Abstract
The honeybee pathogens Nosema ceranae and deformed wing virus (DWV) cause the collapse of honeybee colonies. Therefore, it is plausible that these two pathogens act synergistically to increase colony losses, since N. ceranae causes damage to the mid-gut epithelial ventricular cells and actively suppresses the honeybees’ immune response, either of which could increase the virulence of viral pathogens within the bee. To test this hypothesis we exploited 322 Hawaiian honeybee colonies for which DWV prevalence and load is known. We determined via PCR that N. ceranae was present in 89–95% of these colonies, with no Nosema apis being detected. We found no significant difference in spore counts in colonies infected with DWV and those in which DWV was not detected, either on any of the four islands or across the entire honeybee population. Furthermore, no significant correlation between DWV loads (ΔCT levels) and N. ceranae spore counts was found, so these two pathogens are not acting synergistically. Although the Hawaiian honeybees have the highest known prevalence of N. ceranae in the world, with average number of spores been 2.7 million per bee, no acute Nosema related problems i.e. large-scale colony deaths, have been reported by Hawaiian beekeepers.
Introduction
It is well established that millions of honey bee colonies have been killed due to the global spread of the Varroa mite and its inter-action with deformed wing virus (DWV) (Martin et al., 2012). More recently honeybee colony losses have occurred across America and Europe that cannot be attributable to the Varroa mite, so unknown combinations of stressors are suspected (Potts et al., 2010). These could be various combinations of pathogens (Cox-Foster et al., 2007; Higes et al., 2009) or effects of pesticides and pathogens (vanEngelsdorp et al., 2009). For example, synergistic effects between various pesticides and the microsporidia fungal pathogen Nosema ceranae have been found, which in combination, were found to increase honey bee mortality in laboratory essays (Alaux et al., 2010; Vidau et al., 2011; Pettis et al., 2002). Although at a field scale any synergistic effect between DWV and N. ceranae was lacking (Hedtke et al., 2011). Studies looking at synergistic effects under natural field conditions between two or more pathogens are rare, since it is difficult to find situations where the prevalence and, more importantly, the load of a pathogens is known to exist at different levels within the same population. The accidental introduction of the Varroa mite to the Hawaiian Islands of Oahu and Big Island, but not Maui and Kauai has caused a large change in both the prevalence and load of DWV across only two of these islands (Martin et al., 2012). This provides a unique opportunity to investigate whether DWV and Nosema act synergistically to become more virulent when combined in the same colony. Both DWV (Schroeder and Martin, 2012) and N. ceranae (Higes et al., 2007; 2009) are known to kill honey bee colonies in their own right. Although N. ceranae has been implicated in the large-scale colony losses in Spain (Higes et al., 2009), its impact in other countries remains controversial (Higes et al., 2013). However, a synergistic effect between these two pathogens is highly plausible, since the obligate, spore-forming, intracellular parasite N. ceranae causes extensive damage to the mid-gut epithelial ventricular cells (Fries, 2010; Dussaubat et al., 2012). This could then allow viral pathogens, such as DWV, to pass more easily through the gut wall into the haemolymph which is supported by correlative evidence (Bromenshenk et al., 2010; Costa et al., 2011). The gut wall is an important barrier against viral pathogens, since oral transmission of viruses between bees are significantly less efficient than when a virus is injected directly into the haemolymph (Bailey and Ball, 1991). It has also been shown that N. ceranae can actively suppresses the immune response in honeybees (Chaimanee et al., 2012), which may also make N. ceranae infected colonies more susceptible to viral infections.
To test the hypothesis that Nosema and DWV act synergistically, we used our existing viral data from 322 colonies across the four main Hawaiian islands of Oahu (5 apiaries), Big Island (14 apiaries), Maui (4 apiaries) and Kauai (6 apiaries) (Martin et al., 2012). From these same colonies we surveyed for the prevalence of Nosema apis and N. ceranae via PCR and estimated Nosema load (spore count) in a subset of colonies in which DWV was either detected (DWV+) or not (DWV−).
