Abstract
Folates are essential for life and folate deficiency contributes to a host of health problems including cardiovascular disease, fetal abnormalities, neurologic disorders, and cancer. Antifolates, represented by methotrexate, continue to occupy a unique niche among the modern day pharmacopoeia for cancer along with other pathologic conditions. This review focuses on the biology of the membrane transport system termed the “reduced folate carrier” or RFC with a particular emphasis on RFC structure and function. The ubiquitously expressed RFC is the major transporter for folates in mammalian cells and tissues. Loss of RFC expression or function portends potentially profound physiologic or developmental consequences. For chemotherapeutic antifolates used for cancer, loss of RFC expression or synthesis of mutant RFC protein with impaired function results in antifolate resistance due to incomplete inhibition of cellular enzyme targets and low levels of substrate for polyglutamate synthesis. The functional properties for RFC were first documented nearly 40 years ago in murine leukemia cells. Since 1994, when RFC was first cloned, tremendous advances in the molecular biology of RFC and biochemical approaches for studying the structure of polytopic membrane proteins have led to an increasingly detailed picture of the molecular structure of the carrier, including its membrane topology, its N-glycosylation, identification of functionally and structurally important domains and amino acids, and helix packing associations. Although no crystal structure for RFC is yet available, biochemical and molecular studies, combined with homology modeling, based on homologous bacterial Major Facilitator Superfamily transporters such as LacY, now permit the development of experimentally testable hypotheses designed to establish RFC structure and mechanism.
Keywords: reduced folate carrier, folate, antifolate, membrane transport
I. Introduction
Folate is the generic term for water-soluble members of the B class of vitamins that are required for normal tissue growth and development. Folic acid is the synthetic form of the metabolically important folates found in cells that differ in the level of oxidation of the pteridine ring, the nature of the one-carbon substituent at the N5 and N10 positions, and the extent of γ glutamate conjugation (Stokstad, 1990). The biological importance of reduced folates derives from their essential roles in one-carbon transfer leading to thymidylate, purine nucleotides, serine, and methionine, and in biological methylation reactions from S-adenosylmethionine (Stokstad, 1990).
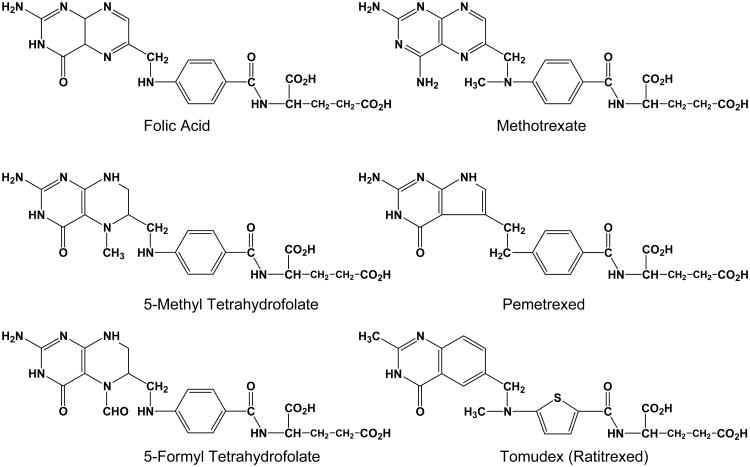
Folates are hydrophilic molecules that are anions at physiologic pH and thus cross biological membranes only poorly by diffusion. Reflecting this, mammalian cells have evolved sophisticated uptake systems for facilitating cellular uptake of folate cofactors (Matherly and Goldman, 2003). The reduced folate carrier (RFC) or SLC19A1 is expressed ubiquitously and is recognized to be the major transport system for folates in mammalian cells and tissues (Matherly and Goldman, 2003). In addition to its generalized role in folate transport, RFC performs certain specialized tissue functions including absorption across intestinal/colonic epithelia (Chiao et al., 1997; Said, 2004; Balamurugan and Said, 2006), transport across the basolateral membrane of renal proximal tubules (Kneuer et al., 2005), transplacental transport of folates (Sweiry and Yudlievich, 1985), and folate transport across the blood-brain barrier (Spector and Johanson, 2006). Reflecting these important physiological roles, low levels of RFC can be envisaged to contribute to a number of pathophysiologic states associated with folate deficiency, including cardiovascular disease, fetal abnormalities, neurologic disorders, and possibly cancer (Matherly, 2004). Susceptibilities to these conditions could be exacerbated with folate deficiency. Importantly, membrane transport by RFC is also important for the antitumor activities of antifolate therapeutics used for cancer chemotherapy such as methotrexate (MTX) and pemetrexed (Matherly et al., 2007) (Figure 1).
Figure 1. Transport substrates for the reduced folate carrier.
Structures are shown for folate and antifolate substrates for RFC.
This review focuses on the biology of the RFC with a particular emphasis on RFC structure and function. The functional properties for RFC were first documented nearly 40 years ago in murine leukemia cells (Goldman et al., 1968). However, it is only since the cloning of the rodent RFCs in 1994 (Dixon et al., 1994; Williams et al., 1994) and the human RFC (hRFC) in 1995 (Moscow et al., 1995; Williams et al., 1995; Wong et al., 1995; Prasad et al., 1995), and the application of molecular biology approaches to engineer RFC for biochemical studies, that a detailed picture of the molecular structure of this physiologically important carrier has emerged, including its membrane topology, its N-glycosylation, and identification of functionally and structurally important domains and amino acids. In this chapter, we review the considerable progress in this area, drawing significantly from the literature for RFC since 1994, along with structural inferences from structurally homologous bacterial Major Facilitator Superfamily (MFS) members for which crystal structures were recently reported (Abramson et al., 2003; Huang et al., 2003; Yin et al., 2006).
II. The Major Facilitator Superfamily of Transporters
The MFS family of transporters is represented in animals, plants, fungi, lower eukoryotes, bacteria, and eukaryotic organelles and transports a diverse assortment of substrates in a uniport, symport, or antiport fashion, including amino acids, neurotransmitters, sugars, vitamins, nucleosides, and organic phosphates (Saier et al., 1999). The MFS includes over 2000 sequenced members (Chang et al., 2004), making it the largest secondary active transporter family. MFS proteins typically contain 400-600 amino acids and a structural motif composed of 2 halves, each half composed of 6 transmembrane-spanning α helixes connected by a large hydrophilic loop, with cytosolic N- and C-termini.
Protein structural information is a prerequisite for understanding the mechanism of membrane transport. It is remarkable that, given their abundance and biological importance, there is a paucity of structural information on the MFS proteins. This in part reflects difficulties in isolating sufficient quantities of purified proteins and in crystallizing membrane proteins for x-ray diffraction. Accordingly, structural insights have relied on an extensive array of sophisticated biochemical and biophysical approaches, along with homology structural modeling from the crystal structures that have been reported.
To date, x-ray crystal structures have been reported for 4 MFS proteins. The first structural evidence approaching atomic resolution was for the oxalate transporter (OxlT) at 6.5 Å, generated by cryo-electron microscopy (Hirai et al., 2002, 2003). In 2003, x-ray crystallographic structures of the MFS proteins, the lactose/proton symporter (LacY) (Abramson et al., 2003) and the inorganic phosphate/glycerol-3-phosphate antiporter (GlpT) (Huang et al., 2003), were reported at resolutions of 3.5 Å and 3.3 Å, respectively. In 2006, a crystal structure of the E. coli multidrug transporter EmrD was reported (Yin et al., 2006). For GlpT, LacY, and OxlT, the proteins were crystallized in cytoplasmic orientations, whereas EmrD appears as an intermediate conformation.
In spite of their very different substrates and mechanisms, and limited sequence homologies, the overall structures of these four MFS transporters are quite similar. In some cases, most notably LacY (Kaback, 2005), the structure data corroborates an abundance of biochemical and biophysical data. All four MFS structures exhibit symmetrical structures in which two bundles of 6 helices surround a large central cavity. In both the GlpT and LacY structures, hydrophilic cavities accommodate the substrate-binding sites formed by helices-I, -II, -IV and -V of the N-terminal domain, and helices-VII, -VIII, -X and -XI of the C-terminal domain (Abramson et al., 2003; Huang et al., 2003). Helices III, VI, IX, and XII do not directly participate in substrate binding and are embedded in the lipid bilayer. The helices that form the hydrophilic cavity are irregular in that they contain numerous proline and glycine residues, likely permitting structural flexibility.
While no crystal structures are available, mammalian MFS proteins have also been studied by biochemical methods and modeling based on the GlpT and LacY structures. Examples include the human glucose transporter (GLUT1) (Hruz and Mueckler, 2001; Salas-Burgos et al., 2004), the human glucose-6-phosphate transporter (Fiser and Sali, 2003; Almqvist et al. 2004), the rat organic cation transporter-1 (OCT1) (Popp et al., 2005), the rabbit organic cation transporter-2 (OCT2) (Zhang et al. 2005), and, as described below, hRFC (Hou et al., 2006; Matherly et al., 2007).
Biochemical and structural data with LacY were used to argue for a model for lactose/H+ symport in which the hydrophilic substrate binding cavity is alternately accessible to either side of the membrane (“alternating access model”) (Abramson et al., 2003). Direct support for this model was recently described in studies in which almost every residue of Lac Y was replaced individually with cysteine and tested for reactivity was N-ethyl maleimide (Kaback et al., 2007). In this report, alkylation of substituted cysteine residues with NEM was alternately increased on the periplasmic side of the LacY sugar binding site in the presence of ligand, accompanying decreased reactivity on the cytoplasmic side, consistent with a model in which the sugar binding site is alternately exposed to either side of the membrane during transport (Kaback et al., 2007). Similar models were proposed for GlpT (Huang et al., 2003) and EmrD (Yin et al., 2006).
III. Folate Transport in Tissue Folate Homeostasis and Physiology—Role of Multiple Transport Systems for Folate Uptake and Efflux
Mammalian cells cannot synthesize folates de novo so these derivatives must be acquired from foods. Although folates are absorbed throughout the intestine, absorption occurs primarily in the duodenum and upper jejunum. Excellent sources of folate include orange juice, liver, dried beans and peas, dark green leafy vegetables, and strawberries. A proton-coupled folate transporter (PCFT; SLC46A1) has been implicated as the major transport system at the acidic pH in the upper small intestine (Qiu et al., 2006). However, the expression of RFC throughout the intestine (Chiao et al., 1997; Wang et al., 2001; Said, 2004; Balamurugan and Said, 2006) implies its contribution to intestinal folate uptake as well, particularly in the lower intestine. Folates are also absorbed by RFC in the colon and monoglutamyl folate synthesized by the intestinal microflora can be nutritionally significant (Rong et al., 1991). Following intestinal absorption, folates are transported across the basolateral membrane [via the multidrug resistance-associated proteins (MRPs) 1 and 3] of enterocytes (Mutch et al., 2003) and delivered via the hepatic portal system to the liver, where they are stored as polyglutamates. Folates are eventually released from the liver (primarily 5-methyl tetrahydrofolate monoglutamate) into the bloodstream, whereupon they are transported via specific transport systems into peripheral tissues.
Specific transport systems for folates include RFC and PCFT, both of which are reported to be widely expressed (Whetstine et al. 2002; Qiu et al. 2006; Zhao and Goldman, 2007), but are functionally distinct in that transport by RFC occurs optimally at neutral pH (∼7.4) whereas transport by PCFT is optimal at acidic pH (∼5.5-6.5) (Matherly and Goldman, 2003; Zhao and Goldman, 2007). Other uptake systems include the high affinity folate receptors (FRs) (α and β), glycosyl-phosphoinositol-linked proteins that accumulate folates via an endocytotic process (Matherly and Goldman, 2003; Salazar and Ratnam, 2007), and the organic anion transporters (OATs) that are expressed in epithelial tissues such as kidney and transport organic anions in addition to folates (Matherly and Goldman, 2003; Zhou et al., 2007; Miyazaki et al., 2004) such as bromosulfopthalein, taurocholate, and probenecid.
Renal tubular secretion and reabsorption of folates in proximal tubules involves specific roles for folate transporters on the basolateral (e.g., OAT1, OAT3, RFC) and apical (e.g., OATP1, FR α, MRP2, MRP4) membranes (Russel et al., 2002; Nozaki et al., 2004). Folates are filtered via the glomerulus and reabsorbed by a FR α-mediated endocytotic process, then transported into the bloodstream by folate transporters localized to the basolateral membrane. Both FR α and RFC are also involved in transplacental transport of folates (Sweiry and Yudlievich, 1985; Barber et al., 1999).
The choroid plexus separates the blood compartment from the cerebral spinal fluid (CSF). 5-Methyl tetrahydrofolate is typically present in CSF at approximately 4-times the concentration found in plasma (Spector and Lorenzo, 1975). FRα is localized to the basal (blood) side of choroid plexus and likely mediates uptake of folates from blood (Spector and Lorenzo, 1975; Suleiman and Spector, 1981). RFC is localized on the apical membrane of the bulbous microvili of the choroid plexus epithelium adjacent to the ventricular membrane (Wang et al., 2001), suggesting its role in transporting folates into the CSF at the apical surface. The recent finding that at least some cases of hereditary folate maladsorption syndrome are accompanied by low levels of central nervous system and plasma folates and a loss of functional PCFT (Qui et al., 2006), suggests a role of PCFT in CNS folate transport along with those of FR α and RFC. The possibility that RFC may also contintribute to hereditary folate maladsorption syndrome has been suggested (Said, 2004). Interestingly, RFC was detected in axons and dendrites, and on the apical membrane of the spinal canal (Wang et al., 2001), suggesting that this carrier is an important mode of folate transport in neuronal cells.
For mice in which RFC was inactivated by targeted homologous recombination (“knockout mice”), RFC was obligatory for development since targeting both alleles was embryonic lethal (Zhao et al., 2001b). However, approximately 10% of RFC-null mice could be brought to live birth by supplementing the dams with folic acid. These mice subsequently died within one or two weeks due to the failure of hematopoietic organs such as the bone marrow, thymus, or spleen (Zhao et al., 2001b), consistent with in vitro immunohistochemistry detection of RFC in the red pulp of the spleen (Wang et al., 2001).
