SETBP1 encodes SET-binding protein 1, a binding partner for the multi-function SET protein. This protein is encoded by the SET nuclear oncogene and is involved in apoptosis, transcription and nucleosome assembly.1 The proposed functional outcome of this interaction is based on in vitro studies that demonstrate a protection of SET protein from protease cleavage that results in inhibition of protein phosphatase 2A activity, leading to higher rates of cell proliferation.1 Initial identification of germline SETBP1 alterations affecting amino-acid residues between 858 and 871 have been described in patients with Schinzel–Giedion syndrome, associated with a congenital phenotype including mental retardation and facial deformities.2
Recently, analysis of exome sequencing data from eight cases of atypical chronic myelogenous leukemia (aCML) led to the identification of recurrent somatic mutations involving SETBP1.3 Mutational frequency was 24% among 70 patients with aCML, and 4% among 82 patients with chronic myelomonocytic leukemia (CMML). The investigators were not able to detect similar mutations among 106 patients with acute myeloid leukemia (AML), 100 with myelodysplastic syndromes (MDS), 42 with chronic myeloid leukemia, 33 with primary myelofibrosis (PMF), 42 with polycythemia vera and 36 with essential thrombocythemia.3 A more recent study identified SETBP1 mutations with an overall prevalence of 3.2% in a total of 658 cases consisting of 195 patients with CMML, 222 with MDS and 241 with secondary acute myeloid leukemia (sAML). SETBP1 mutations were identified in 6.2% of CMML patients, 2.2% of MDS patients and 1.7% of patients with sAML.4
In an effort to further investigate the prevalence and prognostic value of SETBP1 mutations in PMF and CMML, we studied a total of 415 patients with either PMF (n=236) or CMML (n=179). PCR and Sanger sequencing was used for mutation screening in PMF patients (forward primer 5′-ATGCACCCACTTTCAACACA-3′ and Reverse primer 5′-AAAAGGCACCTTTGTCATGG-3′ to generate sequence for amino-acid region 825–1013). For the CMML cohort, we used the ViiA7 quantitative RT-PCR platform (qPCR) and MeltDoctor high-resolution melting assay (Life Technologies, Grand Island, NY, USA) using forward primer 5′-GCGAGATTGGCTCCCTAAAG-3′ and reverse primer 5′-CCAGGGAGCAGAAATCAAAA-3′ to generate sequence for amino-acid region 860–1000. Targeted cases in the CMML cohort were validated using Sanger sequencing to confirm the presence of a mutation.
Among the 236 patients with PMF (median age 63 years; 63% males), Dynamic International Prognostic Scoring System (DIPSS)-plus5 risk distributions were high in 30%, intermediate-2 in 37%, intermediate-1 in 20% and low in 13%. Only six (2.5%) patients displayed SETBP1 mutations including three with D868N, two with G870S and one with I871T (Table 1). These mutations have all been previously described in other myeloid malignancies but not in PMF.3 We found no significant correlations between the presence of SETBP1 mutations and age (P=0.74), sex (P=0.5), DIPSS-plus risk category (P=0.38), red cell transfusion need (P=0.3), hemoglobin <10 g/dl (P=0.34) or karyotype (P=0.48; three normal and three abnormal karyotype). SETBP1 mutations significantly correlated with higher leukocyte count (P=0.047), and borderline significance was seen with lower platelet count (P=0.08). Among 234 patients with concomitant JAK2V617F analysis, SETBP1 mutations were seen in 3 of 136 JAK2V617F-mutated and 3 of 98 unmutated cases (P=0.68). Table 1 outlines the patterns of concomitant mutations in other genes, including MPL, ASXL1, EZH2, SRSF2 and IDH, for all six SETBP1-mutated cases. Three of the six SETBP1-mutated patients were also screened for SF3B1 mutations and were all negative (P=0.61). At a median follow-up of 47 months, 129 (55%) deaths and 22 (9%) leukemic transformations were documented. Although the number of informative cases were too small to be definitive, the differences in either overall (hazard ratio (HR) 1.9; 95% confidence interval (CI) 0.7–5.2) or leukemia-free survival (HR 2.6; 95% CI 0.34–19.4) did not reach statistical significance.
