Abstract
Muscle contractions strongly activate p38 MAP kinases, but the precise contraction-associated sarcoplasmic event(s) (e.g. force production, energetic demands and/or calcium cycling) that activate these kinases are still unclear. We tested the hypothesis that during contraction the phosphorylation of p38 isoforms is sensitive to the increase in ATP demand relative to ATP supply. Energetic demands were inhibited using N-benzyl-p-toluene sulphonamide (BTS, type II actomyosin) and cyclopiazonic acid (CPA, SERCA). Extensor digitorum longus muscles from Swiss Webster mice were incubated in Ringer’s solution (37°C) with or without inhibitors and then stimulated at 10 Hz for 15 min. Muscles were immediately freeze-clamped for metabolite and western blot analysis. BTS and BTS+CPA treatment decreased force production by 85%, as measured by the tension time integral, while CPA alone potentiated force by 310%. In control muscles, contractions resulted in a 73% loss of ATP content and a concomitant 7-fold increase in IMP content, a measure of sustained energetic imbalance. BTS or CPA treatment lessened the loss of ATP, but BTS+CPA treatment completely eliminated the energetic imbalance since ATP and IMP levels were nearly equal to those of non-stimulated muscles. The independent inhibition of cytosolic ATPase activities had no effect on contraction-induced p38 MAPK phosphorylation, but combined treatment prevented the increase in phosphorylation of the γ isoform while the α/βisoforms unaffected. These observations suggest that an energetic signal may trigger phosphorylation of the p38γ isoform while other factors are involved in activating the α/β isoforms, and also may explain how contractions differentially activate signaling pathways.
Keywords: Ultra Performance Liquid Chromatography, skeletal muscle, mitochondria, adaptation, exercise
INTRODUCTION
Skeletal muscle has a remarkable ability to modify gene expression and thus remodel in response to contractile activity (Booth et al., 2002; LaFramboise et al., 2009). The extent of remodeling and the particular genes expressed can vary considerably depending on the exercise protocol (Dudley et al., 1982; Hulmi et al., 2012) or the muscle innervation pattern (Buller et al., 1960). While it is clear that differential gene expression is driven, at least in part, by the activation of particular signaling cascades (Bassel-Duby and Olson, 2006; Röckl et al., 2008), relatively little is known about how contractions can selectively activate different signaling cascades.
Muscle contraction is comprised of a series of major intracellular events including dramatic increases in calcium concentration, tension development and their associated, energetic demands. Therefore, one mechanism to explain the differential activation of signaling molecules is that the intensity of each contraction-induced sarcoplasmic event is interpreted separately and serves as an independent activator of signaling molecules. In fact, this has been shown conclusively in a few instances. For example, cytosolic calcium, which increases many-fold during each contraction cycle, binds directly to calmodulin and leads to activation of calcineurin (a phosphatase that is critical to changes in muscle fiber type) (Parsons, 2004). Similarly, a rise in the high-energy phosphate AMP, coincident with increased energy demand of contraction, activates AMP-activated protein kinase (a molecule involved in both mitochondrial biogenesis and glucose homeostasis) (Witczak et al., 2008). Unfortunately, the contraction-induced sarcoplasmic events that activate many other signaling molecules are not as well characterized.
The p38 mitogen-activated protein kinases (MAPKs) are a family of signaling molecules central to many cellular adaptations, but the sarcoplasmic event(s) that initiate their activation are not precisely known. In skeletal muscle, the MAPKs play a major role in muscle development (Foster et al., 2012; Gillespie et al., 2009; Lovett et al., 2010), mitochondria biogenesis (Hong et al., 2011; Pogozelski et al., 2009), and maintenance of fiber size (Foster et al., 2012; McClung et al., 2010). Four isoforms of p38 MAPK are expressed in skeletal muscle: p38α, p38β, p38δ and p38γ, with p38γ being the most abundant (Goedert et al., 1997; Lechner et al., 1996; Wang et al., 1997). While these isoforms have partial functional redundancy because they share some of the same substrates (Cuenda and Rousseau, 2007), it is now clear that each isoform has a distinct role in muscle. For example, p38γ, but not p38α or p38β, regulates expression of PGC-1α (a transcriptional co-activator sufficient to increase mitochondrial content) in response to muscle contractions (Pogozelski et al., 2009). Similarly, p38α, but not p38γ, is essential for myoblast differentiation in culture (Lovett et al., 2010). Given the distinct roles of the p38 MAPK isoforms, it is plausible that the activation of each isoform is initiated by a different sarcoplasmic event.
The kinase activity of all isoforms of p38 MAPK are activated by dual phosphorylation of a highly conserved three amino acid motif (Thr-Gly-Tyr) (Cuadrado and Nebreda, 2010). In contracting skeletal muscle, a positive relationship exists between total force production and MAPK phosphorylation/activation (Boppart et al., 2001; Martineau and Gardiner, 2001; Ryder et al., 2000; Wretman et al., 2001). However, the precise sarcoplasmic event or events that induce phosphorylation is controversial. Increasing passive tension alone activates p38 MAP kinases in some (Boppart et al., 2001; Hanke et al., 2010) but not all (Martineau and Gardiner, 2001; Wretman et al., 2001) studies. However, Dentel et al. have shown that active tension development during muscle contraction is not required for the phosphorylation of p38 MAPK (Dentel et al., 2005). In addition an increased energetic imbalance imposed by chronic depletion of creatine (Levine et al., 1996) leads to an increased phosphorylation of p38 MAPK in skeletal muscle (Williams et al., 2009). However, which sarcoplasmic events are responsible for inducing phosphorylation of each of the p38 MAPK isoforms during contraction remains unresolved because the major events have not been tested independently.
The purpose of this study was to test whether p38 MAPK isoforms in skeletal muscle are activated during contractions by a persistent imbalance between energetic supply and demand. Nearly all ATP use during skeletal muscle contraction is by actomyosin-ATPase and sarco/endoplasmic reticulum calcium-ATPase (SERCA) (Rall, 1985). Through the use of specific chemical inhibitors of actomyosin-ATPase and SERCA, the effects of force, calcium, and energetic imbalance can be established in vivo by using isolated murine skeletal muscle. Thus, energy utilization both by activation (contraction) and inhibition (chemical reagents) of the specific ATPases associated with physiologic function in muscle can be reliably controlled and interpreted in the context of intracellular signaling pathways, a factor absent in some studies. We tested the hypothesis that the phosphorylation pattern of p38 isoforms is sensitive to the increases in ATP demand relative to ATP supply. Understanding how the events associated with muscle contraction are coupled to the activation of p38 MAPK isoforms may lend insight into how signaling pathways are integrated to elicit many different exercise-induced adaptations.