Results and discussion
Determination of the species of Nosema by PCR followed the methodology described by Higes and colleagues (2008) and Martín-Hernández and colleagues (2012) using a pool of 30 bees collected from the brood area from each colony at the same time the samples for DWV analysis were collected. Across all four major Hawaiian Islands N. ceranae was the only Nosema species detected in the 322 colonies surveyed. Of these 283 colonies (88%) and all but one of the 31 apiaries tested positive for N. ceranae. In five of the eight N. ceranae free colonies as determined by PCR, spores were detected under the microscope. This discrepancy is due to the known high degree of variability between Nosema subsamples (Botías et al., 2012a). This increased the prevalence to 89–95% assuming that spores were absent in all the other PCR-negative samples, or were present in 63% (i.e. five-eighths) of the remaining PCR-negative samples. Based on the PCR results no significant difference in N. ceranae prevalence between the four islands (Kruskal–Wallis, d.f. = 3, H = 7.64, P = 0.054) was found.
We selected two groups from each island from the 283 colonies that tested positive for N. ceranae in which DWV had been previously detected (DWV+) or not (DWV−). The long-term establishment of Varroa on Oahu meant that no DWV− colonies were present. The amount of DWV had been previously determined from a pooled sample of 30 bees and the ΔCT values were used as an indication of viral load in each colony (for details see Martin et al., 2012). Our N. ceranae spore counts were based on pooled samples of 20 bees using the standard methods and were highly variable between colonies, as is typical for this species (Meana et al., 2010; Mulholland et al., 2012), but no significant difference (Kruskal–Wallis, d.f. = 3, H = 1.37, P = 0.7) was detected between the four islands. Across all samples the average and median spore counts were 2.7 million/bee and 1.1 million/bee respectively. We acknowledge that spores counts may not be the most accurate method to determine N. ceranae infection (Meana et al., 2010), but qPCR results that measure the actively reproducing vegetative state have been found to be significantly correlated with spore counts, although the level of correlation varied from strong (r2 = 0.74, Bourgeois et al., 2010) to weak (r2 = 0.26, Traver and Fell, 2011). Although, when high or moderate infection levels are present, 80% of the samples testing positive by qPCR also contained spores (Traver and Fell, 2011). No significant different in spore count between colonies infected with DWV or without DWV could be detected in any of the three islands populations (Mann–Whitney, Big Island U = 10, P = 0.14; Maui U = 22, P = 0.25; Kauai U = 7, P = 0.17) (Fig. 1) or when the data from all islands were combined (U = 150, P = 0.25, n = 41). Again no significant correlation (Spearman Rank, n = 24, r = −0.147, P = 0.5) between DWV viral load data (ΔCT values) and N. ceranae spore counts was detected (Fig. 2). Therefore, while both DWV and N. ceranae are known to be lethal pathogens of honeybees when they occur at high loads (Higes et al., 2009; Martin et al., 2012), we found no significant effect, positive or negative, indicating that there was no synergistic or additive effect between DWV and N. ceranae in the Hawaiian honey bee population. Increased DWV prevalence and load associated with the spread of the Varroa mite corresponded with an increase in colony losses on Oahu and Big Island (Martin et al., 2012). However, the present data show that prevalence and load of N. ceranae were not significantly different from other islands (Maui and Kawai) where DWV prevalence and load remained low and no increase in colony losses were seen. As DWV prevalence and load is closely correlated with the presence of the Varroa mite (Martin et al., 2012), as expected there was also no synergistic effect between the presence of Varroa and N. ceranae. A weak correlation was reported between Varroa and Nosema in Germany, but this finding was based only on presence or absence data (Hedtke et al., 2011).
Figure 1.
