Abstract
Epidemiologic studies have reported inconsistent associations between menarcheal age and ovarian cancer risk. To our knowledge, a meta-analysis for the association between menarcheal age and ovarian cancer has not been reported. Relevant published studies of menarcheal age and ovarian cancer were identified using MEDLINE, EMBASE, and Web of Science through the end of April, 2012. Two authors (T-TG and Q-JW) independently assessed eligibility and extracted data. We pooled the relative risks (RR) from individual studies using a random-effects model and performed heterogeneity and publication bias analyses. A total of 27 observational studies consisting of 22 case-control and 5 cohort studies were included in our analysis. In a pooled analysis of all studies, a statistically significant inverse association was observed between menarcheal age (for the oldest compared with the youngest category) and ovarian cancer risk (RR=0.85; 95% confidence interval (95% CI) 0.75–0.97). The pooled RRs of ovarian cancer for the oldest versus the youngest categories of menarcheal age in prospective and case-control studies were 0.89 (95% CI 0.76–1.03) and 0.84 (95% CI 0.70–0.99), respectively. Inverse associations between menarcheal age and ovarian cancer risk were observed in most sub-groups, but the association was restricted to invasive and borderline serous ovarian cancer. In conclusion, findings from this meta-analysis support that menarcheal age was inversely associated with the risk of ovarian cancer. More large studies are warranted to stratify results by different cancer grading and histotype of ovarian cancer.
Keywords: menarche, meta-analysis, ovarian neoplasms, reproductive factor
Introduction
Ovarian cancer is the third most common malignancy and the second most common cause of cancer death worldwide among gynecologic cancers, with approximately 225,500 new cases diagnosed in 2008.1 In developed countries, ovarian cancer accounts for 31.4% of all common gynecologic cancers and is the second most common cancer overall.1 Consistent evidence indicates that increasing age, family history of breast or ovarian cancer, nulliparity, and exposure to radiation and asbestos are risk factors for ovarian cancer, while the use of oral contraceptives (OC), parity, and tubal ligation decrease ovarian cancer risk.2
Taking into account the effects of pregnancy and OC use on the risk of ovarian cancer, it is reasonable that menarcheal age may also be a risk factor. The “incessant ovulation” hypothesis 3,4 suggests that later menarcheal age might decrease risk of ovarian cancer by decreasing a woman’s lifetime number of ovulations. Moreover, sex hormones (e.g., progesterone, androgen) change in the period of childhood and adolescence and these hormone changes have been proposed to play an important role in the etiology of ovarian cancer.5–7 Nevertheless, despite extensive studies 8–12 with a focus on whether later menarche may be protective for ovarian cancer, the results are inconsistent. In light of the potential role of ovulation and female hormones in ovarian cancer etiology, we performed a meta-analysis on all prospective and case-control studies published through the end of April, 2012 to evaluate the relationship between menarcheal age and the risk of ovarian cancer.
Material and Methods
Literature search
To identify relevant epidemiologic studies, we comprehensively searched MEDLINE, EMBASE, and Web of Science from their inception until April 30, 2012 for epidemiological studies evaluating the association between menarcheal age and the risk of ovarian cancer. Key words and medical subject heading terms searched were as follows: (menarche) AND (ovarian OR ovary) AND (cancer OR neoplasm OR carcinoma OR tumor). The searches were limited to studies of humans and articles published in English. Furthermore, we also searched the reference lists of all the studies that were included in our analysis. We followed standard criteria for conducting and reporting meta-analyses.13
Study selection criteria
Published studies were included if they 1) used a case-control or cohort study design; 2) evaluated the association between menarcheal age and ovarian cancer risk; 3) presented odds ratio (OR), relative risk (RR) or hazard ratio (HR) estimates with 95% confidence intervals (CI), standard errors (SE) or data necessary to calculate these. When multiple publications from the same study were available, we used the publication with the largest number of cases and most applicable information.
Data abstraction and quality assessment
For each eligible study, two investigators (T-TG and Q-JW) independently performed the eligibility evaluation, data abstraction, and quality assessment; disagreements were resolved by consensus. Data abstracted from each study were: author list, year of publication, study region and design, study sample size (number of cases and controls or cohort size), range of follow-up for cohort studies, exposure assessment and menarcheal age categories, study-specific adjusted ORs or RRs with their 95% CIs for the oldest versus youngest category of menarcheal age (if multiple estimates were available, we abstracted the estimate that adjusted for the most covariates), and factors matched by or adjusted for in the design or data analysis.
To assess the study quality, a 9-star system of the Newcastle-Ottawa Scale 14 was used in which a study was judged on 3 broad perspectives: the selection of study groups, comparability of groups, and ascertainment of either the exposure or outcome of interest for case-control or cohort studies, respectively. The full score was 9 and the high quality study was defined as a study with quality scores greater than or equal to 7.