METHODS AND MATERIALS
Materials and Animals
ATPase inhibitors, N-benzyl-p-toluene sulphonamide (BTS) and cyclopiazonic acid (CPA), were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Rabbit polyclonal antibody to phosphorylated p38 (Thr180/Tyr182) was purchased from Cell Signaling Technology (Beverly, MA; catalog #9211). This antibody detects phospho-p38α, p38β, and p38γ isoforms. Total p38 was detected with rabbit antibodies specific for the α isoform (Cell Signaling Technology; catalog #9212), and γ isoform (anti-SAPK3, Upstate Cell Signaling Solutions, Charlottesville, VA; catalog #07-139). Goat anti-rabbit IgG linked to horseradish peroxidase was purchased from Cell Signaling Technology (catalog #7074). All other chemicals were obtained from Sigma-Aldrich.
Adult, male Swiss Webster mice (28-32 g body weight) (Harlan, Indianapolis, IN) were housed in a 12h light/12h dark cycle and given food and water ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee at Michigan State University.
Isolated Muscle Preparations
Mice were anesthetized with an intraperotineal injection of pentobarbital (50 mg/kg). Extensor digitorum longus (EDL) muscles were ligated at the proximal and distal tendons with 5.0 silk sutures, removed from the hindlimbs, and secured in a 37°C water-jacketed organ bath (Radnoti Glass Technology, Inc., Monrovia, CA) at their approximate in vivo resting length. The bath contained 8 ml modified Ringer solution (117 mM NaCl, 4.6 mM KCl, 25 mM NaHCO3, 2.5 mM CaCl2, 1.16 mM MgSO4, and 11 mM glucose, pH 7.4) with 10 mg/L gentamycin and was continuously gassed with 95% O2/5% CO2. Mouse EDL muscles were selected for two reasons. First, these muscles are sufficiently small in diameter so that diffusion of oxygen to the muscle core is adequate to maintain the muscle energetic state, even during prolonged incubations (Crow and Kushmerick, 1982; Wiseman and Kushmerick, 1995). Second, EDL muscles are comprised of greater than 95% type II fibers (Augusto et al., 2004), and BTS is specific for only type II myosin (Cheung et al., 2002).
Muscles were field-stimulated via platinum electrodes using a Grass S88 Stimulator (Grass Instruments, Quincy, MA). After adjusting the muscle to optimal length (Lo) using the length tension relationship, initial maximal twitch force was determined using supramaximal voltage. Only muscles that were physiologically stable and able to generate 5 or more grams of force under supramaximal stimulation prior to the start of any experimental protocol were included in this study.
All muscles were treated with inhibitors and/or vehicle for a total of 60 minutes. To inhibit both cytosolic ATPases, muscles were first treated with BTS (in 20 μl DMSO). Thirty minutes later, CPA was added (in 20 μl DMSO). Muscles were treated in this order because inhibition of SERCA by CPA causes a rise in intracellular calcium levels (Baudet et al., 1993; Robin et al., 2012) that may be sufficient to increase basal tension by activation of actomyosin ATPase. For experiments where a single ATPase was inhibited, 20 μl DMSO was added to the bath during the first 30 minute period, followed by addition of either 20 μl BTS or CPA stock solutions for the remaining 30 minutes. The final concentration of DMSO was 0.5% (v/v) in all experiments. When present, the final concentration of BTS was 75 μM, and the final concentration of CPA was 50 μM.
Inhibitor concentrations were selected based on the minimum concentration necessary to abolish the function of each ATPase. Dentel et al. have previously shown that 75 μM BTS is sufficient to inhibit force production by over 95% within 30 minutes but has no effects on Ca2+ kinetics (Dentel et al., 2005). To determine the optimal concentration of CPA, a dose response curve was constructed using the increase in relaxation time and peak force as indices of SERCA inhibition. Muscles were incubated in different concentrations of CPA, and twitches were performed every 10 minutes for a period of 30 min in total.
Muscles were electrically stimulated with 0.5 msec duration pulses delivered at a frequency of 10 Hz for 15 minutes. A sub-set of muscles were not stimulated, but were instead kept at Lo for an identical duration (15 minutes). Immediately after this 15 minute period, the sutures securing the muscles were cut, and the muscles were rapidly blotted of medium and freeze-clamped between stainless steel tongs pre-cooled in liquid nitrogen to preserve the energetic state and protein phosphorylation status. Muscles were frozen within approximately 3 seconds of removal from the organ bath, and this time was not different between groups. Muscles were stored at −80°C until further analysis.
Metabolite analyses
EDL muscles were cut free of their sutures on a liquid nitrogen cooled aluminum block and weighed while still frozen. Metabolites were extracted in 20 to 30-fold excess ice-cold 0.5 N perchloric acid with rapid homogenization by pre-cooled 1.4 mm stainless steel beads in a Bullet Blender (Next Advance). Extracts were neutralized and perchlorate was removed by the addition of ice-cold KOH and subsequent centrifugation at 4°C. Samples were stored at −80°C until analysis.
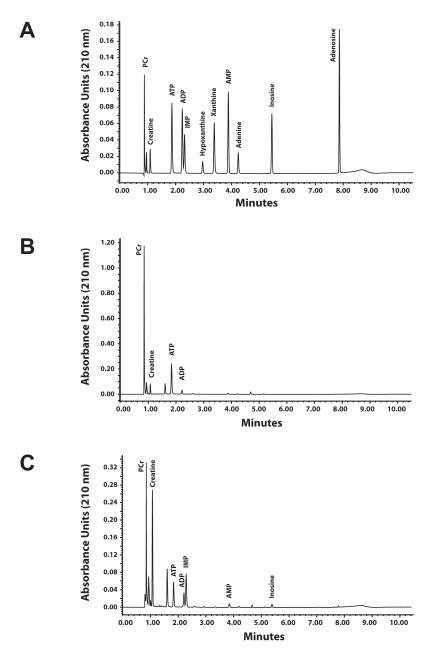
Concentrations of phosphocreatine (PCr), creatine (Cr), adenine nucleotides (ATP, ADP, and AMP), and adenine nucleotide degradation products (IMP, adenosine, adenine, and inosine) were determined by ultra performance liquid chromatography (UPLC) using a Waters Acquity UPLC H-class system. The method is based on a high performance liquid chromatography method from Teerlink et al. (Teerlink et al., 1993). UPLC separation was achieved by gradient reverse-phase chromatography using an Acquity UPLC HSS T3 1.8 μm, 2.1 × 150 mm column (Waters) maintained at 35°C. Mobile phases consisted of 0.2 M KH2PO4 (buffer A) and 50% water / 25% acetonitrile / 25% methanol (buffer B). Flow rate was a constant 0.4 ml /min with the initial conditions 98.5% buffer A and the balance buffer B. Starting at 0.1 minutes, the percent buffer A decreased linearly to 85% at 6 minutes and further decreased to 50% at 7 minutes. The mobile phase was returned to the initial buffer A: buffer B ratio (98.5 : 1.5) at 7.2 minutes, where it remained until 10.5 minutes to re-equilibrate the column, after which a new sample could be injected. Metabolites were identified by comparison of peak retention times of pure, commercially available standards (Sigma-Aldrich), which were separated with baseline resolution in less than 7 minutes (Fig. 1A). Baseline resolution was also achieved with extracts from non-contracted (Fig. 1B) and 15 minute contracted (Fig. 1C) EDL muscles demonstrating that other metabolites did not interfere with this chromatography method. Quantification was by comparing peak area at 210 nm, which was linear with amount injected from 0.2 pmol to 100 pmol with an r2 >0.998 for all metabolites.