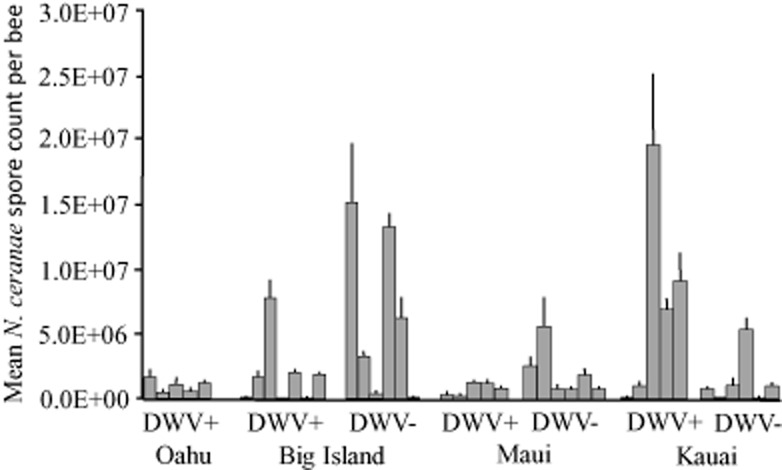
Shows the average Nosema ceranae counts per bee for 40 colonies across the four islands. Each bar represents a colony and error bars indicate one standard deviation. No significant difference in spore counts between colonies in which DWV was detected (DWV+) or not (DWV−) was found on any of the three islands where both groups exists. The DWV titre (mean number of DWV copies per bee and ΔCT value) on each island had been previously calculated as Kauai (103.7, −14), Maui (104.2, −17), Big Island (103.5, −15), and on Oahu (1010, 7) (see Martin et al., 2012 for details).
Figure 2.
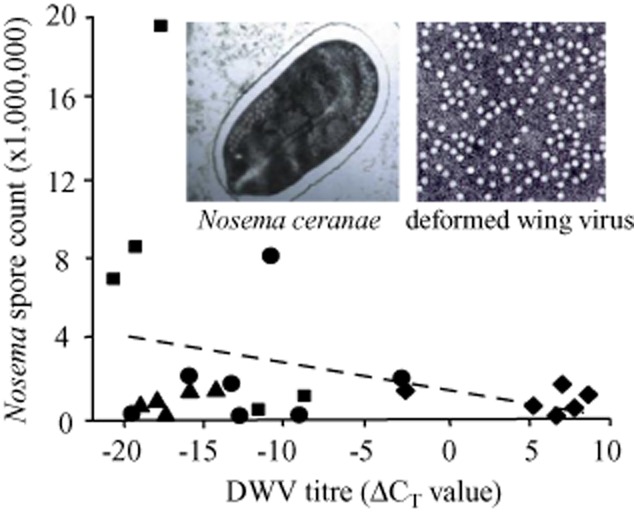
Shows the lack of a significant correlation between Nosema ceranae spore counts and DWV load, across the 23 colonies studied on Oahu (diamond), Maui (triangle), Big Island (circle) and Kauai (square). The higher the ΔCT value the higher the amount of virus in that sample of bees, i.e. colony.
As Nosema has never been seen as a major problem among Hawaiian beekeepers it was generally assumed that Nosema levels would be low, i.e. below the economic threshold of 1 million spores per bee (El-Shemy and Pickard, 1989). Furthermore, due to import restrictions of honeybee colonies into the islands since the 1930s (Eckert, 1950), which were rigorously enforced after the arrival of Varroa on the US mainland in 1987, it was assumed that N. apis would be the dominant species. So it was unexpected that we detected only N. ceranae in Hawaii. It was originally believed that N. ceranae had recently expanded from its natural host Apis ceranae to include Apis mellifera (Higes et al., 2006; Huang et al., 2008), since it appeared to be replacing N. apis in several populations across Europe (Klee et al., 2007). Although more recent European studies found no evidence that replacement is occurring (Paxton et al., 2007; Gisder et al., 2010; Martín-Hernández et al., 2012; Forsgren and Fries, 2013). In the USA there are confirmed infections of N. ceranae dating back to 1985 (Traver et al., 2012, Accession No. FJ416497.1) and it was already widespread in the mid-1990s (Chen et al., 2008). Therefore, the current situation on Hawaii is very similar to that on the US mainland, but the question of how and when N. ceranae first arrived in Hawaii remains open.