Statistical analysis
The study-specific adjusted RRs were used as the common measure of association across studies. For the meta-analysis, we assumed that estimates of ORs from case-control studies and risk, rate or hazard ratios from cohort studies were all valid estimates of the RR, therefore we reported all estimates of association as RRs.15 For studies not using the category with the youngest menarcheal age as the reference, we used the effective count method proposed by Hamling et al 16 to recalculate the RRs using the stratum with the youngest menarcheal age as the reference. For studies that reported results separately for borderline and invasive ovarian cancers, but not combined, we pooled the results using a fixed-effects model to obtain an overall combined estimate before combining with the other studies.
The possible heterogeneity in results across studies was examined by using the Cochran Q and I2 statistics.17 For the Q statistic, a P value less than 0.1 was considered to be representative of statistically significant heterogeneity. The I2 represents the proportion of total variation contributed by between-study variation 17. When substantial heterogeneity was detected, the summary estimate based on the random effects model (DerSimonian and Laird method) 18 was reported which assumes that the studies included in the meta-analysis had varying effect sizes. Otherwise, the summary estimate based on the fixed effects model (the inverse variance method) 19 was reported which assumes that the studies included in the meta-analysis had the same effect size. We used these two effects models to calculate summary RRs and 95% CI for the oldest versus the youngest categories of menarcheal age for the analysis (Stata META command). Heterogeneity between subgroups was evaluated by meta-regression (Stata METAREG command). Summary estimates were calculated for the association between menarcheal age and the risk of ovarian cancer. Subgroup analyses were carried out based on study quality, study design (cohort vs. case-control studies), exposure assessment (self-administered questionnaire vs. trained interviewers), type of control subjects for case-control studies (population-based vs. hospital-based controls), study population (Asians, Americans or Europeans), cancer grading (invasive vs. borderline), cancer histotype (invasive serous vs. borderline serous). We also stratified the meta-analysis by potentially important confounders. Finally, we carried out sensitivity analyses excluding one study at a time to explore whether the results were strongly influenced by a specific study.
Publication bias was evaluated via the Egger’s linear regression,20 the Begg’s rank correlation methods 21 and funnel plots (Stata METABIAS command). A P value less than 0.05 for Egger’s or Begg’s tests was considered representative of significant statistical publication bias. Statistical analyses were performed with Stata (version 11.0; StataCorp, College Station, TX). P values were two sided with a significance level of 0.05.
Results
Literature search
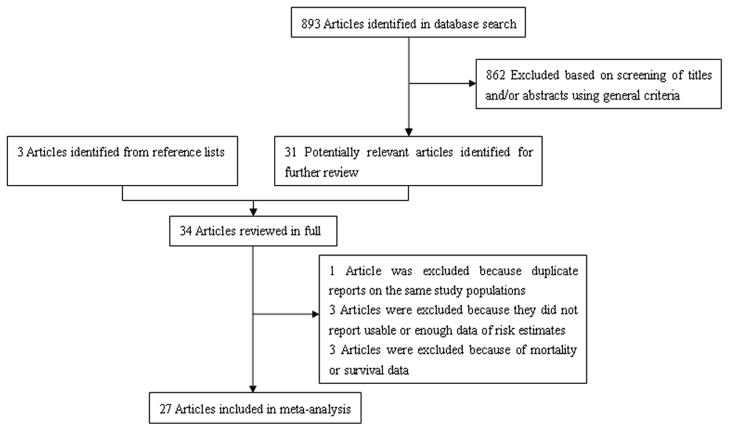
We identified 893 potentially relevant articles from our search of the 3 databases. Of these, 862 articles were excluded after the first screening based on the abstract or title, leaving 31 articles for full-text review. Hand searching of the bibliographic references of these articles identified 3 additional articles,22–24 for a total of 34 articles for full-text review. On this review, one article was excluded because of duplicate reports from the same study population,25 three articles were excluded because they did not report usable or enough data of risk estimates, and 3 articles were excluded because of the use of mortality or survival data. The remaining 27 articles were included in the meta-analysis (Fig. 1).
Fig 1.
Selection of studies for inclusion in meta-analysis
Study characteristics and quality assessment
Characteristics of the 27 included articles are shown in Supp Table 1. These articles, including 9,859 cases and 1,020,516 subjects, were published between 1979 and 2012 and consisted of 5 cohort 8, 9, 26–28 and 21 case-control studies.10–12, 22–24, 29–43 Among the included studies, two studies 30, 31 using the same control population reported the results of borderline and invasive cancer separately so we pooled the results of these 2 studies. Jordan et al 32 reported information on histotype of ovarian cancer but included fewer cases than Purdie et al,36 so we excluded the Jordan et al 32 study in the overall results but included it in the analysis of cancer histotype. Most individual studies were matched on or adjusted for a wide range of potential confounders, including BMI, parity, and use of OC. All included studies identified ovarian cancer cases from a cancer registry or using histological/pathological data confirmed by pathologist. Eight studies 8, 9, 26–31 used self-administered questionnaires and 15 studies 10, 11, 22–24, 33–36, 38–43 use trained interviewers to collect data on menarcheal age.
