Abstract
Background
Treatment for tobacco dependence is not available in many low-resource settings, especially in developing countries.
Purpose
To test the impact of a novel mix of monetary and social incentives on smoking abstinence in rural communities of Thailand.
Design
An RCT of commitment contracts and team incentives for rural smokers to quit smoking. Smokers were not blinded to treatment status, although the assessor of the biochemical urine test was.
Setting/participants
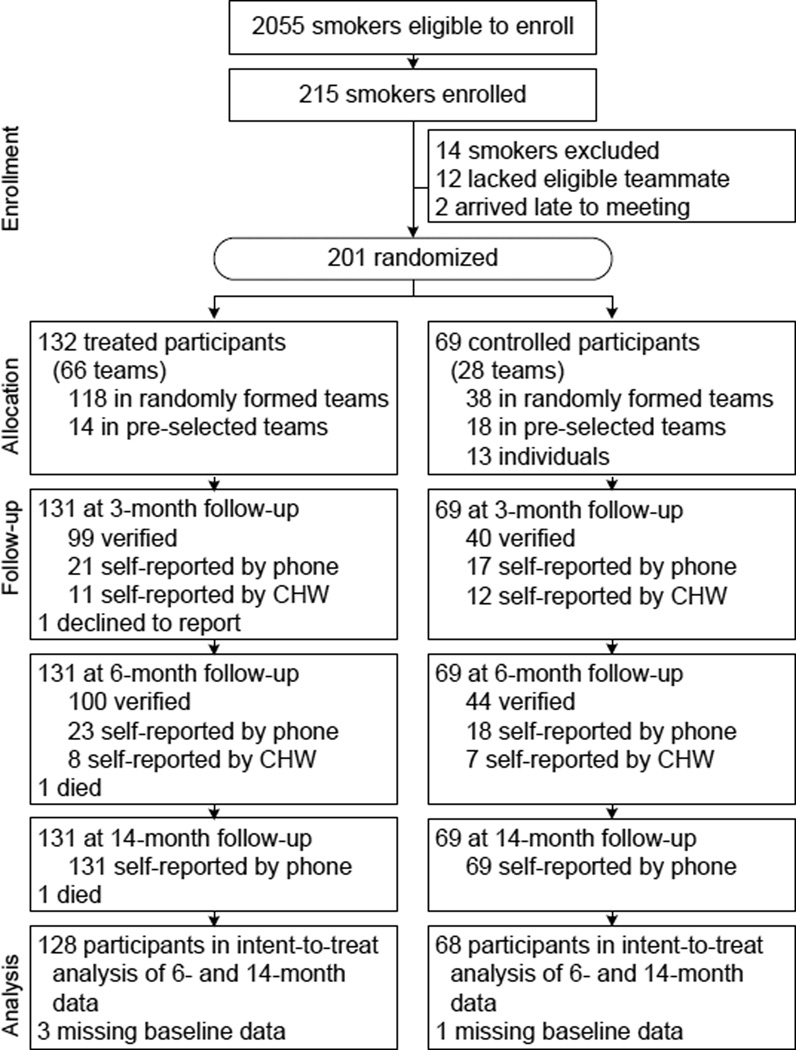
All adult smokers living in the study area were eligible to participate; 215 adult smokers from 42 villages in Nakhon Nayok province, Thailand participated. Fourteen smokers who lacked teammates were dropped.
Intervention
201 smokers were assigned to a two-person team, and then randomly assigned by team (in a 2:1 ratio) with computer-generated random numbers to receive smoking-cessation counseling (control group) or counseling plus offer of a commitment contract, team incentives, and text message reminders for smoking cessation at 3 months (intervention group).
Main outcome measures
The primary outcome was biochemically-verified 7-day abstinence at 6 months, assessed on an intention-to-treat basis. Secondary outcomes include biochemically-verified abstinence at 3 months, self-reported abstinence at 14 months, and the incremental cost per quitter of the intervention, nicotine gum, and varenicline in Thailand. Data were collected in 2010–2011 and analyzed in 2012.
