Abstract
Stereotyped behavior is commonly observed in neurodevelopmental disorders (e.g., autism, intellectual and developmental disability) and in a wide variety of animal species maintained in restricted environments. Stereotyped behavior can also be induced by psychostimulants, an effect potentiated by repeated intermittent exposure to these drugs (behavioral sensitization). The present study evaluated whether similar neuroadaptations in cortical–basal ganglia circuitry underlie the expression and development of spontaneous stereotypy and psychostimulant-induced sensitization. Sensitization was induced in deer mice with the degree of sensitization being dependent on housing condition but not age or environmental context. Environmentally enriched animals showed the least behavioral sensitization. Despite demonstrating robust sensitization in both older and younger animals, independent of context, behavioral sensitization was not associated with any alteration in the development or expression of spontaneous stereotypy in deer mice. Moreover, the frequency of baseline spontaneous stereotypy did not predict response to amphetamine challenge in either sensitized or non-sensitized mice. Thus, the present findings do not support the notion that sensitization-related neuroadaptations in cortical–basal ganglia circuitry are similar to those neuroadaptations that underlie spontaneous or environmentally linked stereotypy.
Keywords: Dopamine, Repetitive behavior, Environmental restriction, Neurodevelopmental disorders
1. Introduction
Stereotyped behavior typically refers tomotor responses of unknown function or purpose that are performed repetitively in a nearly identical manner such that the behavior often appears aberrant or abnormal (Mason and Rushen, 2006; Sprague and Newell, 2006). Stereotypies and related repetitive behaviors are diagnostic for autism spectrum disorders and represent a common component of other developmental, genetic, and neuropsychiatric disorders (Bodfish et al., 2000; Lewis and Bodfish, 1998). Although these restricted, repetitive behaviors have been linked to alterations in cortico–basal ganglia circuitry (Lewis et al., 2007), an understanding of specific mechanisms of action that give rise to the development and expression of stereotypies and related repetitive behaviors in clinical populations remains elusive.
Animal models of aberrant repetitive behavior in neurodevelopmental disorders generally fall into three classes: repetitive behavior associated with targeted insults to the CNS (e.g., gene deletion); repetitive behavior induced by pharmacological agents; and repetitive behavior associated with restricted environments and experience (see Lewis et al., 2007 for a review). Studies of drug-induced (e.g., amphetamine, cocaine) stereotyped behavior have made the largest contribution by far to our knowledge of the neurobiological basis of repetitive motor behaviors. These studies have highlighted the importance of cortical–basal ganglia circuitry and the neurotransmitter dopamine, particularly in models of psychostimulant-induced stereotypy.
Repeated, intermittent psychostimulant (e.g., amphetamine, cocaine) administration has been shown by a number of groups to be associated with the potentiated expression of locomotion and stereotypy (Robinson and Berridge, 2003; Vezina, 2004). This outcome reflects behavioral sensitization, a process by which repeated psychostimulant administration results in a progressive increase in the efficacy of a psychostimulant drug. Intermittent psychostimulant administration has also been shown to sensitize animals to the effects of environmental stressors. Reciprocally, repeated intermittent exposure to stressors can sensitize animals to the effects of psychostimulants. Thus, stressors and psychostimulants exhibit cross-sensitization (Antelman et al., 1980; Nikulina et al., 2004).
Sensitization may have particular relevance for understanding processes such as the escalation of drug use to drug craving and abuse (Robinson and Berridge, 2003), the transition from goal-directed to habitual responding (Graybiel, 2008), and the development of LDOPA- induced dyskinesias in Parkinson's disease (Cenci et al., 1999). Sensitization has been associated with long-lasting functional changes in cortico-striatal circuitry and can thus be viewed as a model of pathological neuroadaptation. For example, preferential activation of striatal striosomes or patches has been reported in sensitized animals at much lower doses of amphetamine than those required to induce this effect in non-sensitized animals (Vanderschuren et al., 2002). As mentioned previously, preferential activation of striosomes versus matrix has been shown to be highly correlated with the occurrence of drug-induced stereotyped behavior (Canales and Graybiel, 2000a). Repeated exposure to psychostimulants also induces alterations that progress from ventral to dorsal areas of the striatum which likely mediates augmented stereotypy. Such findings are certainly consistent with evidence for dopamine modulation of synaptic plasticity in cortico–striatal pathways.
Cabib (2006) has hypothesized that spontaneous stereotypy associated with environmental restriction may be mediated by the same neurobiological mechanisms that give rise to stress-induced or drug-induced sensitization. Such a hypothesis is linked to the notion that confined or restricted environments that give rise to stereotypy are inherently stressful and that stress alters dopamine neurotransmission and, like psychostimulants, can result in neuronal/behavioral sensitization. This sensitization hypothesis of stereotypy would thus predict that any stimulus or experience capable of activating mesoaccumbens dopamine should also be able to promote stereotypy.
The purpose of this study was to determine if exposure to repeated doses of amphetamine, known to induce neuronal/behavioral sensitization, would significantly alter the expression and development of spontaneous repetitive behavior exhibited by deer mice (Peromyscus maniculatus). Both male and female deer mice develop high rates of persistent, spontaneously emitted stereotypy consisting of repetitive vertical jumping and backward somersaulting when housed under standard laboratory conditions (Hadley et al., 2006; Powell et al., 2000; Presti et al., 2002). Support for the importance of environmental restriction in generating stereotypy in deer mice comes from our studies of the attenuation of such behavior by environmental enrichment (see Lewis et al., 2006 for review).
Four experiments were performed to assess whether neuronal/ behavioral sensitization might play a role in the expression or development of spontaneous or non-drug induced stereotypies in deer mice. These experiments included examining the effects of context-independent sensitization on adult animals reared in either conventional (Experiment 1) or enriched (Experiment 2) housing. In addition, younger animals reared in conventional housing were also used to examine either context-independent or context-dependent sensitization (Experiment 3 and 4 respectively).
2. General methods
2.1. Subjects
All deer mice (Peromyscus maniculatus) were obtained from the breeding colony maintained in our laboratory, and maintained on a 16:8-h light/dark cycle with lights off at 10:00 AM. Rodent chow and water were available ad libitum. The room was maintained at 20–25 °C and 50–70% humidity. Mice were weaned at 21 days of age. All procedures were performed in accordance with the guidelines set forth in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Florida Institutional Animal Care and Use Committee.
