron E. Vav regulates activation of Rac but not Cdc42 during FcgammaR-mediated phagocytosis. Mol. Biol. Cell. 2002;13:1215–1226. doi: 10.1091/mbc.02-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall AB, et al. Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcgammaR- and complement-mediated phagocytosis. Immunity. 2006;24:305–316. doi: 10.1016/j.immuni.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Tzircotis G, Braga VMM, Caron E. RhoG is required for both FcγR- and CR3-mediated phagocytosis. J. Cell Sci. 2011;124:2897–2902. doi: 10.1242/jcs.084269. [DOI] [PubMed] [Google Scholar]
- 50.Yamauchi A, et al. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J. Immunol. Baltim. Md 1950. 2004;173:5971–5979. doi: 10.4049/jimmunol.173.10.5971. [DOI] [PubMed] [Google Scholar]
- 51.Botelho RJ, et al. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 2000;151:1353–1368. doi: 10.1083/jcb.151.7.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coppolino MG, et al. Inhibition of phosphatidylinositol-4-phosphate 5-kinase Ialpha impairs localized actin remodeling and suppresses phagocytosis. J. Biol. Chem. 2002;277:43849–43857. doi: 10.1074/jbc.M209046200. [DOI] [PubMed] [Google Scholar]
- 53.Santarius M, Lee CH, Anderson RA. Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem. J. 2006;398:1–13. doi: 10.1042/BJ20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coppolino MG, et al. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J. Cell Sci. 2001;114:4307–4318. doi: 10.1242/jcs.114.23.4307. [DOI] [PubMed] [Google Scholar]
- 55.Herant M, Heinrich V, Dembo M. Mechanics of neutrophil phagocytosis: experiments and quantitative models. J. Cell Sci. 2006;119:1903–1913. doi: 10.1242/jcs.02876. [DOI] [PubMed] [Google Scholar]
- 56.Renkawitz J, Sixt M. Mechanisms of force generation and force transmission during interstitial leukocyte migration. EMBO Rep. 2010;11:744–750. doi: 10.1038/embor.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr. Biol. CB. 2002;12:1413–1418. doi: 10.1016/s0960-9822(02)01069-2. [DOI] [PubMed] [Google Scholar]
- 58.Hackam DJ, Rotstein OD, Schreiber A, Zhang Wj, Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcgamma receptors in macrophages. J. Exp. Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox D, Tseng CC, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- 60.Lecuit T, Lenne P-F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 61.Herant M, Heinrich V, Dembo M. Mechanics of neutrophil phagocytosis: behavior of the cortical tension. J. Cell Sci. 2005;118:1789–1797. doi: 10.1242/jcs.02275. [DOI] [PubMed] [Google Scholar]
- 62.Marion S, et al. The NF-κB signaling protein Bcl10 regulates actin dynamics by controlling AP1 and OCRL-bearing vesicles. Dev. Cell. 2012;23:954–967. doi: 10.1016/j.devcel.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 63.Niedergang F, Colucci-Guyon E, Dubois T, Raposo G, Chavrier P. ADP ribosylation factor 6 is activated and controls membrane delivery during phagocytosis in macrophages. J. Cell Biol. 2003;161:1143–1150. doi: 10.1083/jcb.200210069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Q, Cox D, Tseng CC, Donaldson JG, Greenberg S. A requirement for ARF6 in Fcgamma receptor-mediated phagocytosis in macrophages. J. Biol. Chem. 1998;273:19977–19981. doi: 10.1074/jbc.273.32.19977. [DOI] [PubMed] [Google Scholar]
- 65.Scott CC, et al. Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J. Cell Biol. 2005;169:139–149. doi: 10.1083/jcb.200412162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cox D, et al. Myosin X is a downstream effector of PI(3)K during phagocytosis. Nat. Cell Biol. 2002;4:469–477. doi: 10.1038/ncb805. [DOI] [PubMed] [Google Scholar]