Results
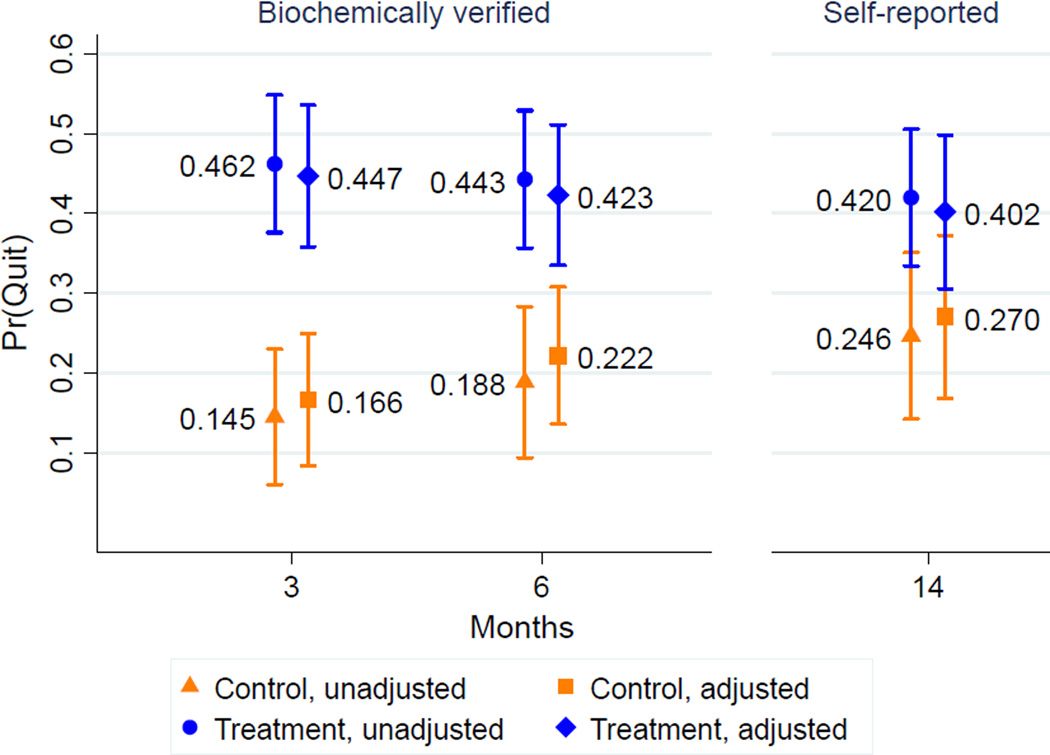
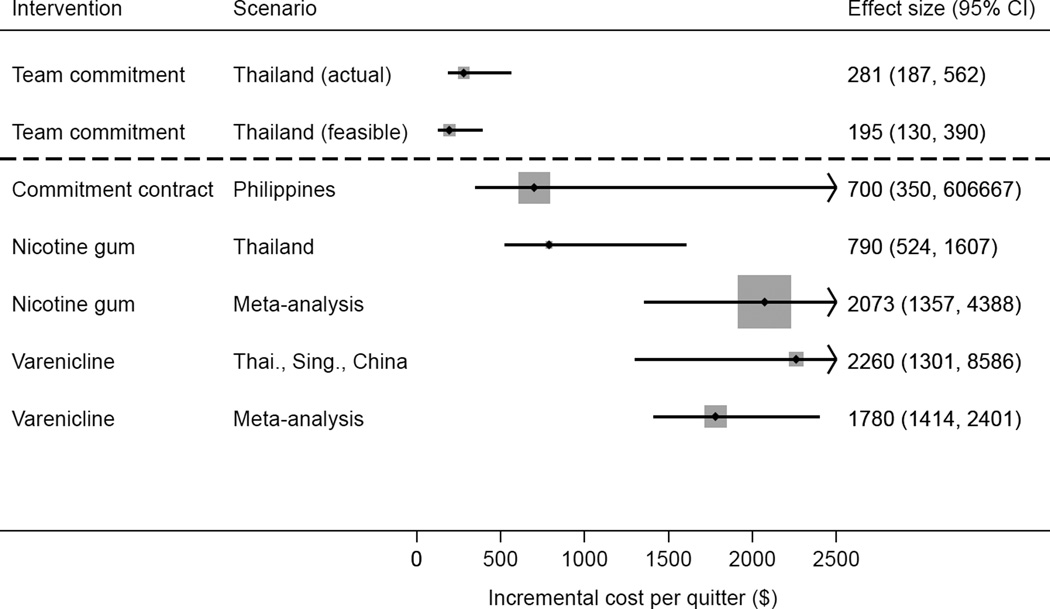
The trial enrolled 215 (10.5%) of 2055 smokers. The abstinence rate was 46.2% (61/132) in the intervention group and 14.5% (10/69) in the control group (adjusted OR 7.5 [3.0–18.6]) at 3 months; 44.3% (58/131) and 18.8% (13/69) at the primary end point of 6 months (adjusted OR 4.2 [1.8–9.7]); and 42.0% (55/131) and 24.6% (17/69) at 14 months (adjusted OR 2.2 [1.0–4.8]). The purchasing-power–parity-adjusted incremental cost per quitter from the intervention is $281 (95% CI=$187, $562), less than for nicotine gum ($1780 [95% CI=$1414, $2401]) or varenicline ($2073 [95% CI=$1357, $4388]) in Thailand.
Conclusions
The intervention enhanced abstinence by 91%–136% at 6 months, relative to the control group, although self-reports at 14 months suggest tapering of the treatment effect. The intervention may offer a viable, cost-effective alternative to current smoking cessation approaches in low-resource settings.
Introduction
Treatment for tobacco dependence is not widely available in low-resource settings in the developed and developing world. Standard treatment options—nicotine replacement therapy, prescription drugs, and professional counseling—are efficacious,1–3 but are not presently feasible in many areas, where trained health professionals are scarce, access to and availability of health services is limited, and treatment is relatively expensive. This study tests a novel intervention that uses social and monetary incentives for delivering smoking cessation services to rural communities in central Thailand.
This study assesses the potential of voluntary, binding financial agreements to promote smoking abstinence. Behavioral economists have recently applied these commitment contracts to health behaviors such as weight loss, exercise, and smoking cessation.4–6 In the most rigorous study of smoking cessation contracts to date, smokers in the Philippines CARES trial deposited money with study staff that was returned at 6 months conditional on quitting. Smoking abstinence at 12 months increased 3.5% points (38%) for depositors compared to a control group that received a pamphlet about quitting.7 Yet, 66% of depositors forfeited their contributions.
