Abstract
Object
The role of genetic polymorphisms in the neurological outcome of patients after carotid endarterectomy (CEA) remains unclear. There are single nucleotide polymorphisms (SNPs) that predispose patients to postoperative cognitive dysfunction (CD). We aim to assess the predictability of three complement cascade-related SNPs for CD in patients having CEAs.
Methods
In 252 patients undergoing CEA, genotyping was performed for the following polymorphisms: complement component 5 (C5) rs17611, mannose-binding lectin 2 (MBL2) rs7096206, and complement factor H (CFH) rs1061170. Differences among genotypes were analyzed via the chi-square test. Patients were evaluated with a neuropsychometric battery for CD 1 day and 1 month after CEA. A multiple logistic regression model was created. All variables with univariate p < 0.20 were included in the final model.
Results
The C5 genotypes A/G (OR 0.26, 95% CI 0.11–0.60, p = 0.002) and G/G (OR 0.22, 95% CI 0.09–0.52, p < 0.001) were significantly associated with lower odds of exhibiting CD at 1 day after CEA compared with A/A. The CFH genotypes C/T (OR 3.37, 95% CI 1.69–6.92, p < 0.001) and C/C (OR 3.67, 95% CI 1.30–10.06, p = 0.012) were significantly associated with higher odds of exhibiting CD at 1 day after CEA compared with T/T. Statin use was also significantly associated with lower odds of exhibiting CD at 1 day after CEA (OR 0.43, 95% CI 0.22–0.84, p = 0.01). No SNPs were significantly associated with CD at 1 month after CEA.
Conclusions
The presence of a deleterious allele in the C5 and CFH SNPs may predispose patients to exhibit CD after CEA. This finding supports previous data demonstrating that the complement cascade system may play an important role in the development of CD. These findings warrant further investigation.
Keywords: carotid endarterectomy, complement, vascular disorders, single nucleotide polymorphism, neuropsychometric, cognitive dysfunction
Although carotid endarterectomy is considered a safe procedure, with a stroke rate of 1%–2%, accumulating evidence indicates that subtle postoperative cognitive dysfunction (CD) occurs in 9%–28% of cases.16,25 The etiology of CD is likely multifactorial, resulting from focal ischemia during clamp time, post-recanalization hyperperfusion, microemboli, and individual genetic factors.13 These potential mechanisms have been investigated in more detail through radiographic correlation, serum marker quantification, atherosclerotic plaque analysis, and genetic studies.
Single-nucleotide polymorphism analyses and genome-wide association studies have identified multiple candidate genes for involvement in the pathophysiology of ischemic stroke. SNPs associated with elevated inflammation increase the incidence of CD following both carotid artery and cardiac surgery. Less-active forms of complement component 3, a protein that decreases the activity of the complement system, correlate with increased CD 1 day after surgery. In addition, less-active forms of complement factor H (CFH), an inhibitor of the common complement pathway, are also associated with increased CD 1 day following CEA.11
Polymorphisms in the complement components C5 and MBL2 have recently been implicated in the risk of ischemic stroke and cardiovascular disease.29 In a larger cohort study, we aim to confirm these prior genetic findings and further delineate the role of the complement cascade by analyzing the C5, CFH, and MBL2 SNPs of the complement cascade and evaluating their utility in the prediction of CD after CEA.
Methods
Patients
We performed a nested cohort study of 252 patients prospectively recruited in this IRB-approved study. Written informed consent was obtained from all patients. This subgroup consists of individuals who consented to genetic testing while enrolled in a larger study investigating the relationship between stroke risk factors and CD. All patients were genotyped for the C5, CFH, and MBL2 polymorphisms. A second group of 155 age- and education-matched patients undergoing lumbar laminectomy or microdiscectomy were contemporaneously recruited to serve as a postoperative reference group for neuropsychometric testing. All patients were native English speakers with no history of drug abuse, Axis I psychiatric disorders, or previous ipsilateral CEA.
Surgery and Anesthesia
Eligible patients were scheduled for elective CEA for high-grade carotid artery stenosis. All patients received general anesthesia with standard hemodynamic and temperature monitoring, as previously described.16 No patient received blood transfusion. The surgical technique, anesthetic management, and indications for CEA have remained constant at our institution, as previously described.16,25
Genotyping
DNA was extracted from buffy coats of whole blood samples and amplified by PCR. PCR was performed with an Eppendorf Mastercycler Gradient thermal cycler. Primers were identified for C5 rs17611, CFH rs1061170, and MBL2 promoter rs7096206. PCR products were treated with QIAquick PCR purification kits (Qiagen). Sequencing was performed using the respective primers in conjunction with BigDye Termination v3.1 cycle sequencing kits (Applied Biosystems). Analysis was performed using an Applied Biosystems 3730xl capillary instrument. Finally, SNPs of interest were determined using Chromas 2.01 software (Digital River).
