Abstract
IL-15 is a powerful T cell growth factor (TCGF) with particular importance for the maintenance of CD8+ T cells. Because costimulation blockade does not result in universal tolerance, we hypothesized that “escape” from costimulation blockade might represent a CD8+ and IL-15/IL-15R+-dependent process. For this analysis, we have used an IL-15 mutant/Fcγ2a protein, a potentially cytolytic protein that is also a high-affinity receptor site specific antagonist for the IL-15Rα receptor protein, as a therapeutic agent. The IL-15-related fusion protein was used as monotherapy or in combination with CTLA4/Fc in murine islet allograft models. As monotherapies, CTLA4/Fc and an IL-15 mutant/Fcγ2a were comparably effective in a semiallogeneic model system, and combined treatment with IL-15 mutant/Fcγ2a plus CTLA4/Fc produced universal permanent engraftment. In a fully MHC-mismatched strain combination known to be refractory to costimulation blockade treatment, combined treatment with both fusion proteins proved to be highly effective; >70% of recipients were tolerized. The analysis revealed that the IL-15 mutant/Fc treatment confers partial protection from both CD4+ and CD8+ T cell graft infiltration. In rejections occurring despite CTLA4/Fc treatment, concomitant treatment with the IL-15 mutant/Fcγ2a protein blocked a CD8+ T cell-dominated rejection processes. This protection was linked to a blunted proliferative response of alloreactive T cells as well silencing of CTL-related gene expression events. Hence, we have demonstrated that targeting the IL-15/IL-15R pathway represents a new and potent strategy to prevent costimulation blockade-resistant CD8+ T cell-driven rejection.
Blockade of B7-CD28 or CD40-CD40L costimulatory interactions blunts T cell-dependent immune activation processes (1–4) such as allograft rejection (5–8). Blockade of the CD28 T cell signal through treatment with CTLA4/Fc can suppress Th1 cytokine production in vivo (9) and deviate the alloimmune response to a Th2 pathway, which is less detrimental than Th1-type immune activation in transplant models (10). In several circumstances, however, costimulatory blockade fails to induce permanent engraftment. A CD8+ T cell-dependent process appears to be responsible for costimulation blockade-resistant rejection (11–15). We find that costimulation blockade does not prolong survival of islet allografts in a CD8+ TCR-transgenic model (our unpublished data). Honey et al. (12) have recently reported that anti-CD154 mAb treatment prolongs engraftment of skin allografts, but the induction of tolerance requires adjunctive treatment with anti-CD8 mAb, or other means of depleting CD8+ T cells (14). Trambley et al. (15) have also demonstrated the importance of GM1+CD8+ T cells in costimulation blockade-resistant rejection.
Clearly, costimulation blockade drastically decreases expression of IL-2 posttransplantation (7, 9). Which T cell growth factors are responsible for the clonal proliferation of alloreactive T cells that cause graft rejection in some costimulation blockade-treated hosts? IL-2 expression is not a prerequisite for allograft rejection as IL-2 knockout (KO)5 mice promptly reject islet and cardiac allografts (16, 17). Immunohistology in the IL-2 KO recipients reveals that CD4+ and CD8+ T cells infiltrate rejecting grafts while robust intragraft expression of the IL-4, IL-7, IL-15, as well as granzyme B genes is evident (Refs. 16 and 18 and our unpublished data). Moreover, mice rendered genetically deficient for both IL-2 and IL-4, the principal T cell growth factors that are produced by T cells, also readily reject allografts (17). Thus, non-T cell-derived cytokines, such as IL-15, may be actively involved in supporting allograft rejection. An analysis of biopsies taken from rejecting human renal allografts has revealed that intragraft gene expression of IL-15 is a far more consistent marker of rejection than IL-2 gene expression (18). Moreover, Smith et al. (19) have recently reported the prolonged survival of heart allografts in a treatment protocol consisting of soluble IL-15Rα chain proteins and nondepleting anti-CD4 mAb. Can expression of IL-15 be linked to CD8-dependent costimulation-resistant rejection? The phenotype of IL-15−/− (20) and IL-15Rα−/− (21) mice demonstrates that IL-15 selectively supports the homeostasis and proliferation of memory CD8+ T cells (22, 23).
Costimulation blockade cannot act to directly decrease IL-15 expression because epithelial and endothelial cells and monocyte/macrophages, not T cells, are the primary cellular sources of this cytokine (24). Since costimulation blockade-resistant rejection may be mediated by CD8+ T cells and IL-15 is essential for the homeostasis and proliferation of CD8+ T cells, we have now tested the hypothesis that targeting the IL-15/IL-15R system in concert with costimulation blockade might provide a new tool for the induction of allograft tolerance.
The trimolecular IL-15R expressed upon T cells includes the constitutively expressed IL-2Rβ and IL-2R common γ-chains shared with the IL-2R. A unique IL-15Rα chain (25, 26) is expressed upon activated, but not resting, mononuclear leukocytes (27). The IL-15Rα is expressed in lesser abundance upon activated epithelial cells, macrophages, and fibroblasts (24). We have previously reported on the specificity of a unique IL-15 mutant/Fcγ2a protein possessing a prolonged circulating half-life, high-affinity IL-15Rα site-specific antagonist function and both complement- and Fc-related cytocidal potential against IL-15Rα+ cells (28). Kim et al. (28) have demonstrated that this IL-15 mutant/Fc fusion protein specifically binds to the IL-15Rα; IL-15 mutant/Fcγ2a binding to activated T cells was blocked by IL-15, but not by IL-2 or anti-IL-2/IL-15Rγ chain Abs. In this report, we also demonstrated that this IL-15 mutant/Fc protein efficiently blocks delayed-type hypersensitivity in mice (28). In the present report, the therapeutic activity of the IL-15-related protein was compared with that of a CTLA4/Fc. CTLA4/Fc treatment is known to promote engraftment and often leads to tolerance (5, 29). Combined treatment with CTLA4/Fc and IL-15 mutant/Fc proteins was also studied. Our data suggest that IL-15R+ cells are an important component of allograft rejection and these cells play a pivotal role in CD8+ T cell-mediated CTLA4/Fc blockade-resistant rejection.
