Abstract
Sweet and bitter taste distinguishes good food sources from potential toxins, but what happens when these tastants are mixed? In this issue, Jeong et al. show that in Drosophila, bitter compounds act through an extracellular odorant binding protein to inhibit sweet-responsive neurons and block the response to sweet taste.
In the 1964 film Mary Poppins, Julie Andrews touts how “a spoonful of sugar helps the medicine go down”, reflecting that addition of sweet taste agonists can mask the presence of bitter compounds, like most medicines. New work from Craig Montell’s lab studying taste behavior in Drosophila melanogastor (Jeong et al., 2013) reveals how flies would not be easily swayed by Mary Poppins into taking their medicine by mixing bitter with sugar. Flies have distinct taste neurons tuned to bitter or sweet compounds, but when sweet and bitter compounds are mixed, the bitter tastants turn off the drive to consume sugar. In this issue of Neuron, Jeong et al. show the surprising mechanism behind how bitters trump sweet; through association with the odorant binding protein OBP49a and suppression of the sweet neuron activity (Jeong et al., 2013).
Taste is a critical sense that allows animals to evaluate the quality of food sources. Sweet taste is associated with the presence of energy-rich sugars, while bitter taste is associated with toxic or noxious compounds that might threaten the health of the animal. Sweet and bitter tastes are mediated by membrane receptors expressed on taste neurons that specifically detect these compounds. Receptors detecting sweet compounds are expressed by different neurons than those detecting bitter compounds, establishing “labeled lines” that allow the brain to distinguish between good, energy-rich foods and potentially toxic ones.
There are approximately 120 taste neurons located in sensilla on the labellum (mouth) of the fly (Figure 1A, B). Each side of the labellum has 31 taste sensilla that are divided into classes based on sensillum length (Figure 1B) (Montell, 2009). L-type and S- type sensilla (for Long and Short sensilla) each house the dendrites of 4 chemosensory neurons, while the I-type (intermediate length) sensilla house the dendrites of 2 neurons. Bitter compounds are detected by neurons in the S-type and I-type sensilla, but not the L-type sensilla (Weiss et al., 2011), while all 3 sensilla types contain neurons activated by sugars. The four chemosensory neurons within the L-type sensilla are tuned respectively to sugar, low salt, high salt, and water (low osmolarity), and each neuron is tuned to only one of these stimuli.
Figure 1. Organization of the taste sensilla on the labellum of the fruit fly.
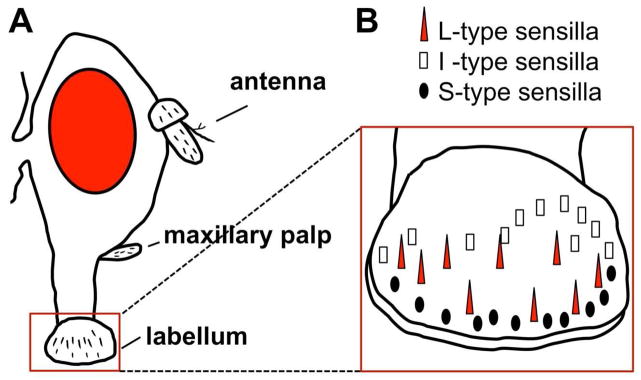
A. Side view of Drosophila head showing olfactory organs (antenna and maxillary palp) and gustatory organ (labellum). B. Side view of the surface of the labellum showing 3 classes of taste sensilla. L-type (red triangle) detect sugars but not bittters, I-type (white rectangle) and S-type (black oval) detect both sugars and bitter compounds.