Neuropsychometric Testing
A reference group was used to account for trauma of surgery, effects of general anesthesia, and practice effect associated with repeated neuropsychometric testing, as previously described.16,25 Patients were examined with a previously described battery of neuropsychometric tests preoperatively and postoperatively at 1 day and 1 month after CEA. The neuropsychometric battery evaluates a variety of cognitive domains—verbal memory, visuospatial organization, motor function, and executive action.
A variety of factors affect the risk of CD in patients after CEA, but only age greater than 75,25 statin use,15 and diabetes mellitus25 have been previously shown to significantly and independently affect the risk of CD. Other factors that might also affect the risk, but have not been shown to independently affect the risk, of CD were evaluated as well. These included years of education, body mass index, history of smoking, hypertension, extensive peripheral vascular disease, symptomatic history of stroke or transient ischemic attack, and duration of cross-clamping of the carotid artery.
Statistical Analysis
Allele and genotype frequencies were compared with values predicted by Hardy-Weinberg equilibrium using a chi-square test. Each patient’s neuropsychometric performance was calculated, as described previously.9,16,26 The tests were grouped by cognitive domain and patients were considered to have CD based on 2 criteria to account for both focal and global/hemispheric deficits: 1) scoring at least 2 SD worse than the reference group in 2 or more cognitive domains or 2) scoring at least 1.5 SD worse than the reference group in all 4 cognitive domains.
Statistical analysis was performed using R environment for statistical computing (R Development Core Team, 2008). For univariate analyses, the Student t-test, the Wilcoxon rank-sum test, the Fisher exact test, the Pearson chi-square test, and simple logistic regression were used where appropriate. The alpha level was adjusted for multiple hypotheses using the Benjamini and Hochberg method to control for the false discovery rate.1 Multiple logistic regression models were constructed to identify independent predictors of CD at 1 day and 1 month after CEA. All factors with p < 0.20 in a simple univariate logistic regression were entered into the final models for 1 day and 1 month after CEA. To control for population stratification and confounding, any variables significantly associated with differences in any of the SNP genotypes were included in the model as well. Model fit and calibration were confirmed with the likelihood ratio test, Hosmer-Lemeshow goodness-of-fit test, and receiver operating characteristic analysis. In the event of missing values for predictor variables, the sample mean was imputed. Significance was set at p ≤ 0.05.
Results
Patients
Baseline patient characteristics and hospital course variables did not differ between genotypes for the C5 and CFH alleles (Tables 1 and 2). In the MBL2 allele, however, the G/G genotype group differed from the other 2 genotype groups in age (mean age in the G/G group 68.9 ± 5.3 years with 5.0% of patients older than 75 years of age vs 70.4 ± 9.2 years with 29.8% older than 75) and statin use (50.0% in the G/G group vs 75.2% in the combined C/C and G/C group) (Table 3).
TABLE 1.
Patient characteristics—C5 genotypes*
| Characteristic | A/A (n = 47) | A/G (n = 130) | G/G (n = 118) |
|---|---|---|---|
| age >75 yrs | 23.4% | 24.6% | 33.9% |
| yrs of education (mean) | 14.4 ± 3.0 | 14.4 ± 3.4 | 14.7 ± 3.1 |
| BMI (mean) | 26.0 ± 4.2 | 27.4 ± 4.6 | 27.4 ± 4.9 |
| history of smoking | 78.7% | 71.5% | 71.2% |
| hypertension | 48.9% | 59.2% | 58.5% |
| statin use | 72.3% | 74.6% | 72.9% |
| diabetes mellitus | 19.1% | 24.6% | 19.5% |
| PVD | 27.7% | 29.2% | 25.4% |
| symptomatic† | 51.1% | 50.0% | 39.0% |
| cross-clamp duration in minutes (mean) | 43.8 ± 18.1 | 47.4 ± 16.6 | 45.2 ± 18.8 |
| CD at 1 day after CEA | 40.4% | 18.5% | 14.4% |
| CD at 1 month after CEA | 16.7% | 18.1% | 6.8% |
| white | 87.2% | 90.8% | 92.4% |
| black | 6.4% | 3.8% | 1.7% |
| Hispanic | 2.1% | 1.5% | 1.7% |
| other | 4.3% | 3.8% | 4.2% |
Values represent percentages of patients unless otherwise indicated. Means are given with SDs.