Materials and Methods
Animals
BALB/c (H-2d), DBA/2 (H-2d), B6AF1 (H-2b/d.k), and C57BL/6 (H-2b) mice, 8–10 wk old, were obtained from Taconic Farms (Germantown, NY). C57BL/6cd4tm1knw (CD4KO, H-2b) and C57BL/6.129S2cd8atm1mak (CD8KO, H-2b) were obtained from The Jackson Laboratory (Bar Harbor, ME).
Islet transplantation
Allogeneic DBA/2 islet cell grafts were transplanted into 8- to 10-wk-old B6AF1 recipient mice rendered diabetic by a single i.p. injection of streptozotocin (225 mg/kg; Sigma, St Louis, MO). Allogeneic BALB/c islet cell grafts were transplanted into 8- to 10-wk-old C57BL/6 recipient mice rendered diabetic by a single i.p. injection of streptozotocin (270 mg/kg). Islet cell transplantation was performed as previously described (30). Briefly, islets were isolated from donor DBA/2 or BALB/c pancreata through collagenase digestion and centrifugation on a discontinuous Ficoll gradient. The crude islet isolates containing islets, vascular tissue, ductal fragments, and lymph nodes were divided into aliquots of ~300 islets and were transplanted under the renal capsule into B6AF1 or C57BL/6 recipients. By intent, we use these crude islet preparations which are more immunogenic than more pure islet preparations (our unpublished observations). Initial allograft function was verified by sequential blood glucose measurements with levels under 200 mg/dl on days 3–5 after transplantation, and graft rejection was defined as a rise in blood glucose levels exceeding 300 mg/dl following a period of primary graft function.
Treatment protocol
Murine CTLA4/Fc (31) and human IL-15 mutant/Fcγ2a (28) proteins were constructed and expressed in our laboratory. CTLA4/Fc protein used in these studies bears active FcR binding and complement-binding domains (29). Treatment of islet allograft recipients with CTLA4/Fc consisted of 0.1 mg/day i.p. for 10 consecutive days after transplantation, which is the optimal treatment period in this model (our unpublished data). Islet allograft recipients received 1.5 µg of IL-15 mutant/Fcγ2a/day i.p. for 21 consecutive days after transplantation. An IgG2a protein, bearing the same Fc sequence as the CTLA4/Fc and IL-15 mutant/Fcγ2a proteins, was used as a control treatment at 1.5 µg/day for 10 days.
Histopathology and immunohistology
The left kidney bearing the islet graft was removed from the recipients after 8 days and embedded in OCT compound (Tissue TCK; Miles Scientific, Elkhart, IN). Cryostat sections of islets (n = 3/group) were fixed in paraformaldehyde-lysine-periodate for analysis of leukocyte Ags and stained by a four-layer peroxidase-antiperoxidase method involving overnight incubation with mAb, followed by mouse Ig-absorbed goat anti-rat Ig, rabbit anti-goat Ig, goat peroxidase-antiperoxidase complexes, and diaminobenzidine substrate. Rat anti-mouse mAbs and isotype-matched control mAbs were purchased from BD PharMingen (San Francisco, CA) and included mAbs to CD4+ (H129.19) and CD8+ (53-6.7) T cells. Sections were counterstained in hematoxylin and mounted. Isotype-matched mAbs and a control Ab were analyzed for endogenous peroxidase activity in each experiment. Samples were assigned a random number and processed and evaluated in a blinded fashion; each sample was evaluated at two to three different levels of sectioning.
PCR analysis
Intragraft mRNA analysis was performed via template RT-PCR as previously described (16). The specific primers used for hybridization to murine IL-2, TCR Cβ, perforin, Fas ligand (FasL), granzyme B, and GAPDH cDNA, the latter as an internal control, have been previously described (16). The PCR amplification was performed in a thermocycler (Gene Amp, PCR system 2400; PerkinElmer/Cetus, Norwalk, CT): denaturing at 94°C for 30 s, annealing at 57°C for IL-2 and FasL, at 60°C for perforin and granzyme B, at 60°C for TCR Cβ, and at 57°C for GAPDH for 30 s, and extension at 72°C for 30 s for each cycle, for a total of 40 cycles. A negative control was included for each PCR amplification and consisted of the omission of cDNA in the PCR mixture. To further confirm data, a semiquantitative competitor template RT-PCR analysis was also performed with IL-2 using the gene-specific relative RT-PCR kit from Ambion (Austin, TX). To quantitate the relative amounts of TCR Cβ gene transcripts between samples, each PCR was performed by coamplifying the cDNA of interest with an internal PCR control. A gene-specific competitive template cDNA was designed and the semiquantitative PCR was performed as described by Steiger et al. (16). After amplification, the samples (10 µl) were separated on ethidium bromide-stained 1.5% agarose gel and the DNA were visualized and photographed using UV transilluminator (Gel Doc 1000; Bio-Rad, Hercules, CA).
