Abstract
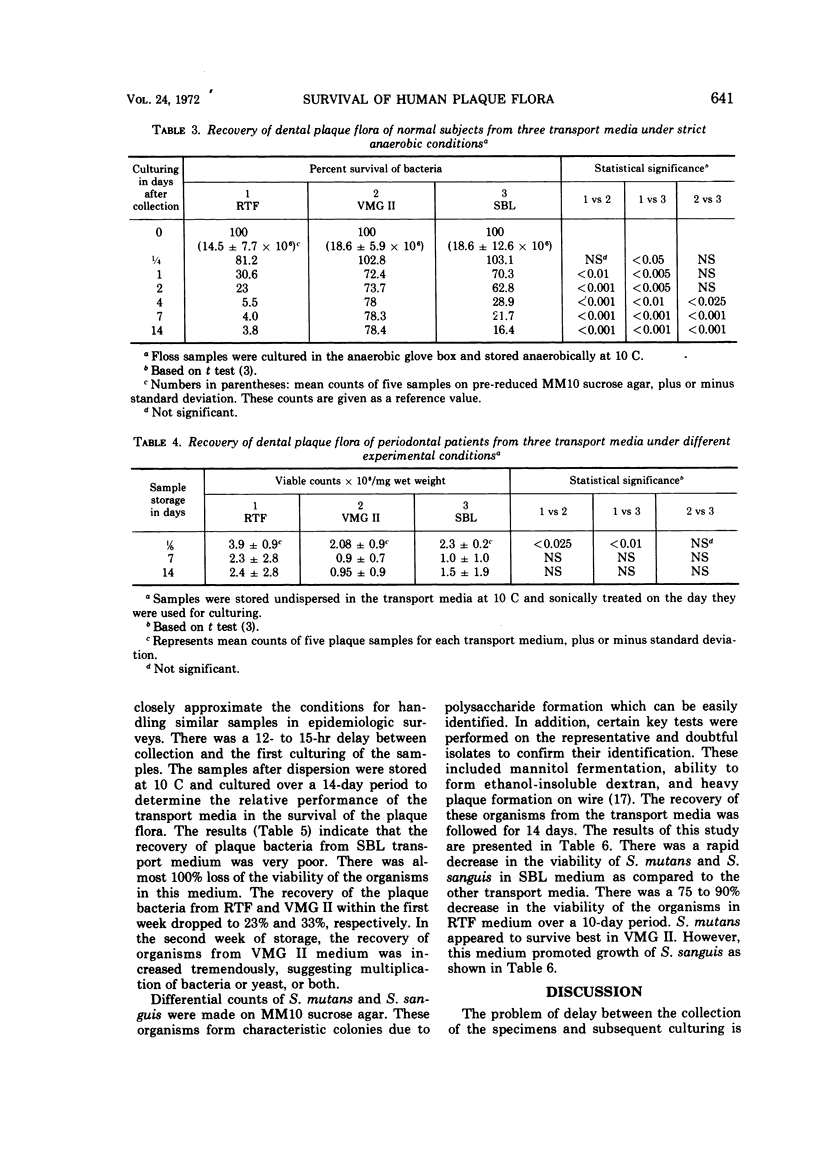
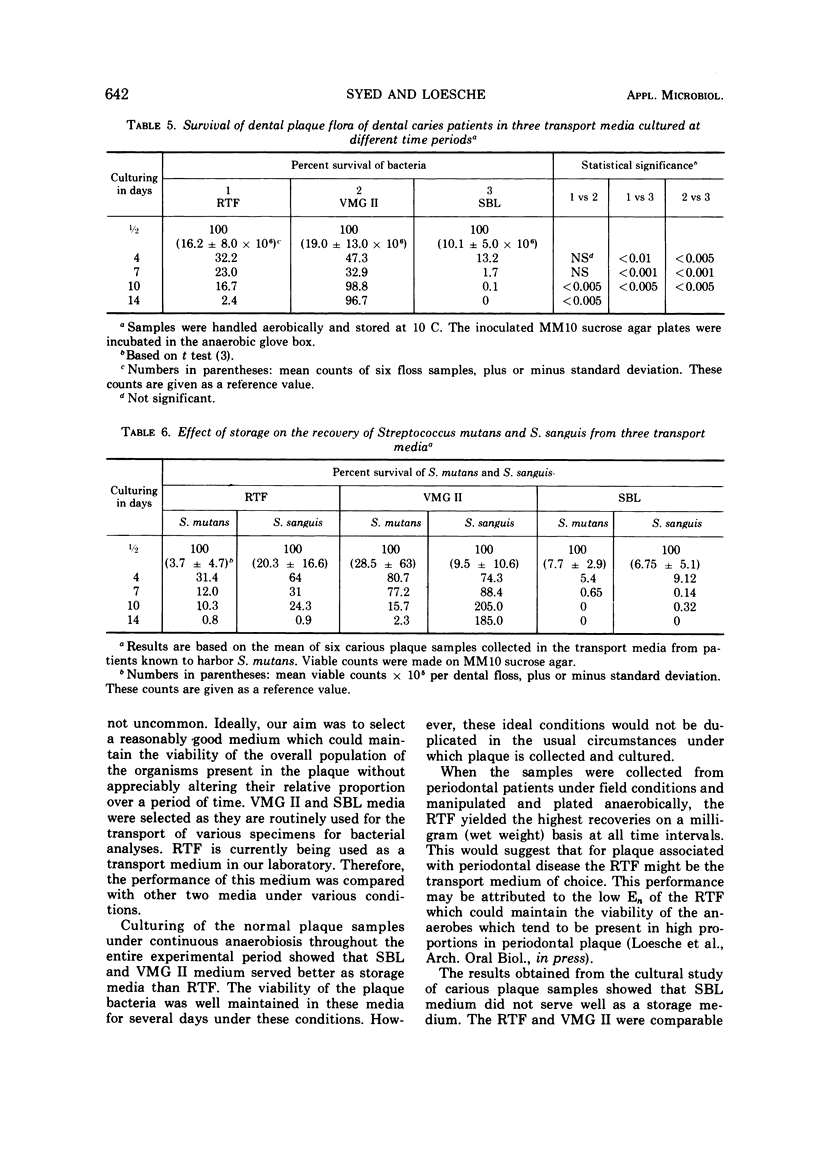
Dental plaque samples from (i) subjects with no apparent oral disease, (ii) mentally retarded subjects with periodontal disease, and (iii) subjects with active caries were collected in three transport media viz. a dithiothreitol poised balanced mineral salt solution designated as reduced transport fluid (RTF), VMG II, and modified Stuart medium (SBL). The samples were dispersed by sonic treatment, diluted in the respective medium in which they were collected, and cultured on MM10 sucrose agar. The efficiency of the transport media in the survival of dental plaque flora was determined by comparing the quantitative recovery (expressed as percentage of the initial viable count) from the specimens stored for various lengths of time. The data showed a great variation in the recovery of the oral bacterial flora from the plaque samples. VMG II and SBL served better than RTF as storage media for non-disease-associated dental plaque cultured under strict anaerobic conditions. Recoveries of bacteria from periodontal plaque specimens stored in RTF were higher than SBL and VMG II under identical conditions. The organisms present in the carious plaque samples appeared to survive much better in RTF and VMG II than in SBL as determined by conventional anaerobic culturing technique. However, VMG II showed a higher recovery of organisms from these specimens with an increase in the storage period, suggesting multiplication of the plaque flora. RTF did not allow the growth of oral bacterial flora under all experimental conditions. On the basis of the relative performance of these media it is suggested that RTF is a satisfactory medium for the transport of oral bacteria present in the samples.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARY S. G., BLAIR E. B. NEW TRANSPORT MEDIUM FOR SHIPMENT OF CLINICAL SPECIMENS. I. FECAL SPECIMENS. J Bacteriol. 1964 Jul;88:96–98. doi: 10.1128/jb.88.1.96-98.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasar B. S. Cultivation of anaerobic intestinal bacteria. J Pathol Bacteriol. 1967 Oct;94(2):417–427. doi: 10.1002/path.1700940223. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M., Socransky S. S. Predominant cultivable micro-organisms inhabiting periodontal pockets. Br Dent J. 1968 Jun 18;124(12):560–564. [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S., SAWYER S., KAPSIMALIS B., MACDONALD J. B. The microbiota of the gingival crevice area of man. II. The predominant cultivable organisms. Arch Oral Biol. 1963 May-Jun;8:281–289. doi: 10.1016/0003-9969(63)90020-7. [DOI] [PubMed] [Google Scholar]
- Gordon D. F., Stutman M., Loesche W. J. Improved isolation of anaerobic bacteria from the gingival crevice area of man. Appl Microbiol. 1971 Jun;21(6):1046–1050. doi: 10.1128/am.21.6.1046-1050.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. H., Dubos R. The anaerobic bacterial flora of the mouse cecum. J Exp Med. 1970 Aug 1;132(2):251–260. doi: 10.1084/jem.132.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gästrin B., Kallings L. O., Marcetic A. The survival time for different bacteria in various transport media. Acta Pathol Microbiol Scand. 1968;74(3):371–380. doi: 10.1111/j.1699-0463.1968.tb03490.x. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Krasse B., Möller A. A method of sampling human dental plaque for certain "caries-inducing" streptococci. Arch Oral Biol. 1968 Aug;13(8):919–927. doi: 10.1016/0003-9969(68)90007-1. [DOI] [PubMed] [Google Scholar]
- Keyes P. H. Research in dental caries. J Am Dent Assoc. 1968 Jun;76(6):1357–1373. doi: 10.14219/jada.archive.1968.0186. [DOI] [PubMed] [Google Scholar]
- Leach P. A., Bullen J. J., Grant I. D. Anaerobic CO 2 cabinet for the cultivation of strict anerobes. Appl Microbiol. 1971 Nov;22(5):824–827. doi: 10.1128/am.22.5.824-827.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn M. T., Crawford J. J. Recovery of anaerobic microorganisms from clinical specimens in prereduced media versus recovery by routine clinical laboratory methods. Appl Microbiol. 1970 Feb;19(2):207–213. doi: 10.1128/am.19.2.207-213.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears R. W., Freter R. Improved isolation of anaerobic bacteria from the mouse cecum by maintaining continuous strict anaerobiosis. Proc Soc Exp Biol Med. 1967 Mar;124(3):903–909. doi: 10.3181/00379727-124-31882. [DOI] [PubMed] [Google Scholar]