RFC knockout mice have begun to provide insights into the possible role of RFC in cancer etiology. For instance, APCmin/+ mice in which one copy of RFC was inactivated developed significantly fewer intestinal adenomas than mice with two functional RFC alleles (Lawrance et al., 2007). Conversely, ablation of one RFC allele in mice decreased plasma S-adenosyl methionine/S-adenosyl homocysteine, increased colonocyte proliferation, increased transcripts for colon cancer-related genes (e.g., Cdh1, Cdx1, Igf2, and Ptgs2) regulated by methylation, and increased susceptibility to carcinogen (azoxymethane), as reflected in the numbers of aberrant crypt foci in colon (Ma et al., 2005). While it is not easy to reconcile the seemingly disparate findings of these reports, they presumably reflect the model system (i.e., carcinogen-treated versus genetically modified Apcmin/+ mouse) or the localization of the tumors (colon versus intestine). Regardless, these results raise the intriguing possibility that levels of RFC can profoundly impact the neoplastic process.
Thus, while the RFC is ubiquitously expressed and plays an integral role in in vivo folate homeostasis and tissue-specific folate transport, this is often in concert with other folate transport systems such as PCFR and/or high affinity FR α. Altered RFC levels and function could easily exacerbate effects of dietary folate deficiency, thereby contributing to cardiovascular disease, fetal abnormalities, neurodegenerative disease, and cancer (Matherly, 2004). Alterations in folate membrane transport by RFC may be further compounded by gene polymorphisms that result in changes in the catalytic activities of folate-dependent interconverting and biosynthetic enzymes such as 5,10-methylene tetrahydrofolate reductase (MTHFR) that impact the intracellular distribution of individual reduced folate forms (Matherly, 2004). The recent generation of a “humanized” mouse in which the hRFC gene locus has replaced the mouse RFC gene (Patterson et al., 2008) should provide an opportunity to study the regulation and function of hRFC in relation to folate homeostasis and polymorphisms in hRFC or other critical genes, and in response to dietary interventions with folic acid.
IV. Role of RFC in Antifolate Chemotherapy
MTX continues to be an important component of the chemotherapeutic arsenal for a number of cancers including pediatric acute lymphoblastic leukemia, osteogenic sarcoma, lymphoma, and breast cancer (Monahan et al., 2001). Raltitrexed is used throughout much of the world outside of the US for advanced colorectal cancer (Chu et al., 2002). In 2004, pemetrexed was approved for pleural mesothelioma in the US (Hazarika et al., 2004), and subsequently as a second line treatment for non-small cell lung cancer (Cohen et al., 2005). MTX has found other clinical applications including treatment of autoimmune diseases and psoriasis (Giannini et al., 1992; Chladek et al., 1998).
These “classical” antifolates are all excellent substrates for cellular uptake by RFC (Goldman and Matherly, 1985; Jansen, 1999; Goldman and Zhao, 2002; Matherly et al., 2007). Membrane transport of antifolates such as MTX is critical to drug activity since sufficient intracellular drug is required to sustain suppression of enzyme targets and to support synthesis of polyglutamates required for high affinity inhibition of intracellular enzymes and sustained drug effects as plasma drug levels decline (Goldman and Matherly, 1985). Not surprisingly, impaired active transport of MTX has been identified as an important mechanism of MTX resistance (Goldman and Matherly, 1985; Zhao and Goldman, 2003; Matherly et al., 2007). Impaired MTX transport was reported as early as 1962 in MTX resistant L5178Y mouse leukemia cells (Fischer, 1962). Decreased MTX transport by RFC has been reported in cultured murine and human tumor cells selected in vitro with antifolate (in some cases with prior exposure to carcinogen) (Schuetz et al., 1988; Zhao et al., 1998a,b; Zhao et al., 1999; Roy et al., 1998; Jansen et al., 1998; Gong et al., 1997; Sadlish et al., 2000; Rothem et al., 2002, 2003; Wong et al., 1999; Drori et al., 2000), and in vivo in MTX resistant murine leukemia cells from mice treated with MTX chemotherapy (Sirotnak et al., 1981). In 42 primary osteosarcoma samples from patients who experienced poor responses to chemotherapy including MTX, 65% showed low level RFC expression (Guo et al., 1999). Similarly, in primary acute lymphoblastic leukemia, low levels of RFC were associated with a poor prognosis (Gorlick et al., 1997; Levy et al., 2003; Ge et al., 2007).
The MTX resistant phenotype is frequently complex and involves elevated or kinetically altered dihydrofolate reductase, and/or decreased synthesis of MTX polyglutamates in addition to impaired MTX transport (Goldman and Matherly, 1985; Zhao and Goldman, 2003). Impaired transport that results in a loss of sensitivity to standard doses of antifolate should, at least in part, be circumvented by increasing extracellular concentrations of drug. This forces the drug into tumor cells expressing mutated or low levels of RFC, and involves alternate uptake routes and/or passive diffusion, to a sufficient extent to inhibit intracellular enzymes and/or to support antifolate polyglutamate synthesis. However, a point is often achieved for which these elevated extracellular antifolate concentrations are no longer capable of significantly increasing intracellular drug levels. This is due to the saturability of RFC, electrical restrictions on net drug accumulation, and the presence of high capacity efflux pumps such as the MRPs. Thus, relatively small increases in intracellular target enzymes or decreased levels of antifolate uptake can result in a requirement for intracellular drug that cannot be achieved clinically.
V. Functional Properties of RFC
Detailed functional studies of RFC transport date back to the late 1960s (Goldman et al., 1968; Sirotnak et al., 1968) and have included a wide range of both rodent and human (mostly tumor) cell lines in culture (Sirotnak, 1985). Whereas folates such as 5-methyl or 5-formyl tetrahydrofolate (leucovorin), and classical antifolates such as MTX, pemetrexed, and raltitrexed are all RFC substrates (Figure 1), most functional studies have used radioactive MTX as a surrogate substrate. This reflects its commercial availability and efficient unidirectional transport over short intervals (due to its rapid and tight binding to intracellular dihydrofolate reductase) (Goldman et al., 1968). Further, in contrast to reduced folates such as 5-formyl tetrahydrofolate, MTX is not appreciably metabolized over the short intervals used for assaying transport. At steady state, bound intracellular MTX can be easily distinguished from free intracellular drug by simple efflux into MTX-free media (Goldman et al., 1968). This permits calculation of transmembrane gradients and uphill transport from the membrane potentials.
For most reduced folate and many antifolate substrates for RFC, uptake is saturable at low micromolar concentrations (Kt∼1-5 μM) (Goldman and Matherly, 1985; Sirotnak, 1985; Jansen, 1999; Matherly and Goldman, 2003). Folic acid is generally a poor substrate for RFC (Kt >100 μM) in physiologic buffers. RFC transport is not stereospecific for 5-methyl tetrahydrofolate (Sirotnak and Donsbach, 1974; White et al., 1978). Since leucovorin is racemic mixture of (6R) and (6S) stereoisomers of 5-formyl tetrahydrofolate, it is of interest that the (6R) isomer of 5-formyl tetrahydrofolate has a far lower affinity than the natural (6S) stereoisomer (Sirotnak et al., 1979). A benzoquinazoline antifolate, GW1843U89, is a surprisingly poor substrate for the murine RFC (Vmax/Kt =0.25) and one of the best known substrates for the human carrier (Vmax/Kt=20.3) (Duch et al., 1993). This is the only example of a substantial substrate disparity between the rodent and human RFCs.
A consistent feature of RFC substrates is their anionic character (Figure 1). For folates, the glutamate is of particular significance in that its α and γ carboxyl groups are ionized at physiologic pH, thus limiting diffusion across biological membranes. For transport by RFC, modifications of the glutamic acid (e.g., 2-amino-4-phosphonobutanoic acid, L-homocysteic acid, ornithine) are generally not well tolerated (Westerhof et al., 1995). However, modifications of the glutamate γ carboxyl group in the antifolates ZD9331 and PT523 are extremely well tolerated (Jansen, 1999). Notably, γ-glutamyl conjugated polyglutamyl folates are not substrates for RFC. Interestingly, for a series of diamino furo[2,3-d]pyrimidine antifolates with substituted (e.g., methyl) α or γ carboxyl groups, analogs with only single α carboxyl and no γ carboxyl were potent inhibitors of MTX transport by hRFC, whereas analogs with only γ carboxyl group but no α carboxyl group were poor inhibitors (Deng et al., 2007). This indicates that the α carboxyl group is essential for binding to hRFC.
Transport by RFC is temperature-dependent and sodium-independent (Goldman and Matherly, 1985; Sirotnak, 1985; Jansen, 1999; Matherly and Goldman, 2003). Although a neutral pH appears to be optimal for RFC transport in leukemia cells (Goldman et al., 1968), in prostate carcinoma (Horne and Reed, 2001) and intestinal epithelial cells (Chiao et al., 1997; Balamurugan et al., 2006) an acidic pH optimum (pH 5.5-6.5) for RFC transport was reported. This low pH transport activity was accompanied by altered specificity for certain substrates (e.g., folic acid). Very recent studies suggest that the low pH transport of (anti)folate substrates in intestine is likely due to expression of PCFT rather than RFC (Qiu et al., 2006; Zhao and Goldman, 2007).
RFC is an anion transporter and thus is highly sensitive to its anionic environment. For instance, replacing anionic buffers with non-anionic Hepes-sucrose buffers results in concentrative MTX uptake (Henderson and Zevely, 1983a). This is due to a decreased competition for binding to RFC in the absence of anions, since MTX influx via RFC is competitively inhibited by inorganic anions such as chloride, bicarbonate, or phosphate in physiological buffers. Likewise, transport is inhibited by structurally diverse organic anions such as adenine nucleotides and thiamine phosphates (Goldman, 1971a).
In studies with membrane vesicles loaded with sulfate or phosphate anions, uptake of MTX in the “trans” compartment was dramatically stimulated via a counter-transport mechanism (Yang et al., 1984). Similarly, influx of radiolabeled MTX by RFC is significantly enhanced (“trans-stimulated”) in cells preloaded with high concentrations of 5-formyl or 5-methyl tetrahydrofolate (Goldman, 1971a, b). In anion-free buffers without glucose, the rate of MTX efflux from cells is inhibited but can be stimulated with both inorganic and organic anions (e.g., folic acid, 5-formyl tetrahydrofolate, AMP, ADP, thiamine pyrophosphate, phosphate, sulfate, and chloride) (Henderson and Zevely, 1980, 1981, 1983b). The anion concentrations required for half-maximal stimulation of efflux were similar to their Ki values for inhibition of influx by RFC.
Thus, large electrochemical anion gradients accelerate the movement of RFC within the plasma membrane and are likely to provide the driving force for the concentrative uptake of folate substrates by the carrier. Most probably, this involves an extrusion of intracellular organic anions into the extracellular medium and down a concentration gradient that somehow drives uptake of folate substrates into cells. Consistent with this model are recent studies with phosphorylated derivatives of thiamine that show that these anionic species are good substrates for RFC in murine L1210 cells, and that efflux of these forms is dramatically enhanced in cells with increased expression of RFC (Zhao et al., 2001a). However, neither the identify of the actual physiologic counter-anion(s), the binding sites for dianionic folate substrates and the putative transport counter-anion(s), nor the mechanism by which the bidirectional fluxes are coupled are firmly established (see below).
As described above, RFC shows a striking structural homology to bacterial transporters of the MFS for which crystal structures have recently been reported [lactose/proton symporter (LacY) (Abramson et al., 2003); inorganic phosphate/glycerol-3-phosphate antiporter (GlpT) (Huang et al., 2003); multidrug transporter EmrD (Yin et al., 2006)]. By analogy with these bacterial proteins, transport of folates by RFC into mammalian cells would be expected to involve a physical movement of the carrier within the plasma membrane accompanied by alternate accessibility of the aqueous substrate-binding cavity to the intra- and extracellular sides of the plasma membrane. For RFC, as noted above, this is driven by extrusion of anions down a large (intra- to extracellular) concentration gradient. As described below, recent studies have begun to explore the three-dimensional structure of the hRFC molecule including identification of substrate binding residues and the transmembrane translocation pathway for anionic folates and antifolates (Hou et al., 2005, 2006).
VI. Biochemistry of RFC
The low levels of RFC in most tissues and mammalian cell lines for many years limited its characterization mostly to functional kinetic assays of folate and antifolate uptake. However, commencing in the early 1980s, a number of novel biochemical strategies were developed to identify and study the RFC protein that would subsequently facilitate its cloning and structural characterization.
In 1984, Sirotnak and coworkers developed a strategy to select L1210 murine leukemia cells with upregulated RFC (Sirotnak et al., 1984). Selection was based on the notion that carrier-mediated uptake of reduced folate cofactors is rate-limiting to their utilization in biosynthetic reactions and involved growing cultures in folate-free culture medium with growth-limiting concentrations of leucovorin. Under these conditions, only cells that exhibited enhanced capacities for reduced folate transport were capable of sustained growth. Analogous RFC transport-upregulated K562 (Matherly et al., 1991), HL60 (Yang et al., 1992), and CCRF-CEM (Jansen et al., 1990) leukemia sublines were generated by selection under folate-limiting growth conditions.
Another key advance involved development of approaches for identifying and quantitating low levels of RFC protein. Specific binding of radiolabeled RFC substrates (5-methyl tetrahydrofolate, methotrexate, or aminopterin) to surface RFC at 0° C (corresponding to the difference between bound ligand in the absence and presence of high concentrations of a competing unlabeled ligand) in intact cells provides an overall estimate of RFC levels (Henderson et al., 1980a). However, it was not until development of affinity ligands for covalently modifying the carrier, that the molecular characteristics of the RFC protein could be studied. A number of RFC affinity reagents have been reported including 8-azidoadenosine-5′-monophosphate (Henderson et al., 1979), 4,4′-diisothiocyanostilbene-2,2′-disulfonate (Henderson and Zevely, 1982), carbodiimide-activated antifolates (Henderson et al., 1980b), 3,3′-dithiobissulfosuccinimidyl propionate (Jansen et al., 1989), N-hydroxysuccinimide (NHS) MTX ester (Herderson and Zevely, 1984), and Nα-(4-amino-4-deoxy-10-methylpteroyl)-Nε-4-azido-5-salicylyl)-L-lysine (APA-ASA-Lys) (Freisheim et al., 1992). Whereas treatment of cells with all of these reagents irreversibly inhibited 3H-MTX uptake by RFC, only APA-ASA-Lys and NHS-MTX showed the sensitivity and specificity needed for radio affinity labeling the carrier.