Like all olfactory and gustatory neurons, the dendrites of the L-type gustatory neurons are bathed in a fluid called sensillum lymph that contains water, ions and secreted proteins produced by the non-neuronal support cells (Figure 2A, 2C). One family of proteins secreted into the lymph are members of the odorant binding protein family, perhaps mis-named because members are expressed in both olfactory and gustatory organs (Galindo and Smith, 2001). Insect odorant binding proteins are encoded by a large gene family (around 50 members in Drosophila) and typically encode small (~14kD) proteins with 3 conserved disulfide bridges. The best-studied OBP is LUSH, an antennal protein required for detection of the male-specific volatile pheromone 11-cis vaccenyl acetate, or cVA (Xu et al., 2005). In the absence of LUSH, cVA sensitivity is dramatically reduced, revealing that the extracellular binding protein is important for sensitivity to pheromone. Furthermore in lush mutants, the spontaneous activity in the cVA-sensing neurons (in the absence of pheromone) plummets from 1 spike per second to 1 spike every 400 seconds, leading to the suggestion that LUSH may be part of the ligand for neuronal membrane receptors on cVA-sensitive neurons. Conformational changes in LUSH structure induced by cVA binding correlate with the ability of LUSH to stimulate pheromone-sensitive neurons in the absence of cVA (Laughlin et al., 2008). Indeed, introduction of mutant LUSH protein locked in a cVA-bound conformation activates cVA-sensitive neurons in the absence of pheromone, but is inactive on any other class of olfactory neuron (Laughlin et al., 2008). This suggests pheromone-sensitive neurons have membrane receptors that detect conformationally activated LUSH (Figure 2B). Such a mechanism could explain the remarkable sensitivity of insect pheromone detection systems that approach single molecule sensitivity (Kaissling, 1998). Do other OBPs work like LUSH?
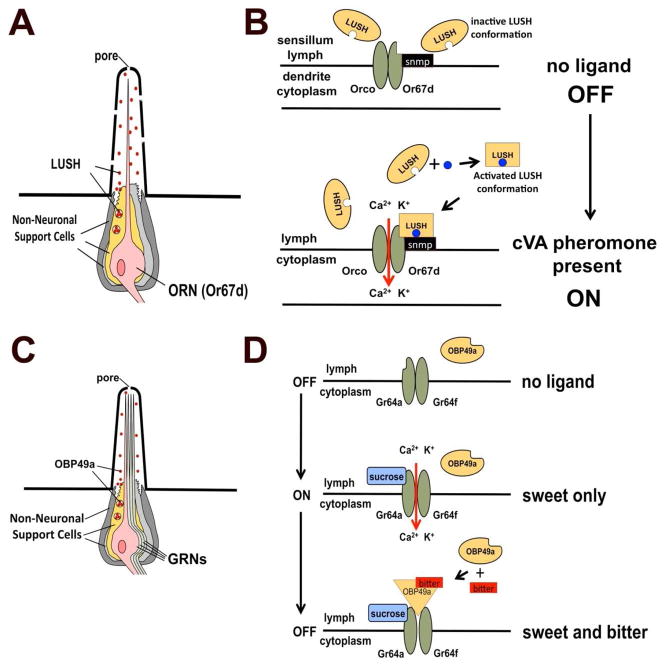
Figure 2. Model for conformational activation of OBPs in smell and taste.
A. Cartoon depicting a sensillum for a cVA-sensing olfactory neuron. Non-neuronal support cells secrete the extracellular odorant binding protein LUSH into the sensillum lymph. B. LUSH is conformationally activated by binding cVA pheromone. Top: LUSH is normally in an inactive state in sensillum lymph in the absence of pheromone. Bottom: In the presence of cVA (blue circles), LUSH undergoes conformational activation upon binding the pheromone (oval to square in the figure). This is recognized by a neuronal odorant receptor complex (a ligand-gated ion channel) composed of Or67d (a tuning odorant receptor found only in cVA-sensing neurons), Orco (a co-receptor for all odorant receptors in Drosophila) and Snmp (sensory neuron membrane protein) that may act as an inhibitory receptor subunit (reviewed in Ronderos and Smith, 2009). C. Structure of the L-type sensilla. Four gustatory receptor neurons extend dendrites into the shaft of the sensillum. Dendrites are surrounded by sensillum lymph, containing OBP49a, secreted by the support cells. D. Model for bitter suppression of sugar-sensing neurons. In sugar-sensing cells, the sucrose receptor complex (requiring Gr64a and Gr64f) is inactive in the absence of sucrose (top). In the presence of sucrose (middle panel) the receptor is activated, allowing entry of calcium and potassium into the sugar-sensing neuron (both ions are more concentrated in the lymph compared to the cytoplasm). In the presence of sweet and bitter compounds (lower panel) the bitter compound binds to OBP49a, and this complex inhibits the receptor even if sucrose is present.
