The prognosis of acute myeloid leukemia (AML) patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) not in complete remission is poor,1, 2, 3 although this treatment option remains the only possible curative approach for these patients.4 A retrospective analysis recently published by European Group for Blood and Marrow Transplantation (EBMT) on primary refractory AML allotransplanted with unrelated donors showed that factors associated with improved survival were the following: having received fewer than three courses of induction therapy, the presence of a lower percentage of bone marrow blast infiltration at transplant and patient cytomegalovirus seropositivity. This allowed the development of a scoring system that identified four groups with survival rates ranging between 44 and 0%.5 However, the largest retrospective analysis on AML patients with active disease at the time of conditioning (1673 patients) has been conducted by the Center for International Blood and Marrow Transplant Research (CIBMTR), which, on five pretransplantation variables (duration of first complete remission (CR) <6 months, circulating blasts, donor other than HLA-identical sibling, Karnofsky score less than 90 and poor-risk cytogenetics), also set up a pre-HSCT score defining a 3-year overall survival (OS) ranging from 42 to 6%.6 Here we report outcome data obtained in Italy in a similar cohort of AML patients (523 patients) allotransplanted with active disease. The primary aim of the study was to externally validate the CIBMTR score in a multicenter, retrospective study setting, evaluating the prognostic power of the score in a wider patient population that included not only those receiving a myeloablative conditioning (MAC) but also those treated with a reduced-intensity conditioning (RIC)7, 8 (as detailed in Supplementary Table 1) and those grafted with a cord blood. Twenty Italian centers belonging to the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) participated in this retrospective observational study. Data were retrieved from the GITMO database, and missing data or specific queries were asked to each center. Overall, 523 patients (no one enrolled into prospective trials) from 20 GITMO centers were included in this study. Patient, disease and transplant characteristics are listed in Table 1. The median age was 47.6 (range 18–72). At time of conditioning, AML was defined as primary refractory (patients not achieving a CR after the first induction chemotherapy), MDS-related (untreated patients with >20% bone marrow blasts), untreated first relapse (patients not receiving a salvage chemotherapy before conditioning), refractory first relapse (patients not achieving a remission after a salvage chemotherapy), second or further relapse (untreated or refractory to further salvage chemotherapy). A marrow blast infiltration >25% or any level of peripheral blood (PB) blasts was found in 42%. Donors were HLA identical sibling or matched unrelated in 69%, a family or unrelated mismatched in 25% and a cord blood unit in 6%. More than 60% of patients received a MAC and 37% received a RIC program. A T-cell depletion was performed in vivo in 37% and ex vivo in 8% of patients as described elsewhere.9, 10, 11