NHS esters of 3H-MTX and 3H-aminopterin have been used extensively for covalently labeling RFC (Henderson et al., 1984; Schuetz et al., 1988; Matherly et al. 1991; Yang et al., 1992; Witt et al., 2004; Hou et al., 2005) since these inhibitors are simple to prepare from NHS and commercially available radioactive antifolates. Both of these reagents show a relatively high specificity for RFC. In studies of transport-upregulated K562 human erythroleukemia cells (designated K562.4CF) treated with NHS-3H-MTX, tritium was incorporated into a broadly migrating ∼76-85 kDa band that was increased (∼7-fold) over parental K562 cells and could be completely blocked by unlabeled MTX or (6S) 5-formyl tetrahydrofolate, establishing specificity (Matherly et al., 1991). Additional experiments confirmed that radioaffinity labeled hRFC protein was glycosylated since treatment of K562.4CF plasma membranes with endo-β-galactosidase resulted in a shift to a substantially lower molecular mass (∼58 kDa). The mouse RFC from L1210 leukemia cells treated with NHS-3H-MTX or NHS-3H-aminopterin typically migrated on SDS gels or gel filtration (with 0.1% SDS) as a 42-48 kDa species (Schuetz et al., 1988; Yang et al. 1992). However, more recent studies with antibody to the mouse RFC identified a 58 kDa RFC protein (Zhao et al., 2000b), suggesting that the smaller molecular mass species must have arisen from proteolytic degradation of a larger RFC form. A very recent study with a series of classical diamino furo [2,3-d] pyrimidine analogs with methyl-substituted α and γ carboxyl groups established that the γ-carboxyl NHS ester of antifolate substrates is far more reactive with nucleophilic amino acid(s) than is the α carboxyl NHS ester (Y. Deng, A. Gangjee, and L.H. Matherly, unpublished observation). This is interesting since the α carboxyl group rather than the γ carboxyl group is essential for high affinity substrate binding to hRFC (Deng et al., 2007).
APA-125I-ASA-Lys is a radioiodinated photoaffinity ligand originally used for labeling dihydrofolate reductase (Price et al., 1986) that was subsequently adapted for labeling RFC (Freisheim et al., 1992; Wong et al., 1995). Ultraviolet activation of a reactive nitrene in APA-125I-ASA-Lys results in a covalent modification of proteins to which it is bound. The advantages of APA-125I-ASA-Lys over NHS-3H-MTX include its increased specificity for MTX-binding proteins, resulting from its decreased reactivity in the absence of ultraviolet irradiation, and its greater sensitivity, reflecting the incorporation of the 125I radionuclide rather tritium. Freisheim and coworkers also used APA-125I-ASA-Lys to label the ∼80-85 kDa glycosylated hRFC protein from transport-upregulated CCRF-CEM cells (Freisheim et al., 1992).
VII. Cloning of RFC cDNAs That Restore Transport To Transport-Impaired Cultured Cells
In 1994, a mouse RFC cDNA was isolated by expression cloning and found to restore MTX transport activity and sensitivity to transport-impaired ZR75-1 human breast cancer cells (Dixon et al., 1994). This was followed by a report from Flintoff and coworkers that a homologous hamster cDNA could restore MTX transport to transport-impaired Chinese hamster ovary (CHO) cells (Williams et al., 1994). By early 1995, there were 4 published reports on the characteristics of the homologous human cDNAs (Moscow et al., 1995; Prasad et al., 1995; Williams et al., 1995; Wong et al., 1995). An identical cDNA from human intestine was reported in 1997 (Nguyen et al., 1997).
These homologous cDNAs consistently restored in vitro antifolate sensitivities and transport properties typical of the endogenously expressed RFCs to transport-impaired cell lines. These include characteristic uptake patterns of radioactive folate and antifolate substrates, inhibition by known substrate competitors (e.g., 5-formyl tetrahydrofolate, raltitrexed), irreversible inhibition by NHS-MTX, and a capacity for trans-stimulation by preloading with reduced folates (Wong et al., 1995, 1997). For transport-impaired CHO or K562 cells transfected with hRFC cDNAs, a cDNA-encoded ∼85-92 kDa protein was detected by photoaffinity labeling with APA-125I-ASA-Lys (Wong et al., 1995, 1997) or antibodies raised to a peptide sequence predicted from the hRFC cDNA sequence (Wong et al., 1998). This species was deglycosylated with N-glycosidase F to ∼65 kDa, in close agreement with the predicted size of the full-length hRFC protein (64,873 daltons) and the size of the deglycosylated NHS-3H-MTX-labeled hRFC protein originally identified in transport-upregulated K562.4CF cells (Matherly et al., 1991).
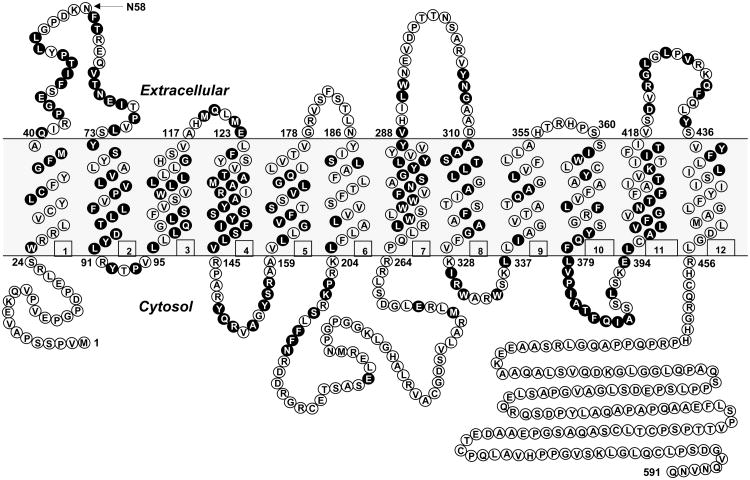
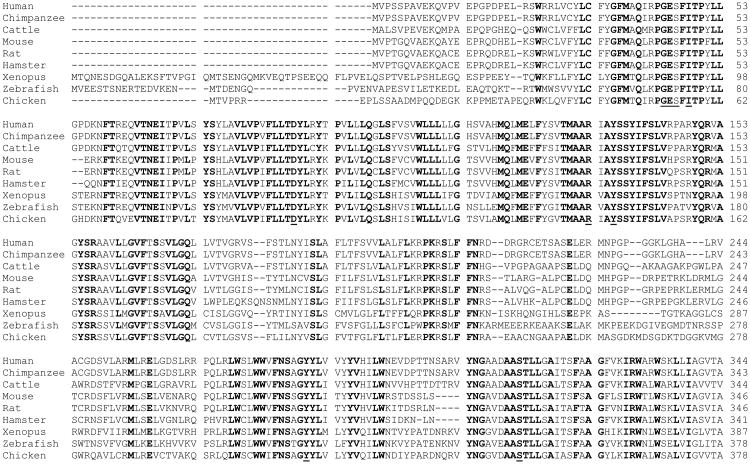
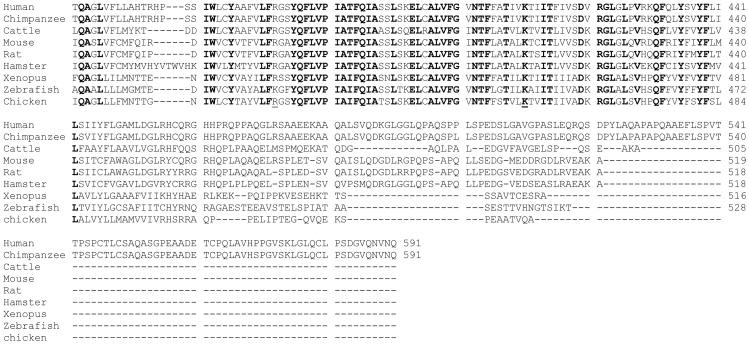
By hydropathy analysis of the predicted amino acid sequence for hRFC, the hRFC cDNA encoded a protein that conformed to a model expected for an integral membrane protein, with up to 12 stretches of mostly hydrophobic, α-helix-promoting amino acids, internally oriented N- and C-termini, an external N-glycosylation site at Asn58, and a large central loop domain connecting transmembrane domains (TMDs) 1-6 and 7-12 (Figure 2). Much of this topology structure has been confirmed experimentally (Ferguson et al., 1999; Liu et al., 2002; Cao and Matherly, 2004; Flintoff et al., 2003) (see below). The predicted amino acid sequence is reasonably conserved between species ranging from Xenopus laevis to mice and humans, with the highest homologies in the TMDs (Figure 3). Sequence homology is substantially decreased in the most Nor C-terminal regions and in the TMD6/TMD7 connecting loop domain. The RFC sequence for primates (humans, chimpanzee) includes 50-86 more amino acids than that for the other species (Figure 3).
Figure 2. Topologic model for hRFC showing conserved residues between 7 species.
Topology model for hRFC, depicting 12 TMDs, internally oriented N and C termini, an externally orientated N-glycosylation site at Asn-58 and a cytosolic loop connecting TMDs 6 and 7. Amino acids conserved between RFCs from different species as summarized in Figure 3 are depicted as black circles.
Figure 3. Species homologies for RFC proteins.
Genbank accession numbers are: Homo sapiens (human) NP 001069921; Pan troglodytes (chimpanzee) XP 001157360; Gallus gallus (chicken,) NP 001006513; Danio rerio (zebrafish) XP 687261; Bos taurus (cow)NP 001069921; Rattus norvegicus (Norway rat) NP 001030309; Cricetulus griseus (Chinese hamster) U17566; Mus musculus (mouse) NP 112473; Xenopus laevis (African clawed frog) NM 001092530.
Glycosylation at Asn58 is responsible for the aberrant migration of hRFC on SDS protein gels (Wong et al., 1998). However, N-glycosylation is not essential for RFC function since the murine RFC is not glycosylated. A transport competent ∼65 kDa hRFC protein was identified by western blots prepared from hRFC-null K562 cells transfected with an hRFC cDNA in which Asn58 was replaced by glutamine (Wong et al., 1998). When tagged with a carboxyl terminal hemagglutinin (HA) epitope (YPYDVPDYASL), Gln58 hRFC transport function was largely preserved, accompanying efficient plasma membrane targeting (Wong et al., 1998). These results suggest that the N-glycosylation of hRFC does not significantly alter either membrane targeting or transport function.
VIII. Topologic Structure of RFC
A. Topologic structure by hemagglutinin epitope accessibility and N-glycosylation scanning mutagenesis
The original report by Cowan and coworkers (Dixon et al., 1994) suggested that the mouse RFC amino acid sequence conformed to a 12 TMD structure, closely resembling that for other MFS proteins such as GLUT1 (Salas-Burgos et al., 2004). The demonstration of N-glycosylation at Asn58 for hRFC provided direct confirmation of an extracellular orientation for the loop domain connecting TMDs 1 and 2 (Figure 2). HA epitope accessibility methods, in which HA epitopes were inserted into the hRFC molecule followed by immunofluorescence detection with HA-specific antibody in the presence and absence of low levels of Triton X-100 (0.1%) were used to map the hRFC topologic structure (Ferguson et al., 1999; Liu et al., 2002). In these studies, intracellular orientations were confirmed by inserting HA epitopes into the hRFC N-terminus (Pro20, Gly17), the connecting loop between TMDs 6 and 7 (Ser225, Glu226), and TMDs 8 and 9 (Ala332). Since HA epitopes in the connecting loops between TMDs 3 and 4 (Gln120) and between TMDs 7 and 8 (Glu294, Pro297) were accessible to antibody without permeabilization, these likely had extracellular orientations. By N-glycosylation scanning mutagenesis, in which an N-glycosylation consensus sequence [NX(S/T)] is inserted into putative loop domains, followed by western blotting of functional constructs to confirm glycosylation status, the TMD 5/6 loop of hRFC was confirmed as having an extracellular orientation (Liu et al., 2002).
B. Topologic structure by scanning cysteine accessibility methods (SCAM)
The development of sulfhydryl reagents with reactivities amenable to use with intact cells, typified by the alkylthiosulfonates and maleimides, has revolutionized the structural analysis of membrane-spanning ion channels and membrane transporters (Karlin and Akabas, 1998; Frillingos et al., 1998). Thus, by inserting cysteine residues into functional “cysteine-less” membrane proteins and treating with cysteine-active reagents, it is possible to establish aqueous accessibilities and, by inference, determine membrane topologies (Loo and Clarke, 1995; Nicoll et al., 1999; Hu and Kaplan, 2000), identify amino acids that line the transmembrane translocation pathway (Loo and Clarke, 2000; Dodd and Christie, 2001; Slotboom et al., 2001), and confirm the spacial relationships between domains (Zeng et al., 1999; Kwaw et al., 2001; Loo and Clarke, 2001).
In hRFC, there are 11 cysteine residues. A functional “cysteine-less” hRFC was generated by deleting 56 carboxyl-terminal amino acids including 4 cysteines (Cys546, 549, 563, and 580) and replacing the remaining 7 cysteines (Cys30, 33, 220, 246, 365, 396, and 458) with serines (Cao and Matherly, 2003). A Myc-His6 epitope was added to the truncated carboxyl terminus and single-cysteine hRFC mutants, including Ser301Cys (TMD7/8 loop), Ala332Cys (TMD8/9), Ser360Cys (TMD9/10), Ala388Cys (TMD10/11), Ser390Cys (TMD10/11), and Arg429Cys (TMD11/12) hRFCs, were expressed in RFC-null CHO cells (Cao and Matherly, 2004). Cells were treated with thiol-reactive biotin maleimide [3-(N-maleimidylpropionyl)biocytin] with or without membrane-impermeant stilbenedisulfonate maleimide (4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid). Plasma membrane proteins were solubilized, the hRFC-Myc-His6 immunoprecipitated, and the immunoprecipitates analyzed on western blots. Biotinylated thiols were detected with peroxidase-linked streptavidin. Consistent with their predicted extracellular orientations (Figure 2), Ser301Cys, Ser360Cys, and Arg429Cys hRFC mutants were all highly reactive with biotin maleimide and labeling was significantly blocked with membrane-impermeant stilbenedisulfonate maleimide. Whereas Ala388Cys and Ser390Cys mutants, located in the middle of the putative conserved TMD10-11 loop domain, were completely unreactive toward biotin maleimide, these positions (as well as Ala332Cys) were all labeled following permeabilization with Streptolysin O. An identical strategy was used by Flintoff et al. (2004) for hamster RFC to localize the connecting loops between TMD1/2 (Ser46), TMD4/5 (Ser152), TMD5/6 (Ser179), TMD6/7 (Cys224), TMD7/8 (Leu300), TMD 9/10 (Tyr355), and TMD11/12 (Lys430), and the C-terminal domain (Leu475).