Of the 5 cohort studies, two were conducted in European countries 8, 9 and one each was conducted in the United Stated,28 Korea,27 and Japan.26 Sample sizes ranged from 45,748 26 to 443,909 people,27 and the number of ovarian cancer cases varied from 86 26 to 878.8 The duration of follow-up in the cohort studies was longer than 9 years in all studies except one.26
Of the 22 case-control studies, five were conducted in the Italy,34, 35, 38, 39, 42 four in the United States,10, 24, 33, 41 two each in China,22, 40 Australia,32, 36 Sweden,30, 31 and Greece,12, 37 and one each in Japan,29 Mexico,11 Poland,43 and United Kingdom.23 The number of cases enrolled in these studies ranged from 84 11 to 1,031,34 and the number of control subjects varied from 172 40 to 3,899.30, 31 Control subjects were drawn from the general population in 7 studies,10, 22, 30, 31, 33, 36, 40 hospitals in 12 studies, 11, 12, 23, 24, 29, 34, 35, 37–39, 42, 43 and both places in 1 study.41
Study-specific quality scores are summarized in Supp Table 2 and Supp Table 3. The quality scores ranged from 4 to 9 with a median score of 7. The median scores of cohort and case-control studies were 8 and 7, respectively. All of the cohort and 13 case-control studies were considered to be high-quality.8–10, 22, 25–31, 33–36, 38, 40, 42
Late vs. Early Age at Menarche
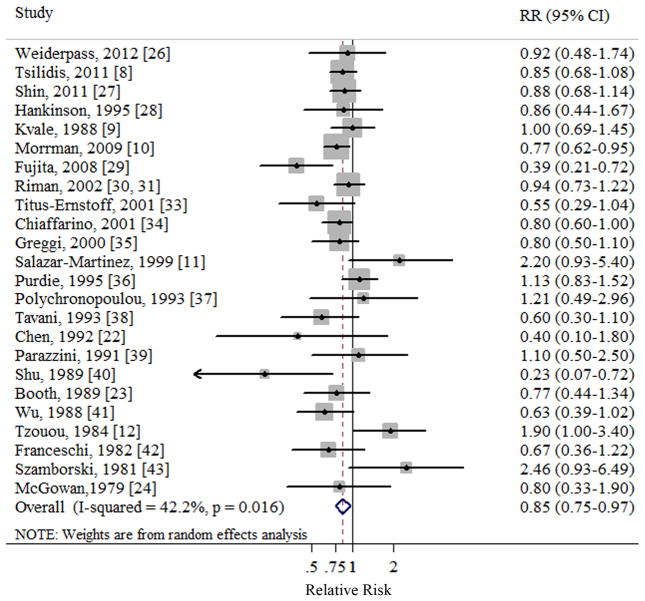
In a random effect pooled analysis of the included studies, the summary RR of ovarian cancer for the oldest versus the youngest categories of menarcheal age was 0.85 (95% CI 0.75–0.97) (Table 1 and Fig. 2). Moderate heterogeneity was observed in the study results (Q=39.81, P=0.016, I2=42.2%). There was no indication of publication bias with Egger’s test (P for bias=0.866) or with Begg’s test (P for bias=0.901).
Table 1.
Summary risk estimates of the association between menarcheal age and ovarian cancer risk
| No. of studies | Summary RR (95% CI) | Q Statistic | I-square Value (%) | Ph* | Ph** | Study Reference | |
|---|---|---|---|---|---|---|---|
| Overall | 25 | 0.85 (0.75–0.97) | 39.81 | 42.2 | 0.016 | — | 8–12, 22–24, 26–31, 33–43 |
| Subgroup analyses | 0.146 | ||||||
| High quality studies (scores ≥7) | 18 | 0.83 (0.76–0.91) | 21.70 | 26.3 | 0.153 | 8–10, 22, 26–31, 33–36, 38–40, 42 | |
| Study Design | 0.673 | ||||||
| Cohort studies | 5 | 0.89 (0.76–1.03) | 0.56 | 0 | 0.968 | 8, 9, 26–28 | |
| Case-control studies | 20 | 0.84 (0.70–0.99) | 38.92 | 53.7 | 0.003 | 10–12, 22–24, 29–31, 33–43 | |
| Exposure Assessment | 0.946 | ||||||
| Trained interviewer | 15 | 0.81 (0.68–0.97) | 24.49 | 42.8 | 0.040 | 10, 11, 22–24, 33–36, 38–43 | |
| Self-administered questionnaire | 8 | 0.87 (0.77–0.99) | 7.48 | 19.8 | 0.279 | 8, 9, 26–31 | |
| Type of Control Subjects | 0.327 | ||||||
| Population based | 7 | 0.79 (0.60–1.04) | 12.68 | 59.9 | 0.029 | 10, 22, 30, 31, 33, 36, 40 | |
| Hospital based | 12 | 0.91 (0.70–1.19) | 24.99 | 56.0 | 0.009 | 11, 12, 23, 24, 29, 34, 35, 37–39, 42, 43 | |
| Case Assessment | — | ||||||
| Cancer registry or medical records | 25 | 0.85 (0.75–0.97) | 39.81 | 42.2 | 0.016 | — | 8–12, 22–24, 26–31, 33–43 |
| Self report | 0 | N/A | N/A | N/A | N/A | N/A | |
| Study Population | 0.879 | ||||||
| Asians | 5 | 0.58 (0.34–0.96) | 10.86 | 63.2 | 0.028 | 22, 26, 27, 29, 40 | |
| Americans | 5 | 0.74 (0.62–0.88) | 1.62 | 0 | 0.806 | 10, 24, 28, 33, 41 | |
| Europeans | 13 | 0.89 (0.80–1.00) | 14.98 | 26.6 | 0.183 | 8, 9, 12, 23, 30, 31, 34, 35, 37–39, 42, 43 | |
| Cancer Grading | 0.103 | ||||||
| Invasive | 8 | 0.75 (0.58–0.95) | 13.42 | 47.8 | 0.063 | 10, 24, 26, 29, 30, 32, 33, 40 | |