The present study aims to strengthen commitment contracts by supplementing monetary commitment with a form of social commitment. Specifically, the study induces peer pressure by offering a pair of smokers (a team) a cash bonus contingent on both people quitting. Peer pressure is a strong force for increasing willpower and motivation.8–10 Buddy interventions that rely on social support are a common adjunct to smoking treatment, but have not consistently enhanced the likelihood of quitting.11,12 Likewise, cash incentives for quitting often fail to induce lasting quits.13 In the present study, however, participants deposit money up front, selecting for smokers who have a desire to be abstinent rather than those who are only financially motivated. In sum, all participants received group counseling, and the intervention group were also offered regular text message reminders and multiple incentives contingent on quitting at 3 months: a small up-front contribution with the option to make additional deposits, a project-matched contribution, and a large team incentive. The combination of reminders and monetary and social incentives is hypothesized to help smokers to quit successfully.
Thailand is an appropriate study setting for two reasons. First, all Thai villages have a network of community health workers (CHWs). The workers served as recruiters and deposit collectors but did not require technical training. Many experts believe that CHWs can help alleviate the health workforce shortage in rural areas.14,15 Second, Thailand has a high demand for quitting,16 due in part to its comprehensive tobacco control policies,17 and commitment contracts rely on smokers having a pre-existing desire to quit. Global tobacco control efforts are expected to spur an increased demand for quitting in the coming decade, which will make low-cost treatment options in the developing world increasingly important.
Methods
Study Site and Participants
This study employs a randomized design undertaken in six subdistricts of Nakhon Nayok province, located 125 km northeast of Bangkok. The villages lie within the catchment area of the province’s major tertiary hospital, where the research team was based. The enumeration area includes 42 villages, each with about 500 residents. The region is agrarian and has a median household income of $10 per day.18 Prior to recruitment, CHWs were paid to conduct a census of smokers in their village, in order to target recruitment efforts and measure trial participation. In Thailand, CHWs have an assigned kum of 10–15 households, in which they conduct health promotion activities. CHWs were asked to survey and recruit smokers living within their kum. The household census yielded an adult smoking prevalence in the study area of 23% for men and 2% for women. Research staff held recruitment meetings within each study village, and CHWs recruited smokers to enter the trial. Eligibility criteria consisted of current smokers aged 20 and older who resided in a study community. Smoking status at enrollment was based on self-report and verified with eyewitness reports by CHWs, collected at enrollment meetings. There was no racial or gender bias in the selection of participants. All residents are Asian, and female smokers are over-represented in the study sample.
During meetings held in study communities from December 2010 to March 2011, the trial enrolled 215 (10.5%) of 2,055 smokers living in the area. Participants came from 30 of 42 eligible villages. In 12 villages, CHWs did not recruit any participants. The meetings were held in public spaces with each village, in order to minimize participants’ time and travel costs. Trial enrollment ended after a meeting was held in each community. All participants provided written informed consent. Ethics review committees at Srinakharinwirot University (Thailand) and University of California at Berkeley (U.S.) approved the study design. The targeted sample size was based on a power analysis, conducted using Optimal Design Software (version 2).
Randomization
The study followed a two-step stratified randomization process: (1) assignment to a two-person team and (2) random allocation to the treatment and control group. In the first step, participants selected a teammate prior to enrollment (pre-selected pairs) or chose to be randomly assigned a teammate at enrollment. Randomly formed teams were stratified by village and gender. This paper does not analyze the differences between pre-selected and randomly assigned teammates, so the nature of teammate selection does not affect the trial’s internal validity.
For village-gender strata with an odd number of at least three non–pre-selected enrollees, the extra person was retained in the sample (n = 13), and faced the same treatment allocation probabilities as other participants. Fourteen individuals from the sample were dropped, 12 of whom belonged to a strata with one person and thus had no probability of being assigned a teammate (e.g., the lone female recruit from a given village) and two of whom arrived late to the enrollment meeting. The final sample included 201 participants, 188 assigned to a dyadic team.
In the second step, teams were allocated to the control group and the treatment group in a 1:2 ratio. At each enrollment meeting, a programmer implemented the random team and allocation sequences using computer-generated random numbers, concealing the sequence from other field staff and participants. The field coordinator received assignments from the programmer and then informed the participants of their allocation. Participants were not masked to their assignment. Note that control group members were also assigned a teammate, either one they pre-selected or a “synthetic teammate” whose identity was never revealed and used only for analysis. Pre-selected teams assigned to the control group were given no instructions regarding whether to interact with their teammate.
Procedures
Prior to randomization, all participants completed a screening questionnaire. A smoking cessation counselor, who was masked to participants’ treatment status, provided a group counseling session. Research staff then announced treatment status assignment, and the control group was dismissed. Treated participants learned their assigned teammate’s identity, met briefly with their teammate to discuss plans (e.g., preferred frequency and nature of their interactions), provided a baseline deposit, and then were dismissed.
The control group had no intervention-related activities following enrollment, aside from a second round of counseling at 3 months. The treatment arm received three additional components, the combination of which constitute team commitment contracts. First, each individual in the treatment group opened a commitment savings account with the project at enrollment. The account had a minimum balance of $1.67 (Thai Baht [THB] 50). For 10 weeks after enrollment, a CHW visited the participant weekly to collect voluntary contributions to the account. A triple-entry receipt system (with copies for the participant, CHW, and field coordinator) tracked contributions, and the project collected deposits and a copy of the receipts from CHWs biweekly. The project added a $5 starter contribution to each person’s account and an extra $5 (THB 150) if the participant reached an account balance of $5 during the deposit period. The participant had the deposits and matching contribution refunded only if the person abstained from smoking as assessed at 3 months.