Abbreviations: BMI = body mass index; PVD = peripheral vascular disease.
Due to previous cerebrovascular accident, stroke, or transient ischemic attack.
TABLE 2.
Patient characteristics—CFH genotypes*
| Variable | C/C (n = 27) | C/T (n = 105) | T/T (n = 163) |
|---|---|---|---|
| age >75 yrs | 14.8% | 27.6% | 30.7% |
| yrs of education (mean) | 13.9 ± 2.9 | 14.7 ± 3.4 | 14.6 ± 3.2 |
| BMI (mean) | 27.3 ± 4.5 | 27.1 ± 4.8 | 27.3 ± 4.7 |
| history of smoking | 85.2% | 69.5% | 72.4% |
| hypertension | 66.7% | 62.9% | 52.1% |
| statin use | 81.5% | 78.1% | 69.3% |
| diabetes mellitus | 14.8% | 25.7% | 20.2% |
| PVD | 14.8% | 31.4% | 27.0% |
| symptomatic† | 59.3% | 50.5% | 40.5% |
| cross-clamp duration in minutes (mean) | 43.1 ± 15.6 | 48.1 ± 18.3 | 45.1 ± 17.6 |
| CD at 1 day after CEA | 33.3% | 27.6% | 13.5% |
| CD at 1 month after CEA | 20.0% | 14.5% | 11.2% |
| white | 88.9% | 90.5% | 91.4% |
| black | 11.1% | 1.9% | 3.1% |
| Hispanic | 0% | 2.9% | 1.2% |
| other | 0% | 4.8% | 4.3% |
Values represent percentages of patients unless otherwise indicated. Means are given with SDs.
Due previous cerebrovascular accident, stroke, or transient ischemic attack.
TABLE 3.
Patient characteristics—MBL2 genotypes*
| Variable | C/C (n = 188) | C/G (n = 87) | G/G (n = 20) |
|---|---|---|---|
| age >75 yrs† | 31.4% | 26.4% | 5.0% |
| yrs of education (mean) | 14.7 ± 3.1 | 14.4 ± 3.6 | 13.9 ± 2.0 |
| BMI (mean) | 26.8 ± 4.5 | 27.9 ± 5.1 | 27.8 ± 4.0 |
| history of smoking | 72.9% | 71.3% | 75.0% |
| hypertension | 60.6% | 49.4% | 60.0% |
| statin use† | 75.5% | 74.7% | 50.0% |
| diabetes mellitus | 21.8% | 20.7% | 25.0% |
| PVD | 28.7% | 28.7% | 10.0% |
| symptomatic‡ | 49.5% | 39.1% | 40.0% |
| cross-clamp duration in minutes (mean) | 46.0 ± 18.2 | 45.7 ± 16.6 | 46.6 ± 18.6 |
| CD at 1 day after CEA | 19.1% | 17.2% | 45.0% |
| CD at 1 month after CEA | 12.2% | 15.4% | 16.7% |
| white | 91.5% | 88.5% | 95.0% |
| black | 2.7% | 4.6% | 5.0% |
| Hispanic | 1.6% | 2.3% | 0% |
| other | 4.3% | 4.6% | 0% |
Values represent percentages of patients unless otherwise indicated. Means are given with SDs.
Significant association with SNP genotype (p < 0.05).
Due to previous cerebrovascular accident, stroke, or transient ischemic attack.