2.2. Stereotypy assessment
Rates of spontaneous stereotypy (hindlimb vertical jumping and backward somersaulting) were assessed using a modified automated photocell detection apparatus obtained from Columbus Instruments (Columbus, OH). The session consisted of the 8 h of the dark cycle. The testing protocol involved removing mice from their home cages and placing them singly in testing cages (22×25×28 cm) made of Plexiglas. The mice were left undisturbed for 2 to 3 h for habituation and recovery from the stress of handling prior to the beginning of the dark cycle. Food and water were provided. All sessions were digitally video-recorded for further identification of behavioral topographies and accuracy of the automated counters. Each animal received a stereotypy score that represented the average stereotypy frequency per hour.
2.2.1. Amphetamine administration and behavioral assessment
Injections of either saline or d-amphetamine (Sigma) were administered in the light cycle and were spaced approximately 10 h apart (9:30 AM and 7:30 PM) over a seven-day period. After a seven day drug-free period, all mice received a single injection of 2.5 mg/kg of d-amphetamine given during the light cycle at approximately 7:30 PM. The behavioral response to this pharmacological challenge was assessed immediately following drug administration. This was done by video-recording individual mice in the test chambers described previously for 1 h following the acute amphetamine challenge. The frequencies of rearing, locomotion, and stereotypies were recorded using either video, Ethovision (Noldus, Netherlands, for Experiment 4), and/or the automated photocell detection apparatus described previously. Seven days after the acute amphetamine challenge, all mice were assessed for stereotypy levels using the method described in a previous section.
2.3. Data analysis
For all four experiments, the effects of a seven-day regimen of amphetamine on the frequency of spontaneous stereotypies were assessed using a two-factor (dose and time) repeated measures analysis of variance (ANOVA). For all four experiments, group differences in behavioral responses recorded over 1 h following acute amphetamine challenge were analyzed by two-tailed t-tests. A one-factor analysis of covariance (ANCOVA) was also used in Experiment 2 to account for differences in ages between the two groups. In addition, the association between baseline stereotypy scores and the motor response to acute amphetamine challenge was analyzed by Pearson correlation. Effects were considered significant when p<0.05.
3. Specific methods and results
3.1. Experiment 1: context-independent sensitization in conventionally housed adult mice
3.1.1. Methods
Forty-six male mice (63–105 days of age) were group-caged (4–5 mice/cage) from weaning in standard rodent cages (48×27×15 cm) before and during the experimental procedures. All mice were tested for baseline levels of stereotypy as described in the previous section. These animals were then randomly assigned to one of three groups (n = 12) and were administered two doses of saline, 2.5, or 5.0 mg/kg of d-amphetamine subcutaneously for seven consecutive days.
3.1.2. Results
Mice in the two amphetamine pre-treatment groups exhibited higher levels of motor activity following drug challenge compared to saline pre-treatment controls (Table 1). Mice pre-treated with 2.5 mg/kg amphetamine exhibited significantly higher levels of rearing one-hour post-injection (t(22) = −2.09, p<0.05) and higher frequency of locomotor activity (mid-line crosses of the test chamber) for the first 30 min post-injection (t(22) = −2.11, p = 0.04) compared to mice treated with saline. For mice pre-treated with the 5.0 mg/kg amphetamine, significantly higher levels of locomotor activity were seen for both one-hour (t(22) = −3.08, p<0.01) and the first 30-min post injection (t(22) = −2.21, p = 0.04), but no significant increase in the frequency of rearing (p = 0.09 for 1 h, p = 0.17 for 30 min). No significant differences were seen between the 2.5 and 5.0 mg/kg pretreatment groups on any of the measures. Drug challenge was also not associated with any group differences in stereotyped vertical jumping (p = 0.85 for 1 h, p = 0.75 for 30 min).
Table 1.
| Rearing | Locomotion | |||
|---|---|---|---|---|
| 30 min | 60 min | 30 min | 60 min | |
| Saline | 139.9 (28.0) | 236.5 (51.8) | 67.6 (15.0) | 129.3 (28.1) |
| 2.5 mg/kg amphetamine | 219.7 (27.4) | 505.9 (117.9)* | 113.5 (14.4)* | 326.3 (105.9) |
| 5.0 mg/kg amphetamine | 205.8 (36.9) | 397.4 (74.3) | 128.8 (23.2)* | 382.2 (77.0)* |
Effects of saline or amphetamine (2.5 or 5.0 mg/kg) pre-treatment on the motor response to a subsequent acute challenge of amphetamine in adult, conventionally housed mice. Values expressed are group means with SEM in parentheses, n = 12 per group.
represents statistical significance at p<0.05 as compared to saline group.