Figure 1.
Ultra-performance liquid chromatography chromatograms. A: Chromatogram of a standard mixture containing 100 pmol each phosphocreatine (PCr), creatine, ATP, ADP, AMP, IMP, adenosine, inosine, and xanthine and 25 pmol each hypoxanthine and adenine. Detection limits are less than 1.0 pmol B: Chromatogram of a perchloric acid extract of a non-contracted EDL muscle incubated at resting length. Injection volume was 2 μl. C: Chromatogram of an extract from an EDL muscle after contracting at 10 Hz for 15 min.
These UPLC measures can provide at least two clear indices of intracellular energetic state. First, the ratio of the concentrations of PCr and Cr is an index of short-term ATP demand. Under conditions where equilibrium assumptions coupling PCr/Cr to the cytosolic ATP/ADP pools are met, this is a measure of the available ATP free energy within the cytosol (Meyer et al., 1984; Wiseman and Kushmerick, 1995). Second, the amount of inosine monophosphate (IMP) reflects longer periods of exercise-induced metabolic demand exceeding supply as the available adenylate pool is deaminatd to IMP (Meyer and Terjung, 1979). The accumulation of IMP occurs within the cytosol, is effectively an irreversible process during contractions (Meyer and Terjung, 1980), and is only slowly converted back to AMP after several minutes of rest in fast-twitch muscle (Meyer and Terjung, 1979). Therefore, over the timeframe of these stimulation protocols, IMP accumulation is a reliable measure of sustained mismatch between ATP supply and ATP demand.
Western blot analysis
Frozen muscles were homogenized in isotonic saline containing glycerol, DTT, SDS, protease inhibitors, and phosphatase inhibitors as previously described (Dentel et al., 2005). Briefly, 50 μg of total protein for each sample was resolved by sodium dodecyl sulfate polyacrylamide electrophoresis (SDS-PAGE) through 10% or 12% (w/v) acrylamide gels and then transferred onto nitrocellulose membranes. Membranes were reversibly stained with Ponceau S to ensure equal loading and transfer of proteins, blocked, and then incubated overnight at 4 °C with primary antibodies diluted 1:1000. After incubation with secondary antibody-HRP conjugate, bound second antibody was detected using the Phototope-HRP Western Blot Chemiluminescent kit (Cell Signaling Technology, Beverly, MA). Chemiluminescent signals were visualized on Amersham ECL Hyperfilm (Amersham Biosciences, Piscataway, NJ), digitally captured, (EDAS 290 gel documentation system, Eastman Kodak, Rochester, NY), and quantified by densitometry (Quantity One Image Analysis software, BioRad, Hercules, CA). The amount of enzyme that is in the phosphorylated state for each p38 MAP kinase isoform was expressed as a ratio to the total p38 MAPK for each isoform. For comparisons between each blot, densitometry was quantified relative to an internal standard comprised of a pooled set of stimulated muscles used on each gel, and an absolute optical density scale was used for quantification from the images as previously described (Dentel et al., 2005).
Statistics
Data are presented as the mean ± standard error (SE). Statistical significance (P < 0.05) was determined by ANOVA. Differences between groups, if found, were assessed post hoc by Tukey-Kramer HSD.
RESULTS
Inhibition of SERCA by CPA
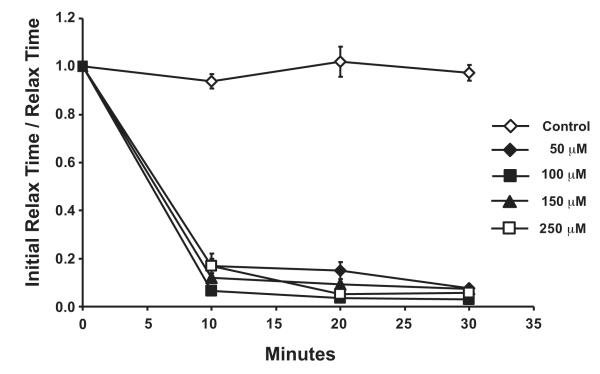
The optimum concentration of CPA to inhibit SERCA function was derived from inhibitor titration experiments. The normalized data show that all concentrations had a time-dependent increase in the total relaxation time (an index of SERCA inhibition) reaching a maximum at 30 min (Fig 2). At the highest concentration (250 μM), CPA caused a decrease in peak twitch force (data not shown). To minimize non-specific effects on force, all subsequent CPA incubation experiments were performed using the 50 μM dose.
Figure 2.
Dose response curves for cyclopiazonic acid (CPA) over time in isolated EDL mouse muscles. Inhibition of SERCA by CPA was evaluated by measuring the total relaxation time for each twitch contraction (in msec), normalized to the percent of initial (Time 0), and then expressed as the reciprocal. The data are presented as mean ± SE.
Specific inhibitor effects on contractile performance
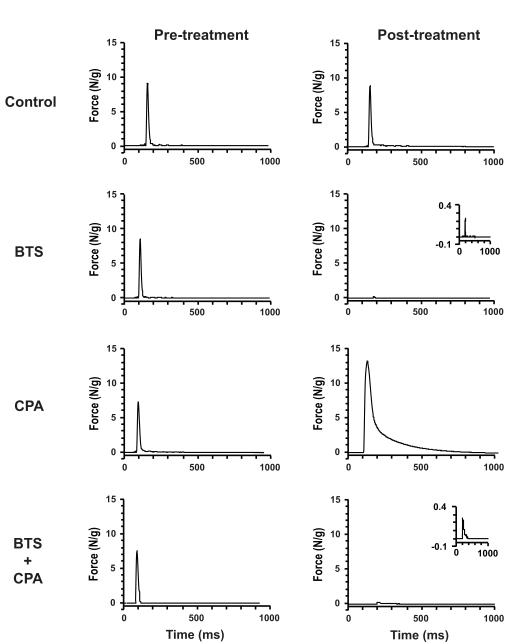
To discriminate the effects of each inhibitor alone or in combination on physiologic function, single twitch characteristics were evaluated both pre- and post-inhibitor treatment (Table 1). In isolated EDL muscles, control treatment (DMSO) had no clear effects on twitch kinetics, and peak force was sustained at better than 10 N/g (Fig 3). In contrast, treatment with BTS caused >95% reduction in twitch peak force and tension time integral with only modest increase in rise time and decrease in relaxation time, consistent with observations reported previously from this laboratory (Dentel et al., 2005) and others (Cheung et al., 2002; Young et al., 2003). Incubation with the SERCA inhibitor CPA caused a 1.8-fold increase in peak force and a dramatic seven-fold increase in the total relaxation time from 28 to almost 200 msec (Table 1), also in agreement with previous reports (Kurebayashi and Ogawa, 1991; Même et al., 1998). The combination of greater peak force and longer relaxation time resulted in a 12-fold increase in the tension-time integral for a single twitch. Treatment with both BTS and CPA had little influence on twitch kinetics but force was suppressed by >90%, presumably because BTS effects predominate. The net effect of using these inhibitors in combination depressed the overall tension-time integral by almost 90%.