Second, if the person and his or her teammate both abstained from smoking within 3 months, each received a cash bonus of $40 (THB 1200), about 16% of median monthly household income. By comparison, Volpp and colleagues,19 who offered some of the largest cash incentives for quitting to date, used incentives that amounted to roughly 27% of household income (authors’ calculations). The incentives were designed to motivate without placing undue pressure on participants, in line with recommendations for the ethical use of cash incentives for healthy behaviors.20,21 Third, the project sent weekly text messages to boost the frequency and intensity of deposits and to increase the strength and salience of partner monitoring and support.
Participants returned to the same meeting site 3 months after enrollment. At that time, all participants received cessation counseling. Treated participants also received monetary rewards if they quit, as described. Quitting is defined as the 7-day point prevalence of biochemically-verified abstinence. Quitters had to self-report abstaining from smoking for at least 7 days and to pass a urine test. Participants were tested 3 months and 6 months after enrollment using the NicCheck™ urine test for cotinine, a metabolite of nicotine. The color-coded test strips give results in 15 minutes. To pass the test, individuals needed a score of 0 (i.e., no detectable cotinine) on a scale from 0 to 14. According to the manufacturer, the test has both a sensitivity and specificity of 97% and a detection period of 3–4 days for a smoker of 5–10 cigarettes per day and 5–6 days for a smoker of 20–30 cigarettes per day. Participants and field staff were not informed of the detection period. The assessor of the urine test was blinded to treatment allocation. Anyone who disputed the test results could request a second test, although field staff encountered very few disputes. Participants went one at a time into public bathroom facilities to provide urine samples. Research staff monitored participants to ensure that they did not carry containers into the bathroom. The same field staff were used at enrollment and at all follow-up periods, allowing them to verify participants’ identity with near certainty. For all participants who did not attend either the 3-month or 6-month meeting, the field coordinator contacted the person by phone or else through a CHW to ascertain the person’s self-reported smoking status. All individuals who reported having quit were visited at home to verify their smoking status by urine test.
At 6 months—3 months after all incentives were awarded—field staff biochemically assessed abstinence. The 6-month visit dates were announced less than a week in advance, reducing the ability of smokers to abstain right before the tests. Brief surveys were administered at the 3-month and 6-month follow-up meetings. Scheduled urine testing at 12 months was replaced by telephone follow-up at 13–16 months (denoted hereafter as 14 months) due to severe flooding in study communities in fall 2011. An inconvenience fee of $3 per follow-up meeting was paid to the control group for attending the 3-month and 6-month follow-up meetings and to the treatment group for attending the 6-month follow-up. Importantly, at both the 6-month and 14-month follow-ups, there are no differential incentives between the control group and treatment group to game the urine test or misreport smoking status. Any difference in abstinence rates at those time points can be attributed to the intervention.
Data Analysis
The primary outcome is biochemically-verified abstinence at 6 months. Secondary outcome measures include study participation, biochemically-verified smoking status at 3 months, and self-reported smoking status at 14 months. Trial participation is an indicator of the feasibility of and demand for the intervention. The difference between smoking status at 3 and 6 months is an indicator of relapse following the intervention. The analysis also includes calculations of the incremental cost per quitter for the present intervention, nicotine gum, and varenicline.
The analysis, conducted in 2012, assesses on an intention-to-treat basis whether quitting varied by treatment status. Adjusted odds ratios are calculated from logistic regression models with treatment status as the key independent variable. Regressions control for the baseline characteristics listed in Table 1, cluster standard errors by team, and correct for heteroskedasticity. Stata (Version 12) was used for statistical analyses.
Table 1.
Baseline characteristics of participants and nonparticipants
| Trial participants |
t-test of (1) vs (2) (p- value) |
||||
|---|---|---|---|---|---|
| Nonparticipants | All |
Control group |
Treatment group |
||
| (1) | (2) | (3) | (4) | (5) | |
| Panel A. Sociodemographic characteristics | |||||
| Male | 0.926 (0.262) | 0.872 (0.334) | 0.868 (0.341) | 0.875 (0.332) | 0.001 |
| Age | 45.21 (15.06) | 51.06 (13.86) | 51.07 (14.04) | 51.05 (13.82) | < 0.001 |
| Monthly household income, in $100s | 3.838 (4.971) | 3.513 (2.809) | 4.011 (5.805) | ||