| Borderline | 4 | 1.18 (0.82–1.70) | 0.33 | 0 | 0.954 | 31–33, 39 | |
| Cancer Histotype | 0.788 | ||||||
| Invasive serous | 2 | 1.01 (0.71–1.44) | 2.53 | 60.4 | 0.112 | 32, 33 | |
| Borderline serous | 2 | 0.81 (0.42–1.47) | 0.37 | 0 | 0.543 | 32, 33 | |
| Adjustment for confounders | |||||||
| Body mass index | 0.657 | ||||||
| Yes | 7 | 0.87 (0.77–0.97) | 5.95 | 15.9 | 0.311 | 8, 10, 11, 26, 27, 30, 31 | |
| No | 18 | 0.82 (0.68–0.99) | 33.78 | 49.7 | 0.009 | 9, 12, 22–24, 28, 29, 33–43 | |
| Parity | 0.167 | ||||||
| Yes | 17 | 0.83 (0.75–0.91) | 21.51 | 30.3 | 0.121 | 8, 9, 10, 22, 26, 28–31, 33–36, 38, 39, 40, 42 | |
| No | 8 | 1.09 (0.78–1.51) | 16.21 | 56.8 | 0.023 | 11, 12, 23, 24, 27, 37, 41, 43 | |
| OC use | 0.519 | ||||||
| Yes | 9 | 0.83 (0.74–0.92) | 2.97 | 0 | 0.888 | 8, 10, 28, 30, 31, 34, 35, 38, 39 | |
| No | 16 | 0.87 (0.69–1.09) | 35.96 | 58.3 | 0.002 | 9, 11, 12, 22–24, 26, 27, 29, 33, 36, 37, 40–43 | |
| Exogenous hormones use | 0.721 | ||||||
| Yes | 4 | 0.89 (0.76–1.05) | 0.34 | 0 | 0.845 | 8, 26, 30, 31 | |
| No | 21 | 0.84 (0.72–0.98) | 39.15 | 48.9 | 0.006 | 9–12, 22–24, 27–29, 33–43 | |
| Menopause status | 0.561 | ||||||
| Yes | 10 | 0.89 (0.79–1.01) | 5.85 | 0 | 0.670 | 8, 11, 26–28, 30, 31, 35, 39, 42 | |
| No | 15 | 0.81 (0.66–0.99) | 33.34 | 58.0 | 0.003 | 9, 10, 12, 22–24, 29, 33, 34, 36–38, 40, 41, 43 | |
| Smoking status | 0.911 | ||||||
| Yes | 6 | 0.84 (0.64–1.11) | 10.76 | 53.5 | 0.056 | 8, 11, 26–29 | |
| No | 19 | 0.86 (0.74–0.99) | 29.04 | 41.5 | 0.034 | 9, 10, 12 22–24, 30, 31, 33–43 | |
RR: relative risk; CI: confidence interval; OC: oral contraceptive.
P value for heterogeneity within each subgroup.
P value for heterogeneity between subgroups with meta-regression analysis.
Fig 2.
Forest plot (random effects model) of menarcheal age and ovarian cancer risk. Squares indicate study-specific relative risks (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; diamond indicates the summary relative risk estimate with its 95% CI. CI: confidence interval; RR: relative risk.
Subgroup and sensitivity analyses
In subgroup analyses, menarcheal age was inversely associated with the risk of ovarian cancer (for the oldest compared with the youngest category) in most subgroups, and there was no evidence of significant heterogeneity between subgroups with meta-regression analyses. (Table 1). Compared with the overall analysis, the pooled estimate was similar when analyses were restricted to the 18 high-quality studies (RR=0.83; 95% CI 0.76–0.91). The pooled RRs of ovarian cancer for the oldest versus youngest categories of menarcheal age of prospective and case-control studies were 0.89 (95% CI 0.76–1.03) and 0.84 (95% CI 0.70–0.99), respectively. When separately analyzed by method of exposure assessment, we found no apparent difference between studies using a self-administered questionnaire (RR=0.81; 95% CI 0.68–0.97) and those using the trained interviewer (RR=0.87; 95% CI 0.77–0.99). Although protective effects of menarcheal age and ovarian cancer were also observed among Asians (RR=0.58; 95% CI 0.34–0.96), Americans (RR=0.74; 95% CI 0.68–0.88), and Europeans (RR=0.89; 95% CI 0.80–1.00), the results from these three populations did not have consistent estimates of the association. When stratified by cancer grading, the analysis of invasive ovarian cancer yielded a RR of 0.75 (95% CI 0.58–0.95) (Table 1). We further focused on the difference between studies where associations with menarcheal age were of primary interest and studies that mainly dealt with other associations. However, the results of the meta-regression did not show the significant difference (data not shown).