Jeong et al. produced mutants in OBP49a and show that, similar to LUSH, OBP49a is required to ‘sensitize’ sweet taste neurons in L-type sensilla to bitter compounds, but surprisingly OBP49a acts to block the ability of sucrose to stimulate sweet-sensing neurons (Figure 2D). OBP49a is expressed specifically in taste sensilla on the labellum (Figure 2C) (Jeong et al., 2013) and is highly conserved among Drosophila species implying it serves a conserved function. Jeong et al. tested OBP49a mutants for defects in taste behavior. Given a choice, flies normally prefer 5mM to 1mM sucrose, assayed by adding different food coloring to each solution, allowing the flies to choose between these solutions and observing the stomach color of the flies after a 90 minute assay. Flies usually have stomachs colored with the dye added to the 5 mM solution. However, if any one of several bitter compounds (including papaverine, berberine, denatonium, quinine, strychnine or caffeine) were mixed with the 5 mM sucrose, normal flies will prefer the untainted 1 mM sucrose, and will have stomachs dyed with the 1mM food coloring. Interestingly, flies lacking OBP49a still prefer 5 mM sucrose tainted with any one of these five bitter compounds. Behavior to another bitter, L-canavanine, was not affected, revealing OBP49a is not required for avoiding all bitter compounds. Responses of L-type neurons to sugar or S-type neurons to bitter compounds are unchanged in the OBP49a mutant when the compounds are assayed alone. Furthermore, bitter compounds did not activate any L-type neurons in either genetic background, confirming that bitter compounds do not activate the sugar neurons in the mutant. What did change was the response of L-type sweet-sensing neurons to sugar when combined with bitter compounds. In wild type flies, the mixture of sweet and bitter strongly inhibits spiking in the sugar-sensitive neuron, while in the OBP49a mutant this neuron responds to sugar as if there were no bitter compounds present! Since there are no bitter-sensing neurons in L-type sensilla, this suppression cannot result from direct inhibition by activity in bitter-sensing neurons. A rescuing transgene expressing OBP49a in the support cells of the L-type sensilla restored normal inhibition of the sugar neuron to bitters in the OBP49a mutants, confirming OBP49a is the essential factor underlying this behavioral defect. Even more remarkable, expressing an OBP49a construct that added an anchoring transmembrane domain to the C-terminus of the normally secreted OBP49a in the sugar neuron also restored wild type inhibition. This implicates a role for extracellular OBP49a in regulating some membrane protein on the L-type sugar-sensing neurons that mediates neuronal firing.
Could OBP49a be acting directly on the sweet receptor, perhaps as a competitive or non-competitive antagonist? Sucrose detection requires co-expression of at least two gustatory receptors, Gr64a and Gr64f (Jiao et al., 2008). To determine if membrane-anchored OBP49a is in close proximity to either of these receptor subunits, they undertook a split-YFP experiment (Ghosh et al., 2000). They fused the N-terminal half of a YFP gene to the N-terminal portion of Gr64a and also to Gr64f (the N-termini of these receptors reside inside the cell) and produced lines of flies expressing each of these transgenes in the sugar-sensing cells. They also produced flies expressing the C-terminal half of YFP fused to the intracellular region of tethered OBP49a in the same neurons. When flies expressing the Gr64a receptor fusion were crossed to the tethered OBP49a-YFP fusion, strong fluorescence was detected in the labellum, suggesting that the intracellular domains of Gr64a and the membrane-tethered OBP49a are in close proximity. These findings are consistent with OBP49a interacting with, and inhibiting sweet responses through Gr64a (Figure 2D). Surprisingly, no fluorescence was detected when the tethered OBP49a-YFP flies were crossed to the Gr64f-YFP fusion, indicating there may be a specific interaction between OBP49a and the Gr64a subunit that permits association of the two YFP fragments. Addition of bitter ligands did not alter the fluorescence in either combination. This could mean OBP49a is always bound to the receptor and only inhibits when bitters are present, or perhaps adding an artificial membrane anchor to OBP49a results in some structural configuration that is only able to interact with Gr49a-YFP. Split YFP experiments have to be interpreted with caution, as these findings only demonstrate that the proteins are in close proximity, and does not implicate or rule out any specific protein-protein interactions between OBP49a and membrane receptor subunits. Additional work remains to demonstrate exactly how this inhibition works at the receptor level.