Table 1. Patients characteristics with univariate and multivariate analysis of prognostic factors for survival.
| Characteristics |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| N (%) | Overall survival at 3 years | P-value | Hazard ratio | 95% CI | P-value | |
| Age at transplant | ||||||
| ⩽47 | 269 (51) | 0.759 | 1.00 | |||
| >47 | 254 (49) | 1.01 | 0.83–1.23 | 0.924 | ||
| Sex | ||||||
| Male | 265 (51) | 0.15 | 0.9088 | 1.00 | ||
| Female | 258 (49) | 0.18 | 0.90 | 0.75–1.07 | 0.233 | |
| Diagnosis | ||||||
| De novo | 370 (71) | 0.16 | 0.0201 | 1.00 | ||
| Secondary to MDS/CMML | 120 (23) | 0.02 | 1.01 | 0.79–1.29 | 0.953 | |
| Secondary to CMN/therapy-related | 28 (5) | 0.08 at 1.5 yrs | 1.87 | 1.21–2.88 | 0.005 | |
| Missing | 5 (1) | 3.80 | 1.49–9.71 | 0.005 | ||
| Disease status at transplant | ||||||
| PRF | 166 (32) | 0.10 | 0.0008 | 1.00 | ||
| Untreated I relapse | 44 (8) | 0.26 | 1.94 | 0.72–5.19 | 0.187 | |
| Untreated MDS-related AML | 27 (5) | 0.57 | 0.85 | 0.33–2.18 | 0.737 | |
| Refractory I relapse | 179 (34) | 0.16 | 1.95 | 0.77–4.92 | 0.156 | |
| > I relapse | 77 (15) | 0.12 | 2.38 | 0.91–6.19 | 0.076 | |
| Missing | 30 (6) | 2.49 | 0.96–6.49 | 0.061 | ||
| Previous transplant in CR | ||||||
| Autologous | 58 (55) | 0.21 | 0.8992 | — | — | — |
| Allogeneic | 47 (45) | 0.12 | — | — | — | |
| Duration first CR | ||||||
| <6 months | 137 (46) | 0.09 | 0.023 | 1.00 | ||
| ⩾6 months | 136 (45) | 0.24 | 1.32 | 1.02–1.70 | 0.032 | |
| Missing | 27 (9) | 1.31 | 0.84–2.03 | 0.230 | ||
| Chemotherapy cycles for primary refractory | ||||||
| 1 | 34 (20) | 0.18 | 0.0062 | 1.00 | ||
| ⩾2 | 126 (76) | 0.07 | 1.68 | 1.12–2.52 | 0.013 | |
| Missing | 6 (4) | 0.86 | 0.34–2.17 | 0.755 | ||
| Cytogenetics/ molecular biology | ||||||
| Favorable/intermediate I | 235 (45) | 0.20 | 0.0484 | 1.00 | ||
| Intermediate II/adverse | 178 (34) | 0.11 | 1.29 | 1.05–1.59 | 0.014 | |
| Missing | 110 (21) | 1.03 | 0.79–1.34 | 0.819 | ||
| Blasts at transplant | ||||||
| BM blasts <25% or no blasts in PB | 197 (38) | 0.26 | 0.0000 | 1.00 | ||
| BM blasts ⩾25% or any level in PB | 218 (42) | 0.12 | 1.46 | 1.19–1.80 | 0.000 | |
| Missing | 108 (20) | 1.30 | 0.98–1.73 | 0.072 | ||
| Karnofsky performance score at transplant | ||||||
| <90 | 177 (34) | 0.11 | 0.0000 | 1.00 | ||
| ⩾90 | 284 (54) | 0.21 | 1.52 | 1.24–1.87 | 0.000 | |
| Missing | 62 (12) | 1.47 | 1.10–1.96 | 0.010 | ||
| Graft type | ||||||
| Bone marrow | 148 (28) | 0.12 | 0.1515 | — | — | — |
| Peripheral stem cells | 342 (65.5) | 0.18 | — | — | — | |
| Cord blood | 33 (6.5) | 0.16 | — | — | — | |
| Donor-recipient HLA-match | ||||||
| Identical sibling /matched unrelated | 362 (69) | 0.19 | 0.0015 | 1.00 | ||
| Cord blood | 33 (6) | 0.16 | 1.59 | 1.08–2.34 | 0.020 | |
| Haplo/mismatched unrelated | 128 (25) | 0.09 | 1.59 | 1.28–1.98 | 0.000 | |
| Donor-recipient sex | ||||||
| M-M/F-F | 270 (52) | 0.18 | 0.6215 | — | — | — |
| M-F | 147 (29) | 0.18 | — | — | — | |
| F–M | 99 (19) | 0.13 | — | — | — | |
| Missing | 7 (1) | — | — | — | ||
| Donor anti-CMV antibodies | ||||||
| Positive | 312 (60) | 0.17 | 0.8963 | 1.00 | ||
| Negative | 160 (31) | 0.18 | 1.03 | 0.84–1.26 | 0.786 | |