- 68.Diakonova M, Bokoch G, Swanson JA. Dynamics of cytoskeletal proteins during Fcgamma receptor-mediated phagocytosis in macrophages. Mol. Biol. Cell. 2002;13:402–411. doi: 10.1091/mbc.01-05-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swanson JA, Johnson MT, Beningo K, Post P, Mooseker M, Araki N. A contractile activity that closes phagosomes in macrophages. J. Cell Sci. 1999;112(Pt 3):307–316. doi: 10.1242/jcs.112.3.307. [DOI] [PubMed] [Google Scholar]
- 70.Allen LA, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J. Exp. Med. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cox D, Dale BM, Kashiwada M, Helgason CD, Greenberg S. A regulatory role for Src homology 2 domain-containing inositol 5’-phosphatase (SHIP) in phagocytosis mediated by Fc gamma receptors and complement receptor 3 (alpha(M)beta(2); CD11b/CD18) J. Exp. Med. 2001;193:61–71. doi: 10.1084/jem.193.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patel PC, Harrison RE. Membrane ruffles capture C3bi-opsonized particles in activated macrophages. Mol. Biol. Cell. 2008;19:4628–4639. doi: 10.1091/mbc.E08-02-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colucci-Guyon E, Niedergang F, Wallar BJ, Peng J, Alberts AS, Chavrier P. A role for mammalian diaphanous-related formins in complement receptor (CR3)-mediated phagocytosis in macrophages. Curr. Biol. CB. 2005;15:2007–2012. doi: 10.1016/j.cub.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 74.Kim J-G. Ras-related GTPases Rap1 and RhoA collectively induce the phagocytosis of serum-opsonized zymosan particles in macrophages. J. Biol. Chem. 2012;287:5145–5155. doi: 10.1074/jbc.M111.257634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewkowicz E, Herit F, Clainche C Le, Bourdoncle P, Perez F, Niedergang F. The microtubule-binding protein CLIP-170 coordinates mDia1 and actin reorganization during CR3-mediated phagocytosis. J. Cell Biol. 2008;183:1287–1298. doi: 10.1083/jcb.200807023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaoui K, Honoré S, Isnardon D, Braguer D, Badache A. Memo-RhoA-mDia1 signaling controls microtubules, the actin network, and adhesion site formation in migrating cells. J. Cell Biol. 2008;183:401–408. doi: 10.1083/jcb.200805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iijima M, Huang YE, Devreotes P. Temporal and spatial regulation of chemotaxis. Dev. Cell. 2002;3:469–478. doi: 10.1016/s1534-5807(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 78.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 79.Janetopoulos C, Firtel RA. Directional sensing during chemotaxis. FEBS Lett. 2008;582:2075–2085. doi: 10.1016/j.febslet.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allen WE, Zicha D, Ridley AJ, Jones GE. A role for Cdc42 in macrophage chemotaxis. J. Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr. Opin. Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 82.Haastert PJM Van. Chemotaxis: insights from the extending pseudopod. J. Cell Sci. 2010;123:3031–3037. doi: 10.1242/jcs.071118. [DOI] [PubMed] [Google Scholar]
- 83.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 85.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- 86.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 87.Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev. Cell. 2009;17:310–322. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 88.Suraneni P, Rubinstein B, Unruh JR, Durnin M, Hanein D, Li R. The Arp2/3 complex is required for lamellipodia extension and directional fibroblast cell migration. J. Cell Biol. 2012;197:239–251. doi: 10.1083/jcb.201112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pixley FJ. Macrophage Migration and Its Regulation by CSF-1. Int. J. Cell Biol. 2012;2012:501962. doi: 10.1155/2012/501962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sasaki Y, Ohsawa K, Kanazawa H, Kohsaka S, Imai Y. Iba1 is an actin-cross-linking protein in macrophages/microglia. Biochem. Biophys. Res. Commun. 2001;286:292–297. doi: 10.1006/bbrc.2001.5388. [DOI] [PubMed] [Google Scholar]