CFSE labeling and analysis of T cell proliferation in vivo
Spleen and lymph node cells from wild-type C57BL/6, CD8−/−, or CD4−/− mice (C57BL/6, H-2b) were harvested, processed, and labeled with CFSE (Molecular Probes, Portland, OR) as described previously (32). CFSE was dissolved in DMSO and added into the cell suspension at a final concentration of 5 nM for 3 min at room temperature. The reaction was stopped by the addition of HBSS/1% FCS. The cells were washed in HBSS/1% FCS and resuspended in the same solution before injection. Recipient BALB/c mice were sublethally irradiated (1000 rad with a GammaCell irradiator, Kanata, Ontario, Canada) before injection of the CFSE-labeled cells via the lateral tail vein. From that day, untreated recipient mice or mice receiving an i.p. injection of CTLA-4/Fc (0.1 mg/mouse) or IL-15 mutant/Fcγ2a protein (1.5 µg/mouse) for 3 days were studied. Adoptive transfer experiments using syngeneic CFSE-labeled lymphocytes was also performed as a control. On day 3, recipient spleen and lymph node cells were removed and cell suspensions were processed as before. Cells were stained with anti-CD4-PE conjugate (L3T4, 2 µg/ml) or a biotinylated mAb against mouse CD8a (53-6.7, 2 µg/ml; BD PharMingen) for 30 min at 4°C. After staining, cells were washed once and resuspended in 0.5 ml of HBSS for analysis by flow cytometry using a BD Biosciences FAC-Sort equipped with CellQuest software (Mountain View, CA). Live events were collected and analyzed by gating on CD4+ or CD8+CFSE+ cells.
Calculation of the frequency of proliferating T cell
Analysis of CD4+ and CD8+ T cell proliferation in response to alloantigen stimulation was performed according to Noorchashm et al. (33). With each round of cell division, the CFSE dye partitions equally between the daughter cells. By using the FACS acquisition software (CellQuest), the total number of cells in each generation of proliferation can be calculated and the number of precursors that generated the daughter cells was determined by using the following formula: y/n2 (y = absolute number of cells in each peak, n = number of cell division). The calculation of the frequency of T cell proliferation was then analyzed by dividing the total number of precursors by the total CFSE-labeled cells.
Results
Survival of islet allografts in B6AF1 and C57BL/6 recipients
To probe for the role of the IL-15/IL-15R network in the allograft response, partially MHC-mismatched DBA/2 (H-2d) islet allografts were transplanted into B6AF1 (H-2b/d.k) mice. Untreated recipients rejected DBA/2 islet allografts with a mean survival time (MST) of 14 days (Table I) while B6AF1 recipient mice treated with CTLA4/Fc or IL-15 mutant/Fcγ2a have a MST of 70 and 77 days, respectively. All B6AF1 recipients of DBA/2 islet allografts treated with a combination of IL-15 mutant/Fcγ2a and CTLA4/Fc were permanently engrafted. Surgical removal of the left kidney bearing the islet allograft was performed on two recipients 150 days after transplantation. Six days later, a second DBA/2 islet allograft was successfully engrafted without rejection in the absence of further immunosuppressive therapy.
Table I.
Survival of DBA/2 islet allografts in B6AF1 recipients treated with CTLA4/Fc and IL-15 mutant/Fc
| Donor | Recipient | Treatmenta | Islet Graft Survival (days) |
|---|---|---|---|
| DBA/2 | B6AF1 | Untreated | 11, 11, 11, 12, 13, 13, 15, 17, 17, 20 |
| DBA/2 | B6AF1 | CTLA4/Fc | 20, 31, 34, 51, >120, >120, >120, >120 |
| DBA/2 | B6AF1 | IL-15m/Fc | 15, 22, 29, 32, >120, >120, >120, >120 |
| DBA/2 | B6AF1 | CTLA4/Fc + IL-15m/Fc | >120, >120, >120, >120 |
Treatment: CTLA4/Fc, 0.1 mg/day, i.p. from days 0 to 11 posttransplantation; IL-15m/Fc, 1.5 µg/day, i.p. from days 0 to 21 posttransplantation.
To test the hypothesis that targeting the IL-15/IL-15R system would block CD8+-driven CTLA4/Fc-resistant rejection, fully MHC-mismatched BALB/c (H-2d) donor islet allografts were transplanted into C57BL/6 (H-2b) recipient mice. In this model, the recipients are refractory to the tolerizing effects of optimal doses of CTLA4/Fc. Untreated C57BL/6 recipients rejected BALB/c islet allografts (MST, 20 days; Fig. 1a). As additional controls, C57BL/6 recipients were also treated with a control IgG2a protein (1.5 µg/ml). In other experiments, C57BL/6 received CTLA4/Fc (0.1 mg/ml) plus IgG2a (1.5 µg/ml). Recipients treated with IgG2a rejected rapidly their allografts (MST, 14 days; Fig. 1a), and the recipients treated with CTLA4/Fc and IgG2a rejected allografts at the same tempo as recipients treated with CTLA4/Fc alone (Fig. 1a) These data confirm that the prolonged engraftment observed with IL-15 mutant/Fcγ2a fusion protein treatment is not produced solely by Fc sequences shared with IgG2a. Engraftment was prolonged in C57BL/6 recipients treated with CTLA4/Fc or with IL-15 mutant/Fcγ2a protein (MST, 30 days; Fig. 1a); some grafts functioned permanently following monotherapy with either agent. A dramatic improvement was noted among C57BL/6 recipients treated with a combination of IL-15 mutant/Fcγ2a and CTLA4/Fc (MST 120 days; Fig. 1a). Surgical removal of the islet allograft in each of five recipients 150 days after transplantation led to hyperglycemia, demonstrating that the euglycemic state was maintained by the islet allografts. Subsequent to removal of the primary allograft, four recipient mice were successfully engrafted with a second BALB/c islet allograft in the absence of further immunosuppressive treatment (Fig. 1b). One recipient received a third-party islet allograft (B10.BR, H-2k) that was rejected at day 12 posttransplantation (Fig. 1b), thereby confirming that the tolerant state was donor strain specific.