Table 1.
Muscle mass and twitch characteristics before (Pre-treatment) and after (Post-treatment) treatment with DMSO (control), 75μM BTS, 50μM CPA, and CPA + BTS. The values represent the average rise time, relaxation time, peak force, and tension time integral +/− SE (n= 6 or 7 per group).
| Pretreatment |
Post Treatment |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Weight (mg) |
Rise Time (ms) |
Relaxation Time (ms) |
Peak Force (N/g) |
TTI (N.s/g) |
Rise Time (ms) |
Relaxation Time (ms) |
Peak Force (N/g) |
TTI (N.s/g) |
|
| Control | 10.3 ± 0.5 | 10.0 ± 0.3 | 20 ± 3 | 12.0 ± 1.0 | 0.15 ± 0.01 | 10.0 ± 0.0 | 19 ± 0 | 10.8 ± 0.7 | 0.165 ± 0.013 |
| BTS | 10.3 ± 0.3 | 112 ± 0.5 | 28 ± 4 | 8.5 ± 0.4 | 0.12 ± 0 01 | 8.5 ± 1.4 | 18 ± 1 | 0.5 ± 0.2 | 0.006 ± 0.002 |
| CPA | 7.8 ± 0.3 | 9.3 ± 0.7 | 28 ± 4 | 8.5 ± 0.9 | 0.14 ± 0.01 | 28.7 ± 3.7 | 198 ± 165 | 15.5 ± 1.4 | 2.045 ± 0.345 |
| BTS+CPA | 13.8 ± 1.0 | 12.0 ± 0.9 | 30 ± 8 | 6.6 ± 0.5 | 0.15 ± 0.03 | 11.7 ± 1.3 | 30 ± 8 | 0.3 ± 0.03 | 0.017 ± 0.003 |
Figure 3.
Representative twitch force recordings of electrically stimulated muscles before (Pre-) and after (Post-) treatment with DMSO (Control) or with the ATPase inhibitors BTS (75 μM), CPA (50 μM), and CPA (50 μM) + BTS (75 μM).
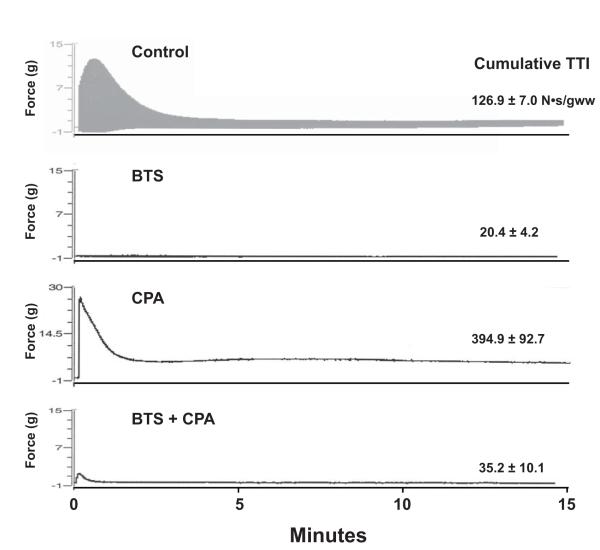
To examine the effects of specific inhibition of ATPase activities during repeated contractions, EDL muscles were stimulated at 10 Hz for 15 minutes. Time courses of typical experiments are presented in Figure 4. Stimulation of EDL muscles in the presence of DMSO alone (Control) potentiated force production during the first few seconds to generate peak force values of nearly 14 grams. However, force fell rapidly within the first 4 minutes of stimulation by 80% and remained at that level for the remainder of the stimulation. The cumulative tension time integral for all twitches over the entire stimulation time course was 127 N.s/gww and was used as an index of the imposed workload. In agreement with the single twitch measurements, treatment with BTS caused a substantial decrease in overall force production by 85%. The cumulative tension time integral for BTS-treated muscles was 20 N.s/gww. In contrast, when CPA treated muscles were stimulated, peak force production increased to over 28 grams because calcium reuptake was inhibited but fell rapidly within 2 minutes presumably due to the higher force production associated with SERCA inhibition. These muscles failed to fully relax between pulses presumably due to inhibition of calcium pumping from the cytosol by SERCA and appeared as one fused contraction. Therefore, CPA treated muscles yielded the highest cumulative tension time integral of all the groups (395 N.s/gww). The combined treatment of muscles with both BTS and CPA resulted in a near total loss of force production similar to that observed with BTS alone (Fig. 4), but because of the increased relaxation time for each twitch, the summed tension time integral for BTS+CPA was larger than BTS treatment alone (35 N.s/gww).
Figure 4.
Representative force recordings of incubated muscles electrically stimulated at 10 Hz for 15 min. Muscles were treated with DMSO (control) (n=6), BTS (n=7), CPA (n=7), or CPA + BTS (n=7) for 60 minutes before stimulation. The cumulative tension time integral (TTI) of each group is presented as mean ± SE and represents the total amount of contractile performance during the entire time course.
High-energy phosphates
In the non-stimulated muscles treated only with vehicle (DMSO), the PCr/Cr ratio, an immediate indicator of the energetic state, was approximately 3:1 (Table 2). This is consistent with previous HPLC measures from perchloric acid extracts of non-contracting mouse muscles (Hancock et al., 2005; Kushmerick et al., 1992; Wiseman and Kushmerick, 1995) and demonstrates that neither the muscle incubation nor treatment with DMSO had any pronounced detrimental effects on muscle energetics. Inosine monophosphate levels, an indicator of long-term energetic mismatch, were 0.35 ± 0.09 μmol/gww, which is comparable to values reported by others in resting fast-twitch muscles from mice (Hancock et al., 2005).
Table 2.