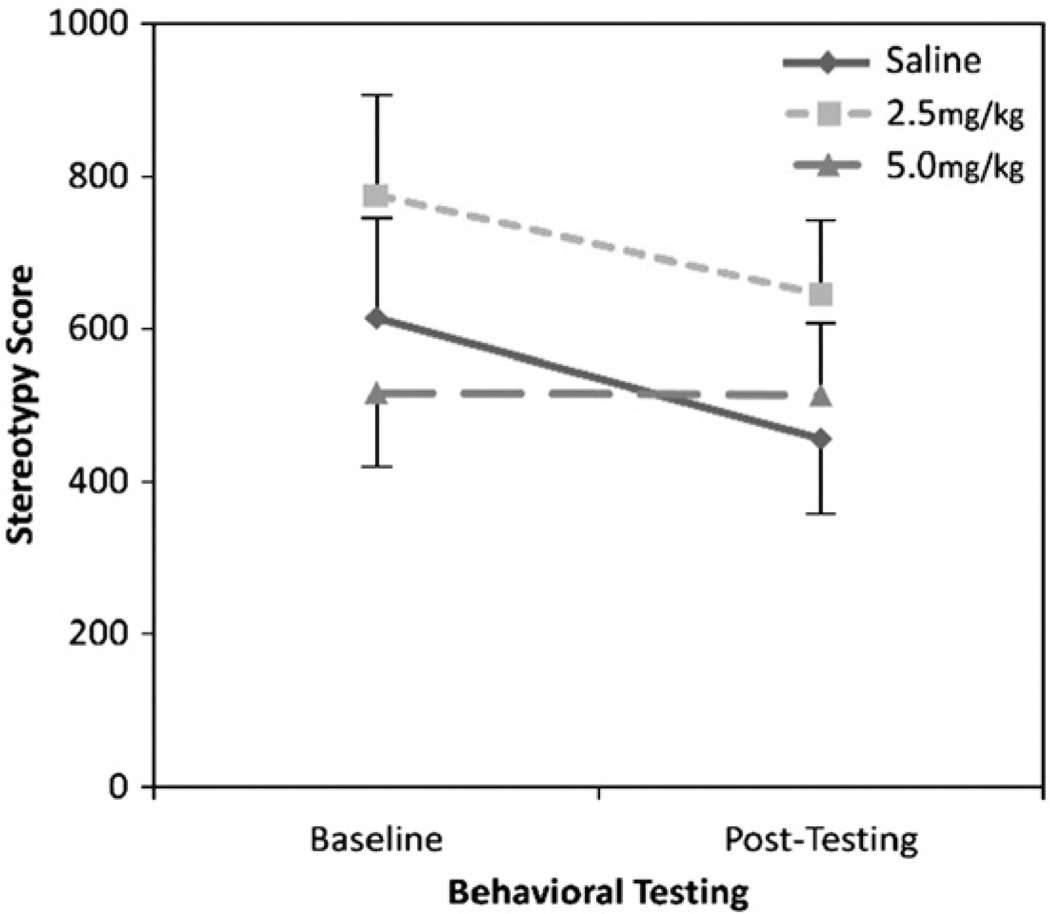
With regard to spontaneous stereotypy, a two-factor ANOVA indicated no main effect of treatment (saline, 2.5, or 5.0 mg/kg amphetamine) (F(2,33) = 1.19, p = 0.32) or time (baseline, post-testing) (F(1,33) = 1.67, p = 0.21) nor was there a treatment-by-time interaction (F(2,33) = 0.41, p = 0.67) (Fig. 1).
Fig. 1.
Rates of spontaneous stereotypy before and after amphetamine administration in adult, conventionally housed mice.
3.2. Experiment 2: context-independent sensitization in environmentally enriched adult mice
The relatively high levels of spontaneous stereotyped behavior typical of adult deer mice may well reflect long-term neuroadaptations, perhaps attenuating subsequent neuroplasticity in these animals. If this is the case, neuronal sensitization using a psychostimulant may not have an appreciable effect on spontaneous stereotypy. To test this potential outcome, Experiment 2 employed adult animals that had been reared in larger, more complex environments (environmental enrichment) which we have shown repeatedly attenuates the development of stereotypy (see Lewis et al., 2006).
3.2.1. Methods
Twenty-two deer mice (46–123 days of age; 11 female and 11 male) were group-caged (5–6 same sex mice/cage) in large dog kennels (122×81×89 cm) from weaning (PND21). This environmentally complex housing consisted of two extra levels of floors constructed of galvanized wire mesh and connected by ramps of the same material. Bedding, a running wheel, shelters, and various other objects were placed in each kennel. In addition to ad libitum food and water, 1 oz. of Cockatiel vita seed was scattered throughout the kennel three times each week to encourage foraging behavior. A running wheel remained undisturbed in the kennel, but other objects were removed and replaced with clean novel objects on a weekly basis. Mice were not handled or disturbed prior to the baseline stereotypy testing.
All mice were tested for baseline levels of stereotypy as described in the previous section. They were then caged in standard rodent cages (5–6 mice/cage) with one shelter to maintain some complexity in the environment. This housing change was necessitated in order to minimize the effect of handling at the time of drug administration. These animals were then randomly assigned to one of two groups (n = 11) and were administered two doses of either saline or 5.0 mg/ kg of d-amphetamine subcutaneously for seven consecutive days as in Experiment 1. The subsequent challenge and behavioral assessment procedures were the same as described in Experiment 1.
3.2.2. Results
Mice in the amphetamine pre-treatment group exhibited higher levels of rearing one-hour post-challenge compared to saline pretreatment controls (t(20) = −2.58, p = 0.02) (Table 2), although no significant differences in the frequency of locomotor activity were observed in response to drug challenge (p = 0.25 for 1 h, p = 0.46 for 30 min). A one-factor ANCOVA confirmed these group effects when differences in age were removed from the model. Drug challenge was also not associated with any increase in stereotyped vertical jumping (p = 0.32 for 1 h, p = 0.29 for 30 min).
Table 2.
| Rearing | Locomotion | |||
|---|---|---|---|---|
| 30 min | 60 min | 30 min | 60 min | |
| Saline | 107.1 (19.0) | 153.6 (32.5) | 66.3 (11.6) | 99.5 (24.6) |
| 5.0 mg/kg amphetamine | 171.4 (26.9) | 300.5 (46.8)* | 79.0 (12.4) | 143.7 (28.1) |
Effects of saline or amphetamine (5.0 mg/kg) pre-treatment on the motor response induced by a subsequent acute challenge of amphetamine in adult, environmentally enriched mice. Values expressed are group means with SEM in parentheses, n = 11 per group.
represents statistical significance at p<0.05 as compared to saline group.