- 91.Kinne RW, Bräuer R, Stuhlmüller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 94.Mouchemore KA, Pixley FJ. CSF-1 signaling in macrophages: pleiotrophy through phosphotyrosine-based signaling pathways. Crit. Rev. Clin. Lab. Sci. 2012;49:49–61. doi: 10.3109/10408363.2012.666845. [DOI] [PubMed] [Google Scholar]
- 95.Sengupta A, Liu WK, Yeung YG, Yeung DC, Frackelton AR, Jr, Stanley ER. Identification and subcellular localization of proteins that are rapidly phosphorylated in tyrosine in response to colony-stimulating factor 1. Proc. Natl. Acad. Sci. U. S. A. 1988;85:8062–8066. doi: 10.1073/pnas.85.21.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joos H, Trouliaris S, Helftenbein G, Niemann H, Tamura T. Tyrosine phosphorylation of the juxtamembrane domain of the v-Fms oncogene product is required for its association with a 55-kDa protein. J. Biol. Chem. 1996;271:24476–24481. doi: 10.1074/jbc.271.40.24476. [DOI] [PubMed] [Google Scholar]
- 97.Wilhelmsen K, Burkhalter S, Geer P van der. C-Cbl binds the CSF-1 receptor at tyrosine 973, a novel phosphorylation site in the receptor’s carboxy-terminus. Oncogene. 2002;21:1079–1089. doi: 10.1038/sj.onc.1205166. [DOI] [PubMed] [Google Scholar]
- 98.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 99.Yeung YG, Wang Y, Einstein DB, Lee PS, Stanley ER. Colony-stimulating factor-1 stimulates the formation of multimeric cytosolic complexes of signaling proteins and cytoskeletal components in macrophages. J. Biol. Chem. 1998;273:17128–17137. doi: 10.1074/jbc.273.27.17128. [DOI] [PubMed] [Google Scholar]
- 100.Munugalavadla V, Borneo J, Ingram DA, Kapur R. p85alpha subunit of class IA PI-3 kinase is crucial for macrophage growth and migration. Blood. 2005;106:103–109. doi: 10.1182/blood-2004-10-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vedham V, Phee H, Coggeshall KM. Vav activation and function as a rac guanine nucleotide exchange factor in macrophage colony-stimulating factor-induced macrophage chemotaxis. Mol. Cell. Biol. 2005;25:4211–4220. doi: 10.1128/MCB.25.10.4211-4220.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vanhaesebroeck B, et al. Distinct PI(3)Ks mediate mitogenic signalling and cell migration in macrophages. Nat. Cell Biol. 1999;1:69–71. doi: 10.1038/9045. [DOI] [PubMed] [Google Scholar]
- 103.Papakonstanti EA, Ridley AJ, Vanhaesebroeck B. The p110delta isoform of PI 3-kinase negatively controls RhoA and PTEN. EMBO J. 2007;26:3050–3061. doi: 10.1038/sj.emboj.7601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mouchemore KA, Sampaio NG, Murrey MW, Stanley ER, Lannutti BJ, Pixley FJ. Specific inhibition of PI3K p110δ inhibits CSF-1-induced macrophage spreading and invasive capacity. FEBS J. 2013 doi: 10.1111/febs.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sampaio NG, et al. Phosphorylation of CSF-1R Y721 mediates its association with PI3K to regulate macrophage motility and enhancement of tumor cell invasion. J. Cell Sci. 2011;124:2021–2031. doi: 10.1242/jcs.075309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J. Cell Sci. 1997;110(Pt 6):707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- 107.Zicha D, et al. Chemotaxis of macrophages is abolished in the Wiskott-Aldrich syndrome. Br. J. Haematol. 1998;101:659–665. doi: 10.1046/j.1365-2141.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 108.Badolato R, et al. Monocytes from Wiskott-Aldrich patients display reduced chemotaxis and lack of cell polarization in response to monocyte chemoattractant protein-1 and formyl-methionyl-leucyl-phenylalanine. J. Immunol. Baltim. Md 1950. 1998;161:1026–1033. [PubMed] [Google Scholar]