Phosphocreatine (PCr), creatine (Cr), adenine nucleotide, IMP and inosine content of resting (Non-stimulated) and 15 minute stimulated (Control, BTS, CPA and BTS+CPA) EDL muscles. Values expressed as μmol/g, mean ± SE.
| PCr | Creatine | [PCr]/[Cr] | ATP | ADP | AMP | IMP | Inosine | |
|---|---|---|---|---|---|---|---|---|
| Non-stimulated | 14.53 ± 0.76 | 5.05 ± 0.27 | 2.94 ± 0.25 | 4.24 ± 0.39 | 0.59 ± 0.03 | 0.16 ± 0.03 | 0.35 ± 0.09 | 0.11 ± 0.03 |
| Control | 3.89 ± 0.24* | 13.84 ± 0.28* | 0.28 ± 0.02* | 1.15 ± 0.15* | 0.61 ± 0.06 | 0.20 ± 0.01 | 2.48 ± 0.09* | 0.25 ± 0.03* |
| BTS | 4.58 ± 0.80* | 13.16 ± 0.29* | 0.35 ± 0.06* | 1.97 ± 0.15* | 0.66 ± 0.02 | 0.18 ± 0.01 | 1.90 ± 0.09*† | 0.16 ± 0.01 |
| CPA | 9.32 ± 0.86*† | 10.14 ± 0.56*† | 0.96 ± 0.14*† | 2.21 ± 0.21*† | 0.60 ± 0.02 | 0.14 ± 0.01 | 2.15 ± 0.21*† | 0.18 ± 0.03 |
| BTS + CPA | 11.84 ± 0.68† | 7.33 ± 0.48*† | 1.68 ± 0.18*† | 3.61 ± 0.18† | 0.68 ± 0.04 | 0.15 ± 0.03 | 0.58 ± 0.08† | 0.07 ± 0.02† |
Significantly different (P<0.05) than Non-stimulated
Significantly different (P<0.05) than Control
Intense electrical stimulation of control-treated EDL muscles at 10 Hz for 15 minutes resulted in a 73% fall in both PCr and ATP concentrations (P< 0.05), with a concomitant 2.7-fold increase in Cr and a 7.1-fold increase in IMP (p<0.05) (Table 2). This contraction protocol also resulted in a modest, but significant (P<0.05), increase in inosine concentration. However, further degradation of the adenine nucleotide pool was not evident since neither adenine (0.12 μmol/gww) nor adenosine (0.10 μmol/gww) was different between contracted and non-contracted muscles. Inhibition of actomyosin ATPase with BTS in conjunction with the same stimulation protocol had no significant effect on the decrement of PCr or ATP levels, and IMP accumulation was nearly identical to that of stimulated muscles in the absence of inhibitors (1.90 ± 0.09 μmol/gww). These observations indicate that the energetic costs of calcium handling are still overwhelming ATP free energy supplies within the 15 min timeframe. Consistent with the observations of BTS experiments, inhibition of SERCA with CPA during stimulation tempered the fall in PCr and ATP, but the accumulation of IMP was near that of stimulated control muscles (2.15 ± 0.21 μmol/gww). This indicates that large energetic demands occur while SERCA is inhibited likely due to the higher myosin ATPase use evident by higher contractile force production from these treatments (Table 1, Fig 4). In contrast, when a combination of BTS and CPA is used during contractions, there is a large decrease in the mismatch between ATP demand and supply as shown by near resting levels of PCr, Cr, and ATP and a modest drop in the PCr/Cr ratio (Table 2). More importantly, the IMP concentration (0.58 ± 0.08 μmol/gww) was no longer different than that found in control non-stimulated muscles.
Phosphorylation of p38 MAPK isoforms
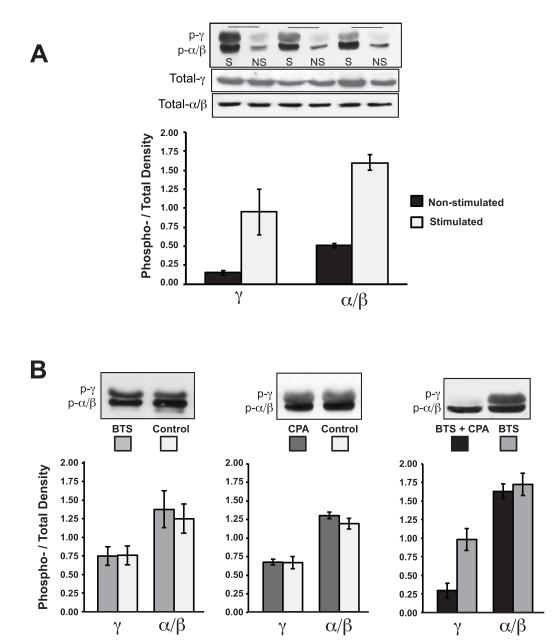
In resting muscles, the ratio of phosphorylated p38α/β to total p38α/β protein was 0.51 ± 0.03 and the ratio of phosphorylated p38γ to total p38γ protein was 0.15 ± 0.04 (Fig.5A). These values were not significantly different when measured in freshly dissected muscles (data not shown) demonstrating that the incubation at Lo had no effect on the phosphorylation state in isolated, superfused preparations. However, stimulation at 10 Hz for 15 min profoundly increased the phosphorylation ratio of the p38 isoforms, 1.6 ± 0.1 for p38α/β and 0.95 ± 0.3 for p38γ (Fig. 5). This represents a 3- and 6-fold increase in the phosphorylation of p38α/β and p38γ relative to the non-stimulated muscles incubated. Western blots of total MAPK present demonstrated that stimulation had no effect on isoform protein expression levels, as expected given the short duration of these experiments. Therefore, the increased amount of phosphorylation of each isoform also reflects a decrease in the non-phosphorylated form.
Figure 5.
The increase in phosphorylation of p38-MAPK isoforms in skeletal muscle with electrical stimulation is prevented by combined treatment with BTS and CPA. A: Muscles were either stimulated (S) at 10 Hz for 15 minutes, or non-stimulated (NS). Protein levels of phospho-p38 MAPK isoforms and total p38 MAPK were determined by western blot. Quantification was based on the fraction of phosphorylated protein relative to the total detected by Western blot analysis (n=3). B: Muscles were treated without (control) or with (BTS and CPA) cytosolic ATPase inhibitors and then stimulated for 15 min. Protein levels of phospho-p38 MAPK isoforms were determined by western blot. Bar graphs represent the mean ± SE; n=4.
To test whether phosphorylation of the p38 isoforms was dependent on force production, muscles were incubated in the absence or presence of BTS and then electrically stimulated. Even though there was a dramatic decrease in force (Table 1, Fig. 3), BTS did not decrease the phosphorylation of the p38 isoforms as compared to control-stimulated muscles (Fig. 5B). This confirms previous findings that p38 phosphorylation is not mediated by active force production nor the specific energy demands of actomyosin ATPases (Dentel et al., 2005). Furthermore, it was previously shown that stimulating muscles at 10 Hz for 15 seconds (an equivalent tension-time integral to BTS-treated muscles stimulated for 15 minutes) did not increase p38 phosphorylation relative to non-stimulated muscle (Dentel et al., 2005). Therefore, even the residual force generated after BTS treatment is not sufficient to activate p38 MAPK.
Sarcoplasmic reticulum-ATPase (SERCA) catalyzes the reuptake of Ca2+ into the SR, which accounts for roughly 30% of the free energy used in contracting muscle (Rall, 1982). To test whether calcium or the energetic costs of Ca2+ handling induces phosphorylation of the p38 isoforms, muscles were treated with CPA. In muscles stimulated at 10 Hz for 15 minutes, the phosphorylation pattern for the p38 isoforms was not significantly different from the control-stimulated muscles (Fig. 5B), demonstrating that neither the higher, sustained cytosolic Ca2+ nor the reduction of energetic demands associated with calcium pumping were sufficient to alter the phosphorylation pattern for any of the p38 isoforms.