In a sensitivity analysis, we sequentially removed one study at a time and re-analyzed the data. The 28 study-specific RRs ranged from a low of 0.84 (95% CI 0.73–0.95) after omission of the study by Purdie et al 36 to a high of 0.87 (95% CI 0.78–0.98) after omission of the study by Fujita et al.29 When we removed two studies 10, 24 which RRs and 95% CI were not reported but calculated from raw data, the results (RR=0.86; 95% CI 0.75–0.99) were similar.
Discussion
To our knowledge, this is the first meta-analysis evaluating an association between menarcheal age and ovarian cancer. Overall, the results of this meta-analysis suggest that menarcheal age (for the oldest category compared with the youngest category) was inversely associated ovarian cancer risk, with an estimated 15% reduction in risk. Inverse associations between menarcheal age and the risk of ovarian cancer were observed in most sub-groups, but were restricted to invasive and borderline serous ovarian cancer.
The inverse association between later menarcheal age and risk of ovarian cancer could be partly explained by the “incessant ovulation” hypothesis 3 and some hormonal changes in the childhood and adolescent period.5–7 Fathalla 3 hypothesized that ovarian carcinogenesis involves some mechanical sequelae of ovulation, such as trauma or mitotic stimuli to the ovarian epithelium. Support for this mechanism includes the high ovarian cancer rates in domestic fowl which were stimulated for daily egg production.3 Similar to pregnancy and OC use, later menarcheal age might decrease the risk of ovarian cancer by decreasing a woman’s lifetime number of ovulations. On the other hand, early menarche is associated with a more rapid onset of ovulatory cycles and a tendency to sustain higher levels of luteal phase estradiol and progesterone.5, 6 Progestins may increase apoptosis in ovarian epithelium, which means women with a later age at menarche may have several extra years of low-level estrogen (or other hormone) stimulation of their ovarian epithelium in the absence of the apoptotic effects of progesterone, possibly increasing the chance of the cells acquiring genetic damage.32 Meanwhile, androgens, which are also relatively abundant in girls with later menarcheal age,6 have been shown to stimulate DNA synthesis and decrease cell death in ovarian cell culture lines.44
In subgroup analyses of study design, the pooled association estimate from case-control studies were relatively similar to those from prospective studies, although only the estimate from case-control studies reached statistical significance (Table 1). Compared with case-control studies, cohort studies are less susceptible to bias (e.g., recall bias, selection bias) because information on exposures is collected before the diagnosis of the disease. However, since menarche is the milestone of puberty initiation, imprecision in recall of age at first menses might not occur in case-control studies. Bean et al 45 previously reported that age at menarche has reasonably good recall accuracy. Furthermore, although both the median quality score of prospective and case-control studies achieved the high-quality study standard (≥7), case-control studies had a lower median quality score than prospective studies (7 vs. 8). On the other hand, the studies varied in the number of potential confounding factors for which they had adjusted. Some cohort studies published in recent years provided detailed information of adjustment for confounders, whereas some early case-control studies adjusted for fewer factors. Hence, the difference in the results between cohort and case-control studies maybe partly attributed to the quality of the study methodologies and adjustment for confounders.
Results from subgroup analysis by study population suggested that the inverse association between menarcheal age and ovarian cancer risk in Asians was somewhat different from Americans and Europeans (RR=0.58, RR=0.74 and RR=0.89, respectively). Such a difference might be attributed to the later mean age at menarche in Asians than found in Americans and Europeans. Weiderpass et al 26 reported a mean menarcheal age of 15 years in a cohort study of 45,662 non-cases conducted in Japan, while two case-control studies conducted in the United Stated 41 and Sweden 30 reported a mean menarcheal age of 13 years in 472 controls and 13.6 years in 3,899 controls, respectively. Similarly, genome-wide association studies (GWAS) 46 which included only women of European ancestry identified some novel genetic loci associated with age at menarche. However, whether the variants and genes that influence age at menarche are the same in women of Asian descent is still unknown.