- 109.Ishihara D, Dovas A, Park H, Isaac BM, Cox D. The chemotactic defect in wiskott-Aldrich syndrome macrophages is due to the reduced persistence of directional protrusions. PLoS ONE. 2012;7:e30033. doi: 10.1371/journal.pone.0030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dovas A, Gevrey J-C, Grossi A, Park H, Abou-Kheir W, Cox D. Regulation of podosome dynamics by WASp phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J. Cell Sci. 2009;122:3873–3882. doi: 10.1242/jcs.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abou-Kheir W, Isaac B, Yamaguchi H, Cox D. Membrane targeting of WAVE2 is not sufficient for WAVE2-dependent actin polymerization: a role for IRSp53 in mediating the interaction between Rac and WAVE2. J. Cell Sci. 2008;121:379–390. doi: 10.1242/jcs.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parent CA, Devreotes PN. A cell’s sense of direction. Science. 1999;284:765–770. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- 113.DeMali KA, Burridge K. Coupling membrane protrusion and cell adhesion. J. Cell Sci. 2003;116:2389–2397. doi: 10.1242/jcs.00605. [DOI] [PubMed] [Google Scholar]
- 114.Borm B, Requardt RP, Herzog V, Kirfel G. Membrane ruffles in cell migration: indicators of inefficient lamellipodia adhesion and compartments of actin filament reorganization. Exp. Cell Res. 2005;302:83–95. doi: 10.1016/j.yexcr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 115.Pixley FJ, Lee PS, Condeelis JS, Stanley ER. Protein tyrosine phosphatase phi regulates paxillin tyrosine phosphorylation and mediates colony-stimulating factor 1-induced morphological changes in macrophages. Mol. Cell. Biol. 2001;21:1795–1809. doi: 10.1128/MCB.21.5.1795-1809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 117.Owen KA, et al. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J. Cell Biol. 2007;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Block MR, et al. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur. J. Cell Biol. 2008;87:491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 119.Dovas A, Cox D. Signaling networks regulating leukocyte podosome dynamics and function. Cell. Signal. 2011;23:1225–1234. doi: 10.1016/j.cellsig.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wheeler AP, Smith SD, Ridley AJ. CSF-1 and PI 3-kinase regulate podosome distribution and assembly in macrophages. Cell Motil. Cytoskeleton. 2006;63:132–140. doi: 10.1002/cm.20111. [DOI] [PubMed] [Google Scholar]
- 121.Monypenny J, et al. Role of WASP in cell polarity and podosome dynamics of myeloid cells. Eur. J. Cell Biol. 2011;90:198–204. doi: 10.1016/j.ejcb.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dehring DAK, et al. Hematopoietic lineage cell-specific protein 1 functions in concert with the Wiskott-Aldrich syndrome protein to promote podosome array organization and chemotaxis in dendritic cells. J. Immunol. Baltim. Md 1950. 2011;186:4805–4818. doi: 10.4049/jimmunol.1003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Srinivasan S, et al. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J. Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J. Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 126.Wheeler AP, Ridley AJ. RhoB affects macrophage adhesion, integrin expression and migration. Exp. Cell Res. 2007;313:3505–3516. doi: 10.1016/j.yexcr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 127.Sellers JR. Myosins: a diverse superfamily. Biochim. Biophys. Acta. 2000;1496:3–22. doi: 10.1016/s0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- 128.Giannone G, et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lim JI, Sabouri-Ghomi M, Machacek M, Waterman CM, Danuser G. Protrusion and actin assembly are coupled to the organization of lamellar contractile structures. Exp. Cell Res. 2010;316:2027–2041. doi: 10.1016/j.yexcr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wilson CA, et al. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465:373–377. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Z-H, Dulyaninova NG, House RP, Almo SC, Bresnick AR. S100A4 regulates macrophage chemotaxis. Mol. Biol. Cell. 2010;21:2598–2610. doi: 10.1091/mbc.E09-07-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Swaney KF, Huang C-H, Devreotes PN. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 2010;39:265–289. doi: 10.1146/annurev.biophys.093008.131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gambardella L, Vermeren S. Molecular players in neutrophil chemotaxis--focus on PI3K and small GTPases. J. Leukoc. Biol. 2013 doi: 10.1189/jlb.1112564. [DOI] [PubMed] [Google Scholar]
- 134.Baruzzi A, Caveggion E, Berton G. Regulation of phagocyte migration and recruitment by Src-family kinases. Cell. Mol. Life Sci. CMLS. 2008;65:2175–2190. doi: 10.1007/s00018-008-8005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Curnock AP, Logan MK, Ward SG. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology. 2002;105:125–136. doi: 10.1046/j.1365-2567.2002.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gevrey J-C, Isaac BM, Cox D. Syk is required for monocyte/macrophage chemotaxis to CX3CL1 (Fractalkine) J. Immunol. Baltim. Md 1950. 2005;175:3737–3745. doi: 10.4049/jimmunol.175.6.3737. [DOI] [PubMed] [Google Scholar]
- 137.Park H, Cox D. Syk regulates multiple signaling pathways leading to CX3CL1 chemotaxis in macrophages. J. Biol. Chem. 2011;286:14762–14769. doi: 10.1074/jbc.M110.185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marzio P Di, Dai WW, Franchin G, Chan AY, Symons M, Sherry B. Role of Rho family GTPases in CCR1- and CCR5-induced actin reorganization in macrophages. Biochem. Biophys. Res. Commun. 2005;331:909–916. doi: 10.1016/j.bbrc.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 139.Weiss-Haljiti C, et al. Involvement of phosphoinositide 3-kinase gamma, Rac,and PAK signaling in chemokine-induced macrophage migration. J. Biol. Chem. 2004;279:43273–43284. doi: 10.1074/jbc.M402924200. [DOI] [PubMed] [Google Scholar]
- 140.Bouma G, Burns SO, Thrasher AJ. Wiskott-Aldrich Syndrome: Immunodeficiency resulting from defective cell migration and impaired immunostimulatory activation. Immunobiology. 2009;214:778–790. doi: 10.1016/j.imbio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang H, et al. Impaired integrin-dependent function in Wiskott-Aldrich syndrome protein-deficient murine and human neutrophils. Immunity. 2006;25:285–295. doi: 10.1016/j.immuni.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kumar S, et al. Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood. 2012;120:3563–3574. doi: 10.1182/blood-2012-04-426981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wilson AK, Gorgas G, Claypool WD, Lanerolle P de. An increase or a decrease in myosin II phosphorylation inhibits macrophage motility. J. Cell Biol. 1991;114:277–283. doi: 10.1083/jcb.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Si J, Ge Y, Zhuang S, Gong R. Inhibiting nonmuscle myosin II impedes inflammatory infiltration and ameliorates progressive renal disease. Lab. Investig. J. Tech. Methods Pathol. 2010;90:448–458. doi: 10.1038/labinvest.2009.142. [DOI] [PubMed] [Google Scholar]
- 145.Nakayama M, et al. Rho-kinase and myosin II activities are required for cell type and environment specific migration. Genes Cells Devoted Mol. Cell. Mech. 2005;10:107–117. doi: 10.1111/j.1365-2443.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- 146.Hanley PJ, et al. Motorized RhoGAP myosin IXb (Myo9b) controls cell shape and motility. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12145–12150. doi: 10.1073/pnas.0911986107. [DOI] [PMC free article] [PubMed] [Google Scholar]