In stimulated muscles treated with both BTS and CPA (Fig. 5B), the phosphorylation of p38 α/β (1.63 ± 0.1) was not different from stimulated muscles that were treated with BTS alone (1.73 ± 0.14). However, the phosphorylation of p38γ did not reach the same level (0.30 ± 0.08) as BTS-treated muscle (0.98 ± 0.15). The finding that combined treatment of BTS and CPA prevented p38γ phosphorylation suggests that activation of the p38γ isoform but not p38α/β isoforms of MAPK could be highly dependent on changes in energetic demand since both major ATPases active in skeletal muscle during contraction were being inhibited. By extension, p38γ cannot be activated by changes in cytosolic calcium because CPA treatment results in large increases in cytosolic calcium (as shown in frog (Même et al., 1998) and murine muscle fibers (Robin et al., 2012) as well as evidenced by the mechanics data in Figure 4), yet muscle treated with both CPA and BTS have no increase in the p38γ signal.
DISCUSSION
The novel result of this study was that the phosphorylation of p38 MAP kinase isoforms in contracting skeletal muscle is differentially activated by an imbalance between ATP supply and demand. The p38γ MAPK isoform appears to have a higher sensitivity to energetics, while the α/β isoforms appear to be less sensitive or not sensitive at all to changes in energetics. Isolated, superfused fast-twitch skeletal muscles were electrically stimulated in the absence and presence of ATPase inhibitors, BTS (a type II actomyosin-ATPase inhibitor) and CPA (a SR-ATPase inhibitor), in an effort to isolate the critical sarcoplasmic event(s) associated with contraction. Stimulating muscle in the presence of BTS alone, which eliminates force production but allows normal calcium transients to proceed, had no effect on the phosphorylation of any of the p38 isoforms when compared to just stimulation alone, confirming a previous report that force production is not responsible for the activation of p38 signaling (Dentel et al., 2005). However, when CPA and BTS were used together, a combination that leads to an increase in intracellular calcium (Robin et al., 2012) but may eliminate up to 97% of the free energy used in exercised muscle (Rall, 1982; Rall, 1985), the increase in p38γ phosphorylation typically found with stimulation was blunted. This demonstrates that p38 MAPK γ is not activated by increased contractile force or intracellular calcium concentration, but instead is responsive to changes in the balance of ATP usage since blocking ATPases also blunts phosphorylation. Conversely, neither CPA, BTS, nor CPA+BTS prevented the phosphorylation of the p38α/β isoforms during stimulation, which suggests that the p38α/β isoforms may respond to another sarcoplasmic event such as calcium release (which is elevated by stimulation, in the presence or absence BTS or CPA) or by processes associated with membrane depolarization.
The present findings that an energetic mismatch during contraction induces p38γ phosphorylation agree well with studies not using ATPase inhibitors that have reported that the degree of p38 phosphorylation is directly related to force production (Martineau and Gardiner, 2001; Wretman et al., 2001), because the energetic cost of contraction scales directly with active tension (Rall, 1982; Rall, 1985). Even without contraction, chronic energetic stress induces p38γ phosphorylation in muscle. When animals are fed a diet of β-guanidopropionic acid () for several weeks, creatine and phosphocreatine are depleted from skeletal muscle, and the cytosolic phosphate potential decreases (Williams et al., 2009; Wiseman and Kushmerick, 1995). Western blot analysis of lysates from the muscles of these animals shows an increase in p38γ phosphorylation (Williams et al., 2009) supporting the conclusions of the present study. Furthermore, recent work from Foster et al. (2012) have shown that lysates from slow twitch muscles have higher levels of phosphorylated MAPK isoforms than the representative fast twitch muscles from these same animals. Taken together with the higher energy turnover in the tonically active slow-twitch muscles relative to fast-twitch, these data support the relationship between energetics and p38γ MAPK from this report.
Previous studies have also reported that increases in passive tension alone are associated with activation p38 MAPK (Boppart et al., 2001; Hanke et al., 2010) in apparent contradiction to the findings of this study. One possible mechanism that would explain this contradiction lies in the sarcoplasmic events that occur when muscles are stretched beyond their normal resting length. Stretch is able to activate mechanosensitive ion channels (Lansman and Franco-Obregón, 2006), which may lead to an increase in intracellular calcium and subsequent ATP use by SERCA and possibly myosin-ATPase. Unfortunately these studies did not control or measure calcium or energetic levels associated with the changes in resting length. Therefore, a mismatch in muscle energetics, not mechanical stretch per se, may induce the phosphorylation of p38 MAPKs in stretched, non-stimulated muscles. A second possible explanation is that p38 γ may be activated by more than one sarcoplasmic event. In other words, both force and energetics may each be capable of activating p38γ. For example, our findings do not exclude the possibility that extreme passive stretching can induce phosphorylation of p38 MAPK. However, force appears to have no quantitative role during intense isometric twitch contractions since stimulated muscles treated with BTS, which do not produce force, had the same amount of phospho-p38γ as stimulated muscles that do produce force (Fig 5B).
The sensitivity of p38γ phosphorylation to energetics as described in the current work may mechanistically also explain the timing of p38 MAP kinase phosphorylation in muscle reported from other studies. For instance, if p38 MAPK γ phosphorylation is dependent on muscle energetics, then the time course of p38γ phosphorylation should match the time course of energetic imbalance within the cytosol of the active muscle. There are several reports within the literature that suggest this is the case. Phosphorylation of p38 MAPK is substantially higher immediately after exercise whether after a long-distance run (Boppart et al., 2000), high intensity interval training session (Bartlett et al., 2012), intense bicycle ergometer trial (Egan et al., 2010), or resistance exercise (Deldicque et al., 2008) but quickly returns to normal levels, consistent with the return of muscle to its resting energetic state.
The results of the current study may also explain, in part, how different training protocols lead to differential activation of p38 MAPK. For example, whether in muscle biopsies of humans doing different heavy resistance protocols (Hulmi et al., 2012), cycle exercise (Gibala et al., 2009), or in isolated muscle (Martineau and Gardiner, 2001; Ryder et al., 2000), the amount of p38 phosphorylation increases with the amount of work done or force produced. More precisely, the amount of p38 MAPK phosphorylation scales with energy demand imposed. Perhaps the most convincing aspect of this contention is revealed when different exercise protocols are matched for total energy expenditure and the resulting p38 phosphorylation is the same (Bartlett et al., 2012; Egan et al., 2010).