Table 1. SETBP1 mutational frequency and distribution in PMF and CMML.
| SETBP1 mutations | PMFn=236 | CMMLn=179 | |
|---|---|---|---|
| SETBP1 mutated | 6/236 (2.5%) | 8/179 (4.5%) | |
| D868N | 3/236 | 5/179 | |
| D868Y | 0/236 | 1/179 | |
| G870S | 2/236 | 1/179 | |
| I871T |
1/236 |
1/179 |
|
|
SETBP1 with concomitant mutations |
PMF |
P-value |
CMML |
| JAK2V617F mutated | 3/136 | 0.68 | —a |
| JAK2V617F unmutated | 3/98 | — | |
| MPL | 0/6 | — | —a |
| ASXL1 | 2/6 | 0.7 | 6/8 |
| EZH2 | 0/6 | 0.52 | —a |
| SRSF2 | 1/6 | 0.65 | 3/8 |
| IDH | 0/6 | 0.6 | —a |
| SF3B1 | 0/3 | 0.61 | 0/8 |
| U2AF35 | —a | —a | 2/8 |
Abbreviations: CMML, chronic myelomonocytic leukemia; PMF, primary myelofibrosis.
Not tested in this patient group.
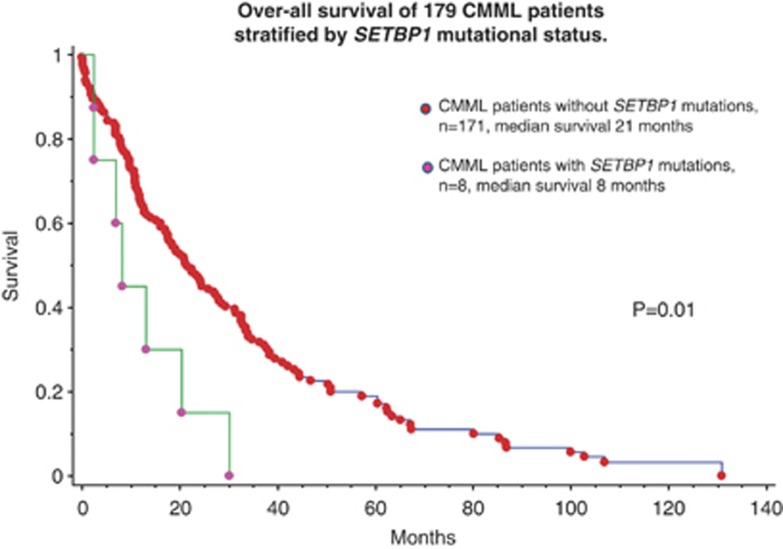
Among the 179 study patients with CMML, median age was 70 years and 122 (68%) were males. Distribution of patients based on the Mayo CMML prognostic model were: 93 (52%) low risk, 45 (25%) intermediate risk and 41 (23%) high risk.6 Eight (4.5%) patients with CMML displayed SETBP1 mutations. These included previously described mutations in seven patients (five with D868N, one with G870S and one with I871T) and a previously undescribed variant affecting amino-acid 868 (D868Y; Table 1). We found no significant correlations between the presence of SETBP1 mutations and age (P=0.4), sex (P=0.6), absolute monocyte count (P=0.77), hemoglobin (P=0.4), platelet count (P=0.34), bone marrow blasts (P=0.8), distribution across the Spanish cytogenetic risk stratification system (P=0.17),7 MD Anderson prognostic scoring system (MDAPS) (P=0.19),8 Mayo prognostic scoring system (P=0.65) and the global MDAPS (P=0.56).9 SETBP1 mutations significantly correlated with higher circulating immature myeloid cells (P=0.03) and circulating blasts (P=0.032), and a borderline significance was noted for leukocyte count (P=0.08). SETBP1-mutated patients with CMML coexpressed mutations involving ASXL1 in six cases (75%), SRSF2 in three (38%), U2AF35 in two (25%) and SF3B1 in none; there was no statistically significant difference between SETBP1-mutated and unmutated cases in their coexpression frequencies. At a median follow-up of 17 months, 134 (75%) deaths and 24 (13%) leukemic transformations were documented. In univariate analysis, SETBP1 mutations were found to have a negative impact on overall survival (P=0.01, HR; 95% CI) (Figure 1). In multivariable analysis, SETBP1 mutations retained their negative prognostic impact against other parameters of prognostic importance that are listed in conventional prognostic models for CMML. Low number of events did not allow accurate statistical evaluation for leukemia-free survival.
Figure 1.