| Education, years | |||||
| 0–3 | 0.469 (0.500) | 0.485 (0.503) | 0.461 (0.500) | ||
| 4–6 | 0.260 (0.440) | 0.324 (0.471) | 0.227 (0.420) | ||
| 7+ | 0.270 (0.445) | 0.191 (0.396) | 0.312 (0.465) | ||
| Currently married | 0.791 (0.408) | 0.794 (0.407) | 0.789 (0.410) | ||
| Buddhist vs Muslim | 0.689 (0.464) | 0.691 (0.465) | 0.688 (0.465) | ||
| Works in agriculture | 0.633 (0.483) | 0.603 (0.493) | 0.648 (0.479) | ||
| Self-rated health is good to excellent vs fair to poor | 0.296 (0.458) | 0.324 (0.471) | 0.281 (0.451) | ||
| Panel B. Smoking characteristics | |||||
| Average number of cigarettes smoked per day | 13.86 (7.407) | 12.79 (9.785) | 14.24 (11.15) | 12.02 (8.926) | 0.077 |
| Type of tobacco used | |||||
| Manufactured cigs. only | 0.301 (0.459) | 0.301 (0.460) | 0.294 (0.459) | 0.305 (0.462) | 0.634 |
| Handrolled cigarettes only | 0.585 (0.493) | 0.480 (0.501) | 0.485 (0.503) | 0.477 (0.501) | 0.010 |
| Both handrolled and manufactured cigs. | 0.114 (0.317) | 0.219 (0.415) | 0.221 (0.418) | 0.219 (0.415) | < 0.001 |
| Number of years since initiated smoking | 20.49 (13.28) | 31.31 (14.87) | 31.93 (14.47) | 30.98 (15.12) | < 0.001 |
| Number of past quit attempts | 2.676 (2.728) | 2.824 (2.938) | 2.598 (2.617) | ||
| Prediction of Pr(Quit) in 3 months | 0.796 (0.208) | 0.7.99 (0.193) | 0.795 (0.217) | ||
| Planning to quit smoking within 6 months vs not | 0.196 (0.397) | 0.821 (0.384) | 0.853 (0.357) | 0.805 (0.398) | < 0.001 |
| Belief that quitting is very important vs not | 0.765 (0.425) | 0.735 (0.444) | 0.781 (0.415) | ||
| Number of other adult smokers in the household | 0.658 (1.033) | 0.632 (1.196) | 0.672 (0.940) | ||
| All of person’s 5 best friends are smokers | 0.515 (0.501) | 0.574 (0.498) | 0.484 (0.502) | ||
| Panel C. Trial characteristics | |||||
| Preselected teammate vs randomly assigned | 0.158 (0.366) | 0.265 (0.444) | 0.102 (0.303) | ||
| N | 1145 | 196 | 128 | 68 | |
Mean and standard deviation (in parentheses) of each variable are reported. The p-values in Column (5) derive from two-sided t-tests of the difference in means between the indicated columns. Only a subset of variables were collected in the census of nonparticipants, i.e., those smokers living in the study area who did not enroll in the trial.
The study estimates the cost per additional quitter for the present intervention, the provision of nicotine gum and varenicline in Thailand, and the use of basic individual commitment contracts. For each intervention, the incremental cost effectiveness refers to additional quitting in the intervention group compared to a control group. The costing for the present intervention uses a programmatic perspective and includes incentives, personnel, and testing supplies, and excludes the subjects’ own costs of quitting and survey costs. The analysis also includes a scenario of the feasible incremental cost per quitter if the project had made three minor changes that would not affect the intervention's effectiveness, namely paying the deposit collectors piece rate rather than a fixed amount, hiring the field coordinator for a full-time equivalent of 2 instead of 3 months, and buying the urine test strips locally. The estimated costs for the pharmacologic interventions are based on each product's costs, as marketed and sold in Thailand. All costs are reported in dollars, adjusted for purchasing power parity (PPP) ($1 = THB 17.1).22 Effectiveness is reported as the average treatment effect from logistic regressions. The exception is for basic commitment contracts, for which the analysis uses the treatment-on-the-treated effect.7 For pharmacologic approaches, effectiveness is derived from available local studies and from multi-country meta-analyses. Additional details are provided in Appendix A, available online at www.ajpmonline.org.
Results
Figure 1 shows the trial profile. According to the household census, 2055 smokers lived in the 42 study communities, although only 86.6% of CHWs returned data-collection forms. The trial enrolled 215 smokers, a participation rate of 10.5% among census takers. Adjusting for random nonreporting in the census (=2055/0.866), the participation rate in the study area is 9.1%, although this likely understates participation, as smokers not counted in the census were not likely invited to join the trial. Smokers enrolled from 30 of 42 study villages. Among those 30 communities, the participation rate is 13.3%. Among smokers who reported pre-trial plans to quit, the participation rate is 39.1%.
Figure 1.
Study profile
Table 1 shows baseline characteristics of participants and nonparticipants living in the study area. Participants are significantly more likely than nonparticipants to be female, older, dual users of handrolled and manufactured cigarettes, smokers of a longer tenure, and more likely to plan to quit smoking. The differences by intention to quit are particularly large (adjusted OR 14.2, 95% CI 7.6–26.2; Table 2). Baseline sociodemographic and smoking characteristics between the treatment and control groups were similar (Table 1).
Table 2.