Hawaii currently has the highest N. ceranae prevalence of any known honeybee population with this study detecting it in 89–95% of the 322 study colonies and well above the average value of 57% for the US mainland (Rennich et al., 2012). In the warmer climates of Southern Europe N. ceranae and not N. apis dominates (Martín-Hernández et al., 2012), which would help explain the dominance of N. ceranae in Hawaii with its subtropical climate. However, N. ceranae was also the dominate species in regions with a cold climate such as Canada and Minnesota (Williams et al., 2008), Germany (Gisder et al., 2010), Scotland (Bollan et al., 2013) and the Balkans (Stevanovic et al., 2011). However, in the cold climate of Sweden and Norway N. apis was dominate (Forsgren and Fries, 2013), indicating that climate is a poor predictor of Nosema species.
The average spore count of 2.7 million per bee in Hawaii, which is typical for many studies, is well below the 10s-100s of millions detected from in-hive bees from CCD colonies (Cox-Foster et al., 2007) or 10s of millions in those that died in Spain (Higes et al., 2008). The spore counts presented in this study are expected to be underestimates, since they were conducted on bees collected from the brood combs (nurse or in-hive bees) that have lower counts than foraging bees (El-Shemy and Pickard, 1989; Higes et al., 2008; Smart and Sheppard, 2012), although Traver and colleagues (2012) found that there was no significant difference between in-hive and foragers in N. ceranae infection levels based on q-PCR results, which detects the earlier vegetative stage.
Despite the almost universal occurrence of N. ceranae across Hawaii combined with high loads, and potential increase in reproductive potential due to high climate temperatures (Martín-Hernández et al., 2009), nosemosis has never been considered a major problem in Hawaii and few beekeepers treat for it when questioned while collecting bee samples for this study. The lack of reported acute effects on Hawaii i.e. colony death is similar to other large-scale studies looking at the impact of N. ceranae on colony losses. For example, in Uruguay Invernizzi and colleagues (2009) found no correlation between the arrival of N. ceranae and either increasing microsporidia loads or increased colony losses. The studies of Traver and Fell (2011) and Traver and colleagues (2012) found no correlation between colony strength and infection level, despite 70% of their 300 study colonies being infected by N. ceranae. Likewise a German study of 220 colonies found no relationship between colony mortality and detectable levels of N. ceranae infection (Gisder et al., 2010) as did a Spanish study involving 77 apiaries (Fernández et al., 2012). It is only in Spain that N. ceranae has been directly associated with large-scale colony losses (Higes et al., 2009). It is currently unclear why these differences exist (Higes et al., 2013). It may be due to N. ceranae strain differences as found in DWV (Martin et al., 2012). Higes and colleagues (2013) observed that colony losses were not generally reported from studies conducted in colder areas; however, colony losses were also not reported from Hawaii with its warmer climate.
Although potentially there could be a synergistic effect between DWV and N. ceranae this idea has not been supported by this study, nor the larger ‘prevalence only’ study of Hedtke and colleagues (2011). Furthermore, the study of Costa and colleagues (2011). found no significant correlation between N. ceranae and DWV at the whole bee level. In the midgut they also found no evidence of synergistic effects, but possibly an antagonistic effect, since a negative correlation between N. ceranae and DWV was found. Dussaubat and colleagues (2012), also mentioned they found no synergistic effect but no details are given. Despite this study showing the Hawaiian honeybee population has the highest prevalence of N. ceranae ever found, why this situation exists is currently unclear. Climatic conditions in Hawaii allow honeybee brood to be produced continuously, so turnover rates of workers and queens are high and this may affect N. ceranae levels (Botías et al., 2012b). Furthermore, N. ceranae could be having an unseen subclinical effect, but further studies are required to discover the real impact, if any, of this pathogen in subtropical climates such as Hawaii.