For the subgroup analysis of menarcheal age and ovarian cancer risk by cancer grade, we only observed a statistically significant inverse association between menarcheal age and invasive ovarian cancer. The protective effect was consistent in all of the included studies 10, 24, 26, 29–33, 40 except one.32 However, fewer studies conducted analyses for borderline ovarian cancer than studies of invasive cancer (4 vs. 8, respectively). Additionally, only one-third of the included studies in our meta-analysis separated ovarian cancer into invasive and borderline. These decreased numbers might diminish the statistical power to detect an association between menarcheal age and borderline ovarian cancer. Previous studies have demonstrated that mucinous and non-mucinous epithelial ovarian cancers might have different etiologies and risk factors.47 However, due to the limited studies focused on the relationship between menarcheal age and ovarian cancer histotype, we could not analyze whether an association would be found after stratification by mucinous and non-mucinous ovarian cancers. Therefore, future epidemiologic studies should focus on whether menarcheal age could be the risk factor for different ovarian cancer grading and histotype.
A strength of this study is the large sample size with 9,859 cases and 1,020,516 subjects. This sample size should have provided sufficient statistical power to detect the putative association between menarcheal age and ovarian cancer. Another strength is the thorough statistical analyses considering a number of subgroups. Several potential limitations of this study are also worth considering. First, this meta-analysis is based on observational studies and therefore the potential study biases and uncontrolled or residual confounding within the individual studies can affect the pooled estimate. Later menarcheal age is often associated with other hormone-dependent or reproductive factors, including lower levels of body mass index (BMI),48 higher prevalence of smoking or environment tobacco smoke,49 lower parity number,50 and earlier menopausal age. However, many but not all of the studies adjusted for potential confounding factors. In addition, an inverse association was still observed when we stratified the results according to adjustment for confounding factors and evidence of meta-regression analyses indicated that adjustment for confounders was not a source of heterogeneity (Table 1). Second, significant heterogeneity and possible publication bias must be considered. There was significant heterogeneity for all studies combined (Q=39.81, P=0.016, I2=42.2%) in the pooled analysis of menarcheal age, but this could be at least partially explained by differences in study quality, study design, exposure assessment, study population, and adjustment for potential confounders (Table 1). Publication bias can be a problem in meta-analyses of published studies, but we found no statistical evidence of publication bias in this meta-analysis and there was also no asymmetry in the funnel plots when inspected visually.
In conclusion, this meta-analysis provided evidence that menarcheal age was inversely associated with the risk of ovarian cancer. Future studies should investigate whether the association between menarcheal age and ovarian cancer risk is modified by other factors (e.g., genetic polymorphisms). Additionally, whether the association differs according to cancer grading or histotype of ovarian cancer warrants further study.
Supplementary Material
(Characteristics of studies of menarcheal age and ovarian cancer risk)
(Methodological quality of the cohort studies included in the meta-analysis)
(Methodological quality of the case-control studies included in the meta-analysis)
Brief description of the novelty and impact.
Ovarian cancer is the third most common malignancy worldwide among gynecologic cancers, with approximately 0.23 million new cases diagnosed in 2008. The association between menarcheal age and ovarian cancer among epidemiologic studies has been inconsistent. This meta-analysis of 27 cohort and case-control studies provides evidence that menarcheal age is inversely associated with the risk of ovarian cancer. More large studies are warranted to stratify results by different cancer grading and histotype of ovarian cancer.
Acknowledgments
Emily Vogtmann was supported by the Cancer Prevention and Control Training Program at the University of Alabama at Birmingham funded through the National Institutes of Health (5R25 CA047888).
Footnotes
Additionally, the authors have declared no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hankinson SE, Danforth KN. Ovarian cancer. In: Schottenfeld D, Fraumeni J, editors. Cancer epidemiology and prevention. 3. New York, NY: Oxford University Press; 2006. pp. 1013–26. [Google Scholar]