Thus, the patterns of biotin maleimide reactivity and protection by stilbenedisulfonate maleimide, combined with previous findings of N-glycosylation at Asn58 and the results of HA epitope insertion and scanning glycosylation mutagenesis (see above), strongly support a 12 TMD topology structure for hRFC with cytosolic orientations for the N- and C-termini and TMD6/7 loop domain (Figure 2).
IX. Insights Into Structural and Functional Determinants of RFC From Studies of Mutant RFC Proteins
An important goal of RFC structure-function studies has been to identify amino acids and/or domains that contribute to binding and/or translocation of folate and antifolate substrates, along with TMD helix packing associations that facilitate folate substrate binding and translocation. For RFC, experimental approaches have included: (i) identifying mutant amino acids in RFC from drug resistant cells selected with antifolate drugs; (ii) targeting specific amino acids for site-directed mutagenesis based on amino acid charge or hydrophilic character, along with homology and membrane topology considerations; (iii) deletion and insertion mutagenesis of potentially functional domains; (iv) radioaffinity labeling with specific radiolabeled affinity inhibitors and identification of the labeled domain/amino acid by selective proteolysis and site-directed mutagenesis; (v) scanning cysteine accessibility methods (SCAM) with thiol reactive reagents; and (vi) protein cross-linking of cysteine-insertion hRFC mutants with homobifunctional thiol-reactive cross-linkers. These studies are described in the following sections.
A. Identification of structurally or functionally important amino acids by selecting for mutant RFCs with antifolate inhibitors
The largest concentration of RFC mutant studies has involved residues localized in or around TMD1, following initial reports of MTX resistant mutants with defective RFC including Glu45Lys (Zhao et al., 1998a) and Ser46Asn (Zhao et al., 1998b) (unless specifically noted, all numbers designate the positions in hRFC).
In the hRFC structure, Glu45 flanks TMD1 and is highly conserved among different species (Figure 3). The first description of the Glu45Lys mutant was in MTX resistant L1210 cells treated with N-methyl-N-nitrosourea and selected in the presence of MTX (Zhao et al., 1998a). This Glu45Lys-RFC phenotype included a global decline in carrier mobility, decreased Kts for folic acid and 5-formyl tetrahydrofolate, respectively, an unchanged Kt for 5-methyl tetrahydrofolate, and a markedly increased Kt for MTX. Virtually identical transport phenotypes were attributed to Glu45Lys hRFC in separate CCRF-CEM sublines selected for MTX resistance (Jansen et al., 1998; Gifford et al., 2002) and in a number of CCRF-CEM sublines selected for resistance to the benzoquinazoline antifolate, GW1843U89 (Drori et al., 2000). When Glu45 was systematically mutated by site-directed mutagenesis, all position 45 mutants were functional for MTX uptake; however, there were substantial differences in maximal transport rates (Vmax) for different substitutions. Increased affinities were measured for 5-formyl tetrahydrofolate and folic acid, accompanying some amino acid replacements (Gln, Arg) but not others (Asp, Leu, Trp) (Zhao et al., 2000c). This implies that amino acid size rather than the charge at position 45 of RFC is the most important determinant of RFC substrate specificity. For certain substrates such as 5-formyl tetrahydrofolate, MTX, or folic acid, adverse effects of particular amino acid replacements on binding affinities were disproportionate to those seen when 5-methyl tetrahydrofolate was used as transport substrate. Although these mutagenesis data do not convincingly argue for a direct role of Glu45 in substrate binding, they, nonetheless, imply that position 45 somehow plays a role in transport. The role of the exofacial stretch of amino acids flanking Glu45 is further considered below.
Ser46 was also suggested to be critical to RFC function, since replacement of this amino acid with asparagine in MTX resistant L1210 cells resulted in a decreased rate of carrier mobility (Vmax) with MTX but no change in MTX Kt (Zhao et al., 1998b). The Vmax effect was substantially greater for MTX than for reduced folates such as 5-formyl tetrahydrofolate or 5-methyl tetrahydrofolate. Further evidence of a functionally important role of Ser46 involves detection of Ser46Ile in CCRF-CEM cells selected for resistance to the benzoquinazoline antifolate GW1843U89 (Drori et al., 2000), and of Ser46Asn in a primary osteosarcoma specimen treated with MTX (Yang et al., 2002).
Gly44Arg was identified in MTX resistant CCRF-CEM T-cell acute lymphoblastic leukemia cells by Wong et al. (1999) and was associated with an 11-fold increased Kt for MTX. The same mutation was reported in a separate CCRF-CEM subline selected for resistance to the hemiphthaloyl ornithine antifolate, PT523 (Drori et al., 2000). An Ile48Phe mutation was detected (in combination with Trp105) in mouse RFC from L1210 cells selected for resistance to 5,10-dideazatetrahydrofolate (Tse et al., 1998). Ile48Phe was associated with a marked increase in the accumulation of folic acid, due to a selective decrease in the Kt for folic acid compared with 5,10-dideazatetrahydrofolate, resulting and an expansion of cellular folate pools.
Mutant studies in murine cells have implicated amino acids localized to other TMDs as functionally or structurally important to RFC function. These include Ser309 (Ser313 in hRFC) and Ser297 (not conserved in hRFC) in TMD8 (Zhao et al., 1999; Roy et al., 1998), Val104 (Val106 in hRFC) (Zhao et al. 2000a) and Trp105 (Trp107 in hRFC) (Tse et al., 1998) in TMD3, and Ala130 (Ala132 in hRFC) (Brigle et al., 1995) in TMD4. In hRFC, Ser127 in TMD4 was implicated as functionally important (Wong et al., 1999). The impact of these mutations ranges from effects on carrier mobility without an effect on the Kt for MTX binding (Ala130Pro in mouse RFC) (Brigle et al., 1995), to decreases in both Kt and Vmax for MTX (Ser127Asn in hRFC) (Wong et al., 1999). For certain mutants (Trp105Gly and Ser309Phe in mouse RFC), different transport phenotypes were seen for different transport substrates (Tse et al., 1998; Zhao et al., 2000), similar to findings for Ser46Asn and Ile48Phe (see above). For instance, Trp105Gly increased transport of folic acid compared to 5,10-dideazatetrahydrofolate by mouse RFC. Ser309Phe in mouse RFC resulted in an increased (∼5-fold) Kt for MTX and 5-formyl tetrahydrofolate without a significant change in the affinities for folic acid and 5-methyl tetrahydrofolate.
B. Identification of structurally or functionally important amino acids in RFC by homology comparisons and site-directed mutagenesis
From membrane topology and comparisons of homologies between hRFC and RFCs from other species, a number of highly conserved charged amino acids are found to map within the TMDs (Figures 2 and 3). These include Asp88 (TMD2), Arg133 (TMD4), Arg373 (TMD10), Lys411 (TMD11), and Asp453 (TMD12). While replacement of Asp88 in hRFC with glutamate partially preserved transport activity for both MTX and 5-formyl tetrahydrofolate, substitution with valine abolished activity. Conversely, valine replacement of Asp453 had only a small effect on carrier activity (Liu et al., 2001). For murine RFC, transport activity was abolished by replacement of conserved Arg131 (Arg133 in hRFC) and Arg363 (Arg373 in hRFC) residues with leucine (Sharina et al., 2001). Similar results were obtained with aliphatic amino acid substitutions at Arg 133 and Arg373 in hRFC (Liu et al., 2001; Deng et al., 2007). With hamster RFC, Arg373 was suggested to be functionally important since systematic replacement at this position with lysine, histidine, glutamine, or alanine progressively decreased the capacity of the position 373 mutant RFCs to complement a transport defective hamster phenotype in supporting colony formation in the presence of low levels of 5-formyl tetrahydrofolate (Sadlish et al., 2002b). In direct transport assays, these substitutions had a much more pronounced adverse effect on carrier translocation (Vmax) than on substrate binding and also decreased RFC stabilities and intracellular trafficking. Replacement of Lys404 in mouse RFC with leucine resulted in a selective loss of binding and transport of reduced folates over MTX (Sharina et al., 2001). For hRFC, low to moderate levels of MTX and 5-formyl tetrahydrofolate transport were detected with Lys411Leu and Lys411Glu; Lys411Arg exhibited an increased affinity and transport of 5-formyl tetrahydrofolate over MTX (Witt et al., 2002).
Thus, a negative charge at position 88 and positive charges at positions 133 and 373 are essential for high levels of MTX transport by RFC. While the situation for Lys411 is more complex and differs with different transport substrates, it nonetheless appears that Lys411 is at least somewhat important for binding hRFC substrates. As described below, very recent results suggest that Lys411 in TMD11 of hRFC is the major target for covalent modification by NHS-MTX (Deng et al., 2007), consistent with a role of this conserved cationic amino acid in binding to the carboxyl group(s) of folate substrates. Finally, recent site-directed mutagenesis results for Ser313 (TMD8), Tyr136 (TMD4), and Tyr281 (TMD7) in hRFC (Hou et al., 2006) imply critical structural or functional roles of these residues. As described above, from the results of RFC mutant studies, important roles for Ser313 and a large portion of TMD4 are likewise suggested.
C. Deletional and insertional mutagenesis of RFC
Another useful strategy to identify and characterize functionally important domains in RFC has involved deletional mutagenesis. Thus, deletion of 27 N-terminal amino acids (residues 1-27) from hRFC (Marchant et al., 2002), or removal of 16 residues (amino acids 7-22) from hamster RFC (Sadlish et al., 2002a) had at most minor effects on membrane targeting or transport function. While deletion of 58 C-terminal residues from hamster RFC (residues 461-518) (Sadlish et al., 2002a) or 39 C-terminal residues from hRFC (residues 453-591) (Marchant et al., 2002) had a slight effect on transport function, for murine RFC, loss of the C-terminus (residues 445 to 512) resulted in a complete loss of membrane targeting (Sharina et al., 2002). As expected, larger deletions (e.g., 302-591, 1-301), including entire TMDs, completely abolished plasma membrane targeting of hRFC (Marchant et al., 2002). Collectively, these studies argue that neither the cytosolic facing N- nor C-terminus is directly involved in substrate binding and that, in general, they only slightly influence membrane targeting and insertion of the carrier.
A prominent feature of the MFS family of transporters involves a connecting loop between TMDs 6 and 7 (Figure 2). Both deletion and insertion mutagenesis strategies have been used to explore the functional and structural role of this region in RFC. Thus, deletion of 31 of the 66 amino acids from the TMD6/TMD7 connecting loop in murine RFC (Sharina et al., 2002), or deletion of up to 45 of the 67 amino acids in the TMD6/TMD7 loop domain from hamster RFC (Sadlish et al., 2002a) preserved membrane targeting and transport activity. However, larger deletions in the TMD6/TMD7 linker domain (57 and 53-55 amino acids, respectively) abolished transport activity.
While deletions of 49 or 60 amino acids from the TMD6/TMD7 linker of hRFC (amino acids 215-263 and 204-263, respectively) completely ablated transport of MTX and 5-formyl tetrahydrofolate, replacement of the deleted segments with non-homologous 73 or 84 amino acid segments from another MFS protein, SLC19A2 (transports thiamine; 18% homologous to hRFC for the TMD6/TMD7 linker region) restored transport (Liu et al., 2003). Interestingly, maximal transport activities for these insertional mutants were absolutely dependent on the presence of the highly conserved 204-214 peptide and deletion of the 204-214 segment alone completely abolished transport (Liu et al., 2003).
Thus, the primary purpose of the TMD6/TMD7 linker domain is to ensure the optimal spacing between the 2 halves of the RFC protein for carrier function. This appears to be essentially independent of amino acid sequence, although an important role for amino acids 204-214 is implied. Most recently, TMD1-6 and TMD7-12 hRFC half molecules were co-transfected into hRFC-null K562 cells (Witt et al., 2004). Co-expressed hRFC half molecules were targeted to the membrane surface where they restored transport activity with normal kinetics, showed sensitivity to inhibition by NHS-MTX, and exhibited a capacity for trans-stimulation by preloading with 5-formyl tetrahydrofolate (Witt et al., 2004).
D. Localization of a substrate binding domain by radioaffinity labeling
The ability to restore functional RFC transport by co-expression of hRFC half molecules provided a unique tool to localize substrate binding domains. Thus, co-expression and NHS-3H-MTX radioaffinity labeling of hRFC TMD1-6 and TMD7-12 half molecules localized covalent labeling to TMD7-12 (Witt et al., 2004). Treatment of radioaffinity labeled TMD7-12 with 2-nitro-5-thiocyanato benzoic acid cleaved adjacent to cysteine residues and localized binding of the radioligand to between amino acids 394 and 457, corresponding to TMDs 11 and 12 (Hou et al., 2005). More recent results localized affinity labeling of NHS-MTX to Lys411 in TMD11 since Lys411Ala could effectively transport MTX yet abolished MTX transport inhibition by unlabeled NHS-MTX and covalent incorporation from NHS-3H-MTX (Deng et al. 2007). While this directly implicates Lys411 in carboxyl binding for RFC substrates, its role is paradoxical since Lys411 in hRFC could be replaced by non-conservative amino acid substitutions with comparatively modest effects on transport (Witt et al., 2002; Deng et al., 2007).