Selective activation of p38 isoforms is determined by the formation of functional complexes between the isoforms and their upstream kinases, MAPK kinase-3, MAPK kinase-4, and MAPK kinase-6 (MKK3, MKK4 and MKK6, respectively) (Enslen et al., 2000; Keesler et al., 1998). MKK6 can phosphorylate all p38 isoforms, whereas MKK3 can phosphorylate p38α and γ, but not p38β. p38 is fully activated by dual phosphorylation within the T-loop at residues Thr180 and Tyr182. Selective phosphorylation of these sites is dependent upon the activating kinase. MKK3 phosphorylates only Thr180 whereas MKK6 phosphorylates both Thr180 and Tyr182 (Morrison and Davis, 2003; Wang et al., 1998). In the current study, the primary antibody used to detect the phosphorylated p38 isoforms identifies only the dually phosphorylated protein. It is conceivable that the differential phosphorylation of the p38 isoforms in the presence of both ATPase inhibitors is due in part through modulating MKK6 activity; specifically through its docking interaction with p38α/β and p38γ, albeit the exact mechanism is unknown. The present study was not designed to establish the upstream phosphorylation cascade, but an intriguing possibility is that activation of the p38 signaling cascade may also require phosphorylation by AMP activated protein kinase. AMPK is activated by many of the same exercise protocols that have been demonstrated to activate p38 (Bartlett et al., 2012; Egan et al., 2010; Gibala et al., 2009) and is directly activated by AMP, found when energy demand is higher than energy supply. Identifying the kinases that link the energetic imbalance and activation of p38γ would require specific inhibitors of the upstream kinases and is an exciting area of future study.
The findings of this study may also help extend the known signaling cascades that lead to specific exercise-induced adaptations. In a recent study, Pogozelski et al. studied skeletal muscle isoform specific p38 knockout mice to determine the role of p38 MAPK in exercise-induced muscle adaptation (Pogozelski et al., 2009). The authors found that p38γ knockout mice show a decrease in capillary density and PGC-1α expression. Therefore, they concluded that regulation of PGC-1α and VEGF through p38γ phosphorylation induces expression of mitochondrial biogenesis and angiogenesis, but the other p38 MAPK isoforms are not involved. Taken together with the results from this study, these observations support the broader perspective that an important feedback loop exists within skeletal muscle that links the energy demand of contractions directly to mitochondrial biogenesis and includes p38γ MAPK.
Acknowledgements
The authors wish to thank Cynthia Stanich and Roop Jayaraman for assistance with muscle mechanic studies and Drs. David Ankrapp and Samuel Blanchard for their assistance with Western blot quantification and data analysis. This work was supported by National Space Biomedical Research Institute grant MA-00210 and NIH DK 095210.
REFERENCES
- Augusto V, Padovani CR, Campos GER. Skeletal muscle fiber types in C57BL6J mice. Braz J Morphol Sci. 2004;21(2):89–94. [Google Scholar]
- Bartlett JD, Hwa Joo C, Jeong T-S, Louhelainen J, Cochran AJ, Gibala MJ, Gregson W, Close GL, Drust B, Morton JP. Matched work high-intensity interval and continuous running induce similar increases in PGC-1α mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J Appl Physiol. 2012;112(7):1135–1143. doi: 10.1152/japplphysiol.01040.2011. [DOI] [PubMed] [Google Scholar]
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- Baudet S, Shaoulian R, Bers DM. Effects of thapsigargin and cyclopiazonic acid on twitch force and sarcoplasmic reticulum Ca2+ content of rabbit ventricular muscle. Circ Res. 1993;73(5):813–819. doi: 10.1161/01.res.73.5.813. [DOI] [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Spangenburg EE. Exercise and gene expression: physiological regulation of the human genome through physical activity. J Physiol. 2002;543(Pt 2):399–411. doi: 10.1113/jphysiol.2002.019265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boppart MD, Asp S, Wojtaszewski JF, Fielding RA, Mohr T, Goodyear LJ. Marathon running transiently increases c-Jun NH2-terminal kinase and p38 activities in human skeletal muscle. J Physiol. 2000;526(Pt 3):663–669. doi: 10.1111/j.1469-7793.2000.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boppart MD, Hirshman MF, Sakamoto K, Fielding RA, Goodyear LJ. Static stretch increases c-Jun NH2-terminal kinase activity and p38 phosphorylation in rat skeletal muscle. Am J Physiol: Cell Physiol. 2001;280(2):C352–C358. doi: 10.1152/ajpcell.2001.280.2.C352. [DOI] [PubMed] [Google Scholar]
- Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4(1):83–88. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773(8):1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M. Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol. 2008;104(2):371–378. doi: 10.1152/japplphysiol.00873.2007. [DOI] [PubMed] [Google Scholar]
- Dentel JN, Blanchard SG, Ankrapp DP, McCabe LR, Wiseman RW. Inhibition of cross-bridge formation has no effect on contraction-associated phosphorylation of p38 MAPK in mouse skeletal muscle. Am J Physiol: Cell Physiol. 2005;288(4):C824–830. doi: 10.1152/ajpcell.00500.2004. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol. 1982;53(4):844–850. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O’Gorman DJ. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol. 2010;588(Pt 10):1779–1790. doi: 10.1113/jphysiol.2010.188011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enslen H, Brancho DM, Davis RJ. Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J. 2000;19(6):1301–1311. doi: 10.1093/emboj/19.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WH, Tidball JG, Wang Y. p38γ activity is required for maintenance of slow skeletal muscle size. Muscle Nerve. 2012;45(2):266–273. doi: 10.1002/mus.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol. 2009;106(3):929–934. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- Gillespie MA, Le Grand F, Scimè A, Kuang S, von Maltzahn J, Seale V, Cuenda A, Ranish JA, Rudnicki MA. p38-{gamma}-dependent gene silencing restricts entry into the myogenic differentiation program. J Cell Biol. 2009;187(7):991–1005. doi: 10.1083/jcb.200907037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Cuenda A, Craxton M, Jakes R, Cohen P. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 1997;16(12):3563–3571. doi: 10.1093/emboj/16.12.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CR, Janssen E, Terjung RL. Skeletal muscle contractile performance and ADP accumulation in adenylate kinase-deficient mice. Am J Physiol: Cell. 2005;288(6):C1287–1297. doi: 10.1152/ajpcell.00567.2004. [DOI] [PubMed] [Google Scholar]
- Hanke N, Kubis HP, Scheibe RJ, Berthold-Losleben M, Husing O, Meissner JD, Gros G. Passive mechanical forces upregulate the fast myosin heavy chain IId/x via integrin and p38 MAP kinase activation in a primary muscle cell culture. Am J Physiol Cell Physiol. 2010;298(4):C910–920. doi: 10.1152/ajpcell.00265.2009. [DOI] [PubMed] [Google Scholar]