Incidence of CD by Genotype
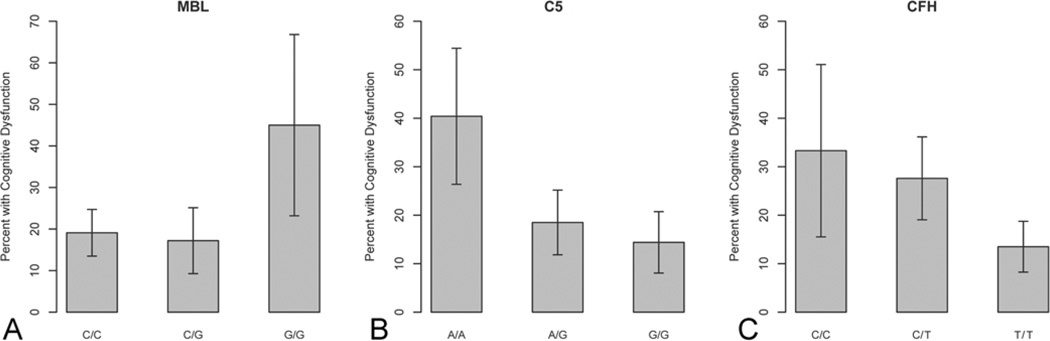
Each SNP had one genotype with an incidence of CD 1 day postoperatively that differed significantly from the other 2 genotypes (Fig. 1). Patients with the G/G MBL2 genotype had a 45.0% incidence of CD, as opposed to 17.2% in the patients with the C/G genotype (p = 0.015, α=0.017) and 19.1% in those with the C/C genotype (p = 0.018, α = 0.033). Similarly, 40.4% of the patients with the C5 genotype A/A had CD 1 day after CEA as compared with 18.5% of those with the A/G genotype (p = 0.004, α = 0.033) and 14.4% of those with the G/G genotype (p < 0.001, α = 0.017). The incidence of CD among patients with the T/T CFH genotype was 13.5%, which was significantly lower than that of patients with the C/T (27.6%, p = 0.006, α = 0.017) or C/C (33.3%, p = 0.020, α = 0.033) genotypes. There were no significant differences in the incidence of CD at 1 month after CEA among the genotypes for CFH, C5, or MBL2.
Fig. 1.
Graphs showing the results of univariate analysis. The incidence of CD 1 day after CEA differed significantly among the genotypes of each SNP: the G/G MBL2 genotype had a significantly higher incidence of CD compared to the C/G and C/C genotypes (A), the A/A C5 genotype had a significantly higher incidence of CD compared to the A/G and G/G genotypes (B), and the T/T CFH genotype had a significantly lower incidence of CD compared to the C/T and C/C genotypes (C). Error bars are standard error.
Multiple Logistic Regression Models
Variables associated with CD at p < 0.20 in univariate regression included years of education, statin use, and the CFH, C5, and MBL2 SNPs. Age greater than 75 years and statin use were both significantly associated with the MBL2 SNP genotype.
The final logistic regression model for 1 day after CEA included years of education, statin use, age greater than 75 years, and the CFH, C5, and MBL2 SNPs (Table 4). The A/G and G/G genotypes for C5 were both associated with significantly decreased odds of CD compared with the A/A genotype (OR 0.26, 95% CI 0.11–0.60, p = 0.002 and OR 0.22, 95% CI 0.09–0.52, p < 0.001, respectively). The C/T and C/C CFH genotypes were associated with significantly increased odds of CD compared with the T/T genotype (OR 3.37, 95% CI 1.69–6.92, p < 0.001 and OR 3.56, 95% CI 1.30–10.06, p = 0.012, respectively). Statin use was also associated with decreased odds of CD (OR 0.43, 95% CI 0.22–0.84, p = 0.014). The final logistic regression model for 1 month after CEA included years of age greater than 75 years, years of education, statin use, hypertension, and the C5 SNP. None of the included variables were significantly associated with CD at 1 month after CEA.
TABLE 4.
Univariate and multivariate logistic regression model—CD at 1 day after CEA
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| age >75 yrs | 1.01 (0.53–1.87) | 0.970 | 1.26 (0.62–2.52) | 0.515 |
| yrs of education | 0.92 (0.84–1.01) | 0.097 | 0.93 (0.84–1.03) | 0.191 |
| BMI | 0.99 (0.93–1.05) | 0.821 | ||
| history of smoking | 0.95 (0.51–1.82) | 0.865 | ||
| hypertension | 1.26 (0.71–2.27) | 0.443 | ||
| statin use | 0.49 (0.27–0.90) | 0.021 | 0.43 (0.22–0.84) | 0.014 |
| diabetes mellitus | 1.42 (0.72–2.69) | 0.297 | ||
| PVD | 1.06 (0.55–1.96) | 0.865 | ||
| symptomatic* | 1.35 (0.76–2.38) | 0.305 | ||
| cross-clamp duration | 0.99 (0.97–1.01) | 0.350 | ||
| MBL2 (ref = G/G) | ||||
| C/G | 0.25 (0.09–0.73) | 0.010 | 0.58 (0.19–1.86) | 0.354 |
| C/C | 0.29 (0.11–0.77) | 0.011 | 0.60 (0.20–1.79) | 0.347 |
| C5 (ref = A/A) | ||||
| A/G | 0.33 (0.16–0.70) | 0.003 | 0.26 (0.11–0.60) | 0.002 |
| G/G | 0.25 (0.11–0.54) | <0.001 | 0.22 (0.09–0.52) | <0.001 |
| CFH (ref = T/T) | ||||
| C/T | 2.45 (1.32–4.59) | 0.005 | 3.37 (1.69–6.92) | <0.001 |
| C/C | 3.20 (1.24–7.92) | 0.013 | 3.67 (1.30–10.06) | 0.012 |
Due to previous cerebrovascular accident, stroke, or transient ischemic attack.