Correlates of trial participation and smoking abstinence
| Participation | Abstinence at 3 mos. | Abstinence at 6 mos. | Abstinence at 14 mos. | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Treatment | 7.51 | (3.03, 18.59) | 4.16 | (1.77, 9.74) | 2.19 | (0.99, 4.84) | ||
| Male | 0.27 | (0.13, 0.58) | 1.07 | (0.23, 4.89) | 0.80 | (0.23, 2.84) | 1.16 | (0.30, 4.57) |
| Age | 0.94 | (0.92, 0.97) | 1.04 | (0.85, 1.27) | 1.01 | (0.83, 1.23) | 0.99 | 0.82, 1.21) |
| Age-squared | 0.94 | (0.92, 0.97) | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) |
| Monthly household income | 0.90 | (0.70, 1.15) | 0.97 | (0.80, 1.17) | 1.10 | (0.79, 1.54) | ||
| Income-squared | 1.00 | (0.99, 1.01) | 1.00 | (0.99, 1.01) | 0.99 | (0.97, 1.01) | ||
| Education 0–3 years (ref) | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) | ||
| Education 4–6 years | 0.37 | (0.11, 1.31) | 0.55 | (0.14, 2.22) | 1.21 | (0.41, 3.59) | ||
| Education 7+ years | 0.87 | 0.24, 3.19) | 1.11 | (0.32, 3.80) | 1.21 | (0.41, 3.60) | ||
| Currently married | 2.05 | (0.71, 5.93) | 1.30 | (0.48, 3.51) | 1.55 | (0.57, 4.23) | ||
| Buddhist | 2.04 | (0.34, 12.24) | 1.64 | (0.30, 8.95) | 2.19 | (0.43, 11.28) | ||
| Works in agriculture | 7.51 | (3.03, 18.59) | 4.16 | (1.77, 9.74) | 2.19 | (0.99, 4.84) | ||
| Health good to excellent | 1.07 | (0.23, 4.89) | 0.80 | (0.23, 2.84) | 1.16 | (0.30, 4.57) | ||
| Average cigarettes per day | 0.99 | (0.96, 1.01) | 0.79 | (0.70, 0.89) | 0.81 | (0.72, 0.91) | 0.92 | 0.84, 1.01) |
| Cigarettes per day-squared | 0.99 | (0.96, 1.01) | 1.01 | 1.00, 1.01) | 1.00 | (1.00, 1.01) | 1.00 | (1.00, 1.00) |
| Manufactured cigs. only (ref) | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) | ||
| Handrolled cigs. only | 0.70 | (0.39, 1.24) | 0.89 | (0.32, 2.46) | 1.19 | (0.41, 3.43) | 0.85 | (0.31, 2.37) |
| Both hand. and man. cigs. | 3.27 | (1.86, 5.74) | 1.49 | (0.42, 5.30) | 2.35 | (0.72, 7.75) | 2.08 | (0.69, 6.25) |
| Years since initiated smoking | 1.11 | (1.08, 1.14) | 0.95 | (0.88, 1.03) | 0.93 | (0.86, 1.01) | 0.96 | (0.90, 1.02) |
| Past quit attempts | 1.05 | (0.90, 1.22) | 0.97 | (0.81, 1.15) | 1.07 | (0.93, 1.24) | ||
| Quit expectations in 3 months | 1.15 | (0.93, 1.42) | 1.18 | (0.95, 1.48) | 1.14 | (0.92, 1.40) | ||
| Planning to quit within 6 mos. | 14.17 | (7.66, 26.20) | 0.80 | (0.23, 2.73) | 1.04 | (0.29, 3.73) | 0.93 | (0.30, 2.89) |
| Quitting very important | 3.17 | (0.87, 11.53) | 2.18 | (0.68, 6.92) | 1.88 | (0.75, 4.72) | ||
| Adult household smokers | 0.17 | (0.04, 0.83) | 0.10 | (0.02, 0.50) | 0.40 | (0.07, 2.17) | ||
| Other smokers in household | 0.89 | (0.32, 2.46) | 1.19 | (0.41, 3.43) | 0.85 | (0.31, 2.37) | ||
| All of best friends smoke | 1.49 | (0.42, 5.30) | 2.35 | (0.72, 7.75) | 2.08 | (0.69, 6.25) | ||
| Pre-selected teammate | 0.95 | (0.88, 1.03) | 0.93 | (0.86, 1.01) | 0.96 | (0.90, 1.02) | ||
| Number of individuals | 1359 | 197 | 196 | 196 | ||||
| Number of clusters | 38 | 119 | 119 | 119 | ||||
| Pseudo R-squared | 0.34 | 0.29 | 0.32 | 0.21 | ||||
Note: Adjusted ORs are calculated from a logistic regression model of individual data. 95% CIs, clustered at the village level for the participation model and at the team level for the smoking abstinence models, are in parentheses. The smoking abstinence models include quadratic terms for age, income, and average cigarettes smoked for day and indicator variables for subdistrict and the health counselor at baseline. Only a subset of variables were available among nonparticipants for the analysis of participation.
Smoking status at 6 months was biochemically-verified for 75.8% (100/132) of the treatment group and 63.8% (44/69) of the control group, including for all self-reported quitters. Figure 2 shows the unadjusted quit probability by treatment status. At 3 months, 46.2% (61/132) of the treatment group and 14.5% (10/69) of the control group had quit. The share of contract users who quit at the end of the intervention period was significantly greater than the 34.1% in the Philippines CARES trial (t (131)=2.78, p<0.003). At the primary end point of 6 months, 44.3% (58/131) of the treatment group and 18.8% (13/69) of the control group had quit. During the 3 months after incentives ended, 9 treated participants (14.8%) relapsed. Thirteen treated participants (21.3%) relapsed between 3 and 14 months.
Figure 2.
Predicted probability of smoking abstinence, by month and treatment status
Analyses of intervention effects on quitting are performed on participants who had complete baseline data (Table 2). Controlling for baseline factors, the intervention increased quitting at 3 months (adjusted OR 7.5, 95% CI 3.0–18.6) and 6 months (adjusted OR 4.2, 95% CI 1.8–9.7). The intervention’s effects persisted to 14 months (42.0% quit), based on unconfirmed self-reports, although the share of control group members reporting having quit increased (24.6%), such that the treatment effect is only marginally significant (adjusted OR 2.2, 95% CI 0.99–4.8). Figure 2 shows the predicted probabilities of quitting based on these regressions. In a sub-analysis, treated participants who received any text message reminders (n=50) were not significantly more likely to quit at 3 months than treated participants who did not, most of whom had no phone (results not shown).