Does OBP49a actually bind to tastants? Jeong et al. purified OBP49a from flies and bound it to sensor chips and used surface plasmon resonance to examine what tastants bind to OBP49a. They found bitter chemicals bound to OBP49a in a dose-dependent manner, but sucrose did not. Together, these data support a model in which OBP49a binds to bitter tastants and inhibits the firing of the sugar-sensing neurons, possibly by direct interactions with the neuronal sweet taste receptors (Figure 2D). OBP49a is expressed in all sensilla on the labellum, so while L-type sensilla were studied by Jeong et al. to rule out potential cross talk between the bitter-sensing neurons and sweet-sensing neurons in the same sensilla, it is likely this mechanism is also present in the S-type and I-type type sensilla as well. This would be consistent with the potent effects observed in the OBP49a mutants on bitter avoidance behavior. These data support the controversial view that members of the odorant binding protein family can directly interact with membrane receptors in a ligand dependent manner and influence neuronal activity.
To fully understand how OBP49a functions, some structural studies are in order. OBP49a is 30% larger that most mature OBP proteins and contains 12 cysteines instead of 6 (Nagnan-Le Meillor and Jacquin-Joly, 2003). This suggests it may have a larger, more complex ligand-interaction domain that might be important for detecting structurally diverse bitter compounds and signaling their presence to the neurons. It will be important to determine the X-ray crystal structure of OBP49a alone and with various bitter compounds bound to establish if there is a shared conformational change induced by the diverse bitter compounds that is distinct from the unliganded OBP, similar to what has been shown for cVA pheromone and LUSH (Laughlin et al., 2008). Such a conformational shift could pinpoint domains that might interact with the taste receptor. Indeed, demonstrating bitter-dependent binding of OBP49a to the sweet receptor would also be important.
Finally, it is fascinating that detection of bitter compounds with neurons located in S-type and I-type sensilla is not enough to deter flies from potential toxins mixed with sugars. OBP49a represents an independent bitter detection mechanism that has evolved to override the activation of sugar neurons in the presence of bitter chemicals. It is likely that the sugar input into the CNS elicits a strong feeding signal that needs to be blocked when bitters are present to prevent feeding behavior, and this two-pronged mechanism prevents ‘the spoonful of sugar’ from facilitating ingestion of potentially toxic bitter “medicines”.
This is a commentary on article Jeong YT, Shim J, Oh SR, Yoon HI, Kim CH, Moon SJ, Montell C. An odorant-binding protein required for suppression of sweet taste by bitter chemicals. Neuron. 2013;79(4):725-37.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Galindo K, Smith DP. A large family of divergent odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics. 2001;159:1059–1072. doi: 10.1093/genetics/159.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh I, Hamilton AD, Regan L. Antiparallel leucine zipper-directed mprotein reassembly: Application to the green fluorescent protein. j AmerChem Soc. 2000;122:5658–5659. [Google Scholar]
- Jeong YT, Shim J, Oh SR, Yoon HI, Kim CH, Moon SJ, Montell C. An odorant binding required for suppression of sweet taste by bitter chemicals. Neuron. 2013 doi: 10.1016/j.neuron.2013.06.025. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr Biol. 2008;18:1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaissling KE. Pheromone deactivation catalyzed by receptor molecules: a quantitative kinetic model. Chem Senses. 1998;23:385–395. doi: 10.1093/chemse/23.4.385. [DOI] [PubMed] [Google Scholar]
- Laughlin JD, Ha TS, Jones DN, Smith DP. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008;133:1255–1265. doi: 10.1016/j.cell.2008.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagnan-Le Meillour P, Jacquin-Joly E. Biochemistry and diversity of insect odorant-binding proteins. In: Bloomquist GJ, Vogt RG, editors. Insect Pheromone Biochemistry and Molecular Biology. Elsevier/Academic Press; 2003. pp. 509–538. [Google Scholar]
- Ronderos DS, Smith DP. Diverse signaling mechanisms mediate volatile odorant detection in Drosophila. Fly (Austin) 2009;3:290–297. doi: 10.4161/fly.9801. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Atkinson R, Jones DNM, Smith DP. Drosophila OBP LUSH is required for activity of Pheromone-sensitive neurons. Neuron. 2005;45:193–200. doi: 10.1016/j.neuron.2004.12.031. [DOI] [PubMed] [Google Scholar]