E. Scanning cysteine accessibility methods for mapping the substrate translocation pathway
The availability of a functional cysteine-less hRFC (Cao and Matherly, 2003) permitted corroboration of mutagenesis and affinity labeling results by SCAM, which identified amino acids that were aqueous accessible and were likely involved in forming the putative membrane translocation pathway for anionic folate substrates. Thus, for hRFC, 282 cysteines were individually inserted into TMDs 1-12 of a cysteine-less hRFC template and hRFC cysteine mutants were expressed in hRFC-null HeLa cells (Hou et al., 2005, 2006). Altogether, 272 of the 282 single cysteine mutants were functional for MTX transport, the only exceptions being Arg133, Ile134, Ala135, Tyr136, and Ser138 in TMD4, Gly163 in TMD5, Tyr281 in TMD7, Ser313 in TMD8, Arg373 in TMD10, and Gly401 in TMD11. For the 272 functional mutants, aqueous accessibilities of the cysteine insertions were confirmed by monitoring losses of transport activity and the protective effects of substrate (i.e., leucovorin) in the presence of the membrane-impermeable hydrophilic sodium (2-sulphonatoethyl) methanethiosulfonate (MTSES). The results of these studies strongly supported a role for amino acids localized to TMDs 4, 5, 7, 8, 10, and 11 in forming the putative substrate binding pocket of hRFC, in excellent agreement with the results of RFC mutant studies.
Exofacial residues flanking TMD1 including positions 40, 44, and 48 (but not 45 or 46), corresponding to a region suggested to be functionally important from mutant studies (see above), were likewise implicated as involved in substrate binding by earlier SCAM experiments with MTSES and cysteine insertion hRFC mutants expressed in transport defective CHO cells (Cao and Matherly, 2003). Similarly, biotinylation by biotin maleimide of cysteines inserted at positions 41, 46, 70, and 71, including the exofacial spanning region connecting TMDs 1 and 2 in hamster RFC, was prevented by prior treatment with MTX or leucovorin, suggesting that these sites form part of a substrate binding domain (Flintoff et al., 2003).
A three dimensional model based in part on SCAM biochemical data for hRFC is shown in Figure 4 that depicts TMDs 1, 2, 4, 5, 7, 8, 10, and 11 as components of the putative aqueous membrane-spanning translocation pathway flanked by TMDs 3, 6, 9, and 12. These results are consistent with the published crystal structures of the LacY and GlpT MFS homologs (Abramson et al., 2003; Huang et al., 2003). Based on data showing the nearly complete ablation of transport activity upon cysteine substitutions into cysteine-less hRFC, it appears that a number of amino acids are structurally or functionally important including Arg133, Ile134, Ala135, Tyr136, and Ser138 in TMD4, Tyr281 in TMD7, Ser313 in TMD8, and Arg373 in TMD10. A functionally important role was also suggested for Lys411 in TMD11 (Sharina et al., 2001; Witt et al., 2002). Studies with the activated NHS-MTX ester established covalent modification at this site, thus implicating Lys411 in binding the glutamate portion of folate substrates (Deng et al., 2007).
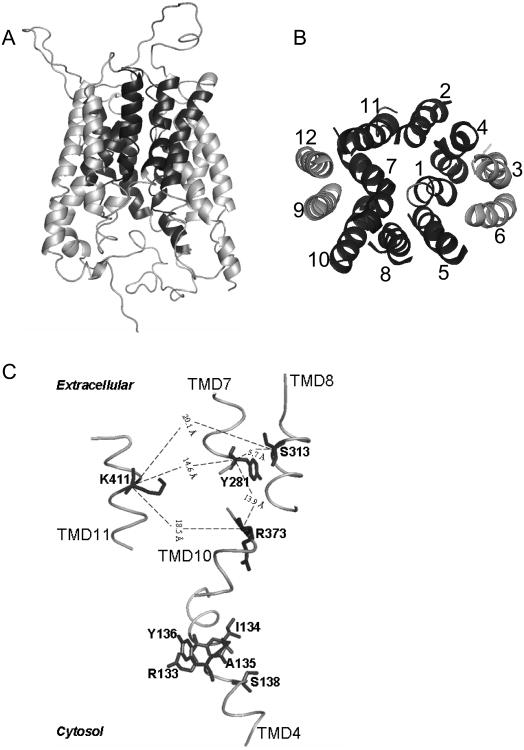
Figure 4. Proposed 3-D models of hRFC based on solved crystal structures of LacY and GlpT and SCAM analysis, and the hypothesized substrate binding site of hRFC.
A three dimensional hypothetical model for hRFC is presented based on structure alignments between hRFC and LacY and GlpT and fine-tuned based on experimental SCAM data. Modeling was performed with the Modeller 8v1 auto mode (Marti-Renom, 2000). All models were drawn by PyMol (DeLano, 2002). Panel A depicts a side view of hRFC for which the extended C-terminal segment is truncated at Lys-479. TMDs 1, 2, 4, and 5 of the N-terminal region and TMDs 7, 8, 10, and 11 of the C-terminal region are hypothesized to be involved in formation of the hydrophilic cavity for anionic substrate binding (colored as black). TMDs 3, 6, 9, and 12 are likely buried in the lipid bilayer and do not directly participate in substrate binding (colored as grey). Panel B depicts a cytosolic view of only the TMD segments (numbered 1-12 as in Figure 2) of the hRFC molecule so that the order of helix packing can be easily seen. TMD shading is the same as in Panel A. Panel C shows an enhanced view of the hypothetical substrate binding site, including Lys-411, Ser-313, Tyr-281, and Arg-373, as described in the text. Other residues that may contribute to the substrate binding pocket are also shown and include Arg-133, Ile-134, Ala-135, Tyr-136, and Ser-138. The physical distances between α carboxyl groups of Lys-411, Ser-313, Tyr-281 and Arg-373 are shown in Å. Adapted from Hou et al. (2006).
Notably, all these amino acids are highly conserved between species as diverse as Xenopus, zebrafish, mice, humans, and cattle (Figure 3). Compelling evidence from RFC mutant studies (see above) established that Ser313 and Arg373 are functionally important and may contribute to substrate binding specificity (Zhao et al., 1999; Sharina et al., 2001; Sadlish et al., 2002b; Hou et al., 2006; Deng et al., 2007). Lys411, Ser313, and Arg373 may easily comprise a hydrophilic binding pocket for anionic folate substrates (Hou et al., 2006) (Figure 4). Reflecting its juxtaposition to both Ser313 and Arg373 in this model, Tyr281 may also participate in substrate binding. The finding that individual cysteine replacements of Arg133, Ile134, Ala135, Tyr136, and Ser138 abolished transport activity is entirely consistent with previous reports that functionally important amino acids (Ser127, Ala132, Arg133) were localized to TMD4 (Brigle et al., 1995; Wong et al., 1999; Liu et al., 2001; Sharina et al., 2001).
The role of the highly conserved residues at positions 40, 44, 45, 46, and 48 in RFC transport remains a paradox. Whereas mutant studies suggested a possible functional importance for amino acids located at positions 44, 45, 46, and 48 in hRFC (Wong et al., 1999; Zhao et al., 1998a,b; Tse et al., 1998), only positions 40, 44, and 48 were implicated as contributing to a substrate binding domain by SCAM (Cao et al., 2003). Similarly, in hamster RFC, positions 41, 46, and 49 flanking TMD1 along with positions 70 and 71 in or flanking TMD2 were implicated in substrate binding by the ability of RFC substrate to protect from the inhibitory effects of biotin maleimide (Flintoff et al., 2003).
F. Mapping helix packing associations in hRFC
Characterizing conserved charged residues in or flanking TMDs by mutant analysis can shed light on tertiary structural elements in membrane proteins including interactions between distal domains. Interpretation is in part based on the notion of an energetic unfavorability associated with uncompensated charged amino acids localized within the lipid bilayer. However, should there be a salt bridge between residues of opposite charge, charged amino acids localized to hydrophobic environments can be substantially stabilized (Barril et al., 1998). Salt bridges between oppositely charged residues in separate domains can serve to orient TMDs for membrane insertion and/or for optimal transport function (Dunten et al., 1993; Merickel et al., 1997).
For hRFC, neutralization of the positive charge on Arg133 (TMD4) by substitution with leucine or the negative charge on Asp88 (TMD2) by replacement with valine abolished transport activity (Liu et al., 2001). However, when both mutations were present in the same construct (i.e., Asp88Leu/Arg133Val), transport activity was restored. This suggests that disruption of the charge-pair by replacing either Arg133 or Asp88, individually, with a neutral amino acid results in an unstable, unpaired charge. However, simultaneous neutralization of both charged amino acids results in a restoration of high levels of transport activity. These results strongly imply that Arg133 and Asp88 form a salt bridge complex that stabilizes the association between TMDs 2 and 4 in the hRFC tertiary structure. Other reports have also explored RFC tertiary structure at the level of putative charge-pairs. For murine RFC, a structural or functional interaction between Glu45 (flanks TMD1) and Lys404 (TMD11) was suggested since the properties of the double Glu45Lys/Lys404Glu murine RFC mutant more closely resembled the properties of the Glu45Lys mutant than those for the Lys404Glu mutant (Zhao et al., 2003). Further, a cross-linking analysis of hamster RFC suggested that Arg373 (in TMD10) is in close proximity to Glu394 (flanks TMD11), implying juxtaposition of these domains and the possible formation of a charge-pair (Sadlish et al., 2002b).
Yu et al. (1995) have described a method for assessing TMD helix packing in polytopic membrane proteins based on disulfide formation between paired cysteine residues in purified segments of the visual pigment rhodopsin. This method was subsequently adapted to assess helix proximities and tilts of a MFS transporter (Kaback et al., 1999).
For hRFC, in situ site-directed thiol cross-linking was applied to study the proximities and tilts of neighboring transmembrane helices 2, 5, 8, and 11 (Z. Hou and L.H. Matherly, manuscript submitted), based on their proposed orientations toward the putative hRFC hydrophilic cavity and their relative proximities in 3-dimensional models (Hou et al., 2006; Matherly et al., 2007) (Figure 4). As described above, Ser313 in TMD8 was previously implicated in the binding of antifolate substrates for RFC (Zhao et al., 1999; Deng et al., 2007) and was identified as “irreplaceable” by scanning cysteine mutagenesis (Hou et al., 2006). TMD8 abuts TMD5 and, by SCAM, both these regions are aqueous-accessible and contribute to the substrate-binding pocket in hRFC (Hou et al., 2006). TMD11 includes Lys411, the primary target for covalent radioaffinity labeling with NHS-3H-MTX (Deng et al., 2007), and is aqueous-accessible by SCAM (Hou et al., 2005). Finally, TMD2 flanks TMD11 and, by homology with LacY (Abramson et al., 2003), lines the substrate translocation pathway and includes at least one residue (Asp88) that is essential for transport (Liu et al., 2001).
In initial studies, cysteine-less hRFC was expressed as two (TMD1-6 and TMD7-12) half molecules, each with a cysteine residue inserted at a defined position in the TMD1-6 (TMDs 2 or 5) or TMD7-12 (TMDs 8 or 11) portions. Altogether, nineteen cysteine-substituted TMD1-6/TMD7-12 pairs (175/311, 174/314, 172/315, 171/317, 168/318, 167/321, 164/322, 163/325, 161/326 and 160/326 in TMDs 5/8; 74/415, 74/412, 74/411, 75/408, 78/405, 81/404, 82/404, 84/404, and 85/404 in TMDs 2/11) were selected for co-expression and cross-linking with homobifunctional cross-linkers of different lengths [1,1-methanediyl bismethanethiosulfonate, 3 Å; o-phenylenedimaleimide, 6 Å; p-phenylenedimaleimide, 10 Å; 1,6-bis(maleimido)hexane, 16 Å], so to assess helix proximities and tilts in relation to the putative hRFC hydrophilic cavity. The results unequivocally establish that the helices of TMDs 5 and 8 are relatively close together at their extracellular ends (within 10 Å), then tilt away from each other toward the cytoplasmic ends; TMDs 2 and 11 are in close proximity at both their extracellular and cytoplasmic ends (within 10 Å). Pro82 in TMD2 may cause a bend in TMD2, resulting in a lack of cross-linking between the middle segments of TMDs 2 and 11.
X. Conclusions
Folates are essential for life and folate deficiency contributes to a host of health problems including cardiovascular disease, fetal abnormalities, neurologic disorders, and cancer (Lucock, 2000; Matherly, 2004). Antifolates, represented by MTX, continue to occupy a unique niche among the modern day pharmacopoeia for cancer along with other pathologic conditions (Matherly et al., 2007).
This review focuses on the biology of the membrane transport system termed the “reduced folate carrier” or RFC with a particular emphasis on RFC structure and function. The ubiquitously expressed RFC is the major transporter for folates in mammalian cells and tissues (Matherly and Goldman, 2003). Loss of RFC expression or function portends potentially profound physiologic, and developmental consequences (Matherly, 2004). For chemotherapeutic antifolates used for cancer, loss of RFC expression or synthesis of mutant RFC protein results in antifolate resistance due to incomplete inhibition of cellular enzyme targets and low levels of antifolate substrate for polyglutamate synthesis (Goldman and Matherly, 1985; Goldman and Zhao, 2003).
Protein structural information is a prerequisite for understanding mechanisms of membrane transport. However, for mammalian MFS transporters, this information has not been widely available due to difficulties in isolating sufficient quantities of purified proteins and in crystallizing proteins for x-ray diffraction studies. Since 1994, when RFC was first cloned, tremendous advances in molecular biology and biochemical approaches for studying the structures of polytopic membrane proteins have led to an increasingly detailed picture of the molecular structure of the carrier, including its membrane topology, its N-glycosylation, identification of functionally and structurally important domains and amino acids, and helix packing associations. Although no crystal structure for RFC is yet available, biochemical and molecular studies, combined with homology modeling based on homologous bacterial MFS transporters such as LacY, now permit the development of experimentally testable hypotheses designed to establish RFC structure and mechanism.
Of course, significant challenges remain. For instance, it is essential to further identify critical amino acids and domains that comprise the substrate binding sites and translocation pathways by biochemical studies and eventually by x-ray crystallography. Mechanistic studies are needed to further characterize the “energetics” of RFC transport, namely how counter transport by an unidentified physiologic counter-anion drives folate substrate accumulation against a concentration gradient, including the relationship between counter-anion and substrate binding. Greater focus in RFC protein structure studies needs to be on key substrate-specific determinants of binding and translocation, as a prelude to the design of new antifolate inhibitors that rely on RFC for cellular entry, or with substantially enhanced transport by other folate transporters such as FRs or PCFT over RFC. It will be necessary to extend static structural studies of helix packing by protein cross-linking to dynamic structural changes involving functionally important TMD helices that accompany substrate binding and translocation. Likewise, it will be important to expand simple considerations of secondary and tertiary structures for RFC to potential oligomeric quaternary associations, including possible homomeric and heteromeric protein-protein associations that may be significant to transport mechanism or regulation. Indeed, insights from RFC structure-function studies may eventually foster the development of strategies for biochemically modulating the carrier which could be therapeutically exploited in the context of nutritional interventions or antifolate chemotherapy.