FIGURE 1.
Survival of islet allografts with a combined treatment of CTLA4/Fc and IL-15 mutant/Fcγ2a. a, Islets from BALB/c (H-2d) donors were transplanted under the renal capsule of C57BL/6 (H-2b) recipients. Recipients were treated with CTLA4/Fc (0.1 mg), IL-15 mutant/Fcγ2a (1.5 µg), or a combination of both proteins as indicated in Materials and Methods. Untreated recipients (■) or recipients treated with a control IgG2a protein (1.5 µg) rejected allografts with a MST of 15 days and 14 days, respectively (□). Graft rejection was delayed by 30 days in recipients treated with CTLA4/Fc (◊), CTLA4/Fc plus IgG2a (♦), or IL-15 m/Fcγ2a (○), whereas in recipients receiving the combined treatment, the MST was of 120 days (●). b, Second islet transplantation. Nephrectomy of the left kidney was performed on five recipients 150 days after transplantation. Then four nephrectomized mice received a second BALB/c islet allograft under the right renal capsule (following the same procedure) without further immunosuppression. All of them accepted the second transplant for more than 50 days, demonstrating a state of tolerance (●). One mouse received a third part islet allograft (B10. BR, H-2k; ○) which was rejected at day 12, confirming that the immune response is intact.
T cell infiltration in the BALB/c donor allografts placed into C57BL/6 recipients
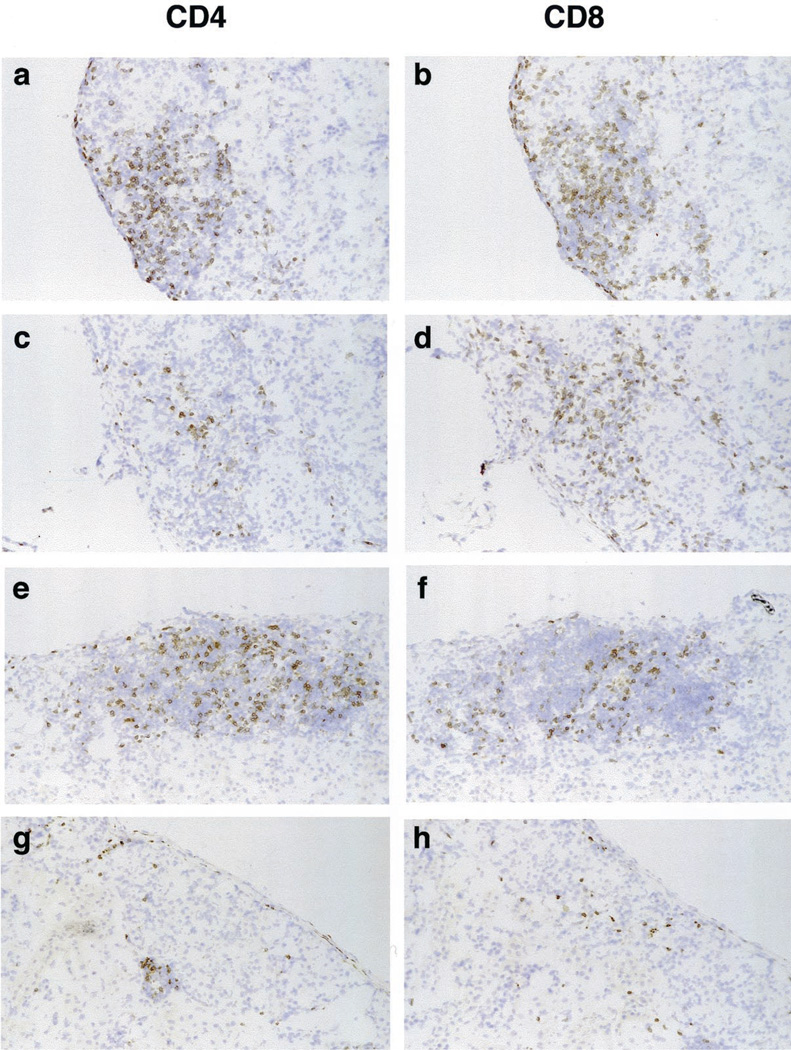
Histologic assessment of the allografts revealed a dense mononuclear leukocytic infiltrate in untreated recipients (Fig. 2a) and less intense infiltration was present in CTLA/Fc- or IL-15 mutant/Fc-treated recipients (Fig. 2, b and c) at day 8 posttransplantation. This day was chosen for study because it is the time at which graft infiltration by host mononuclear leukocytes peaks in untreated control recipients. In the combined treatment group, cellular infiltration was dramatically reduced and islet architecture was well preserved (Fig. 2d). In untreated recipients, dense graft infiltration by mononuclear leukocytes, composed predominantly of CD4+ and CD8+ T cells, was noted (Fig. 3, a and b). By comparison (and as anticipated), a decrease in graft infiltration by CD4+ T cells was noted in recipients treated with CTLA4/Fc (Fig. 3c) while infiltration by CD8+ T cells was similar to that detected in untreated mice (Fig. 3d). This result is in keeping with the hypothesis that costimulation-resistant rejection is a CD8+ T cell-dependent process. In contrast, in recipients treated with IL-15 mutant/Fcγ2a (Fig. 3, e and f), a decrease in CD8+ T cell infiltration without a major change in CD4+ T cell infiltration was evident. Note that recipient mice treated with both CTLA4/Fc and IL-15 mutant/Fcγ2a (Fig. 3, g and h) show a near absence of CD4+ and CD8+ graft-infiltrating T cells.
FIGURE 2.