Discussion
Postoperative CD is a well-documented neurological phenomenon that occurs after a variety of vascular and nonvascular procedures, including cardiac bypass surgery,18,34 CEA,14,23 carotid stenting,9,35 carotid angioplasty, 32 diagnostic coronary angiography,19 and noncardiac surgery.26 The pathophysiology, incidence, and time course of CD vary depending on the specific surgery in question. Cognitive dysfunction associated with noncardiac surgery occurs in 25% of patients at 1 week postoperatively and 10% at 3 months and is associated with increasing age, duration of anesthesia, low education level, a second operation, postoperative infections, and respiratory complications.26 The incidence of CD following CEA has been reported to be 25% at 1 day after the procedure and 9% at 1 month, while more long-term reports of results at 1–5 years after treatment range from no change to improvement.5 Risk factors for CD following CEA include age greater than 75 years and diabetes,25,35 whereas statin use is associated with less CD.15
Cognitive dysfunction after CEA is thought to be, in part, due to a systemic surgery-induced inflammatory response that damages multiple organ systems, including the brain, through neuroinflammation mediated by cytokines including interleukin-1 and tumor necrosis factor-α.33 Cognitive dysfunction following CEA is also considered secondary to general anesthesia, transient hypotension, microemboli, and hyperperfusion following revascularization.
Multiple markers related to cardiovascular disease and inflammation have been associated with CD following CEA. Markers examined by our group that have been shown to correlate with CD include S100β,3 monocyte chemoattractant protein 1 (MCP-1),20 induced nitric oxide synthase (iNOS),36 APOE-E4,17 and matrix metalloproteinase 9 (MMP9).10 We have also identified specific SNPs that have correlated with CD. The presence of at least one APOE-E4 allele has been associated with increased risk of CD at 1 month after CEA. A SNP in the promoter region of the iNOS gene leading to increased function also correlated with less CD at 1 month. This mechanism may be related to cerebral preconditioning, permitting neurons and/or glia to withstand a temporary period of nonlethal ischemia.
Polymorphisms associated with the complement system were first implicated in CD by Gigante et al.,11 when they found that an activating SNP on C3a and a deactivating SNP on the complement inhibitor gene CFH were independently associated with CD 1 day following CEA. Other studies have been performed examining the role of complement genetics in acute cerebrovascular disease. In 135 stroke patients, an MBL2-activating SNP correlated positively with increased MBL2 levels and worse outcome at 3 months following ischemic stroke.2 In addition, Mocco et al.24 found that C3a and C5a are acutely elevated in the peripheral blood of acute stroke patients. Levels of C3 and C3a are elevated in cryptogenic and large-vessel–disease stroke,30 and a recent SNP study examining 16 SNPs at the C3 locus in 844 patients with ischemic stroke compared with 668 healthy community-based controls found a positive association with the SNPs rs2277984 and rs3745565 (OR 1.20, 95% CI 1.02–1.41, p = 0.02 and OR 0.73, 95% CI 0.59–0.92, p = 0.006, respectively). 27
In this study, we found that SNPs associated with multiple complement cascade components are associated with CD at 1 day, but not 1 month, after CEA. The less-active G allele of the C5 SNP rs17611 was found to decrease the likelihood of CD if at least one G allele was present. The homozygous A/A allele has been associated with elevated C5a serum concentration levels. Consistent with the findings in our study, increased C5a levels and the 2416AA C5a genotype have recently been associated with increased risk of cardiovascular disease29 and ischemic stroke.12 The C5a molecule is inflammatory and directly simulates adhesion molecule production and the generation of chemokines by endothelial cells, thereby promotion proinflammatory mechanisms on many cellular levels.4
CFH is a negative regulator of the alternative pathway of complement. The CFH Y402H polymorphism has been well studied in macular degeneration, with the C allele conferring increased risk.7 This allele has been correlated with increased complement activation and increased mortality after intracerebral hemorrhage, and it has been previously correlated with CD in a smaller population of patients treated with CEA.11 Here we have confirmed our earlier findings in this larger patient population. Recent basic science work has shown that the protective CFH allele protein product directly binds oxidized phospholipids, thereby decreasing systemic oxidative stress.28 More work is necessary to explore this proposed mechanism in CD.