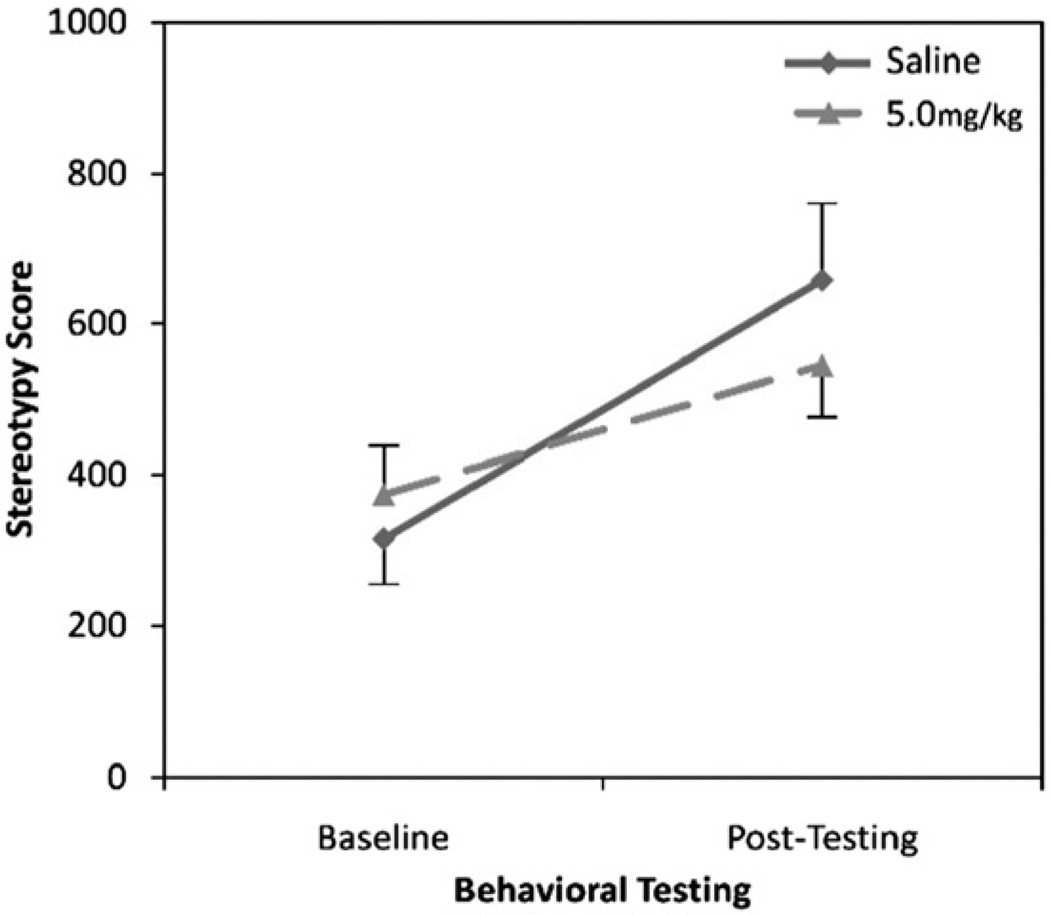
With regard to spontaneous stereotypy, a two-factor ANOVA indicated a main effect of time (baseline, post-testing; F(1,20) = 18.03, p<0.001), but no main effect of treatment (saline, 5.0 mg/kg amphetamine) (F(1,20) = 0.09, p = 0.76) and no treatment-by-time interaction (F(1,20) = 2.02, p = 0.17) (Fig. 2). Spontaneous stereotypy in both groups increased after amphetamine administration. This might be due to the change in housing condition from the large kennels to the smaller laboratory cages with shelters.
Fig. 2.
Rates of spontaneous stereotypy before and after amphetamine administration in adult, environmentally enriched mice.
3.3. Experiment 3: context-independent sensitization in conventionally housed young mice
This experiment used conventional housing but was conducted with younger animals at an age that we have shown predates the expression of asymptotic adult levels of stereotypy. Use of younger animals was hypothesized to increase the potential effect of intermittent amphetamine on neuroadaptations in cortico–basal ganglia circuitry.
3.3.1. Methods
Twenty-four male deer mice (30 days of age) were group-caged (2–3 mice/cage) in standard rodent cages before and during the experimental procedures. All mice were tested for baseline levels of stereotypy as described previously. These animals were then randomly assigned to one of two groups and were administered either saline (n = 12) or 2.5 mg/kg d-amphetamine (n = 11) using the identical protocol described in Experiment 1. In addition, on a subsequent occasion approximately 12–14 days later, all mice were assessed for a second time to determine the effects of amphetamine sensitization at an age (approximately PND60) when rates of spontaneous stereotypy in deer mice reach asymptote (unpublished observations).
3.3.2. Results
Mice in the amphetamine pre-treatment group exhibited higher levels of both rearing (t(21) = −2.71,p = 0.01) and locomotor activity (t(21) = −2.20, p = 0.04) for the first 30-min post-challenge compared to saline pre-treatment controls (Table 3). Drug challenge was also associated with an increase in stereotyped vertical jumping which was significant for the one-hour post-injection data (t(21) = −2.33, p = 0.03).
Table 3.
| Rearing | Locomotion | |||
|---|---|---|---|---|
| 30 min | 60 min | 30 min | 60 min | |
| Saline | 118.3 (24.5) | 272.0 (87.2) | 63.4 (13.0) | 170.3 (68.0) |
| 2.5 mg/kg amphetamine | 220.0 (28.6)* | 422.9 (97.1) | 101.3 (11.1)* | 230.3 (46.9) |
Effects of saline or amphetamine (2.5 mg/kg) pre-treatment on the motor response induced by a subsequent acute, context-independent challenge of amphetamine in younger, conventionally housed mice. Values expressed are group means with SEM in parentheses, n = 12/11.
represents statistical significance at p<0.05 as compared to saline group.