- Hong T, Ning J, Yang X, Liu H-Y, Han J, Liu Z, Cao W. Fine-tuned regulation of the PGC-1α gene transcription by different intracellular signaling pathways. Am J Physiol: Endocrinol Metab. 2011;300(3):E500–507. doi: 10.1152/ajpendo.00225.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulmi JJ, Walker S, Ahtiainen JP, Nyman K, Kraemer WJ, Häkkinen K. Molecular signaling in muscle is affected by the specificity of resistance exercise protocol. Scand Journal Med Sci Sports. 2012;22(2):240–248. doi: 10.1111/j.1600-0838.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- Keesler GA, Bray J, Hunt J, Johnson DA, Gleason T, Yao Z, Wang SW, Parker C, Yamane H, Cole C, Lichenstein HS. Purification and activation of recombinant p38 isoforms alpha, beta, gamma, and delta. Protein Expression Purif. 1998;14(2):221–228. doi: 10.1006/prep.1998.0947. [DOI] [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Discrimination of Ca(2+)-ATPase activity of the sarcoplasmic reticulum from actomyosin-type ATPase activity of myofibrils in skinned mammalian skeletal muscle fibres: distinct effects of cyclopiazonic acid on the two ATPase activities. J Muscle Res Cell Motil. 1991;12(4):355–365. doi: 10.1007/BF01738590. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Moerland TS, Wiseman RW. Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP, and Pi. Proc Natl Acad Sci USA. 1992;89(16):7521–7525. doi: 10.1073/pnas.89.16.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFramboise WA, Jayaraman RC, Bombach KL, Ankrapp DP, Krill-Burger JM, Sciulli CM, Petrosko P, Wiseman RW. Acute molecular response of mouse hindlimb muscles to chronic stimulation. Am J Physiol: Cell Physiol. 2009;297(3):C556–570. doi: 10.1152/ajpcell.00046.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman JB, Franco-Obregón A. Mechanosensitive ion channels in skeletal muscle: a link in the membrane pathology of muscular dystrophy. Clin Exp Pharmacol Physiol. 2006;33(7):649–656. doi: 10.1111/j.1440-1681.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- Lechner C, Zahalka MA, Giot JF, Mo̊ller NP, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc Natl Acad Sci USA. 1996;93(9):4355–4359. doi: 10.1073/pnas.93.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Tikunov B, Henson D, LaManca J, Sweeney HL. Creatine depletion elicits structural, biochemical, and physiological adaptations in rat costal diaphragm. Am J Physiol. 1996;271(5 Pt 1):C1480–1486. doi: 10.1152/ajpcell.1996.271.5.C1480. [DOI] [PubMed] [Google Scholar]
- Lovett FA, Cosgrove RA, Gonzalez I, Pell JM. Essential role for p38alpha MAPK but not p38gamma MAPK in Igf2 expression and myoblast differentiation. Endocrinology. 2010;151(9):4368–4380. doi: 10.1210/en.2010-0209. [DOI] [PubMed] [Google Scholar]
- Martineau LC, Gardiner PF. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J Appl Physiol. 2001;91(2):693–702. doi: 10.1152/jappl.2001.91.2.693. [DOI] [PubMed] [Google Scholar]
- McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol: Cell Physiol. 2010;298(3):C542–549. doi: 10.1152/ajpcell.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Même W, Huchet-Cadiou C, Léoty C. Cyclopiazonic acid-induced changes in the contraction and Ca2+ transient of frog fast-twitch skeletal muscle. Am J Physiol. 1998;274(1 Pt 1):C253–261. doi: 10.1152/ajpcell.1998.274.1.C253. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the “phosphocreatine shuttle”. Am J Physiol. 1984;246(5 Pt 1):C365–377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Terjung RL. Differences in ammonia and adenylate metabolism in contracting fast and slow muscle. Am J Physiol. 1979;237(3):C111–118. doi: 10.1152/ajpcell.1979.237.3.C111. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Terjung RL. AMP deamination and IMP reamination in working skeletal muscle. Am J Physiol. 1980;239(1):C32–38. doi: 10.1152/ajpcell.1980.239.1.C32. [DOI] [PubMed] [Google Scholar]
- Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Parsons SA. Genetic Loss of Calcineurin Blocks Mechanical Overload-induced Skeletal Muscle Fiber Type Switching but Not Hypertrophy. J Biol Chem. 2004;279(25):26192–26200. doi: 10.1074/jbc.M313800200. [DOI] [PubMed] [Google Scholar]
- Pogozelski AR, Geng T, Li P, Yin X, Lira VA, Zhang M, Chi J-T, Yan Z. p38gamma mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS ONE. 2009;4(11):e7934. doi: 10.1371/journal.pone.0007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall JA. Energetics of Ca2+ cycling during skeletal muscle contraction. Fed Proc. 1982;41(2):155–160. [PubMed] [Google Scholar]
- Rall JA. Energetic aspects of skeletal muscle contraction: implications of fiber types. Exercise Sport Sci Rev. 1985;13:33–74. [PubMed] [Google Scholar]
- Robin G, Berthier C, Allard B. Sarcoplasmic reticulum Ca2+ permeation explored from the lumen side in mdx muscle fibers under voltage control. J Gen Physiol. 2012;139(3):209–218. doi: 10.1085/jgp.201110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röckl KSC, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB life. 2008;60(3):145–153. doi: 10.1002/iub.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder JW, Fahlman R, Wallberg-Henriksson H, Alessi DR, Krook A, Zierath JR. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement Of the mitogen- and stress-activated protein kinase 1. J Biol Chem. 2000;275(2):1457–1462. doi: 10.1074/jbc.275.2.1457. [DOI] [PubMed] [Google Scholar]
- Teerlink T, Hennekes M, Bussemaker J, Groeneveld J. Simultaneous determination of creatine compounds and adenine nucleotides in myocardial tissue by high-performance liquid chromatography. Anal Biochem. 1993;214(1):278–283. doi: 10.1006/abio.1993.1488. [DOI] [PubMed] [Google Scholar]
- Wang XS, Diener K, Manthey CL, Wang S, Rosenzweig B, Bray J, Delaney J, Cole CN, Chan-Hui PY, Mantlo N, Lichenstein HS, Zukowski M, Yao Z. Molecular cloning and characterization of a novel p38 mitogen-activated protein kinase. J Biol Chem. 1997;272(38):23668–23674. doi: 10.1074/jbc.272.38.23668. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang S, Sah VP, Ross J, Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273(4):2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- Williams DB, Sutherland LN, Bomhof MR, Basaraba SAU, Thrush AB, Dyck DJ, Field CJ, Wright DC. Muscle-specific differences in the response of mitochondrial proteins to beta-GPA feeding: an evaluation of potential mechanisms. Am J Physiol: Endocrinol Metab. 2009;296(6):E1400–1408. doi: 10.1152/ajpendo.90913.2008. [DOI] [PubMed] [Google Scholar]
- Wiseman RW, Kushmerick MJ. Creatine kinase equilibration follows solution thermodynamics in skeletal muscle. 31P NMR studies using creatine analogs. J Biol Chem. 1995;270(21):12428–12438. doi: 10.1074/jbc.270.21.12428. [DOI] [PubMed] [Google Scholar]
- Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci. 2008;65(23):3737–3755. doi: 10.1007/s00018-008-8244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wretman C, Lionikas A, Widegren U, Lännergren J, Westerblad H, Henriksson J. Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle. J Physiol. 2001;535(Pt 1):155–164. doi: 10.1111/j.1469-7793.2001.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I, Harwood C, Rome L. Cross-bridge blocker BTS permits direct measurement of SR Ca2+ pump ATP utilization in toadfish swimbladder muscle fibers. Am J Physiol: Cell Physiol. 2003;285(4):C781–C787. doi: 10.1152/ajpcell.00025.2003. [DOI] [PubMed] [Google Scholar]