Histology of serial sections of islet allografts isolated 8 days after islet allograft transplantation. A dense tissue infiltration by various mononuclear cells with destruction of islets is observed in untreated recipients (a). Recipients receiving CTLA4/Fc or IL-15 mutant/Fcγ2a evidenced a decreased tissue infiltration by mononuclear leukocytes with some islets preserved (b and c, respectively). Graft sections from recipients treated with a combination of CTLA4/Fc and IL-15 mutant/Fcγ2a reveal almost normal histology with minimal mononuclear cell infiltration and intact islets (d). Cryostat sections, hematoxylin counterstain; original magnification, ×100. These are representative sections from two allograft from each treatment group.
FIGURE 3.
Immunohistologic evaluation of islet allografts isolated 8 days after islet transplantation. Untreated recipients show dense tissue infiltration by mononuclear leukocytes surrounding and invading the islets with a predominance of CD4+ and CD8+ T cells (a and b). Recipients treated with CTLA4/Fc (c and d) or IL-15 mutant/Fcγ2a (e and f) show a decreased mononuclear cell infiltrate with preservation of the tissue structure. Recipient mice receiving a combined treatment present rare CD4+ and CD8+ T cell infiltrates (g and h). Cryostat sections, hematoxylin counterstain; original magnification, ×100. These are representative sections from two recipients from each treatment group.
The effect of treatment upon intragraft IL-2 and TCR Cβ gene expression was analyzed by RT-PCR at day 8 posttransplantation. As the constant chain of TCR Cβ is constitutively expressed by T cells, a substantial decrease in the frequency of graft-infiltrating T cells in the allograft should be accompanied by a decrease in the intragraft TCR Cβ gene expression. In recipients treated with IL-15 mutant/Fcγ2a or the combination of IL-15 mutant/Fcγ2a protein and CTLA4/Fc, a marked decrease in expression of the IL-2 gene and TCR Cβ gene was observed (data not shown).
To further test the hypothesis that CD8+ T cells play a major role in costimulation blockade-resistant rejection in this model, we assessed CTL-selective gene expression events in islet allografts isolated 8 days posttransplantation (Fig. 4). The granzyme B, perforin, and FasL genes are uniformly expressed in tissue samples obtained from untreated recipients or from recipients treated with CTLA4/Fc (Fig. 4). In contrast, reduced expression of the CTL-selective genes was observed in recipients treated with IL-15 mutant/Fcγ2a or in recipients treated with IL-15 mutant/Fcγ2a plus CTLA4/Fc (Fig. 4).
FIGURE 4.
Cytolytic gene transcripts were analyzed by RT-PCR. Tissues were isolated on day 8 after transplantation in untreated, CTLA4/Fc-treated, IL-15 mutant/Fcγ2a-treated, or CTLA4/Fc plus IL-15 mutant/Fcγ2a-treated recipients. Three allografts from each group were analyzed. Each lane represents an individual mouse, the first lane being the negative control. GAPDH primers were used as an internal control. Densitometric scanning quantification of gene expression for perforin, FasL, and granzyme B normalized to GAPDH was performed and the results are as follows: untreated mice: 0.733 ± 0.25, 0.362 ± 0.15, and 2.72 ± 0.57, respectively; CTLA4/Fc-treated mice: 0.98 ± 0.71, 0.56 ± 0.27, and 1.09 ± 0.24, respectively; IL-15 mutant/Fcγ2a-treated mice: 0.094 ± 0.09, 0.03 ± 0.03, and 0.13 ± 0.15, respectively; CTLA4/Fc plus IL-15 mutant/Fcγ2a-treated mice: 0.123 ± 0.15, 0.017 ± 0.02, and 0, respectively. Molecular weight markers are in the left part of the gel.
IL-15 mutant/Fc treatment inhibits alloreactive proliferating CD8+ T cells in vivo
To determine whether treatment with the IL-15 mutant/Fcγ2a protein blunts the proliferation of alloreactive CD8+ T cells in vivo, we labeled splenic lymphocytes from C57BL/6 CD4−/− mice with CFSE. The dye-labeled C57BL/6 CD4−/− lymphocytes were injected i.v. into irradiated untreated or treated (for 3 days) BALB/c mice. On the third day following adoptive cell transfer, CFSE-stained leukocytes from hosts were recovered and stained with a biotinylated anti-CD8 mAb. Since CFSE partitions equally between daughter cells following cell division, the pattern and frequency of proliferation of alloreactive CD8+ T cells in vivo can be analyzed via the CFSE staining pattern (31). In untreated hosts, ~23% of CFSE-labeled allogeneic CD8+ T cells proliferated in the host spleen (Fig. 5b). In CTLA4/Fc-treated hosts, CD8+ T cells (~21%) divide for multiple generations (Fig. 5b). In contrast, treatment with IL-15 mutant/Fc alone or in combination with CTLA4/Fc markedly inhibited proliferation of alloreactive CD8+ T cells with only 15 and 14%, respectively, of CFSE-labeled CD8+ T cells proliferating in vivo (Fig. 5b). Using syngeneic controls (CFSE-labeled C57BL/6 CD4−/− lymphocytes injected into C57BL/6 mice) proliferation of CD8+ T cells was not detected (data not shown). By contrast, when similar experiments were performed with splenic lymphocytes from C57BL/6 CD8−/− mice to analyze the fate of CD4+ T cells, we observed that CTLA4/Fc treatment markedly decreased the frequency of proliferating CFSE-labeled alloreactive CD4+ T cells in vivo (Fig. 5a). Treatment with IL-15 mutant/Fcγ2a reduces the frequency of proliferating CD4+ and CD8+ T cells, although a lesser effect on CD4+ T cells as compared with CD8+ T cells was noted. Similar results were observed when wild-type C57BL/6 mice were used as donor (data not shown). IL-15 mutant/Fcγ2a may represent an important therapeutic agent capable of controlling the proliferation of alloreactive CD8+ (costimulation-resistant) T cells. Proliferation of CD4+, but not CD8+, T cells is controlled by B7 blockade agents.