| Missing | 51 (9) | 1.35 | 0.81–2.24 | 0.248 | ||
| Patient anti-CMV antibodies | ||||||
| Positive | 421 (80) | 0.18 | 0.1908 | 1.00 | ||
| Negative | 61 (12) | 0.17 | 1.42 | 1.07–1.88 | 0.015 | |
| Missing | 41 (8) | 0.81 | 0.45–1.45 | 0.473 | ||
| Conditioning regimen | ||||||
| RIC | 191 (37) | 0.16 | 0.9511 | 1.00 | ||
| MAC | 324 (62) | 0.17 | 0.96 | 0.79–1.17 | 0.690 | |
| Missing | 8 (2) | 1.12 | 0.50–2.53 | 0.780 | ||
| Type of conditioning | ||||||
| Busulfan/TBI> 600 Gy | 288 (55) | 0.13 | 0.1614 | — | — | — |
| Others | 219 (42) | 0.19 | — | — | — | |
| Missing | 16 (3) | — | — | — | ||
| GVHD prophylaxis | ||||||
| Ex vivo T-cell depletion | 42 (8) | 0.05 | 0.0370 | — | — | — |
| (Tacrolimus or CsA)+MTX±other | 307 (59) | 0.18 | — | — | — | |
| (Tacrolimus or CsA)±other | 77 (15) | 0.17 | — | — | — | |
| Other | 50 (10) | 0.25 | — | — | — | |
| Missing | 47 (9) | — | — | — | ||
| T-cell depletion in vivo | ||||||
| No T-cell depletion in vivo | 249 (48) | 0.19 | 0.1220 | — | — | — |
| ATG/ALG/Campath | 196 (37) | 0.17 | — | — | — | |
| Missing | 78 (15) | — | — | — | ||
| Acute GVHD | ||||||
| No | 273 (52) | 0.15 | — | — | — | |
| Yes | 228 (44) | — | — | — | ||
| Grade 1 | 91 (40) | 0.21 | 0.0000 | — | — | — |
| Grade 2 | 71 (31) | 0.28 | — | — | — | |
| Grade 3 | 41 (18) | 0.07 | — | — | — | |
| Grade 4 | 25 (11) | 0.04 | — | — | — | |
| Missing | 22 (4) | — | — | — | ||
| Chronic GVHD | ||||||
| No | 286 (55) | 0.10 | 0.0000 | — | — | — |
| Yes | 126 (24) | 0.39 | — | — | — | |
| Missing | 111 (21) | |||||
Abbreviations: ALG, anti-lymphocyte-globuline; AML, acute myeloid leukemia; ATG, anti-tymocyte-globuline; BM, bone marrow; CMML, chronic myelomonocytic leukemia; CMN, chronic myeloproliferative neoplasm; CMV, cytomegalovirus; CR, complete remission; CsA, cyclosporine; GVHD, graft versus host disease; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MTX, methotrexate; PB, peripheral blood; PRF, primary refractory; RIC, reduced-intensity conditioning; TBI, total body irradiation.
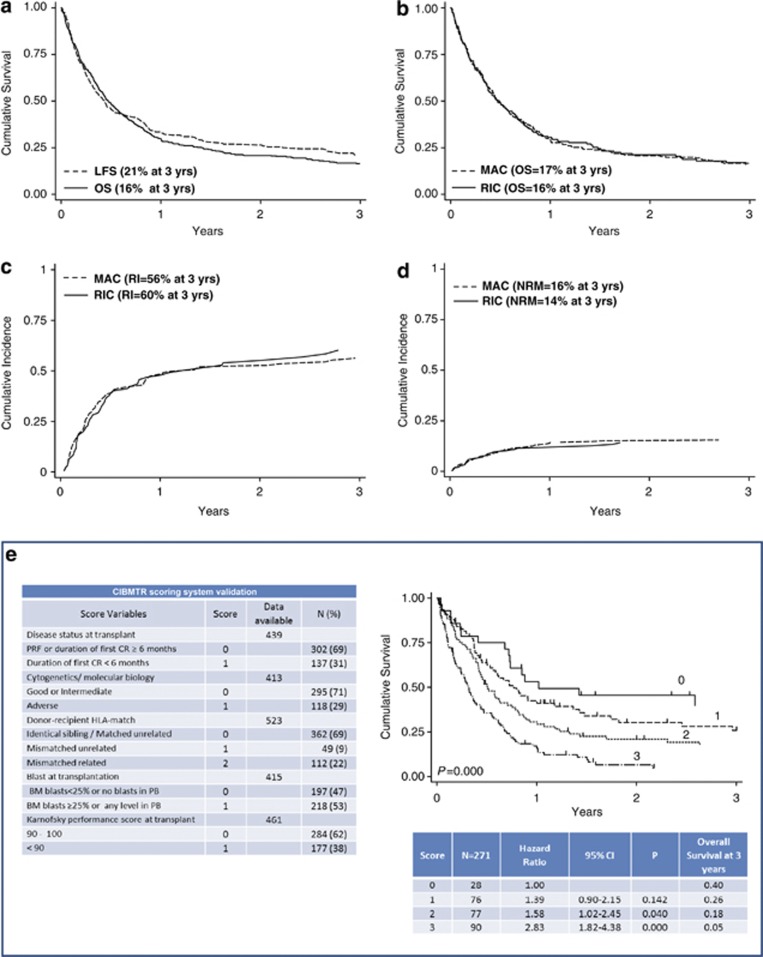
Neutrophil and platelet engraftment was achieved in 87% of patients after a median of 17 (9–63) and 18 (2–117) days, respectively. Acute graft versus host disease (GVHD) was registered in 46% of patients (grade ⩾2 in 60% of cases), whereas chronic GVHD occurred in 31% (judged as extended in half of cases). The 1-year cumulative incidence of acute GVHD was 39%, being 28% for grade 1–2 and 11% for grade 3–4, whereas that of chronic GVHD was 20%. In all, 75 patients (14%) died early, within 45 days from allotransplant, 282 patients (54%) achieved CR after allotransplant. Of these latter patients 155 (55%) relapsed after a median time of 3.7 months (0.4–83). Among the 427 patients who died after HSCT (82%), 91 were leukemia free. The median follow-up of the whole patient cohort was 5.3 months (0.10–133), whereas that of survivors was 26 months (1–133) with 96 patients alive and 77 leukemia free. At 3-years, the cumulative incidence of non-relapse mortality (NRM) was 16%. The leukemia-free survival (LFS, calculated from the time of CR after transplantation to death for any cause or relapse)12 was 21%, whereas the OS was 16% (Figure 1).