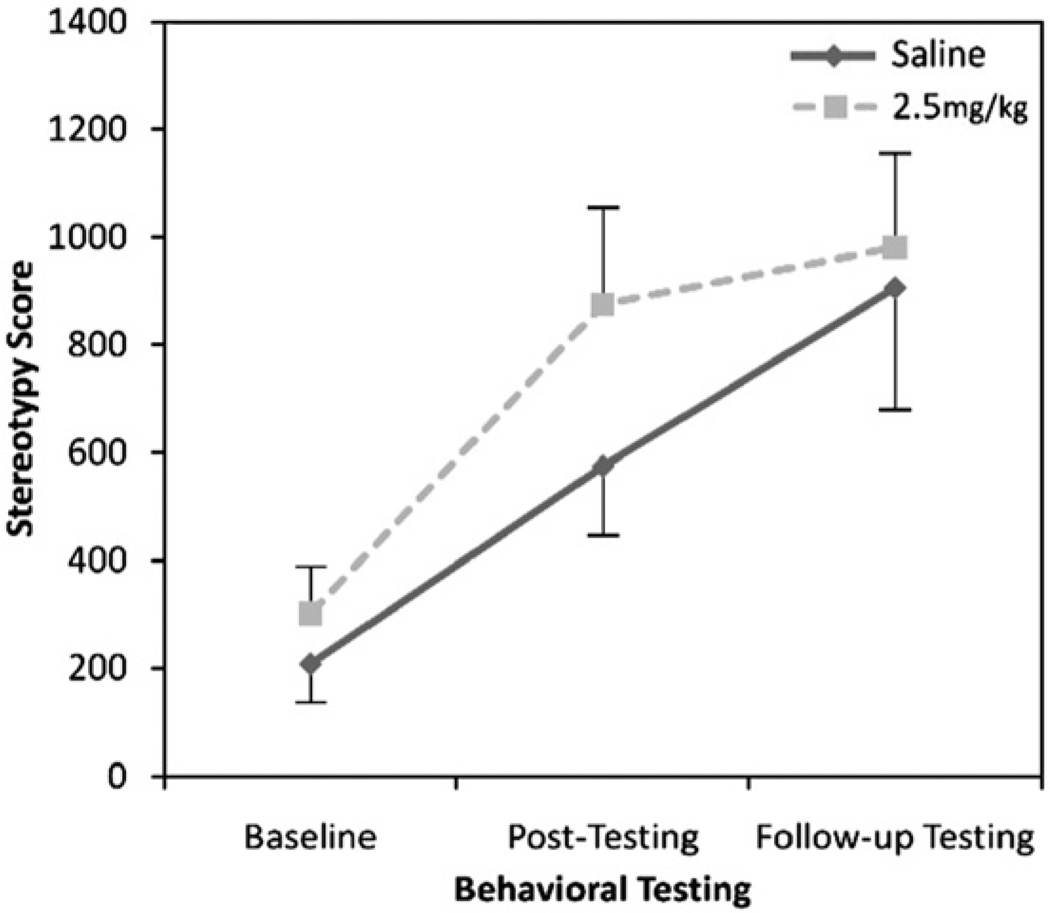
With regard to spontaneous stereotypy, a two-factor ANOVA indicated no main effect of treatment (saline, 2.5 mg/kg amphetamine) (F(1,21) = 1.14, p = 0.30) but a significant effect of time (baseline, post-testing, follow-up testing; F(2,42) = 19.66, p<0.001) (Fig. 3) as expected from our previous observations in younger mice. There was no treatment-by-time interaction (F(2,42) = 0.85, p = 0.44).
Fig. 3.
Rates of spontaneous stereotypy before and after context-independent amphetamine administration in younger, conventionally housed mice.
3.4. Experiment 4: context-dependent sensitization in young mice
The behavioral expression of sensitization has been shown to be influenced by the environmental context in which it has been established. Thus, animals repeatedly injected with the drug in a consistent and distinct environment exhibit an increased behavioral reactivity to the drug compared to animals receiving the same drug pre-treatment outside of the test apparatus. Thus, Experiment 4 used younger, conventionally housed animals but employed a context-dependent sensitization protocol.
3.4.1. Methods
Twenty-one male deer mice (30 days of age) were group-housed in standard rodent cages before and during the experimental procedures. All mice were tested for baseline levels of stereotypy as described previously. Mice were randomly assigned to either the saline (n = 11) or 2.5 mg/kg of d-amphetamine (n = 10) group. The same protocol for injections was followed as described in Experiment 3 (twice per day for 7 days) with one important difference. After each injection, the mice were immediately placed singly in testing cages for 1 h rather than being returned to their home cage.
Following a seven day drug free period, all mice received an acute challenge of 2.5 mg/kg d-amphetamine in the same testing environment as previously used for the prior injections of amphetamine or saline. Behavioral responses to acute amphetamine were recorded for 1 h and included the frequency of rearing, locomotion (total distance travelled, cm), and stereotypy. Data on total distance travelled was acquired using the EthoVision system (Noldus, Wageningen, Netherlands) which is a fully automated video tracking system. Rearing and stereotypy were assessed using methods as described in the previous experiments. Spontaneous stereotypy was then assessed again a week following acute amphetamine challenge when the animals were approximately PND60.
3.4.2. Results
Amphetamine pre-treated mice exhibited significantly greater locomotor activity (distance travelled) for one-hour post-injection (t(19) = −2.48,p = 0.02) and for the first 30minpost-injection (t(19) = −2.26, p = 0.04), but no significant increase was found in the frequency of rearing (p = 0.11 for 1 h, p = 0.17 for 30 min) compared to saline pretreated mice (Table 4). Drug challenge was not associated with an increase in stereotyped vertical jumping (p = 0.08 for 1 h, p = 0.07 for 30 min).
Table 4.
| Rearing | Locomotion | |||
|---|---|---|---|---|
| 30 min | 60 min | 30 min | 60 min | |
| Saline | 60.5 (16.3) | 64.7 (18.3) | 2399.2 (431.4) | 3471.0 (562.1) |
| 2.5 mg/kg amphetamine | 107.5 (29.3) | 150.2 (49.5) | 5180.6 (1206.2)* | 7917.8 (1781.8)* |
Effects of saline or amphetamine (2.5 mg/kg) pre-treatment on the motor response induced by a subsequent acute, context-dependent challenge of amphetamine in younger, conventionally housed mice. Locomotion is expressed as total distance traveled (cm). Values expressed are group means with SEM in parentheses, n = 11/10.
represents statistical significance at p<0.05 as compared to saline group.