FIGURE 5.
Analysis of alloreactive CD4+ and CD8+ T cell proliferation in vivo. CFSE-labeled CD8−/− C57BL/6 (a) and CD4−/− C57BL/6 (b) lymphocytes were injected through the lateral vein into irradiated BALB/c recipient mice. Recipient mice were then untreated or treated with CTLA4/Fc (0.1 mg), IL-15 mutant/Fcγ2a (1.5 µg) or a combination of both fusion proteins for 3 days. a, CFSE-labeled allogeneic CD4+ T cells from the host spleen. b, CFSE-labeled allogeneic CD8+ T cells from the host spleen. Data are represented as percentage of proliferation of CFSE-labeled CD4+ and CD8+ T cells. Data are representative of four individual experiments.
Discussion
T cell costimulation blockade treatment with CTLA4/Fc or Abs against CD154 have often produced permanent engraftment and donor-specific tolerance (5–8). Unfortunately, recent reports have underlined the importance of activated CD8+ T cells in a costimulation-resistant rejection process observed in various transplant models such as skin, intestine, and heart (11–15). It is notable that costimulatory blockade prolongs allograft survival in CD8 KO mice (11, 14) because in those models in which tolerance is not produced by costimulation blockade, tolerance can be achieved with provision of anti-CD8 mAb or other treatments that clear alloreactive CD8+ T cells (12–15). Apparently costimulatory blockade-resistant activation of alloreactive CD8+ T cells can cause allograft rejection.
Since the molecular targets of costimulation blockade are primarily expressed upon CD4+, and not upon CD8+ T cells, the reason that CD8+ T cells play a prominent role in costimulation blockade escape rejection process is enigmatic. Studies using IL-15−/− mice and IL-15Rα−/− mice (19, 20) have emphasized the role of IL-15 in support of the activation and maintenance of CD8+ T cells (22). Because costimulation blockade results in diminished expression of IL-2 by CD4+ T cells (7, 9), we hypothesized that escape from costimulation blockade might represent an IL-15/IL-15R-dependent process. Moreover, costimulation blockade does not target epithelial cells, the principle source of IL-15 (23). Thus, we wanted to study the effect of IL-15 mutant/Fcγ2a on an islet allograft model and its potential to enhance the effectiveness of costimulatory blockade.
For our experiments, we first chose a partial MHC-mismatch combination (H-2d–H-2b/d.k) to examine the effect of the IL-15 mutant/Fcγ2a. In this islet allograft model, the administration of IL-15 mutant/Fcγ2a, a protein that targets the IL-15Rα chain expressed upon activated T cells, prevents allograft rejection in 50% of the recipient mice (Table I). Identical results were obtained with CTLA4/Fc administration. It is notable that combined treatment with CTLA4/Fc and IL-15 mutant/Fcγ2a produced permanent engraftment and tolerance in all recipients. Next, we chose to study a fully MHC-mismatched strain combination and used a recipient strain (C57BL/6) that is refractory to costimulatory blockade therapy (15). A modest beneficial effect of CTLA4/Fc treatment was observed (MST, 30 days vs 13 days in the controls; Fig. 1a) and prolonged graft survival was evident in IL-15 mutant/Fcγ2a-treated recipients (Fig. 1a). Treatment with a control IgG2a protein did not prolong engraftment (MST, 14 days). Furthermore, combined treatment with CTLA4/Fc and IgG2a does yield an improvement upon the effect of CTLA4/Fc monotherapy (Fig. 1a). Moreover, the bioavailability of CTLA4/Fc or IL-15 mutant/Fcγ2a is not altered when the fusion proteins are administered as combined treatment (CTLA4/Fc half-life = 96 h and IL-15 mutant/Fcγ2a half-life = 6–8 h). Thus, the beneficial effects observed with CTLA4/Fc and/or IL-15 mutant/Fcγ2a are not dependent on solely the γ2a Fc peptide sequence present in the fusion proteins. We have previously demonstrated that the IL-15 mutant/Fcγ2a fusion protein specifically binds to IL-15Rα but not the common γ-chain (28). Binding of the IL-15 mutant/Fcγ2a was inhibited by IL-15 but not inhibited by IL-2 or anti-common γ-chain Abs (28).
To begin to test our primary hypothesis concerning the role of IL-15/IL-15R-dependent CD8+ processes in rejections occurring despite use of CTLA4/Fc, we treated a group of recipient mice with combined CTLA4/Fc and IL-15 mutant/Fcγ2a therapy. Permanent engraftment was obtained in most treated recipients (Fig. 1a). Further analysis demonstrated that these permanently engrafted mice were rendered specifically tolerant to donor strain grafts (Fig. 1b). CTLA4/Fc treatment had a far more dramatic effect upon CD4+ T cell infiltration into the graft than upon CD8+ T cell infiltration. While the principal effect of IL-15 mutant/Fcγ2a treatment in the combined CTLA4/Fc plus IL-15 mutant treatment group was exacted upon infiltration of CD8+ T cells into the graft (Fig. 3). The prolongation of islet allograft survival in recipient mice receiving both CTLA4/Fc and IL-15 mutant/Fcγ2a was accompanied by a decrease in T cell infiltration in graft tissues (Fig. 2). Immunohistologic analysis suggests that the potential benefit of a combined treatment is related to the drastic decrease of tissue infiltration by both alloreactive CD4+ and CD8+ T cells (Fig. 3). Molecular markers for activated CD8+ T cells, such as CTL genes, have been identified within the infiltrate of rejecting allografts (28), and expression of these CTL genes (granzyme B, FasL, perforin) has been associated with acute renal allograft rejection (18). It is thus interesting to observe that the expression of CTL genes are markedly decreased in IL-15 mutant/Fcγ2a-treated mice (Fig. 4), further attesting to the effect of IL-15 mutant/Fcγ2a treatment upon alloactivated CD8+/CTLs.