Acknowledgments
This work was supported by Grant CA53535 from the National Cancer Institute, National Institutes of Health.
References
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- Almqvist J, Huang Y, Hovmoller S, Wang DN. Homology modeling of the human microsomal glucose 6-phosphate transporter explains the mutations that cause the glycogen storage disease type Ib. Biochemistry. 2004;43:9289–9297. doi: 10.1021/bi049334h. [DOI] [PubMed] [Google Scholar]
- Balamurugan K, Said HM. Role of reduced folate carrier in intestinal folate uptake. Am J Physiol Cell Physiol. 2006;291:C189–193. doi: 10.1152/ajpcell.00594.2005. [DOI] [PubMed] [Google Scholar]
- Barber RC, Bennett GD, Greer KA, Finnell RH. Expression of patterns of folate binding proteins one and two in the developing mouse embryo. Mol Genet Metab. 1999;66:31–39. doi: 10.1006/mgme.1998.2772. [DOI] [PubMed] [Google Scholar]
- Barril X, Aleman C, Orozco M, Luque FJ. Salt bridge interactions: stability of the ionic and neutral complexes in the gas phase, in solution, and in proteins. Proteins. 1998;32:67–79. doi: 10.1002/(sici)1097-0134(19980701)32:1<67::aid-prot8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Brigle KE, Spinella MJ, Sierra EE, Goldman ID. Characterization of a mutation in the reduced folate carrier in a transport defective L1210 murine leukemia cell line. J Biol Chem. 1995;270:22974–22979. doi: 10.1074/jbc.270.39.22974. [DOI] [PubMed] [Google Scholar]
- Cao W, Matherly LH. Characterization of a cysteine-less human reduced folate carrier: localization of a substrate binding domain by cysteine scanning mutagenesis and cysteine accessibility methods. Biochem J. 2003;374:27–36. doi: 10.1042/BJ20030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Matherly LH. Analysis of the membrane topology for transmembrane domains 7-12 of the human reduced folate carrier by scanning cysteine accessibility methods. Biochem J. 2004;378:201–206. doi: 10.1042/BJ20031288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AB, Lin R, Studley WK, Tran CV, Saier MH. Phylogeny as a guide to structure and function of membrane transport proteins. Mol Membr Biol. 2004;21:171–181. doi: 10.1080/09687680410001720830. [DOI] [PubMed] [Google Scholar]
- Chiao JH, Roy K, Tolner B, Yang CH, Sirotnak FM. RFC-1 gene expression regulates folate absorption in mouse small intestine. J Biol Chem. 1997;273:11165–11170. doi: 10.1074/jbc.272.17.11165. [DOI] [PubMed] [Google Scholar]
- Chládek J, Martínková J, Simková M, Vanecková J, Koudelková V, Nozicková M. Pharmacokinetics of low doses of methotrexate in patients with psoriasis over the early period of treatment. Eur J Clin Pharmacol. 1998;53:437–444. doi: 10.1007/s002280050404. [DOI] [PubMed] [Google Scholar]
- Chu E, Callender MA, Farrell MP, Schmitz JC. Thymidylate synthase inhibitors as anticancer agents: from bench to bedside. Cancer Chemother Pharmacol. 2003;52(1):S80–89. doi: 10.1007/s00280-003-0625-9. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Johnson JR, Wang YC, Sridhara R, Pazdur R. FDA drug approval: pemetrexed for injection (Alimta) for the treatment of non-small cell lung cancer. Oncologist. 2005;10:363–368. doi: 10.1634/theoncologist.10-6-363. [DOI] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL Molecular Graphics System (2002) DeLano Scientific; San Carlos, CA, USA: 2002. [Google Scholar]
- Deng Y, Hou Z, Cherian C, Wu J, Wang L, Gangjee A, Matherly LH. Identification of human reduced folate carrier (hRFC) substrate binding by site-directed mutagenesis and affinity labeling strategies. Abstracts, American Association for Cancer Research. 2007;48:1355. [Google Scholar]
- Dixon KH, Lanpher BC, Chiu J, Kelley K, Cowan KH. A novel cDNA restores reduced folate carrier activity and methotrexate sensitivity to transport deficient cells. J Biol Chem. 1994;269:17–20. [PubMed] [Google Scholar]
- Dodd JR, Christie DL. Cysteine 144 in the third transmembrane domain of the creatine transporter is located close to a substrate-binding site. J Biol Chem. 2001;276:46983–46988. doi: 10.1074/jbc.M107137200. [DOI] [PubMed] [Google Scholar]
- Drori S, Jansen G, Mauritz R, Peters GJ, Assaraf YG. Clustering of mutations in the first transmembrane domain of the human reduced folate carrier in GW1843U89-resistant leukemia cells with impaired antifolate transport and augmented folate uptake. J Biol Chem. 2000;275:30855–30863. doi: 10.1074/jbc.M003988200. [DOI] [PubMed] [Google Scholar]
- Duch DS, Banks S, Dev IK, Dickerson SH, Ferone R, Heath LS, Humphreys J, Knick V, Pendergast W, Singer S, Smith GK, Waters K, Wilson HR. Biochemical and cellular pharmacology of 1843U89, a novel benzoquinazoline inhibitor of thymidylate synthase. Cancer Res. 1993;53:810–818. [PubMed] [Google Scholar]
- Dunten RL, Sahin-Toth M, Kaback HR. Role of the charge pair aspartic acid-237-lysine-358 in the lactose permease of Escherichia coli. Biochemistry. 1993;32:3139–3145. doi: 10.1021/bi00063a028. [DOI] [PubMed] [Google Scholar]
- Ferguson PL, Flintoff WF. Topological and functional analysis of the human reduced folate carrier by hemagglutinin epitope insertion. J Biol Chem. 1999;274:16269–18278. doi: 10.1074/jbc.274.23.16269. [DOI] [PubMed] [Google Scholar]
- Fischer GA. Detective transport of amethopterin (methotrexate) as a mechanism of resistance to the antimetabolite in L5178Y leukemic cells. Biochem Pharmacol. 1962;11:1233–1234. doi: 10.1016/0006-2952(62)90200-9. [DOI] [PubMed] [Google Scholar]
- Flintoff WF, Williams FM, Sadlish H. The region between the transmembrane domains 1 and 2 of the reduced folate carrier forms part of the substrate-binding pocket. J Biol Chem. 2003;278:40867–40876. doi: 10.1074/jbc.M302102200. [DOI] [PubMed] [Google Scholar]
- Fiser A, Sali A. Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol. 2003;374:461–491. doi: 10.1016/S0076-6879(03)74020-8. [DOI] [PubMed] [Google Scholar]
- Freisheim JH, Ratnam M, McAlinden TP, Prasad KMR, Williams FE, Westerhof GR, Schornagel JH, Jansen G. Molecular events in the membrane transport of methotrexate in human CCRF-CEM leukemia cell lines. Adv Enzyme Regul. 1992;32:17–31. doi: 10.1016/0065-2571(92)90006-l. [DOI] [PubMed] [Google Scholar]
- Frillingos S, Sahin-Toth M, Wu J, Kaback HR. Cys-scanning mutagenesis: a novel approach to structure function relationships in polytopic membrane proteins. FASEB J. 1998;12:1281–1299. doi: 10.1096/fasebj.12.13.1281. [DOI] [PubMed] [Google Scholar]
- Ge Y, Haska CL, LaFiura K, Devidas M, Linda SB, Liu M, Thomas R, Taub JW, Matherly LH. Prognostic role of the reduced folate carrier, the major membrane transporter for methotrexate, in childhood acute lymphoblastic leukemia: a report from the Children's Oncology Group. Clin Cancer Res. 2007;13:451–457. doi: 10.1158/1078-0432.CCR-06-2145. [DOI] [PubMed] [Google Scholar]
- Giannini EH, Brewer EJ, Kuzmina N, Shaikov A, Maximov A, Vorontsov I, Fink CW, Newman AJ, Cassidy JT, Zemel LS. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.A.-U.S.S.R. double-blind, placebo-controlled trial. The Pediatric Rheumatology Collaborative Study Group and the Cooperative Children's Study Group. N Engl J Med. 1992;326:1043–1049. doi: 10.1056/NEJM199204163261602. [DOI] [PubMed] [Google Scholar]
- Gifford AJ, Haber M, Witt TL, Whetstine JR, Taub JW, Matherly LH, Norris MD. Role of the E45K reduced folate carrier gene mutation in methotrexate resistance in human leukemia cells. Leukemia. 2002;16:2379–2387. doi: 10.1038/sj.leu.2402655. [DOI] [PubMed] [Google Scholar]
- Goldman ID. The characteristics of the membrane transport of amethopterin and the naturally occurring folates. Ann NY Acad Sci. 1971a;186:400–422. doi: 10.1111/j.1749-6632.1971.tb46996.x. [DOI] [PubMed] [Google Scholar]
- Goldman ID. A model system for the study of heteroexchange diffusion: methotrexate-folate interactions in L1210 leukemia and Ehrlich ascites tumor cells. Biochim Biophys Acta. 1971b;233:624–634. doi: 10.1016/0005-2736(71)90162-3. [DOI] [PubMed] [Google Scholar]
- Goldman ID, Matherly LH. The cellular pharmacology of methotrexate. Pharmacol Ther. 1985;28:77–100. doi: 10.1016/0163-7258(85)90083-x. [DOI] [PubMed] [Google Scholar]
- Goldman ID, Zhao R. Molecular, biochemical, and cellular pharmacology of pemetrexed. Semin Oncol. 2002;29:3–17. doi: 10.1053/sonc.2002.37461. [DOI] [PubMed] [Google Scholar]
- Goldman ID, Lichtenstein NS, Oliverio VT. Carrier-mediated transport of the folic acid analogue methotrexate, in the L1210 leukemia cell. J Biol Chem. 1968;243:5007–5017. [PubMed] [Google Scholar]
- Gong M, Yess J, Connolly T, Ivy SP, Ohnuma T, Cowan KH, Moscow JA. Molecular mechanism of antifolate transport-deficiency in a methotrexate-resistant MOLT-3 human leukemia cell line. Blood. 1997;89:2494–2499. [PubMed] [Google Scholar]
- Gorlick R, Goker E, Trippett T, Steinherz P, Elisseyeff Y, Mazumdar M, Flintoff WF, Bertino JR. Defective transport is a common mechanism of acquired methotrexate resistance in acute lymphocytic leukemia and is associated with decreased reduced folate carrier expression. Blood. 1997;89:1013–1018. [PubMed] [Google Scholar]
- Guo W, Healey JH, Meyers PA, Ladanyi M, Huvos AG, Bertino JR, Gorlick R. Mechanisms of methotrexate resistance in osteosarcoma. Clin Cancer Res. 1999;5:621–627. [PubMed] [Google Scholar]
- Hazarika M, White RM, Johnson JR, Pazdur R. FDA drug approval summaries: pemetrexed (Alimta) Oncologist. 2004;9:482–488. doi: 10.1634/theoncologist.9-5-482. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Zevely EM. Anion exchange mechanism for transport of methotrexate in L1210 cells. Biochem Biophys Res Commun. 1981;99:163–169. doi: 10.1016/0006-291x(81)91727-7. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Zevely EM. Transport of methotrexate in L1210 cells: effect of ions on the rate and extent of uptake. Arch Biochem Biophys. 1980;200:149–155. doi: 10.1016/0003-9861(80)90341-0. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Zevely EM. Functional correlations between the methotrexate and general anion transport systems of L1210 cells. Biochem Int. 1982;4:493–502. [Google Scholar]
- Henderson GB, Zevely EM. Use of non-physiological buffer systems in the analysis of methotrexate transport in L1210 cells. Biochem Int. 1983a;6:507–515. [PubMed] [Google Scholar]
- Henderson GB, Zevely EM. Structural requirements for anion substrates of the methotrexate transport system of L1210 cells. Arch Biochem Biophys. 1983b;221:438–446. doi: 10.1016/0003-9861(83)90162-5. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Zevely EM. Affinity labeling of the 5-methyltetrahydrofolate/methotrexate transport protein of L1210 cells by treatment with an N-hydroxysuccinimide ester of [3H]methotrexate. J Biol Chem. 1984;259:4558–4562. [PubMed] [Google Scholar]
- Henderson GB, Grzelakowska-Sztabert B, Zevely EM, Huennekens FM. Binding properties of the 5-methyltetrahydrofolate/methotrexate transport system in L1210 cells. Arch Biochem Biophys. 1980a;202:144–149. doi: 10.1016/0003-9861(80)90416-6. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Zevely EM, Huennekens FM. Photoinactivation of the methotrexate transpoprt system of L1210 cells by 8-azidoadenosine-5′-monophosphate. J Biol Chem. 1979;254:9973–9975. [PubMed] [Google Scholar]
- Henderson GB, Zevely EM, Huennekens FM. Irreversible inactivation of the methotrexate transport systems in L1210 cells by carbodiimide activated substrates. J Biol Chem. 1980b;255:4826–4833. [PubMed] [Google Scholar]
- Hirai T, Heymann JA, Maloney PC, Subramaniam S. Structural model for 12-helix transporters belonging to the major facilitator superfamily. J Bacteriol. 2003;185:1712–1718. doi: 10.1128/JB.185.5.1712-1718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Heymann JA, Shi D, Sarker R, Maloney PC, Subramaniam S. Three-dimensional structure of a bacterial oxalate transporter. Nat Struct Biol. 2002;9:597–600. doi: 10.1038/nsb821. [DOI] [PubMed] [Google Scholar]
- Horne DW, Reed KA. Transport of methotrexate into PC-3 human prostate cancer cells. Arch Biochem Biophys. 2001;394:39–44. doi: 10.1006/abbi.2001.2528. [DOI] [PubMed] [Google Scholar]
- Hou Z, Stapels SE, Haska CL, Matherly LH. Localization of a substrate binding domain of the human reduced folate carrier to transmembrane domain 11 by radioaffinity labeling and cysteine-substituted accessibility methods. J Biol Chem. 2005;280:36206–30213. doi: 10.1074/jbc.M507295200. [DOI] [PubMed] [Google Scholar]
- Hou Z, Ye J, Haska CL, Matherly LH. Transmembrane domains 4, 5, 7, 8, and 10 of the human reduced folate carrier are important structural or functional components of the transmembrane channel for folate substrates. J Biol Chem. 2006;281:33588–33596. doi: 10.1074/jbc.M607049200. [DOI] [PubMed] [Google Scholar]
- Hruz PW, Mueckler MM. Structural analysis of the GLUT1 facilitative glucose transporter (review) Mol Membr Biol. 2001;18:183–193. doi: 10.1080/09687680110072140. [DOI] [PubMed] [Google Scholar]