Acknowledgments
Many thanks to the Hawaiian beekeepers for allowing their honeybees to be sampled. S.J.M. was funded by funding from NERC (NE/H013164/1), OECD, the CB-Dennis Trust and Apis_m. SN and E.M.V. were funded by USDA-NIFA Tropical and Subtropical Agricultural Research (TSTAR) Program (2010-34135-21499), support from the Hawaii Department of Agriculture.
References
- Alaux C, Brunet JL, Dussaubat F, Mondet S, Tchamitchan S, Cousin M, et al. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera. Environ Microbiol. 2010;12:774–782. doi: 10.1111/j.1462-2920.2009.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey L, Ball BV. Honey Bee Pathology. 2nd edn. London, UK: Academic Press; 1991. [Google Scholar]
- Bollan KA, Hothersall JD, Moffat C, Durkacz J, Saranzewa N, Wright GA, et al. The microsporidian parasites Nosema ceranae and Nosema apis are widespread in honeybee (Apis mellifera) colonies across Scotland. Parasitol Res. 2013;112:751–759. doi: 10.1007/s00436-012-3195-0. doi: 10.1007/s00436-012-3195-0. [DOI] [PubMed] [Google Scholar]
- Botías C, Martín-Hernández R, Meana A, Higes M. Critical aspects of the Nosema spp. diagnostic sampling in honey bee (Apis mellifera L.) colonies. Parasitol Res. 2012a;110:2557–2561. doi: 10.1007/s00436-011-2760-2. [DOI] [PubMed] [Google Scholar]
- Botías C, Martín-Hernández R, Días J, García-Palencia P, Matabuena M, Juarranz A, et al. The effect of induced queen replacement on Nosema spp. infection in honey bee (Apis mellifera iberiensis) colonies. Environ Microbiol. 2012b;14:845–859. doi: 10.1111/j.1462-2920.2011.02647.x. [DOI] [PubMed] [Google Scholar]
- Bourgeois AL, Rinderer TE, Beaman LD, Danka RG. Genetic detection and quantification of Nosema apis and N. ceranae in the honey bee. J Invertebr Pathol. 2010;103:53–58. doi: 10.1016/j.jip.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Bromenshenk JJ, Henderson CB, Wick CH, Stanford MF, Zulich AW, Jabbour RE, et al. Iridovirus and microsporidian linked to honey bee colony decline. PLoS ONE. 2010;5:e13181. doi: 10.1371/journal.pone.0013181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaimanee V, Chantawannakul P, Chen Y, Evans JD, Pettis JS. Differential expression of immune genes of adult honey bee (Apis mellifera) after inoculated by Nosema ceranae. J Insect Physiol. 2012;58:1090–1095. doi: 10.1016/j.jinsphys.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Chen Y, Evans JD, Smith IB, Pettis JS. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J Invertebr Pathol. 2008;97:186–188. doi: 10.1016/j.jip.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Costa C, Tanner G, Lodesani M, Maistrello L, Neumann P. Negative correlation between Nosema ceranae spore loads and deformed wing virus infection levels in adult honey bee workers. J Invertebr Pathol. 2011;108:224–225. doi: 10.1016/j.jip.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Cox-Foster DL, Conlan S, Holmes EC, Palacios G, Evans JD, Moran NA, et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science. 2007;318:283–287. doi: 10.1126/science.1146498. [DOI] [PubMed] [Google Scholar]
- Dussaubat C, Brunet JL, Higes M, Colbourne JK, López J, Choi JH, et al. Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS ONE. 2012;7:e37017. doi: 10.1371/journal.pone.0037017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert JE. The development of resistance to American foulbrood by honey bees in Hawaii. J Econ Entomol. 1950;43:562–564. [Google Scholar]