To further analyze the effect of costimulation blockade and IL-15 mutant/Fcγ2a, we studied alloantigen-driven proliferative responses in vivo using the CFSE dye system. CTLA4/Fc treatment has no effect on the CD8+ T cell proliferative response to alloantigen, whereas IL-15 mutant/Fcγ2a has a potent inhibitory effect on the proliferation of alloreactive CD8+ T cells (Fig. 5). Because IL-15 is of essential importance for the homeostasis and proliferation of CD8+ T cells (22, 23), we cannot exclude the possibility that IL-15 mutant/Fcγ2a treatment decreases the frequency of proliferating CD8+ T cell by promoting apoptosis of these responder cells. In contrast, the alloantigen-driven response of CD4+ T cells was decreased by either CTLA4/Fc or IL-15 mutant/Fcγ2a monotherapy (Fig. 5b). The effect of CTLA4/Fc upon CD4+ T cells is particularly potent (Fig. 5a). The IL-15 mutant/Fcγ2a, but not CTLA4/Fc, treatment targets CD8+ T cells (Fig. 5). Consequently combined IL-15 mutant/Fcγ2a plus CTLA4/Fc treatment exert additive immunosuppressive effects in controlling allograft rejection (Fig. 5 and Table I).
We now confirm and extend previous observations that costimulation blockade treatment reduces the frequency of proliferating alloreactive CD4+ T cells in an allograft model (34), but is unable to inhibit the proliferation of alloreactive CD8+ T cells in vivo (13). The resistance of CD8+ T cells to costimulation blockade is related to the inability of CTLA4/Fc (35, 36) and anti-CD154 mAb (36) treatments to control alloactivated CD8+ T cells and is probably related to the restricted expression of CD28 and CD154 to CD4+ T cells (37). To obtain prolonged engraftment of heart allografts, treatment with a nondepleting anti-CD4 mAb alone is not sufficient. The successful use of soluble IL-15 receptor α-chain proteins as an adjunct to anti-CD4 treatment (19) is consistent with our hypothesis that blocking both CD4+- and IL-15/IL-15R-dependent CD8+ T cell activation is required to gain long-term graft acceptance.
IL-15 mutant/Fcγ2a treatment mediates important effects on allograft survival that are exerted, at least in part, through the inhibition of the activation and proliferation of alloreactive CD8+ T cells (Figs. 3–5 and Table I). This finding emphasizes the role of the IL-15/IL-15R pathway as an important element in the rejection process. Moreover, we have recently noted that the initial five to six waves of the T cell proliferation to alloantigens in vivo are IL-15, not IL-2, dependent (38). The induction of permanent tolerance is still an elusive goal in clinical organ transplantation. By targeting IL-15/IL-15R+ cells to prevent costimulation blockade-resistant rejection, IL-15 mutant/Fcγ2a appears to provide a promising new agent capable of interrupting allograft rejection mediated by CD8+ T cells. The combined effects of costimulation blockade plus targeting the IL-15/IL-15R pathways to target both CD4+ and CD8+ T cells may represent an effective approach to achieve permanent tolerance.
Acknowledgments
We thank Yian Tian for her excellent technical assistance. We also thank E. Csizmadia for technical assistance with immunohistologic staining.
Footnotes
This work was supported by a postdoctoral fellowship grant from the Fondation des Bourses en Médecine et Biologie and the Swiss National Science Foundation (to S.F.-L.), by grants from Juvenile Diabetes Research Foundation International 1-1999-317 (to X.X.Z.), the Juvenile Diabetes Research Foundation Islet Transplantation Center at Harvard Medical School, and by National Institute of Health Grants 1PO AI GF41521 and ROI AI42298 (to T.B.S.).
Abbreviations used in this paper: KO, knockout; FasL, Fas ligand; MST, mean survival time.