Figure 1.
(a) Kaplan–Meier estimates of overall survival, (OS, solid line, n=523) and leukemia-free survival (LFS, dotted line, n=282). (b) Kaplan-Meier estimates of overall survival for patients receiving a myeloablative (MAC, dotted line, n=332) or a reduced-intensity (RIC, solid line, n=191) conditioning regimen. (c) Incidence of relapse in patients receiving a MAC (dotted line) or RIC (solid line) conditioning regimen. (d) Non-relapse mortality (NRM) in patients receiving a MAC (dotted line) or RIC (solid line) conditioning regimen. (e) A CIBMTR scoring system validation with overall survival according to the risk score. The hazard ratio and the overall survival for score 2 and 3 proved significantly worse than 1 and 2.
Eight pre-HSCT variables that negatively influenced survival were identified by univariate and multivariate analysis: an AML secondary to a previous CMN or a therapy-related AML (P=0.005), a relapsed AML with a first CR duration <6 months (P=0.032), a primary refractory AML after ⩾2 chemotherapy cycles pre-HSCT (P=0.013), an intermediate II/adverse cytogenetics (P=0.014), BM blasts ⩾25% or any level of PB at HSCT (P=0.000), a Karnofsky performance score <90 (P=0.000), a mismatched related/unrelated donor (P=0.020) and the presence of patient anti-CMV antibodies (P=0.015) (Table 1). To elucidate the impact of the conditioning regimen on main outcomes, the clinical characteristics of patients who received a RIC (n=191) were compared with those of patients receiving a MAC transplant (n=324). A stratified analysis according to the conditioning regimen was developed, and pre-transplantation variables of the two patients groups were compared using the Fisher exact test for categorical variables. Patients receiving a RIC transplant were older (P=0.000), and more frequently were grafted with PB stem cells (P=0.000) or a mismatched donor (P=0.002) (data not shown). Nonetheless, the intensity of the conditioning regimen did not show an impact on 3-year OS, as well as on the relapse and NRM (Figure 1).
The OS of our patient cohort was finally analyzed according to the risk categories defined by the CIBMTR score (Table 1). In the more favorable prognostic group of 28 patients (10.5%) (score 0), the OS at 3 years was 40% (HR 1.00). Similarly to what observed in the original CIBMTR cohort, in the intermediate-I risk group (score 1) (n=76, 28%) the OS at 3 years was 26% (HR 1.39, P=0.142), whereas in the intermediate-II risk group (score 2) (n=77, 28.5%) and the poor risk group (score 3) (n=90 patients, 33%) the OS was 18% (HR 1.58, P=0.040) and 5% (HR 2.83, P=0.000), respectively (Figure 1).
Therefore, the long-term overall- and event- free survival observed in this group of patients are remarkably in keeping with those reported by CIBMTR6 and EBMT.5 In the GITMO database, the five easy-to-apply pre-HSCT variables defining the CIBMTR score were available contemporarily for only 52% of the patients analyzed, so that the score could be attributable only to a total of 271 patients. Nonetheless, we can reasonably confirm that the CIBMTR score is an effective and reproducible approach for predicting survival of this group of AML patients at poor prognosis.