In experimental stroke, the complement system is partially activated through the lectin pathway. MBL is a C-type collectin homologous to C1q that can activate the complement cascade through the lectin pathway. The SNP studied here is a polymorphism, with replacement of G by C in the promoter region of the MBL2 gene on chromosome 10 that leads to an X to Y amino acid change resulting in a low expression of MBL2 and reduced serum concentration in the range of a 25%–50% decrease per deleterious allele.21 Deficient MBL leads to a deficiency of immune defense and increased risk of various infections. 8,22 Although the univariate analysis suggested that the MBL2 GG genotype might correlate with CD, this result was not maintained in the multivariate analysis. We found the MBL2 C allele correlated with both age greater than 75 and statin use in the univariate analysis. We have previously found statin use to protect against CD following CEA for asymptomatic carotid artery stenosis,15 and this class of medications is currently under investigation as neuroprotective agents in a number of cerebrovascular conditions.6,31 Lower age in this group may have also been confounded by statin use, which may have delayed either the development of atherosclerosis or presentation with neurological symptoms.
This study was limited by a number of factors. First, all patients were enrolled at a single institution, and bias may have been introduced by patient selection or institutional standards regarding surgical or anesthetic technique. Second, given the small size of our cohort, additional studies are needed to validate, further characterize, and define the medical relevance of these SNPs. Third, the genetic contribution to CD was significant 1 day after CEA, but was not present 1 month after CEA, limiting the applicability of these results. However, it is reasonable to consider that patients with the high-risk genotypes may demonstrate an exaggerated inflammatory response to the surgical stimulus of CEA and maintain elevated proinflammatory cytokine levels as well as increased complement activation 1 day after surgery. This proinflammatory state likely resolves by the 1-month followup, and therefore, the association is no longer evident.
Conclusions
The activating C5 allele and a deactivating CFH allele both correlate positively with CD following CEA, whereas an activating MBL2 allele does not. This study further delineates the complement cascade as a detrimental participant in postoperative CD following carotid artery revascularization.
Acknowledgments
The authors thank Dr. Margaret Wood for her support of this study and Dr. Marielba Zerlin, the National Center for Advancing Translational Sciences, and KBioscience for their laboratory assistance.
Eric J. Heyer, E. Sander Connolly, and Joanna L. Mergeche were supported in part by National Institute on Aging grant R01 AG17604–9. This study was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR000040, formerly the National Center for Research Resources grant UL1 RR024156.
Abbreviations used in this paper
- CD
cognitive dysfunction
- CEA
carotid endarterectomy
- CFH
complement factor H
- C5
complement component 5
- iNOS
induced nitric oxide synthase
- MBL2
mannose-binding lectin 2
- MCP
monocyte chemoattractant protein
- MMP
matrix metalloproteinase
- OR
odds ratio
- PCR
polymerase chain reaction
- SNP
single-nucleotide polymorphism
Footnotes
Disclosure
None of the authors have conflicts of interest to disclose.
Author contributions to the study and manuscript preparation include the following. Conception and design: Heyer, Connolly. Acquisition of data: Heyer, Malone, Mergeche. Analysis and interpretation of data: Heyer, Kellner, Malone, Bruce, Mergeche, Connolly. Drafting the article: Heyer, Kellner, Mergeche. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Heyer. Statistical analysis: Heyer, Bruce. Administrative/technical/material support: Mergeche, Ward. Study supervision: Heyer, Connolly.