References
- 1.June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol. Today. 1994;15:321. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 2.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T-cell costimulation. Annu. Rev. Immunol. 1996;14:233. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 3.Grewal IS, Flavell RA. CD40 and CD154 in cell mediated immunity. Annu. Rev. Immunol. 1998;16:111. doi: 10.1146/annurev.immunol.16.1.111. R. A. [DOI] [PubMed] [Google Scholar]
- 4.Marengere LE, Waterhouse P, Duncan GS, Mittrucker H-W, Feng GS, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase in association with CTLA-4. Science. 1996;272:1170. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 5.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 6.Turka LA, Linsley PS, Lin H, Brady W, Leiden JM, Wei RQ, Gibson ML, Zheng XG, Myrdal S, Gordon D, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc. Natl. Acad. Sci. USA. 1992;89:11102. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen CP, Elwood ET, Alexender DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Rae Cho H, Aruffo A, Hollenbaugh D, Linsley PS, et al. Long-term acceptance of skin and cardiac allograft after blocking CD40 and CD28 pathways. Nature. 1996;381:434. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 8.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Jr, Knechtle SJ. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc. Natl. Acad. Sci. USA. 1997;94:8789. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayegh MH, Akalin E, Hancock WW, Russell ME, Carpenter CB, Linsley PS, Turka LA. CD28–B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J. Exp. Med. 1995;181:1869. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh C-S, Heimberger AB, Gold JS, O’Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukin 4 and 10 in ab T-cell-receptor transgenic system. Proc. Natl. Acad. Sci. USA. 1992;89:6065. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newell KA, He G, Guo Z, Kim O, Szot GL, Rulifson I, Zhou P, Hart J, Thistlethwaite JR, Bluestone JA. Blockade of the CD28/B7 costimulatory pathway inhibits intestinal allograft rejection mediated by CD4+ but not CD8+ T cells. J. Immunol. 1999;163:2358. [PubMed] [Google Scholar]
- 12.Honey K, Cobbold SP, Waldmann H. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. J. Immunol. 1999;163:4805. [PubMed] [Google Scholar]
- 13.Jones ND, Van Maurik A, Hara M, Spriewald BM, Witzke O, Morris PJ, Wood KJ. CD40-CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J. Immunol. 2000;165:1111. doi: 10.4049/jimmunol.165.2.1111. [DOI] [PubMed] [Google Scholar]
- 14.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J. Immunol. 2000;164:512. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- 15.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze S-Y, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1+ CD8+ T-cells play a critical role in costimulation blockade-resistant allograft rejection. J. Clin. Invest. 1999;104:1715. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steiger JP, N W, Steurer W, Moscovitch-Lopatin M, Strom TB. IL-2 knockout recipient mice reject islet cell allograft. J. Immunol. 1995;155:489. [PubMed] [Google Scholar]
- 17.Li XC, Roy-Chaudhury P, Hancock WW, Manfro R, Zand MS, Li Y, Zheng XX, Nickerson PW, Steiger J, Malek TR, Strom TB. IL-2 and IL-4 double knockout mice reject islet allografts: a role for novel T cell growth factors in allograft rejection. J. Immunol. 1998;161:890. [PubMed] [Google Scholar]
- 18.Strehlau J, Pavlakis M, Lipman M, Shapiro M, Vasconcellos L, Harmon W, Strom TB. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc. Natl. Acad. Sci. USA. 1997;94:695. doi: 10.1073/pnas.94.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith XG, Bolton EM, Ruchatz H, Wei X-Q, Liew FY, Bradley JA. Selective blockade of IL-15 by soluble IL-15 receptor α chain enhances cardiac allograft survival. J. Immunol. 2000;165:3444. doi: 10.4049/jimmunol.165.6.3444. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T-cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000;191:771. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T-cells in vivo by IL-15. Immunity. 1998;8:591. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 23.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T-cells by opposing cytokines. Science. 2000;288:675. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 24.Bamford RN, Tagaya Y, Waldman TA. Interleukin 15: what it does and how it is controlled. Immunologist. 1997;5:52. [Google Scholar]
- 25.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, Dubose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-5 binding protein that is structurally related to the α chain of the IL-2 receptor. EMBO J. 1995;14:3654. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of β and γ chains of the IL-2 receptor by a novel cytokine IL-15. EMBO J. 1994;13:2822. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chae D-W, Nosaka Y, Strom TB, Maslinski W. Distribution of IL-15 receptor α-chains on human peripheral blood mononuclear cells and effect of immunosuppressive drugs on receptor expression. J. Immunol. 1996;157:2813. [PubMed] [Google Scholar]
- 28.Kim Y, Maslinski W, Zheng XX, Tesch GH, Kelley VR, Strom TB. Targeting the IL-15 receptor with an antagonist IL-15/Fc protein blocks delayed type hypersensitivity. J. Immunol. 1998;160:5742. [PMC free article] [PubMed] [Google Scholar]
- 29.Steurer W, Nickerson PW, Steele AW, Steiger J, Zheng XX, Strom TB. Ex vivo coating of islet cell allografts with murine CTLA4/Fc promotes graft tolerance. J. Immunol. 1995;155:1165. [PubMed] [Google Scholar]
- 30.O’Connell PJ, Pacheco-Silva A, Nickerson PW, Muggia RA, Bastos M, Kelley VR, Strom TB. Unmodified pancreatic islet allograft rejection results in the preferential expression of certain T cell activation transcripts. J. Immunol. 1993;150:1093. [PubMed] [Google Scholar]
- 31.Tivol EA, Boyd SD, McKeon S, Borriello F, Nickerson P, Strom TB, Sharpe AH. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J. Immunol. 1997;158:5091. [PubMed] [Google Scholar]
- 32.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry (CFSE) J. Immunol. Methods. 1994;171:131. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 33.Noorchashm H, Lieu YK, Rostami SY, Song HK, Greeley SAS, Bazel S, Barker CF, Naji A. A direct method for the calculation of alloreactive CD4+ T cell precursor frequency (CFSE) Transplantation. 1999;67:1281. doi: 10.1097/00007890-199905150-00015. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T-cells and induction of peripheral allograft tolerance. Nat. Med. 1999;5:1298. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 35.Bachmann MF, Waterhouse P, Speiser DE, McKall-Faienza K, Mak TW, Ohashi PS. Normal responsiveness of CTLA-4-deficient anti-viral cytotoxic T cells. J. Immunol. 1998;160:95. [PubMed] [Google Scholar]
- 36.Chambers CA, Sullivan TJ, Truong T, Allison JP. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells. Eur. J. Immunol. 1998;28:3137. doi: 10.1002/(SICI)1521-4141(199810)28:10<3137::AID-IMMU3137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 37.Hermann P, van-Kooten C, Gaillard C, Banchereau J, Blanchard D. CD40 ligand-positive CD8+ T cell clones allow B cell growth and differentiation. Eur. J. Immunol. 1995;25:2972. doi: 10.1002/eji.1830251039. [DOI] [PubMed] [Google Scholar]
- 38.Li XC, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek TR, Strom TB. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat. Med. 2001;7:114. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]