Of those in the treatment group, 27.3% (36/132) earned the team bonus. Team outcomes were not evenly dispersed between the treatment and control groups. In the control group, 3.6% of individuals were in teams in which both members quit at 6 months, 32.1% in teams in which one quit and one smoked, and 64.3% in teams in which both failed to quit. In contrast, the distribution in the treatment group is significantly different: 26.2%, 36.9%, and 36.9%, respectively (χ2(2)=17.1, p<0.001). Interestingly, pre-selected teams were not more likely to quit than were randomly-formed teams (Table 2).
Figure 3 shows a forest plot of the incremental cost-effectiveness results. The team commitment intervention cost $281 per additional quitter (95% CI 187–562). With minor changes listed in Table 3, the intervention could feasibly be conducted for $195 per additional quitter (95% CI 130–390). In comparison, the individual commitment contracts fielded in the Philippines CARES trial cost $700 per additional quitter.7 Cost differences result from the reliance on CHWs, rather than professional staff, to implement the present intervention and a 3-month deposit period instead of 6 months. The cost per quitter for a 12-week course of nicotine gum in Thailand is $2260 (95% CI 1301–8586) using effectiveness data from Thailand,22 and $1780 (95% CI 1414–2401) using effectiveness data from a multi-country meta-analysis.23 The analogous estimates for a 12-week course of varenicline in Thailand are $790 (95% CI 524–1607) using effectiveness data from Asian smokers and $2073 (95% CI 1357–4388) using effectiveness data from a multi-country meta-analysis.24
Figure 3.
Incremental cost per quitter, by type of intervention
Thai, Thailand; Sing., Singapore
Discussion
The team commitment intervention increased the likelihood of quitting among adult smokers living in rural communities of central Thailand by 91%–136% relative to the control group, according to biochemically-verified results at 6 months. Few studies have assessed smoking cessation interventions targeted to rural populations in the developing world, despite the large share of their deaths attributable to tobacco use. The effectiveness of the behavioral intervention is on par with pharmacotherapy. Meta-analyses find that the risk ratios of abstinence at 6+ months for varenicline and nicotine replacement therapy, compared to placebo or a control group, are 2.27 (95% CI 2.02–2.55) and 1.58 (95% CI 1.55–1.66),1,25 whereas team commitment had a risk ratio of 2.35 (95% CI 1.39–3.98) at 6 months. Cash incentives for quitting smoking has rarely increased long-term smoking abstinence,13 although team incentives may be effective in combination with commitment contracts. Relative to basic commitment contracts tested in the Philippines,7 team commitment contracts reduced the failure rate of users, highlighting the potential of stronger commitment through team incentives to promote quitting.
A sizable fraction of the smoking population in the study area signed up to use the team commitment contracts. The intervention translated into a decrease in smoking rates of 2%–5% points in the study area. A change of such magnitude could lead to a multiplier effect if quitting spreads through social networks, as some researchers assert.26 The study also found low relapse rates among participants. Coordinated quit attempts of friends within the same community may reduce recidivism, potentially by changing the norms of tobacco use within a smoker's social network.
The incremental cost-effectiveness analysis indicates that the present intervention performed favorably relative to the smoking treatments most used in Thailand and relative to other economic evaluations of smoking cessation therapies.27 This study does not calculate the cost per lives saved or per disability-adjusted life year (DALY) averted, but given the available estimates of DALYs averted from NRT and other tobacco control interventions,28 the team commitment intervention likely meets the WHO standard for “very cost effective” in Thailand, defined as being less than gross domestic product ($8600, PPP-adjusted, in 2011).29 The health gains from the intervention are large if existing estimates of the benefits from smoking cessation transfer to the Thai context. Smoking cessation among men aged 55 (the closest average age to the study population) extended life expectancy by nearly 5 years in the U.S.30 Life expectancy at birth in Thailand was 70 in 2009, according to official WHO estimates.
This study has several limitations. First, external validity is a concern for a small trial fielded in 42 communities. Smoking prevalence in the communities matches national estimates for rural areas, and the communities are culturally and economically diverse; however, the communities were sampled out of convenience, not to represent a broader geographic area. More generally, one might worry that Thailand’s high demand for quitting and comprehensive tobacco control regulations make it a special case, although smoking patterns in other developing countries are likely to follow suit as a result of tobacco control reforms already underway. Second, the two-arm trial cannot disentangle the causal pathways through which the intervention acted. The next step will involve a larger evaluation to determine the relative contribution of potential mechanisms—financial commitment, peer pressure, social support, regular reminders, and monetary rewards—to team commitment’s success.
This study shows that a simple team-based behavioral economics intervention enhanced the likelihood of smoking cessation in rural communities. Team commitment contracts are flexible enough to be offered in a variety of settings, including in clinics and workplaces. The contracts require minimal start-up capital, and community health workers who are deployed throughout the developing world require no additional training to market the contracts. Team commitment contracts may offer a viable, cost-effective alternative to current smoking treatment approaches in low-resource settings.
Supplementary Material
Acknowledgments
The authors thank Parichart Sukanthamala for outstanding field assistance and Tawima Sirirassamee and Chaturon Tangsangwornthamma for advice while in the field. All errors are our own. The full study protocol is available from the Corresponding Author.