References
- 1.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 2.Cervera A, Planas AM, Justicia C, Urra X, Jensenius JC, Torres F, et al. Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke. PLoS ONE. 2010;5:e8433. doi: 10.1371/journal.pone.0008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connolly ES, Jr, Winfree CJ, Rampersad A, Sharma R, Mack WJ, Mocco J, et al. Serum S100B protein levels are correlated with subclinical neurocognitive declines after carotid endarterectomy. Neurosurgery. 2001;49:1076–1083. doi: 10.1097/00006123-200111000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czermak BJ, Lentsch AB, Bless NM, Schmal H, Friedl HP, Ward PA. Role of complement in in vitro and in vivo lung inflammatory reactions. J Leukoc Biol. 1998;64:40–48. doi: 10.1002/jlb.64.1.40. [DOI] [PubMed] [Google Scholar]
- 5.Czerny M, Schuch P, Sodeck G, Bálassy C, Hoelzenbein T, Juraszek A, et al. Sustained cognitive benefit 5 years after carotid endarterectomy. J Vasc Surg. 2010;51:1139–1144. doi: 10.1016/j.jvs.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 6.Elkind MS, Sacco RL, Macarthur RB, Peerschke E, Neils G, Andrews H, et al. High-dose lovastatin for acute ischemic stroke: results of the phase I dose escalation neuroprotection with statin therapy for acute recovery trial (NeuSTART) Cerebrovasc Dis. 2009;28:266–275. doi: 10.1159/000228709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangnon RE, Lee KE, Klein BE, Iyengar SK, Sivakumaran TA, Klein R. Effect of the Y402H variant in the complement factor H gene on the incidence and progression of age-related macular degeneration: results from multistate models applied to the Beaver Dam Eye Study. Arch Ophthalmol. 2012;130:1169–1176. doi: 10.1001/archophthalmol.2012.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garred P, Madsen HO, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet. 1995;346:941–943. doi: 10.1016/s0140-6736(95)91559-1. [DOI] [PubMed] [Google Scholar]
- 9.Gaudet JG, Meyers PM, McKinsey JF, Lavine SD, Gray W, Mitchell E, et al. Incidence of moderate to severe cognitive dysfunction in patients treated with carotid artery stenting. Neurosurgery. 2009;65:325–330. doi: 10.1227/01.NEU.0000349920.69637.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudet JG, Yocum GT, Lee SS, Granat A, Mikami M, Connolly ES, Jr, et al. MMP-9 levels in elderly patients with cognitive dysfunction after carotid surgery. J Clin Neurosci. 2010;17:436–440. doi: 10.1016/j.jocn.2009.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gigante PR, Kotchetkov IS, Kellner CP, Haque R, Ducruet AF, Hwang BY, et al. Polymorphisms in complement component 3 (C3F) and complement factor H (Y402H) increase the risk of postoperative neurocognitive dysfunction following carotid endarterectomy. J Neurol Neurosurg Psychiatry. 2011;82:247–253. doi: 10.1136/jnnp.2010.211144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greisenegger S, Zehetmayer S, Bauer P, Endler G, Ferrari J, Lang W, et al. Polymorphisms in inflammatory genes and the risk of ischemic stroke and transient ischemic attack: results of a multilocus genotyping assay. Clin Chem. 2009;55:134–138. doi: 10.1373/clinchem.2008.112151. [DOI] [PubMed] [Google Scholar]
- 13.Heyer EJ, Adams DC, Solomon RA, Todd GJ, Quest DO, Mc-Mahon DJ, et al. Neuropsychometric changes in patients after carotid endarterectomy. Stroke. 1998;29:1110–1115. doi: 10.1161/01.str.29.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heyer EJ, Gold MI, Kirby EW, Zurica J, Mitchell E, Halazun HJ, et al. A study of cognitive dysfunction in patients having carotid endarterectomy performed with regional anesthesia. Anesth Analg. 2008;107:636–642. doi: 10.1213/ane.0b013e3181770d84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyer EJ, Mergeche JL, Bruce SS, Ward JT, Stern Y, Anastasian ZH, et al. Statins reduce neurologic injury in asymptomatic carotid endarterectomy patients. Stroke. 2013;44:1150–1152. doi: 10.1161/STROKEAHA.111.000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyer EJ, Sharma R, Rampersad A, Winfree CJ, Mack WJ, Solomon RA, et al. A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59:217–222. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heyer EJ, Wilson DA, Sahlein DH, Mocco J, Williams SC, Sciacca R, et al. APOE-epsilon4 predisposes to cognitive dysfunction following uncomplicated carotid endarterectomy. Neurology. 2005;65:1759–1763. doi: 10.1212/01.wnl.0000184579.23624.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito A, Goto T, Maekawa K, Baba T, Mishima Y, Ushijima K. Postoperative neurological complications and risk factors for pre-existing silent brain infarction in elderly patients undergoing coronary artery bypass grafting. J Anesth. 2012;26:405–411. doi: 10.1007/s00540-012-1327-4. [DOI] [PubMed] [Google Scholar]