- Hu YK, Kaplan JH. Site-directed chemical labeling of extracellular loops in a membrane protein. The topology of the Na,K-ATPase alpha-subunit. J Biol Chem. 2000;275:19185–19191. doi: 10.1074/jbc.M000641200. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- Jansen G. Receptor- and carrier-mediated transport systems for folates and antifolates. Exploitation for folate chemotherapy and immunotherapy. In: Jackman AL, editor. Anticancer Development Guide: Antifolate Drugs in Cancer Therapy. Humana Press Inc.; Totowa, NJ: 1999. pp. 293–321. [Google Scholar]
- Jansen G, Mauritz R, Drori S, Sprecher H, Kathmann I, Bunni M, Priest DG, Noordhuis P, Schornagel JH, Pinedo HM, Peters GJ, Assaraf YG. A structurally altered human reduced folate carrier with increased folic acid transport mediates a novel mechanism of antifolate resistance. J Biol Chem. 1998;273:30189–30198. doi: 10.1074/jbc.273.46.30189. [DOI] [PubMed] [Google Scholar]
- Jansen G, Westerhof GR, Jarmuszewski MJ, Kathmann I, Rijksen G, Schornagel JH. Methotrexate transport in variant human CCRF-CEM leukemia cells with elevated levels of the reduced folate carrier. Selective effect on carrier-mediated transport of physiological concentrations of reduced folates. J Biol Chem. 1990;265:18272–18277. [PubMed] [Google Scholar]
- Jansen G, Westerhof GR, Rijksen G, Schornagel JH. Interaction of N-hydroxy(sulfo)succinimide active esters with the reduced folate/methotrexate transport system from human leukemic CCRF-CEM cells. Biochim Biophys Acta. 1989;875:266–270. doi: 10.1016/0005-2736(89)90411-2. [DOI] [PubMed] [Google Scholar]
- Kaback HR. Structure and mechanism of the lactose permease. CR Biologies. 2005;328:557–567. doi: 10.1016/j.crvi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Kaback HR, Wu J. What to do while awaiting crystals of membrane transport protein and therafter. Acc Chem Res. 1999;32:805–13. [Google Scholar]
- Kaback HR, Dunten R, Frillingos S, Venkatesan P, Kwaw I, Zhang W, Ermolova N. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci USA. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- Kneuer C, Honscha KU, Honscha W. Rat reduced folate carrier-1 is localized basolaterally in MDCK kidney epithelial cells and contributes to the secretory transport. Cell Tissue Res. 2005;320:517–524. doi: 10.1007/s00441-005-1092-x. [DOI] [PubMed] [Google Scholar]
- Kwaw I, Zen KC, Hu Y, Kaback HR. Site-directed sulfhydryl labeling of the lactose permease of Escherichia coli: helices IV and V that contain the major determinants for substrate binding. Biochemistry. 2001;40:10491–10499. doi: 10.1021/bi010866x. [DOI] [PubMed] [Google Scholar]
- Lawrance AK, Deng L, Brody LC, Finnell RH, Shane B, Rozen R. Genetic and nutritional deficiencies in folate metabolism influence tumorigenicity in Apc(min/+) mice. J Nutr Biochem. 2007;18:305–312. doi: 10.1016/j.jnutbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Levy AS, Sather HN, Steinherz PG, Sowers R, La M, Moscow JA, Gaynon PS, Uckun FM, Bertino JR, Gorlick R. Reduced folate carrier and dihydrofolate reductase expression in acute lymphoblastic leukemia may predict outcome: a Children's Cancer Group study. J Pediatric Hematol Oncol. 2003;25:688–695. doi: 10.1097/00043426-200309000-00004. [DOI] [PubMed] [Google Scholar]
- Liu X, Matherly LH. Analysis of membrane topology of the human reduced folate carrier protein by hemagglutinin epitope insertion and scanning glycosylation insertion mutagenesis. Biochim Biophys Acta. 2002;1564:333–342. doi: 10.1016/s0005-2736(02)00467-4. [DOI] [PubMed] [Google Scholar]
- Liu XY, Matherly LH. Functional interactions between Arginine-133 and Aspartate-88 in the human reduced folate carrier: evidence for a charge-pair association. Biochem J. 2001;358:511–516. doi: 10.1042/0264-6021:3580511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Witt TL, Matherly LH. Restoration of high level transport activity by human reduced folate carrier/ThTr1 chimeric transporters: role of the transmembrane domain 6/7 linker region in reduced folate carrier function. Biochem J. 2003;369:31–37. doi: 10.1042/BJ20020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Membrane topology of a cysteine-less mutant of human P-glycoprotein. J Biol Chem. 1995;270:843–848. doi: 10.1074/jbc.270.2.843. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Identification of residues within the drug-binding domain of the human multidrug resistance P-glycoprotein by cysteine-scanning mutagenesis and reaction with dibromobimane. J Biol Chem. 2000;275:39272–39278. doi: 10.1074/jbc.M007741200. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Determining the dimensions of the drug-binding domain of human P-glycoprotein using thiol cross-linking compounds as molecular rulers. J Biol Chem. 2001;276:36877–36880. doi: 10.1074/jbc.C100467200. [DOI] [PubMed] [Google Scholar]
- Lucock M. Folic acid: Nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metabol. 2000;71:121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- Ma DW, Finnell RH, Davidson LA, Callaway ES, Spiegelstein O, Piedrahita JA, Salbaum JM, Kappen C, Weeks BR, James J, Bozinov D, Lupton JR, Chapkin RS. Folate transport gene inactivation in mice increases sensitivity to colon carcinogenesis. Cancer Res. 2005;65:887–897. [PMC free article] [PubMed] [Google Scholar]
- Marchant JS, Subramanian VS, Parker I, Said HM. Intracellular trafficking and membrane targeting mechanisms of the human reduced folate carrier in mammalian epithelial cells. J Biol Chem. 2002;277:33325–33333. doi: 10.1074/jbc.M205955200. [DOI] [PubMed] [Google Scholar]
- Marti-Renom MA, Stuart AC, Fiser A, Sanchez R, Melo F, Sali A. Comparative protein structure modeling of genes and genomes. Annual Review of Biophysics and Biomolecular Structure. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- Matherly LH. Human reduced folate carrier gene and transcript variants: functional, physiologic, and pharmacologic consequences. Current Pharmacogenetics. 2004;2:287–298. [Google Scholar]
- Matherly LH, Hou Z, Deng Y. Human reduced folate carrier: translation of basic biology to cancer etiology and therapy. Cancer and Metastasis Reviews. 2007;26:111–128. doi: 10.1007/s10555-007-9046-2. [DOI] [PubMed] [Google Scholar]
- Matherly LH, Goldman ID. Membrane transport of folates. Vitamins and Hormones. 2003;66:403–456. doi: 10.1016/s0083-6729(03)01012-4. [DOI] [PubMed] [Google Scholar]
- Matherly LH, Czajkowski CA, Angeles SM. Identification of a highly glycosylated methotrexate membrane carrier in K562 erythroleukemia cells up-regulated for tetrahydrofolate cofactor and methotrexate transport. Cancer Res. 1991;51:3420–3426. [PubMed] [Google Scholar]
- Merickel A, Kaback HR, Edwards RH. Charged residues in transmembrane domains II and XI of a vesicular monoamine transporter form a charge pair that promotes high affinity substrate recognition. J Biol Chem. 1997;272:5403–5408. doi: 10.1074/jbc.272.9.5403. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Sekine T, Endou H. The multispecific organic anion transporter family: properties and pharmacological significance. Trends Pharmacol Sci. 2004;25:654–662. doi: 10.1016/j.tips.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Monahan BP, Allegra CJ. Antifolates. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy. 4th. Lippincott-Raven; Philadelphia: 2001. pp. 109–148. [Google Scholar]
- Moscow JA, Gong MK, He R, Sgagias MK, Dixon KH, Anzick SL, Meltzer PS, Cowan KH. Isolation of a gene encoding a human reduced folate carrier (RFC1) and analysis of its expression in transport-deficient, methotrexate-resistant human breast cancer cells. Cancer Res. 1995;55:3790–3794. [PubMed] [Google Scholar]
- Mutch DM, Anderle P, Fiaux M, Mansourian R, Vidal K, Wahli W, Williamson G, Roberts MA. Regional variations in ABC transporter expression along the mouse intestinal tract. Physiol Genomics. 2004;17:11–20. doi: 10.1152/physiolgenomics.00150.2003. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Dyer DL, Dunning DD, Rubin SA, Grant KE, Said HM. Human intestinal folate transport: cloning, expression, and distribution of complementary RNA. Gastroenterology. 1997;112:783–791. doi: 10.1053/gast.1997.v112.pm9041240. [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Ottolia M, Lu L, Lu Y, Philipson KD. A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J Biol Chem. 1999;274:910–917. doi: 10.1074/jbc.274.2.910. [DOI] [PubMed] [Google Scholar]
- Nozaki Y, Kusuhara H, Endou H, Sugiyama Y. Quantitative evaluation of the drug–drug interactions between methotrexate and nonsteroidal anti-inflammatory drugs in the renal uptake process based on the contribution of organic anion transporters and reduced folate carrier. J Pharmacol Exp Ther. 2004;309:226–234. doi: 10.1124/jpet.103.061812. [DOI] [PubMed] [Google Scholar]
- Patterson D, Graham C, Cherian C, Matherly LH. A humanized mouse model for the reduced folate carrier. Molecular Genetics and Metabolism. 2008 doi: 10.1016/j.ymgme.2007.09.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp C, Gorboulev V, Muller TD, Gorbunov D, Shatskaya N, Koepsell H. Amino acids critical for substrate affinity of rat organic cation transporter 1 line the substrate binding region in a model derived from the tertiary structure of lactose permease. Mol Pharmacol. 2005;67:1600–1611. doi: 10.1124/mol.104.008839. [DOI] [PubMed] [Google Scholar]
- Prasad PD, Ramamoorthy S, Leibach FH, Ganapathy V. Molecular cloning of the human placental folate transporter. Biochem Biophys Res Commun. 1995;206:681–687. doi: 10.1006/bbrc.1995.1096. [DOI] [PubMed] [Google Scholar]
- Price EM, Sams L, Harpring KM, Kempton RJ, Freisheim JH. Photoaffinity analogues of methotrexate as probes for dihydrofolate reductase structure and function. Biochem Pharmacol. 1986;35:4341–4343. doi: 10.1016/0006-2952(86)90716-1. [DOI] [PubMed] [Google Scholar]
- Russel FG, Masereeux R, van Aubel RA. Molecular aspects of renal anionic drug transport. Annu Rev Physiol. 2002;64(2002):563–594. doi: 10.1146/annurev.physiol.64.081501.155913. [DOI] [PubMed] [Google Scholar]
- Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Rong N, Selhub J, Goldman BR, Rosenberg IH. Bacterially synthesized folate in rat large intestine is incorporated into host tissue folate polyglutamates. J Nutr. 1991;121:1955–1959. doi: 10.1093/jn/121.12.1955. [DOI] [PubMed] [Google Scholar]
- Rothem L, Aronheim A, Assaraf YG. Alterations in the expression of transcription factors and the reduced folate carrier as a novel mechanism of antifolate resistance in human leukemia cells. J Biol Chem. 2003;278:8935–8941. doi: 10.1074/jbc.M209578200. [DOI] [PubMed] [Google Scholar]
- Rothem L, Ifergan I, Kaufman Y, Priest DG, Jansen G, Assaraf YG. Resistance to multiple novel antifolates is mediated via defective drug transport resulting from clustered mutations in the reduced folate carrier gene in human leukemia cell lines. Biochem J. 2002;367:741–750. doi: 10.1042/BJ20020801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, Tolner B, Chiao JH, Sirotnak FM. A single amino acid difference within the folate transporter encoded by the murine RFC-1 gene selectively alters its interaction with folate analogues. Implications for intrinsic antifolate resistance and directional orientation of the transporter within the plasma membrane of tumor cells. J Biol Chem. 1998;273:2526–2531. doi: 10.1074/jbc.273.5.2526. [DOI] [PubMed] [Google Scholar]
- Russel FG, Masereeux R, van Aubel RA. Molecular aspects of renal anionic drug transport. Annu Rev Physiol. 2002;64:563–594. doi: 10.1146/annurev.physiol.64.081501.155913. [DOI] [PubMed] [Google Scholar]
- Sadlish H, Murray RC, Williams FM, Flintoff WF. Mutations in the reduced-folate carrier affect protein localization and stability. Biochem J. 2000;346:509–518. [PMC free article] [PubMed] [Google Scholar]
- Sadlish H, Williams FM, Flintoff WF. Cytoplasmic domains of the reduced folate carrier are essential for trafficking, but not function. Biochem J. 2002a;364:777–786. doi: 10.1042/BJ20011361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlish H, Williams FM, Flintoff WF. Functional role of arginine 373 in substrate translocation by the reduced folate carrier. J Biol Chem. 2002b;277:42105–42112. doi: 10.1074/jbc.M206459200. [DOI] [PubMed] [Google Scholar]
- Said HM. Recent advances in carrier mediated intestinal absorption of water soluble vitamins. Annual Review of Physiol. 2004;66:419–446. doi: 10.1146/annurev.physiol.66.032102.144611. [DOI] [PubMed] [Google Scholar]
- Saier MH, Jr, Beatty JT, Goffeau A, Harley KT, Heijne WH, Huang SC, Jack DL, Jahn PS, Lew K, Liu J, Pao SS, Paulsen IT, Tseng TT, Virk PS. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1:257–279. [PubMed] [Google Scholar]