- 3.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 4.Purdie DM, Bain CJ, Siskind V, Webb PM, Green AC. Ovulation and risk of epithelial ovarian cancer. Int J Cancer. 2003;104:228–32. doi: 10.1002/ijc.10927. [DOI] [PubMed] [Google Scholar]
- 5.Vihko R, Apter D. Endocrine characteristics of adolescent menstrual cycles: impact of early menarche. J Steroid Biochem. 1984;20:231–6. doi: 10.1016/0022-4731(84)90209-7. [DOI] [PubMed] [Google Scholar]
- 6.Apter D, Vihko R. Early menarche, a risk factor for breast cancer, indicates early onset of ovulatory cycles. J Clin Endocrinol Metab. 1983;57:82–6. doi: 10.1210/jcem-57-1-82. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez GC, Walmer DK, Cline M, Krigman H, Lessey BA, Whitaker RS, Dodge R, Hughes CL. Effect of progestin on the ovarian epithelium of macaques: cancer prevention through apoptosis? J Soc Gynecol Investig. 1998;5:271–6. doi: 10.1016/s1071-5576(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 8.Tsilidis KK, Allen NE, Key TJ, Dossus L, Lukanova A, Bakken K, Lund E, Fournier A, Overvad K, Hansen L, Tjonneland A, Fedirko V, et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2011;105:1436–42. doi: 10.1038/bjc.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kvale G, Heuch I, Nilssen S, Beral V. Reproductive factors and risk of ovarian cancer: a prospective study. Int J Cancer. 1988;42:246–51. doi: 10.1002/ijc.2910420217. [DOI] [PubMed] [Google Scholar]
- 10.Moorman PG, Palmieri RT, Akushevich L, Berchuck A, Schildkraut JM. Ovarian Cancer Risk Factors in African-American and White Women. Am J Epidemiol. 2009;170:598–606. doi: 10.1093/aje/kwp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar-Martinez E, Lazcano-Ponce EC, Gonzalez LG, Escudero-De LRP, Salmeron-Castro J, Hernandez-Avila M. Reproductive factors of ovarian and endometrial cancer risk in a high fertility population in Mexico. Cancer Res. 1999;59:3658–62. [PubMed] [Google Scholar]
- 12.Tzonou A, Day NE, Trichopoulos D, Walker A, Saliaraki M, Papapostolou M, Polychronopoulou A. The epidemiology of ovarian cancer in Greece: a case-control study. Eur J Cancer Clin Oncol. 1984;20:1045–52. doi: 10.1016/0277-5379(84)90107-x. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 15.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 16.Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954–70. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–3. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 22.Chen Y, Wu PC, Lang JH, Ge WJ, Hartge P, Brinton LA. Risk factors for epithelial ovarian cancer in Beijing, China. Int J Epidemiol. 1992;21:23–9. doi: 10.1093/ije/21.1.23. [DOI] [PubMed] [Google Scholar]
- 23.Booth M, Beral V, Smith P. Risk factors for ovarian cancer: a case-control study. Br J Cancer. 1989;60:592–8. doi: 10.1038/bjc.1989.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGowan L, Parent L, Lednar W, Norris HJ. The woman at risk for developing ovarian cancer. Gynecol Oncol. 1979;7:325–44. doi: 10.1016/0090-8258(79)90111-2. [DOI] [PubMed] [Google Scholar]
- 25.Parazzini F, La Vecchia C, Negri E, Gentile A. Menstrual factors and the risk of epithelial ovarian cancer. J Clin Epidemiol. 1989;42:443–8. doi: 10.1016/0895-4356(89)90134-0. [DOI] [PubMed] [Google Scholar]
- 26.Weiderpass E, Sandin S, Inoue M, Shimazu T, Iwasaki M, Sasazuki S, Sawada N, Yamaji T, Tsugane S. Risk factors for epithelial ovarian cancer in Japan - results from the Japan Public Health Center-based Prospective Study cohort. Int J Oncol. 2012;40:21–30. doi: 10.3892/ijo.2011.1194. [DOI] [PubMed] [Google Scholar]
- 27.Shin A, Song YM, Yoo KY, Sung J. Menstrual factors and cancer risk among Korean women. Int J Epidemiol. 2011;40:1261–8. doi: 10.1093/ije/dyr121. [DOI] [PubMed] [Google Scholar]
- 28.Hankinson SE, Colditz GA, Hunter DJ, Willett WC, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. A Prospective-study of reproductive factors and risk of epithelial ovarian-cancer. Cancer. 1995;76:284–90. doi: 10.1002/1097-0142(19950715)76:2<284::aid-cncr2820760219>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Fujita M, Tase T, Kakugawa Y, Hoshi S, Nishino Y, Nagase S, Ito K, Niikura H, Yaegashi N, Minami Y. Smoking, Earlier Menarche and Low Parity as Independent Risk Factors for Gynecologic Cancers in Japanese: A Case-Control Study. Tohoku J Exp Med. 2008;216:297–307. doi: 10.1620/tjem.216.297. [DOI] [PubMed] [Google Scholar]
- 30.Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, Persson IR. Risk factors for invasive epithelial ovarian cancer: results from a Swedish case-control study. Am J Epidemiol. 2002;156:363–73. doi: 10.1093/aje/kwf048. [DOI] [PubMed] [Google Scholar]
- 31.Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, Persson IR. Risk factors for epithelial borderline ovarian tumors: Results of a Swedish case-control study. Gynecol Oncol. 2001;83:575–85. doi: 10.1006/gyno.2001.6451. [DOI] [PubMed] [Google Scholar]