However, some important differences between patients analyzed by CIBMTR, EBMT and GITMO must be underlined. First, in the CIBMTR study, only patients who received a total body irradiation or busulfan-based MAC regimen were analyzed, whereas patients receiving a Fludarabine-based or any other RIC regimen were excluded. In the GITMO cohort, a RIC was given to 37% of patients. In addition, we included also patients receiving a cord blood transplant (6%), as well as patients with an untreated, MDS-related AML (5%). In the EBMT experience, patients were limited only to those with a primary refractory AML and those who received an unrelated donor transplant. Despite these differences, our results confirm the EBMT analysis as to the negative impact of a heavy leukemic bone marrow infiltration and the role of the total number of chemotherapy cycles before the conditioning regimen. The prognostic role played by CMV was also underlined in both analysis, although the GITMO results point out the negative impact of a positive serology of the patient while the EBMT suggests that of the negative patient serology. In this study, patients with an AML secondary to a previous CMN or therapy-related had a remarkably poor outcome, and this turned out to be a novel significant adverse prognostic factor. However, the poor outcome of AML developing in patients with a previous history of chronic myeloproliferative disorders is not surprising.13
Importantly, by univariate and multivariate analysis, the conditioning intensity did not have an impact on 3-year OS and LFS on the entire GITMO cohort. Although the retrospective nature of the study suggests caution, this result may represent a new finding and it is tempting to speculate that for chemo-resistant disease the MAC may not be effective anyhow, so that only patients with an active graft versus leukemia reaction may actually benefit from the transplant.
In conclusion, we have validated the CIBMTR prognostic score in this relatively large patient population with active AML at allotransplant. It may therefore be possible to identify the patients with advanced AML, who may benefit more from an allogeneic transplant, and this may be relevant for patient counseling. The fact that RIC regimens could also be effective is encouraging for the older patient population, who may be eligible for this procedure.
Acknowledgments
This work was supported in part by grants from Associazione Italiana per la Ricerca contro il Cancro (AIRC) and Associazione Italiana Lotta alla Leucemia (AIL).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Greinix HT, Reiter E, Keil F, Fischer G, Lechner K, Dieckmann K, et al. Leukemia-free survival and mortality in patients with refractory or relapsed acute leukemia given marrow transplants from sibling and unrelated donors. Bone Marrow Transplant. 1998;21:673–678. doi: 10.1038/sj.bmt.1701152. [DOI] [PubMed] [Google Scholar]
- Michallet M, Thomas X, Vernant JP, Kuentz M, Socie G, Esperou-Bourdeau H, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Societe Francaise de Greffe de Moelle (SFGM) Bone Marrow Transplant. 2000;26:1157–1163. doi: 10.1038/sj.bmt.1702690. [DOI] [PubMed] [Google Scholar]
- Wong R, Shahjahan M, Wang X, Thall PF, De Lima M, Khouri I, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:108–114. doi: 10.1016/j.bbmt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Estey E. Treatment of refractory AML. Leukemia. 1996;10:932–936. [PubMed] [Google Scholar]
- Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H, et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia. 2011;25:808–813. doi: 10.1038/leu.2011.13. [DOI] [PubMed] [Google Scholar]
- Duval M, Klein JP, He W, Cahn J-Y, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaldi A, Bacigalupo A, Fanin R, Ciceri F, Bonifazi F, Falda M, et al. Outcome of patients activating an unrelated donor search: the impact of transplant with reduced intensity conditioning in a large cohort of consecutive high-risk patients. Leukemia. 2012;26:1779–1785. doi: 10.1038/leu.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty M, de Lavallade H, Ladaique P, Faucher C, Vey N, Coso D, et al. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: a donor vs no donor comparison. Leukemia. 2005;19:916–920. doi: 10.1038/sj.leu.2403770. [DOI] [PubMed] [Google Scholar]
- Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- Iacobelli S. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2013;48 (Suppl 1:S1–S37. doi: 10.1038/bmt.2012.282. [DOI] [PubMed] [Google Scholar]
- Larson RA. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia. Best Pract Res Clin Haematol. 2007;20:29–37. doi: 10.1016/j.beha.2006.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.