- El-Shemy AAM, Pickard RS. Nosema apis infection levels in honeybees of known age. J Apic Res. 1989;28:101–106. [Google Scholar]
- vanEngelsdorp D, Evans JD, Saegerman C, Mullin C, Haubruge E, Nguyen BK, et al. Colony collapse disorder: a descriptive study. PLoS ONE. 2009;4:e6481. doi: 10.1371/journal.pone.0006481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández JM, Puerta F, Cousinou M, Dios-Palomares R, Campano F, Redondo L. Asymptomatic presence of Nosema spp. in Spanish commercial apiaries. J Invertebr Pathol. 2012;111:106–110. doi: 10.1016/j.jip.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Forsgren E, Fries I. Temporal study of Nosema spp. in a cold climate. Environ Microbiol Rep. 2013;5:78–82. doi: 10.1111/j.1758-2229.2012.00386.x. [DOI] [PubMed] [Google Scholar]
- Fries I. Nosema ceranae in European honey bees (Apis mellifera. J Invertebr Pathol. 2010;103:S73–S79. doi: 10.1016/j.jip.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Gisder S, Hedtke K, Mockel N, Frielitz M-C, Linde A, Genersch E. Five-year cohort study of Nosema spp. in Germany: does climate shape virulence and assertiveness of Nosema ceranae. Appl Environ Microbiol. 2010;76:3032–3038. doi: 10.1128/AEM.03097-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedtke K, Jensen PM, Bruun A, Genersch E. Evidence for emerging parasites and pathogens influencing outbreaks of stress-related diseases like chalkbrood. J Invertebr Pathol. 2011;108:167–173. doi: 10.1016/j.jip.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Higes M, Martín R, Meana A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J Invertebr Pathol. 2006;92:81–83. doi: 10.1016/j.jip.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Higes M, Garcia-Palencia P, Martin-Hernandez R, Meana A. Experimental infectionof Apis mellifera honeybees with Nosema ceranae (Microsporidia) J Invertebr Pathol. 2007;94:211–217. doi: 10.1016/j.jip.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Higes M, Martín-Hernández R, Botías C, Bailón EG, González-Porto AV, Barrios L, et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol. 2008;10:2659–2669. doi: 10.1111/j.1462-2920.2008.01687.x. [DOI] [PubMed] [Google Scholar]
- Higes M, Martín-Hernández R, Garrido-Bailón E, González-Porto AV, García-Palencia P, Meana A, et al. Honeybee colony collapse due to Nosema ceranae in professional apiaries. Environ Microbiol Rep. 2009;1:110–113. doi: 10.1111/j.1758-2229.2009.00014.x. [DOI] [PubMed] [Google Scholar]
- Higes M, Meana A, Bartolomé C, Botías C, Martín-Hernández R. Nosema ceranae (Microsporida), a controversial 21st century honey bee pathogen. Environ Microbiol Rep. 2013;5:17–29. doi: 10.1111/1758-2229.12024. doi: 10.1111/1758-2229.12024. [DOI] [PubMed] [Google Scholar]
- Huang W-F, Bocquet M, Lee K-C, Sung IH, Jiang J-H, Chen Y-W, Wang C-H. The comparison of rDNA spacer regions of Nosema ceranae isolates from different hosts and locations. J Invertebr Pathol. 2008;97:9–13. doi: 10.1016/j.jip.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Invernizzi C, Abuda C, Tomascoa I, Harriet J, Ramalloc G, Campáb J, et al. Presence of Nosema ceranae in honeybees (Apis mellifera) in Uruguay. J Invertebr Pathol. 2009;101:150–153. doi: 10.1016/j.jip.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Klee J, Besana A, Genersch E, Gisder S, Nanetti A, Tam DQ, et al. Widespread dispersal of the microsporidium Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J Invertebr Pathol. 2007;96:1–10. doi: 10.1016/j.jip.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Highfield AC, Brettell L, Villalobos EM, Budge GC, Powell M, et al. Global honeybee viral landscape altered by a parasitic mite. Science. 2012;336:1304–1306. doi: 10.1126/science.1220941. [DOI] [PubMed] [Google Scholar]