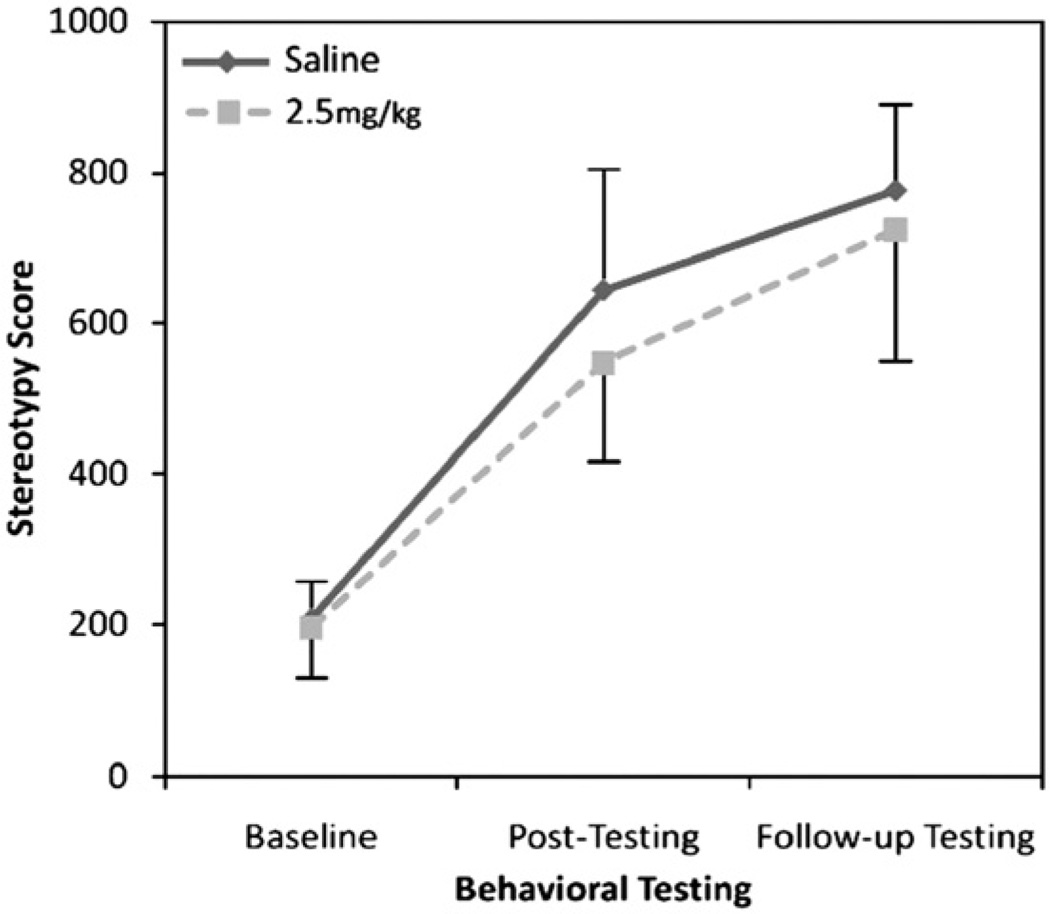
With regard to spontaneous stereotypy, no main effect of treatment (saline, 2.5 mg/kg amphetamine) (F(1,19) = 0.18, p = 0.67) was observed but there was a significant effect of time (baseline, post-testing, follow-up testing; F(2,38) = 23.9, p<0.001) (Fig. 4). There was no treatment-by-time interaction (F(2,38) = 0.07, p = 0.93).
Fig. 4.
Rates of spontaneous stereotypy before and after context-dependent amphetamine administration in younger, conventionally housed mice.
3.5. Spontaneous stereotypy and response to amphetamine challenge
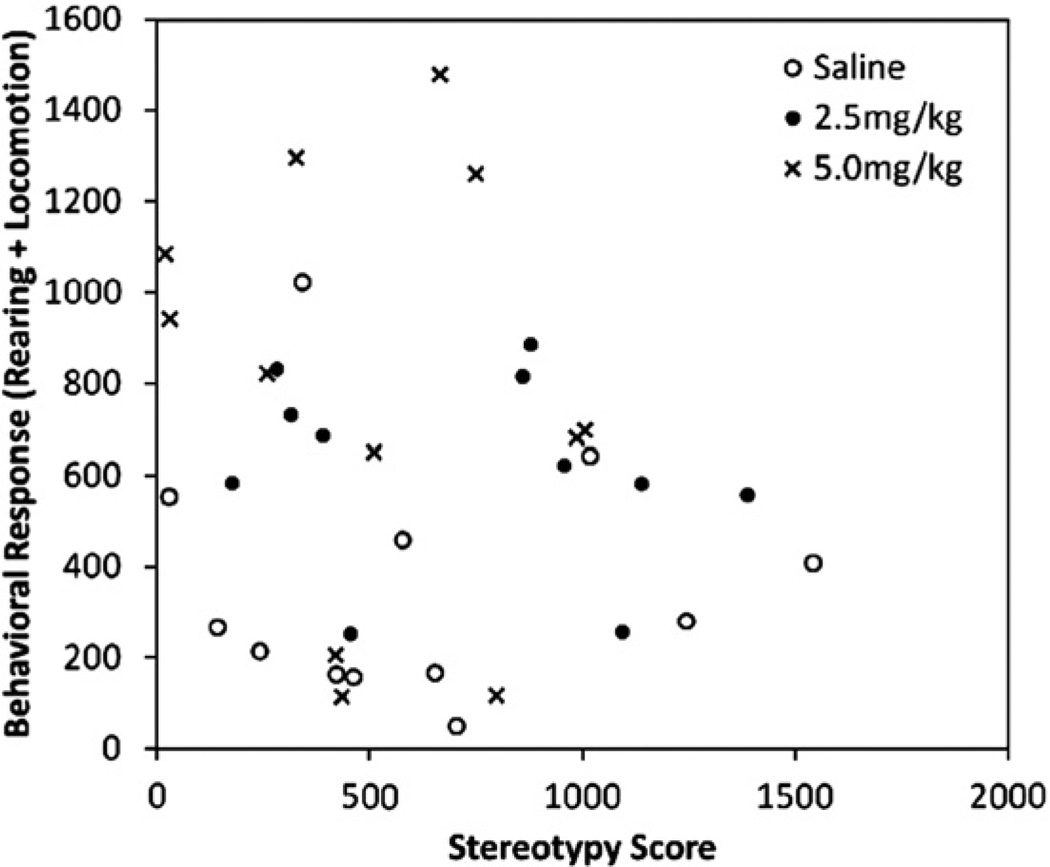
We also examined the association between baseline levels of stereotypy and response to the acute challenge of amphetamine. No systematic relationship was found between baseline levels of stereotypy and drug challenge induced motor activity (rearing plus locomotion) in adult, conventionally housed mice (r = 0.15, p = 0.38) (Experiment 1) (Fig. 5). Similarly, no association was found in adult, environmentally enriched mice (r = −0.24, p = 0.28) (Experiment 2) and younger mice (r = −0.13, p = 0.55; r<0.01, p = 0.98) (Experiments 3 and 4 respectively). No systematic association was seen between baseline levels of stereotypy and response to acute amphetamine in amphetamine pre-treated mice from all four experiments. In addition, this was also the case when only saline pre-treated control mice were used.