The study was funded by grants from the U.S. National Institute on Aging (P30-AG012839, T32-AG000246) and the U.S. National Institute for Child Health and Development (R21-HD056581). The study’s sponsors had no role in study design, data collection, analysis, interpretation, or writing of the report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have completed the ICMJE uniform disclosure form (available on request from the corresponding author) and declare: JSW and WHD have no financial disclosures; and SR has undertaken research and consultancies for and received honoraria for speaking at sponsored meetings of GlaxoSmithKline, Pfizer, and Prosp Pharma, manufacturers of smoking cessation medications.
No other financial disclosures were reported by the authors of this paper.
References
- 1.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1(1) doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of Varenicline, an α4β2 Nicotinic Acetylcholine Receptor Partial Agonist, vs Placebo or Sustained-Release Bupropion for Smoking Cessation: A Randomized Controlled Trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 3.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2005;2:1–23. doi: 10.1002/14651858.CD001292.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Bryan G, Karlan D, Nelson S. Annu Rev Econom. 2010;2:671–698. [Google Scholar]
- 5.John L, Loewenstein G, Troxel A, Norton L, Fassbender J, Volpp K. Financial Incentives for Extended Weight Loss: A Randomized, Controlled Trial. J Gen Intern Med. 2011;26:621–626. doi: 10.1007/s11606-010-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldhaber-Fiebert JD, Blumenkranz E, Garber AM. Committing to Exercise: Contract Design for Virtuous Habit Formation. NBER Work Pap Ser. 2011:16624. [Google Scholar]
- 7.Giné X, Karlan D, Zinman J. Put your money where your butt is: A commitment contract for smoking cessation. Am Econ J Appl Econ. 2010;2(4):213–235. [Google Scholar]
- 8.Asch SE. Effects of group pressure upon the modification and distortion of judgment. In: Guetzkow H, editor. Groups, Leadership and Men. Carnegie: Pittsburgh; 1951. pp. 177–190. [Google Scholar]
- 9.Cialdini RB. Influence: The Psychology of Persuasion. New York NY: Harper Collins Publishers; 2007. [Google Scholar]
- 10.Babcock P, Bedard K, Charness G, Harman J, Royer H. Letting down the team? Evidence of social effects of team incentives. NBER Work Pap Ser. 2011:16687. [Google Scholar]
- 11.May S, West R, Hajek P, McEwen A, McRobbie H. Randomized controlled trial of a social support (`buddy') intervention for smoking cessation. Patient Educ Couns. 2006;64(1–3):235–241. doi: 10.1016/j.pec.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Park EW, Schultz JK, Tudiver FG, Campbell T, Becker LA. Enhancing partner support to improve smoking cessation. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD002928.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Cahill K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database Syst Rev. 2011;4:CD004307. doi: 10.1002/14651858.CD004307.pub4. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Geneva, Switzerland: 2010. Global Experience of Community Health Workers for Delivery of Health Related Millennium Development Goals: A Systematic Review, Country Case Studies, and Recommendations for Integration into National Health Systems. [Google Scholar]
- 15.World Health Organization. Geneva, Switzerland: Working together for Health—the World Health Report 2006. [Google Scholar]
- 16.White JS, Ross H. Role of smoker response in muting the health effects of cigarette taxes. Health Econ. Forthcoming (accepted). [Google Scholar]
- 17.Levy DT, Benjakul S, Ross H, Ritthiphakdee B. The role of tobacco control policies in reducing smoking and deaths in a middle income nation: Results from the Thailand SimSmoke simulation model. Tob Control. 2008;17:53–59. doi: 10.1136/tc.2007.022319. [DOI] [PubMed] [Google Scholar]
- 18.Thailand National Statistics Office. Household Socio-Economic Survey, 2008. web.nso.go.th/indicator/eco_ied08.pdf.
- 19.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. NEJM. 2009;360(7):699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- 20.Lunze K, Paasche-Orlow MK. Financial incentives for healthy behavior: Ethical safeguards for behavioral economics. Am J Prev Med. 2013;44(6):659–665. doi: 10.1016/j.amepre.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Singer E, Bossarte RM. Incentives for survey participation: When are they “coercive”? Am J Prev Med. 2006;31(5):411–418. doi: 10.1016/j.amepre.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1:CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Rungruanghiranya S, Ekpanyaskul C, Hattapornsawan Y, et al. Effect of Nicotine Polyestex Gum on Smoking Cessation and Quality of Life. J Med Assoc Thai. 2008;91:1656–1662. [PubMed] [Google Scholar]
- 24.Wang C, Xiao D, Chan KPW, Pothirat C, Garza D, Davies S. Varenicline for smoking cessation: A placebo-controlled, randomized study. Respirology. 2009;14:384–392. doi: 10.1111/j.1440-1843.2008.01476.x. [DOI] [PubMed] [Google Scholar]
- 25.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;4:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med. 2008;358(21):2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prah Ruger J, Lazar CM. Economic evaluations of pharmaco- and behavioral therapies for smoking cessation: A critical review of empirical research. Annu Rev Public Health. 2012;33:279–305. doi: 10.1146/annurev-publhealth-031811-124553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranson MK, Jha P, Chaloupka FJ, Nguyen SN. The effectiveness and cost-effectiveness of price increases and other tobacco control policies. In: Jha P, Chaloupka FJ, editors. Tobacco Control in Developing Countries. 2000. pp. 427–447. [Google Scholar]
- 29.The World Bank. World DataBank. 2012 databank.worldbank.org/ddp/home.do. [Google Scholar]
- 30.Taylor DH, Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of Smoking Cessation for Longevity. Am J Public Health. 2002;92(6):990–996. doi: 10.2105/ajph.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.