- Martín-Hernández R, Meana A, García-Palencia P, Marín P, Botías C, Garrido-Bailón E, et al. Effect of temperature on the biotic potential of honeybee microsporidia. Appl Environ Microbiol. 2009;75:2554–2557. doi: 10.1128/AEM.02908-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Hernández R, Botías C, Bailón EG, Martínez-Salvador A, Prieto L, Meana A, Higes M. Microsporidia infecting Apis mellifera: coexistence or competition. Is Nosema ceranae replacing Nosema apis. Environ Microbiol. 2012;14:2127–2138. doi: 10.1111/j.1462-2920.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- Meana A, Martín-Hernández R, Higes M. The reliability of spore counts to diagnose Nosema ceranae infections in honey bees. J Apic Res. 2010;49:212–214. [Google Scholar]
- Mulholland GE, Traver BE, Johnson NG, Fell RD. Individual variability of Nosema ceranae infections in Apis mellifera colonies. Insects. 2012;3:1143–1155. doi: 10.3390/insects3041143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton RJ, Klee J, Korpela S, Fries I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie. 2007;38:558–565. [Google Scholar]
- Pettis J, vanEngelsdorp D, Johnson J, Dively G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften. 2012;99:153–158. doi: 10.1007/s00114-011-0881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Rennich K, Pettis J, VanEngelsdorp D, Bozarth R, Eversole H, Roccasecca K, et al. 2012. 2011–2012 National honey bee pests and diseases survey report [WWW document]. URL http://www.aphis.usda.gov/plant_health/plant_pest_info/honey_bees/downloads/2011_National_Survey_Report.pdf.
- Schroeder DC, Martin SJ. Deformed wing virus: the main suspect in unexplained honeybee deaths worldwide. Virulence. 2012;3:1–3. doi: 10.4161/viru.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart MD, Sheppard WS. Nosema ceranae in age cohorts of the western honey bee (Apis mellifera. J Invertebr Pathol. 2012;109:148–151. doi: 10.1016/j.jip.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Stevanovic J, Stanimirovic Z, Genersch E, Kovacevic SR, Ljubenkovic J, Radakovic M, Aleksic N. Dominance of Nosema ceranae in honey bees in the Balkan countries in the absence of symptoms of colony collapse disorder. Apidologie. 2011;42:49–58. [Google Scholar]
- Traver BE, Fell RD. Prevalence and infection intensity of Nosema in honey bee (Apis mellifera L.) colonies in Virginia. J Invertebr Pathol. 2011;107:43–49. doi: 10.1016/j.jip.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Traver BE, Williams MR, Fell RD. Comparison of within hive sampling and seasonal activity of Nosema ceranae in honey bee colonies. J Invertebr Pathol. 2012;109:187–193. doi: 10.1016/j.jip.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Vidau C, Diogon M, Aufauvre J, Fontbonne R, Vigues B, Brunet J-L, et al. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS ONE. 2011;6:e21550. doi: 10.1371/journal.pone.0021550. doi: 10.1371/journal.pone.00215504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR, Shafer ABA, Rogers REL, Shutler D, Stewart DT. First detection of Nosema ceranae, a microsporidian parasite of European honeybees (Apis mellifera), in Canada and central USA. J Invertebr Pathol. 2008;97:189–192. doi: 10.1016/j.jip.2007.08.005. [DOI] [PubMed] [Google Scholar]