- Salas-Burgos A, Iserovich P, Zunia F, Vera JC, Fischbarg J. Predicting the three dimensional structure of the human facilitative glucose transporter Glut1 by a novel evolutionary homology strategy: Insights on the molecular mechanism of substrate migration, and binding sites for glucose and inhibitory molecules. Biophys J. 2004;87:2990–2999. doi: 10.1529/biophysj.104.047886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007;26:141–152. doi: 10.1007/s10555-007-9048-0. [DOI] [PubMed] [Google Scholar]
- Schuetz JD, Matherly LH, Westin EH, Goldman ID. Evidence for a functional defect in the translocation of the methotrexate transport carrier in a methotrexate-resistant murine L1210 leukemia cell line. J Biol Chem. 1988;263:9840–9847. [PubMed] [Google Scholar]
- Sharina IG, Zhao R, Wang Y, Babani S, Goldman ID. Mutational analysis of the functional role of conserved Arginine and Lysine residues in transmembrane domains of the murine reduced folate carrier. Mol Pharmacol. 2001;59:1022–1028. doi: 10.1124/mol.59.5.1022. [DOI] [PubMed] [Google Scholar]
- Sharina IG, Zhao R, Wang Y, Babani S, Goldman ID. Role of the C-terminus and the long cytoplasmic loop in reduced folate carrier expression and function. Biochem Pharmacol. 2002;63:1717–1724. doi: 10.1016/s0006-2952(02)00955-3. [DOI] [PubMed] [Google Scholar]
- Sirotnak FM. Obligate genetic expression in tumor cells of a fetal membrane property mediating “folate” transport: biological significance and implications for improved therapy of human cancer. Cancer Res. 1985;45:3992–4000. [PubMed] [Google Scholar]
- Sirotnak FM, Chello PL, Moccio DM, Kisliuk RL, Combepine G, Gaumont Y, Montgomery JA. Stereospecificity at carbon 6 of formyltetrahydrofolate as a competitive inhibitor of transport and cytotoxicity of methotrexate in vitro. Biochem Pharmacol. 1979;28:2993–2997. doi: 10.1016/0006-2952(79)90599-9. [DOI] [PubMed] [Google Scholar]
- Sirotnak FM, Kurita S, Hutchison DJ. On the nature of a transport alteration determining resistance to amethopterin in the L1210 leukemia. Cancer Res. 1968;28:75–80. [PubMed] [Google Scholar]
- Sirotnak FM, Moccio DM, Yang CH. A novel class of genetic variants of the L1210 cell up-regulated for folate analogue transport inward. Isolation, characterization, and degree of metabolic instability of the system. J Biol Chem. 1984;259:13139–13144. [PubMed] [Google Scholar]
- Sirotnak FM, Moccio DM, Kelleher LE, Goutas LJ. Relative frequency and kinetic properties of transport-defective phenotypes among methotrexate resistant L1210 clonal cell lines derived in vivo. Cancer Res. 1981;41:4442–4452. [PubMed] [Google Scholar]
- Sirotnak FM, Donsbach RC. Stereochemical characteristics of the folate-antifolate transport mechanism in L1210 leukemia cells. Cancer Res. 1974;34:371–377. [PubMed] [Google Scholar]
- Slotboom DJ, Konings WN, Lolkema JS. Cysteine-scanning mutagenesis reveals a highly amphipathic, pore-lining membrane-spanning helix in the glutamate transporter GltT. J Biol Chem. 2001;276:10775–10781. doi: 10.1074/jbc.M011064200. [DOI] [PubMed] [Google Scholar]
- Spector C, Johanson C. Micronutrient and urate transport in choroid plexus and kidney: Implications for drug therapy. Pharmaceutical Res. 2006;23:2515–2524. doi: 10.1007/s11095-006-9091-5. [DOI] [PubMed] [Google Scholar]
- Spector R, Lorenzo AV. Folate transport by the choroid plexus in vitro. Science. 1975;187:540–542. doi: 10.1126/science.1167256. [DOI] [PubMed] [Google Scholar]
- Stokstad ELR. Historical perspective on key advances in the biochemistry and physiology of folates. In: Picciano MF, Stokstad ELR, Gregory JF, editors. Folic Acid Metabolism in Health and Disease. New York: Wiley-Liss; 1990. pp. 1–21. [Google Scholar]
- Suleiman SA, Spector R. Purification and characterization of a folate binding protein from porcine choroid plexus. Arch Biochem Biophys. 1981;208:87–94. doi: 10.1016/0003-9861(81)90126-0. [DOI] [PubMed] [Google Scholar]
- Sweiry JH, Yudlievich DL. Transport of folates at maternal and fetal sides of the placenta. lack of inhibition by methotrexate. Biochim Biophys Acta. 1985;821:497–501. doi: 10.1016/0005-2736(85)90055-0. [DOI] [PubMed] [Google Scholar]
- Tse A, Brigle K, Taylor SM, Moran RG. Mutations in the reduced folate carrier gene which confer dominant resistance to 5,10-dideazatetrahydrofolate. J Biol Chem. 1998;273:25953–25960. doi: 10.1074/jbc.273.40.25953. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao R, Russell RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim Biophys Acta. 2001;1513:49–54. doi: 10.1016/s0005-2736(01)00340-6. [DOI] [PubMed] [Google Scholar]
- Westerhof GR, Schornagel JH, Kathmann I, Jackman AL, Rosowsky A, Forsch RA, Hynes JB, Boyle FT, Peters GJ, Pinedo HM, Jansen G. Carrier- and receptor-mediated transport of folate antagonists targeting folate-dependent enzymes: correlates of molecular-structure and biological activity. Mol Pharmacol. 1995;48:459–471. [PubMed] [Google Scholar]
- Whetstine JR, Flatley RM, Matherly LH. The human reduced folate carrier gene is ubiquitously and differentially expressed in normal human tissues: identification of seven non-coding exons and characterization of a novel promoter. Biochem J. 2002;367:629–640. doi: 10.1042/BJ20020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JC, Bailey BD, Goldman ID. Lack of stereospecificity at carbon 6 of methyltetrahydrofolate transport in Ehrlich ascites tumor cells. J Biol Chem. 1978;253:242–245. [PubMed] [Google Scholar]
- Williams FMR, Flintoff WF. Isolation of a human cDNA that complements a mutant hamster cell defective in methotrexate uptake. J Biol Chem. 1995;270:2987–2992. doi: 10.1074/jbc.270.7.2987. [DOI] [PubMed] [Google Scholar]
- Williams FMR, Murray RC, Underhill TM, Flintoff WF. Isolation of a hamster cDNA clone coding for a function involved in methotrexate uptake. J Biol Chem. 1994;269:5810–5816. [PubMed] [Google Scholar]
- Witt TL, Matherly LH. Identification of lysine-411 in the human reduced folate carrier as an important determinant of substrate selectivity and carrier function by systematic site-directed mutagenesis. Biochim Biophys Acta. 2002;1567:56–62. doi: 10.1016/s0005-2736(02)00583-7. [DOI] [PubMed] [Google Scholar]
- Witt TL, Stapels S, Matherly LH. Restoration of transport activity by co-expression of human reduced folate carrier half molecules in transport impaired K562 cells: localization of a substrate binding domain to transmembrane domains 7-12. J Biol Chem. 2004;279:46755–46763. doi: 10.1074/jbc.M408696200. [DOI] [PubMed] [Google Scholar]
- Wong SC, McQuade R, Proefke SA, Bhushan A, Matherly LH. Human K562 transfectants expressing high levels of reduced folate carrier but exhibiting low transport activity. Biochem Pharmacol. 1997;53:199–206. doi: 10.1016/s0006-2952(96)00710-1. [DOI] [PubMed] [Google Scholar]
- Wong SC, Proefke SA, Bhushan A, Matherly LH. Isolation of human cDNAs that restore methotrexate sensitivity and reduced folate carrier activity in methotrexate transport- defective Chinese hamster ovary cells. J Biol Chem. 1995;270:17468–17475. doi: 10.1074/jbc.270.29.17468. [DOI] [PubMed] [Google Scholar]
- Wong SC, Zhang L, Proefke SA, Matherly LH. Effects of the loss of capacity for N-glycosylation on the transport activity and cellular localization of the human reduced folate carrier. Biochim Biophys Acta. 1998;1375:6–12. doi: 10.1016/s0005-2736(98)00118-7. [DOI] [PubMed] [Google Scholar]
- Wong SC, Zhang L, Witt TL, Proefke SA, Bhushan A, Matherly LH. Impaired membrane transport in methotrexate-resistant CCRF-CEM cells involves early translation termination and increased turnover of a mutant reduced folate carrier. J Biol Chem. 1999;274:10388–10394. doi: 10.1074/jbc.274.15.10388. [DOI] [PubMed] [Google Scholar]
- Yang CH, Pain J, Sirotnak FM. Alteration of folate analogue transport inward after induced maturation of HL-60 leukemia cells. Molecular properties of the transporter in an overproducing variant and evidence for down-regulation of its synthesis in maturating cells. J Biol Chem. 1992;267:6628–6634. [PubMed] [Google Scholar]
- Yang CH, Sirotnak FM, Dembo M. Interaction between anions and the reduced folate/methotrexate transport system in L1210 cell plasma membrane vesicles: directional symmetry and anion specificity for differential mobility of loaded and unloaded carrier. J Membr Biol. 1984;70:285–292. doi: 10.1007/BF01871067. [DOI] [PubMed] [Google Scholar]
- Yang R, Sowers R, Mazza B, Healey JH, Huvos A, Grier H, Bernstein M, Beardsley GP, Krailo MD, Devidas M, Bertino JR, Meyers P, Gorlick R. Sequence alterations in the reduced folate carrier are observed in osteosarcoma tumor samples. Clin Cancer Res. 2002;9:837–844. [PubMed] [Google Scholar]
- Yin Y, He X, Szewczyk P, Nguyen T, Chang G. Structure of the multidrug transporter EmrD from Escherichia coli. Science. 2006;312:741–744. doi: 10.1126/science.1125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Kono M, McKee TD, Oprian DD. A general method for mapping tertiary contacts between amino acid residues in membrane-embedded proteins. Biochemistry. 1995;34:14963–14969. doi: 10.1021/bi00046a002. [DOI] [PubMed] [Google Scholar]
- Zeng FY, Hopp A, Soldner A, Wess J. Use of a disulfide cross-linking strategy to study muscarinic receptor structure and mechanisms of activation. J Biol Chem. 1999;274:16629–16640. doi: 10.1074/jbc.274.23.16629. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shirahatti NV, Mahadevan D, Wright SH. A conserved glutamate residue in transmembrane helix 10 influences substrate specificity of rabbit OCT2 (SLC22A2) J Biol Chem. 2005;280:34813–34822. doi: 10.1074/jbc.M506342200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22:7431–7457. doi: 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. The molecular identity and characterization of a Proton-coupled Folate Transporter--PCFT; biological ramifications and impact on the activity of pemetrexed. Cancer Metastasis Rev. 2007;26:129–139. doi: 10.1007/s10555-007-9047-1. [DOI] [PubMed] [Google Scholar]
- Zhao R, Assaraf YG, Goldman ID. A reduced carrier mutation produces substrate-dependent alterations in carrier mobility in murine leukemia cells and methotrexate resistance with conservation of growth in 5-formyltetrahydrofolate. J Biol Chem. 1998b;273:7873–7879. doi: 10.1074/jbc.273.14.7873. [DOI] [PubMed] [Google Scholar]
- Zhao R, Assaraf YG, Goldman ID. A mutated murine reduced folate carrier (RFC1) with increased affinity for folic acid, decreased affinity for methotrexate, and an obligatory anion requirement for transport function. J Biol Chem. 1998a;273:19065–19071. doi: 10.1074/jbc.273.30.19065. [DOI] [PubMed] [Google Scholar]
- Zhao R, Gao F, Goldman ID. Discrimination among reduced folates and methotrexate as transport substrates by a phenylalanine substitution for serine within the predicted eighth transmembrane domain of the reduced folate carrier. Biochem Pharmacol. 1999;58:1615–1624. doi: 10.1016/s0006-2952(99)00257-9. [DOI] [PubMed] [Google Scholar]
- Zhao R, Gao F, Babani S, Goldman ID. Sensitivity to 5,10-dideazatetrahydrofolate is fully conserved in a murine leukemia cell line highly resistant to methotrexate due to impaired transport mediated by the reduced folate carrier. Clin Cancer Res. 2000a;6:3304–3311. [PubMed] [Google Scholar]
- Zhao R, Gao F, Liu L, Goldman ID. The reduced folate carrier in L1210 murine leukemia cells Is a 58 kDa protein. Biochim Biophys Acta. 2000b;1466:7–10. doi: 10.1016/s0005-2736(00)00190-5. [DOI] [PubMed] [Google Scholar]
- Zhao R, Gao F, Wang PJ, Goldman ID. Role of the amino acid 45 residue in reduced folate carrier function and ion-dependent transport as characterized by site-directed mutagenesis. Mol Pharmacol. 2000c;57:317–323. [PubMed] [Google Scholar]
- Zhao R, Gao F, Wang Y, Diaz GA, Gelb BD, Goldman ID. Impact of the reduced folate carrier on the accumulation of active thiamin metabolites in murine leukemia cells. J Biol Chem. 2001a;276:1114–1118. doi: 10.1074/jbc.M007919200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Russell RG, Wang Y, Liu L, Gao F, Kneitz B, Edelman W, Goldman ID. Rescue of embryonic lethality in reduced folate carrier-deficient mice by maternal folic acid supplementation reveals early neonatal failure of hematopoietic organs. J Biol Chem. 2001b;276:10224–10228. doi: 10.1074/jbc.c000905200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Wang Y, Gao F, Goldman ID. Residues 45 and 404 in the murine reduced folate carrier may interact to alter carrier binding and mobility. Biochim Biophys Acta. 2003;1613:49–56. doi: 10.1016/s0005-2736(03)00136-6. [DOI] [PubMed] [Google Scholar]
- Zhou F, You G. Molecular insights into the structure-function relationship of organic anion transporters OATs. Pharm Res. 2007;24:28–36. doi: 10.1007/s11095-006-9144-9. [DOI] [PubMed] [Google Scholar]