Fig. 5.
The association between baseline spontaneous stereotypy and the behavioral (rearing plus locomotion) response to an acute amphetamine challenge in adult conventionally housed mice (Experiment 1).
4. Discussion
In the present study, we sought to determine if we could behaviorally sensitize deer mice using amphetamine and whether such sensitization would significantly impact the expression or development of spontaneous (non-drug related) stereotypy in these mice. Experiment 1 employed context-independent sensitization in adult mice. Amphetamine pre-treated mice (both doses) showed significant increases in motor activity relative to saline controls following drug challenge. Despite behavioral evidence for sensitization, amphetamine pre-treated mice were not different from saline controls in their expression of spontaneous stereotypy. Environmentally enriched adult animals exhibited less evidence of sensitization, with amphetamine pre-treatment resulting in a significant increase in only rearing at 1 h following drug challenge. As in Experiment 1, no group differences in spontaneous stereotypy were found. The last two experiments with younger mice demonstrated significant behavioral sensitization, although significant context-independent sensitization (Experiment 3) was seen only at the 30 min, whereas context-dependent sensitization (Experiment 4) was seen at the 60 min time point following challenge. In any case, sensitization did not result in any difference in the expression and development of spontaneous stereotypy.
The present results show that repeated, intermittent psychostimulant exposure can successfully induce behavioral sensitization in the genus Peromyscus. In addition, significant behavioral sensitization can be induced in deer mice using either a context-independent or a context-dependent protocol. This finding is somewhat at odds with previous reports demonstrating that repeated psychostimulant administration in the testing context produces a much more robust locomotor sensitization (Anagnostaras et al., 2002; Anagnostaras and Robinson,1996; Badiani et al.,1995; Cabib,1993; Mattson et al., 2008). Behavioral sensitization was least apparent under context-independent conditions in adult environmentally enriched deer mice. The relative absence of amphetamine-induced sensitization in environmentally enriched mice is consistent with a previous report showing that enrichment had a neuroprotective effect with regard to amphetamine-induced sensitization (Bardo et al., 1995). In addition, context-dependent sensitization appeared to have a more robust effect than context-independent sensitization in younger mice, given the large increase in locomotor activity observed at 30 and 60 min.
Locomotion and rearing were used as indices of sensitized motor activity. Interestingly, vertical jumping following drug-challenge was typically not significantly increased except in younger amphetamine pre-treated mice tested in context-independent conditions (Experiment 3). In our previous work, neither systemically nor intrastriatally administered apomorphine increased spontaneous cage stereotypies in deer mice acutely, although other repetitive behaviors (e.g., stereotyped sniffing) were observed (Presti et al., 2002, 2004). We have similar unpublished observations with systemic amphetamine in drug-naive deer mice. The present results indicate that a psychostimulant can increase environmentally related stereotypies in drug-sensitized animals.
Despite clear evidence of behavioral sensitization in Experiments 1, 3, and 4, no differences were found in levels of spontaneous stereotypy tested one-week post challenge in mice exposed to amphetamine pre-treatment when compared to controls. The only systematic differences in spontaneous stereotypy were observed in younger mice (Experiments 3 and 4) and these differences were associated with developmental age.
Neuronal sensitization has been advanced as a mechanism responsible, at least in part, for the development of environmentally related stereotypies (Cabib, 2006; Dantzer, 1986). Little evidence has been available to evaluate such an assertion, however. Cabib and Bonaventura (1997) examined the ability of food restriction to induce stereotypy and to induce sensitization to psychostimulants in two inbred mouse strains. Food restriction was found to induce cage stereotypies in drug-naive mice as well as behavioral sensitization to amphetamine. These effects were observed in DBA but not C57BL/6 mice, however. These investigators concluded that the parallel strain-dependent susceptibility to cage stereotypy and behavioral sensitization provides evidence for a common neurobiological mechanism. Although these findings provide some indirect support for a common mechanism, few other data are available. The present findings, as far as we are aware, are the first effort to assess directly the effects of neuronal/behavioral sensitization on non-drug induced stereotypy. As we have indicated, sensitized animals did not express spontaneous stereotypies at a higher rate than non-sensitized controls. Moreover, if spontaneous stereotypy is a consequence of neuronal/behavioral sensitization, then mice exhibiting high levels of such repetitive behavior should exhibit a potentiated response to drug challenge. The level of baseline stereotypy, however, did not predict response to acute amphetamine in either pre-treatment group. We should hasten to add, though, that a number of theorists have advanced a preeminent role for stress, and stress-induced sensitization, in the genesis of stereotypy. Although there is evidence to support the cross-sensitization of stress and psychostimulants (Conversi et al., 2008), we only employed stimulant-induced sensitization. Future experiments should employ a stress-induced sensitization model to examine further the relationship of stereotypy and sensitization.
Neuronal/behavioral sensitization is associated with long-lasting changes in brain function in striatum and nucleus accumbens as well as prefrontal cortex (Ehrlich et al., 2002; Mattson et al., 2008; Robinson and Berridge, 2003; Vanderschuren and Kalivas, 2000). For example, behavioral sensitization results in the potentiation of dopamine efflux in the nucleus accumbens by a number of different drugs (Robinson and Berridge, 2000). In addition, sensitization results in D1 dopamine receptor supersensitivity in the ventral striatum, altered gene expression in the caudate nucleus (Canales and Graybiel, 2000b) and alterations in striatal glutamate signaling (Vanderschuren and Kalivas, 2000). Sensitization is also associated with morphological changes including persistent alterations in dendritic length and branching in the cortex and the striatum (Robinson and Berridge, 2000). The potentiated stereotyped behavior that is observed after chronic, intermittent amphetamine or cocaine is strongly predicted, across several species, by a preferential activation of striosomal striatal cells (Canales and Graybiel, 2000a). These and many other changes that have been documented reflect a process of pathological neuroadaptation in cortical-basal ganglia circuitry.