Overall survival of 179 CMML patients stratified by SETBP1 mutational status.
Increased expression of SETBP1 has been reported occurring in 27% of patients with AML.1 Similarly, increased expression of SETBP1 has been associated with decreased expression of SETBP1-embedded regulatory micro-RNA miR_4319 in a patient with PMF progressing to AML.10 Accordingly, using published primer sets11 and GAPDH controls, we measured levels of gene expression using qPCR and the SYBR green mastermix (Life Technologies) in 20 PMF patients who were studied for the presence of SETBP1 mutations, including 4 who harbored the mutation. SETBP1 expression levels in 19 of the 20 PMF patients were similar to normal controls (n=4) and the single patient with >5-fold increased expression of SETBP1 was wild-type for SETBP1. The role of SETBP1 in disease progression, including leukemic transformation, is currently poorly understood, although it was recently reported that constitutive expression of SETBP1 in an in vivo murine system may be involved in conferring self-renewal properties to leukemic stem cells.12 Regardless, the strong prognostic value of the particular mutation in CMML, as suggested by the current study as well as that of Damm et al., raises the possibility of its incorporation into current prognostic models.
The authors declare no conflict of interest.
References
- Cristobal I, Blanco FJ, Garcia-Orti L, Marcotegui N, Vicente C, Rifon J, et al. SETBP1 overexpression is a novel leukemogenic mechanism that predicts adverse outcome in elderly patients with acute myeloid leukemia. Blood. 2010;115:615–625. doi: 10.1182/blood-2009-06-227363. [DOI] [PubMed] [Google Scholar]
- Hoischen A, van Bon BW, Gilissen C, Arts P, van Lier B, Steehouwer M, et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat Genet. 2010;42:483–485. doi: 10.1038/ng.581. [DOI] [PubMed] [Google Scholar]
- Piazza R, Valletta S, Winkelmann N, Redaelli S, Spinelli R, Pirola A, et al. Recurrent SETBP1 mutations in atypical chronic myeloid leukemia. Nat Genet. 2013;45:18–24. doi: 10.1038/ng.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm F, Itzykson R, Kosmider O, Droin N, Renneville A, Chesnais V, et al. SETBP1 mutations in 658 patients with myelodysplastic syndromes, chronic myelomonocytic leukemia and secondary acute myeloid leukemias. Leukemia. 2013;27:1401–1403. doi: 10.1038/leu.2013.35. [DOI] [PubMed] [Google Scholar]
- Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–397. doi: 10.1200/JCO.2010.32.2446. [DOI] [PubMed] [Google Scholar]
- Patnaik MM, Padron E, Laborde RR, Lasho TL, Finke CM, Hanson CA, et al. Mayo prognostic model for WHO-Defined chronic myelomonocytic leukemia-ASXL1 and spliceosome component mutations and outcomes. Leukemia. 2013;27:1504–1510. doi: 10.1038/leu.2013.88. [DOI] [PubMed] [Google Scholar]
- Such E, Cervera J, Costa D, Solé F, Vallespí T, Luño E, et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica. 2011;96:375–383. doi: 10.3324/haematol.2010.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onida F, Kantarjian HM, Smith TL, Ball G, Keating MJ, Estey EH, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood. 2002;99:840–849. doi: 10.1182/blood.v99.3.840. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, O'Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano F, Anelli L, Zagaria A, Coccaro N, Casieri P, Minervini A, et al. SETBP1 and miR_4319 dysregulation in primary myelofibrosis progression to acute myeloid leukemia. J Hematol Oncol. 2012;5:48. doi: 10.1186/1756-8722-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filges I, Shimojima K, Okamoto N, Röthlisberger B, Weber P, Huber AR, et al. Reduced expression by SETBP1 haploinsufficiency causes developmental and expressive language delay indicating a phenotype distinct from Schinzel-Giedion syndrome. J Med Genet. 2011;48:117–122. doi: 10.1136/jmg.2010.084582. [DOI] [PubMed] [Google Scholar]
- Oakley K, Han Y, Vishwakarma BA, Chu S, Bhatia R, Gudmundsson KO, et al. Setbp1 promotes the self-renewal of murine myeloid progenitors via activation of Hoxa9 and Hoxa10. Blood. 2012;119:6099–6108. doi: 10.1182/blood-2011-10-388710. [DOI] [PMC free article] [PubMed] [Google Scholar]