- 19.Kim IC, Hur SH, Park NH, Jun DH, Cho YK, Nam CW, et al. Incidence and predictors of silent embolic cerebral infarction following diagnostic coronary angiography. Int J Cardiol. 2011;148:179–182. doi: 10.1016/j.ijcard.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 20.Mack WJ, Ducruet AF, Hickman ZL, Zurica J, Starke RM, Garrett MC, et al. Elevation of monocyte chemoattractant protein-1 in patients experiencing neurocognitive decline following carotid endarterectomy. Acta Neurochir (Wien) 2008;150:779–784. doi: 10.1007/s00701-008-1618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madsen HO, Garred P, Thiel S, Kurtzhals JA, Lamm LU, Ryder LP, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–3020. [PubMed] [Google Scholar]
- 22.Madsen HO, Videm V, Svejgaard A, Svennevig JL, Garred P. Association of mannose-binding-lectin deficiency with severe atherosclerosis. Lancet. 1998;352:959–960. doi: 10.1016/S0140-6736(05)61513-9. [DOI] [PubMed] [Google Scholar]
- 23.Mazul-Sunko B, Hromatko I, Tadinac M, Sekulić A, Ivanec Z, Gvozdenović A, et al. Subclinical neurocognitive dysfunction after carotid endarterectomy—the impact of shunting. J Neurosurg Anesthesiol. 2010;22:195–201. doi: 10.1097/ANA.0b013e3181d5e421. [DOI] [PubMed] [Google Scholar]
- 24.Mocco J, Wilson DA, Komotar RJ, Sughrue ME, Coates K, Sacco RL, et al. Alterations in plasma complement levels after human ischemic stroke. Neurosurgery. 2006;59:28–33. doi: 10.1227/01.NEU.0000219221.14280.65. [DOI] [PubMed] [Google Scholar]
- 25.Mocco J, Wilson DA, Komotar RJ, Zurica J, Mack WJ, Halazun HJ, et al. Predictors of neurocognitive decline after carotid endarterectomy. Neurosurgery. 2006;58:844–850. doi: 10.1227/01.NEU.0000209638.62401.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 27.Olsson S, Stokowska A, Holmegaard L, Jood K, Blomstrand C, Pekna M, et al. Genetic variation in complement component C3 shows association with ischaemic stroke. Eur J Neurol. 2011;18:1272–1274. doi: 10.1111/j.1468-1331.2011.03377.x. [DOI] [PubMed] [Google Scholar]
- 28.Shaw PX, Zhang L, Zhang M, Du H, Zhao L, Lee C, et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc Natl Acad Sci U S A. 2012;109:13757–13762. doi: 10.1073/pnas.1121309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speidl WS, Exner M, Amighi J, Kastl SP, Zorn G, Maurer G, et al. Complement component C5a predicts future cardiovascular events in patients with advanced atherosclerosis. Eur Heart J. 2005;26:2294–2299. doi: 10.1093/eurheartj/ehi339. [DOI] [PubMed] [Google Scholar]
- 30.Stokowska A, Olsson S, Holmegaard L, Jood K, Blomstrand C, Jern C, et al. Plasma C3 and C3a levels in cryptogenic and large-vessel disease stroke: associations with outcome. Cerebrovasc Dis. 2011;32:114–122. doi: 10.1159/000328238. [DOI] [PubMed] [Google Scholar]
- 31.Tapia-Perez JH, Rupa R, Zilke R, Gehring S, Voellger B, Schneider T. Continued statin therapy could improve the outcome after spontaneous intracerebral hemorrhage. Neurosurg Rev. 2013;36:279–287. doi: 10.1007/s10143-012-0431-0. [DOI] [PubMed] [Google Scholar]
- 32.Tedesco MM, Lee JT, Dalman RL, Lane B, Loh C, Haukoos JS, et al. Postprocedural microembolic events following carotid surgery and carotid angioplasty and stenting. J Vasc Surg. 2007;46:244–250. doi: 10.1016/j.jvs.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 33.Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dijk D, Jansen EW, Hijman R, Nierich AP, Diephuis JC, Moons KG, et al. Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: a randomized trial. JAMA. 2002;287:1405–1412. doi: 10.1001/jama.287.11.1405. [DOI] [PubMed] [Google Scholar]
- 35.Wasser K, Hildebrandt H, Gröschel S, Stojanovic T, Schmidt H, Gröschel K, et al. Age-dependent effects of carotid endarterectomy or stenting on cognitive performance. J Neurol. 2012;259:2309–2318. doi: 10.1007/s00415-012-6491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yocum GT, Gaudet JG, Lee SS, Stern Y, Teverbaugh LA, Sciacca RR, et al. Inducible nitric oxide synthase promoter polymorphism affords protection against cognitive dysfunction after carotid endarterectomy. Stroke. 2009;40:1597–1603. doi: 10.1161/STROKEAHA.108.541177. [DOI] [PMC free article] [PubMed] [Google Scholar]