Our previous studies (see Lewis et al., 2007 for a review) have highlighted the importance of this circuitry in the development and expression of spontaneous stereotypy in deer mice. Thus, one important question is whether sensitization-related neuroadaptations in cortical-basal ganglia circuitry are similar to those neuroadaptations that underlie spontaneous or environmentally linked stereotypy. Our results lead to the conclusion that stereotypy associated with environmental restriction and behavioral sensitization do not appear to share common mechanisms. As indicated earlier, drugs that induce stereotypies do not always enhance an animal's spontaneous cage-induced stereotypies and often elicit stereotypies that are quite different in form. Moreover, sensitized animals did not display greater levels of spontaneous stereotypy. In addition, greater levels of stereotypy did not predict an augmented (sensitized) response to a psychostimulant in drug-naive animals. Taken together, it can be concluded that the hypothesis that spontaneous stereotyped behavior, at least in deer mice, reflects neuronal/behavioral sensitization is not supported by the current findings. Whether sensitization-related neural mechanisms may play a role in aberrant repetitive behavior observed in neurodevelopmental disorder like autism remains an open question.
References
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110(6):1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Schallert T, Robinson TE. Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology. 2002;26(6):703–715. doi: 10.1016/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207(4428):329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Badiani A, Anagnostaras SG, Robinson TE. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology (Berl) 1995;117(4):443–452. doi: 10.1007/BF02246217. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bowling S, Rowlett J, Manderscheid P, Buxton S, Dwoskin L. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Cabib S. Strain-dependent behavioral sensitization to amphetamine: role of environmental influences. Behav Pharmacol. 1993;4(4):367–374. [PubMed] [Google Scholar]
- Cabib S. The neurobiology of stereotypy II: The role of stress. In: Mason G, Rushen J, editors. Stereotypic animal behaviour. 2nd ed. Oxford: CAB International; 2006. [Google Scholar]
- Cabib S, Bonaventura N. Paralell strain-dependent susceptibility to environmentally-induced stereotypies and stress-induced behavioural sensitization in mice. Physiol Behav. 1997;61:499–506. doi: 10.1016/s0031-9384(96)00463-5. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000a;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. Patterns of gene expression and behavior induced by chronic dopamine treatments. Ann Neurol. 2000b;47:S53–S59. [PubMed] [Google Scholar]
- Cenci MA, Tranberg A, Andersson M, Hilbertson A. Changes in the regional and compartmental distribution of FosB- and JunB-like immunoreactivity induced in the dopamine denervated rat striatum by acute or chronic L-DOPA treatment. Neuroscience. 1999;94:515–527. doi: 10.1016/s0306-4522(99)00294-8. [DOI] [PubMed] [Google Scholar]
- Conversi D, Bonito-Oliva A, Orsini C, Colelli V, Cabib S. DeltaFosB accumulation in ventro-medial caudate underlies the induction but not the expression of behavioural sensitization by both repeated amphetamine and stress. Eur J Neurosci. 2008;27:191–201. doi: 10.1111/j.1460-9568.2007.06003.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Behavioral, physiological, and functional aspects of stereotyped behavior: a review and re-interpretation. J Anim Sci. 1986;62:1776–1786. doi: 10.2527/jas1986.6261776x. [DOI] [PubMed] [Google Scholar]
- Ehrlich ME, Sommer J, Canas E, Unterwald EM. Periadolescent mice show enhanced DeltaFosB upregulation in response to cocaine and amphetamine. J Neurosci. 2002;22(21):9155–9159. doi: 10.1523/JNEUROSCI.22-21-09155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Hadley C, Hadley B, Ephraim S, Yang M, Lewis MH. Spontaneous stereotypy and environmental enrichment in deer mice (Peromyscus maniculatus): reversibility of experience. Appl Anim Behav Sci. 2006;97:312–322. [Google Scholar]
- Lewis MH, Bodfish JW. Repetitive behavior in autism. Ment Retard Dev Disabil Res Rev. 1998;4:80–89. [Google Scholar]
- Lewis MH, Presti MF, Lewis JB, Turner CA. The neurobiology of stereotypy I: Environmental complexity. In: Mason G, Rushen J, editors. Stereotypic animal behaviour. 2nd ed. Oxford: CAB International; 2006. [Google Scholar]
- Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason G, Rushen J, editors. Stereotypic animal behaviour. 2nd ed. Oxford: CAB International; 2006. [Google Scholar]
- Mattson BJ, Koya E, Simmons DE, Mitchell TB, Berkow A, Crombag HS, et al. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur J Neurosci. 2008;27(1):202–212. doi: 10.1111/j.1460-9568.2007.05984.x. [DOI] [PubMed] [Google Scholar]
- Nikulina E, Covington H, Ganschow L, Hammer R, Miczek K. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Powell SB, Newman HA, McDonald TA, Bugenhagen P, Lewis MH. Development of spontaneous stereotyped behavior in deer mice: effects of early and late exposure to a more complex environment. Dev Psychobiol. 2000;37(2):100–108. [PubMed] [Google Scholar]
- Presti MF, Powell SB, Lewis MH. Dissociation between spontaneously emitted and apomorphine-induced stereotypy in Peromyscus maniculatus bairdii. Physiol Behav. 2002;75(3):347–353. doi: 10.1016/s0031-9384(02)00641-8. [DOI] [PubMed] [Google Scholar]
- Presti MF, Gibney BC, Lewis MH. Effects of intrastriatal administration of selective dopaminergic ligands on spontaneous stereotypy in mice. Physiol Behav. 2004;80(4):433–439. doi: 10.1016/j.physbeh.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addition: an incentive-sensitization view. Addition. 2000;95:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Sprague RL, Newell KM, editors. Stereotypies: brain and behavior relationships. Washington, DC: American Psychological Association; 2006. [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of bhavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151(2–3):99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L, Schoffelmeer A, Van Leeuwen S, Hof L, Jonker A, Voorn P. Compartment specific changes in striatal neuronal activity during expression of amphetamine sensitization are the result of drug hypersensitivity. Eur J Neurosci. 2002;16:2462–2468. doi: 10.1046/j.1460-9568.2002.02308.x. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27(8):827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]