- 32.Jordan SJ, Webb PM, Green AC. Height, age at menarche, and risk of epithelial ovarian cancer. Cancer Epidem Biomar. 2005;14:2045–8. doi: 10.1158/1055-9965.EPI-05-0085. [DOI] [PubMed] [Google Scholar]
- 33.Titus-Ernstoff L, Perez K, Cramer DW, Harlow BL, Baron JA, Greenberg ER. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer. 2001;84:714–21. doi: 10.1054/bjoc.2000.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiaffarino F, Pelucchi C, Parazzini F, Negri E, Franceschi S, Talamini R, Conti E, Montella M, La Vecchia C. Reproductive and hormonal factors and ovarian cancer. Ann Oncol. 2001;12:337–41. doi: 10.1023/a:1011128408146. [DOI] [PubMed] [Google Scholar]
- 35.Greggi S, Parazzini F, Paratore MP, Chatenoud L, Legge F, Mancuso S, La Vecchia C. Risk factors for ovarian cancer in central Italy. Gynecol Oncol. 2000;79:50–4. doi: 10.1006/gyno.2000.5909. [DOI] [PubMed] [Google Scholar]
- 36.Purdie D, Green A, Bain C, Siskind V, Ward B, Hacker N, Quinn M, Wright G, Russell P, Susil B. Reproductive and other factors and risk of epithelial ovarian cancer: an Australian case-control study. Int J Cancer. 1995;62:678–84. doi: 10.1002/ijc.2910620606. [DOI] [PubMed] [Google Scholar]
- 37.Polychronopoulou A, Tzonou A, Hsieh CC, Kaprinis G, Rebelakos A, Toupadaki N, Trichopoulos D. Reproductive variables, tobacco, ethanol, coffee and somatometry as risk factors for ovarian cancer. Int J Cancer. 1993;55:402–7. doi: 10.1002/ijc.2910550312. [DOI] [PubMed] [Google Scholar]
- 38.Tavani A, Negri E, Franceschi S, Parazzini F, Lavecchia C. Risk factors for epithelial ovarian cancer in women under age 45. Eur J Cancer. 1993;29A:1297–1301. doi: 10.1016/0959-8049(93)90077-s. [DOI] [PubMed] [Google Scholar]
- 39.Parazzini F, Restelli C, Lavecchia C, Negri E, Chiari S, Maggi R, Mangioni C. Risk-factors for epithelial ovarian-tumors of borderline malignancy. Int J Epidemiol. 1991;20:871–7. doi: 10.1093/ije/20.4.871. [DOI] [PubMed] [Google Scholar]
- 40.Shu XO, Brinton LA, Gao YT, Yuan JM. Population-based case-control study of ovarian cancer in Shanghai. Cancer Res. 1989;49:3670–4. [PubMed] [Google Scholar]
- 41.Wu ML, Whittemore AS, Paffenbarger RJ, Sarles DL, Kampert JB, Grosser S, Jung DL, Ballon S, Hendrickson M, Mohle-Boetani J. Personal and environmental characteristics related to epithelial ovarian cancer. I. Reproductive and menstrual events and oral contraceptive use. Am J Epidemiol. 1988;128:1216–27. doi: 10.1093/oxfordjournals.aje.a115076. [DOI] [PubMed] [Google Scholar]
- 42.Franceschi S, La Vecchia C, Helmrich SP, Mangioni C, Tognoni G. Risk factors for epithelial ovarian cancer in Italy. Am J Epidemiol. 1982;115:714–9. doi: 10.1093/oxfordjournals.aje.a113353. [DOI] [PubMed] [Google Scholar]
- 43.Szamborski J, Czerwinski W, Gadomska H, Kowalski M, Wacker-Pujdak B. Case control study of high-risk factors in ovarian carcinomas. Gynecol Oncol. 1981;11:8–16. doi: 10.1016/0090-8258(81)90002-0. [DOI] [PubMed] [Google Scholar]
- 44.Edmondson RJ, Monaghan JM, Davies BR. The human ovarian surface epithelium is an androgen responsive tissue. Br J Cancer. 2002;86:879–85. doi: 10.1038/sj.bjc.6600154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bean JA, Leeper JD, Wallace RB, Sherman BM, Jagger H. Variations in the reporting of menstrual histories. Am J Epidemiol. 1979;109:181–5. doi: 10.1093/oxfordjournals.aje.a112673. [DOI] [PubMed] [Google Scholar]
- 46.Liu YZ, Guo YF, Wang L, Tan LJ, Liu XG, Pei YF, Yan H, Xiong DH, Deng FY, Yu N, Zhang YP, Zhang L, et al. Genome-wide association analyses identify SPOCK as a key novel gene underlying age at menarche. PLoS Genet. 2009;5:e1000420. doi: 10.1371/journal.pgen.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purdie DM, Webb PM, Siskind V, Bain CJ, Green AC. The different etiologies of mucinous and nonmucinous epithelial ovarian cancers. Gynecol Oncol. 2003;88:S145–8. doi: 10.1006/gyno.2002.6706. [DOI] [PubMed] [Google Scholar]
- 48.Banerjee I, Chakraborty S, Bhattacharyya NG, Bandyopadhyay S, Saiyed HN, Mukherjee D. A cohort study of correlation between body mass index and age at menarche in healthy Bengali girls. J Indian Med Assoc. 2007;105:75–8. [PubMed] [Google Scholar]
- 49.Ferris JS, Flom JD, Tehranifar P, Mayne ST, Terry MB. Prenatal and childhood environmental tobacco smoke exposure and age at menarche. Paediatr Perinat Epidemiol. 2010;24:515–23. doi: 10.1111/j.1365-3016.2010.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okada Y, Yagi S, Katsu N, Mabuchi Y, Yokota S. Proceedings: Correlation among the age at menarche, the number of parity and the age at menopause. Nihon Naibunpi Gakkai Zasshi. 1974;50:144. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(Characteristics of studies of menarcheal age and ovarian cancer risk)
(Methodological quality of the cohort studies included in the meta-analysis)
(Methodological quality of the case-control studies included in the meta-analysis)