Abstract
Abr deactivates Rac, a master molecular switch that positively regulates many immune cell functions, by converting it to its GDP-bound conformation. Here we report that in the absence of Abr function, cockroach allergen (CRA)-immunized mice experienced a fatal asthma attack when challenged with CRA. The asthma in abr−/− mice was characterized by increased pulmonary mucus production, elevated serum IgE and leukocyte airway infiltration. Decreased pulmonary compliance was further documented by increased airway resistance upon methacholine challenge. Peribronchial and bronchio-alveolar lavage eosinophils, key cells associated with allergic asthma, were increased in abr−/− mice, but adoptive transfer of this cell type from immunized mice to naïve controls followed by CRA challenge showed that eosinophils are not primarily responsible for differences in airway resistance between controls and abr null mutants. CD4+ T cell numbers in the airways of CRA-challenged abr−/− mice were also significantly increased compared to controls, as were the Th2 T cell-secreted cytokines IL-4 and IL-5 in total lung. Interestingly, when control and abr−/− CD4+ T cells from CRA-immunized mice were transferred to wild type animals, airway resistance upon challenge with CRA was significantly higher in mice transplanted with T cells lacking Abr function. CD4+ T cells from CRA-immunized and challenged abr−/− mice contained elevated levels of activated Rac-GTP, compared to wild type controls. Functionally, abr−/− CD4+ T cells from CRA-exposed mice showed significantly enhanced chemotaxis towards CCL21. These results identify Abr-regulated CD4+ T cell migration as an important component of severe cockroach allergen evoked allergic asthma in mice.
Keywords: knockout, null mutant mouse, active Bcr-related, GTPase activating protein, GAP, CD4+ T cells, eosinophil, adoptive transfer, airway hyper-responsiveness, chemotaxis
Introduction
The human ABR3 (active BCR-related) gene on chromosome 17p13.3 encodes a 98 kDa protein that consists of four modular domains (1). Of these, the function of the domain with homology to GTPase activating proteins (GAPs) for the Rho family of GTPases has been investigated in most detail (2, 3). Members of the Rho family including Rac (Ras-related C3 botulinum toxin substrate) act as molecular switches, cycling from an active GTP-bound state to an inactive GDP-bound state. The active and inactive states have different conformations, with the GTP-bound active form of Rac responsible for the interaction with and activation of downstream effectors. For example, binding of RacGTP to a major effector, the serine-threonine kinase Pak1 (p21 protein {Cdc42/Rac}-activated kinase 1), induces a conformational change in Pak1 that activates its kinase activity (4). RacGTP activates glycogen phosphorylase and stimulates T cell proliferation by a similar mechanism (5).
Rac activity is crucial for the positive activation of numerous processes involving reorganization of the actin cytoskeleton in immune cells (diapedesis, chemotaxis, phagocytosis) and for activation of the NADPH oxidase expressed by myeloid and other cells (6). There are only 3 Rac proteins that perform these numerous functions. The specificity of these small G-proteins is accomplished through tightly controlled regulation of their activation and deactivation cycle. A family of proteins called G-nucleotide exchange factors (GEFs) is responsible for activation, and over 69 human Rho family GEFs are known to exist. Deactivation is catalyzed by the GAPs, of which also approximately 70 have been identified and which include Abr (7, 8). The different GAPs and GEFs distinguish each other in tissue or cell type specific expression, subcellular location and upstream activation. A major field of research is, therefore, to determine which GAPs and GEFs regulate which functions of Rac in which cells, tissues and pathologies (9).
Studies by ourselves and others using purified proteins established that Abr has a specificity for deactivating Rac proteins, although it also was active on Cdc42 (cell cycle control protein 42 homolog) in vitro (3, 10). To definitively determine the function of Abr in vivo, we generated mice lacking abr. Experiments with these mice as well as transfection of Abr into cultured cells and assays for deactivation of Rac or Cdc42 were all consistent with this GAP only catalyzing the deactivation of Rac and not of other Rho family members in vivo (11). Mice lacking Abr are phenotypically normal. However, when challenged in experimental models of sepsis (12) and of pulmonary hypertension (13), consequences of the lack of a functional Abr protein became evident in the form of significantly exacerbated pathology.
Asthma is a serious health problem that appears to be increasing in incidence. Acute asthma attacks are responsible for many emergency room visits, and can cause death. In 2009, 24.6 million patients with asthma were reported in the USA alone, of which around 8 million children (14). Studies indicate that exposure to cockroach allergen (CRA) plays an important role in asthma (15–17). CRA consists of proteins derived from cockroach saliva, feces, exoskeleton and dead bodies. Due to the ubiquitous existence of cockroaches and their widespread household infestation in urban dwellings, CRA poses a serious risk for allergic asthma (18).
A murine model for human atopic asthma has been developed on the basis of sensitization and exposure to CRA (19, 20). Since CRA is associated with human asthma, the CRA-induced asthma model in mice is clinically relevant (19–21). Here, using this model in mice lacking Abr, we demonstrate that Abr is responsible for keeping severe pathological manifestations of asthma in check through regulation of the influx of CD4+ T cells.
Materials and Methods
Animals
abr−/− mice were generated previously (22) and were maintained on an FVBJ inbred background. All animal studies were approved by the Institutional Animal Care and Use Committee of the Saban Research Institute, Children’s Hospital of Los Angeles. For each set of experiments, only age- and gender-matched mice were compared.
CRA sensitization and challenge
Mice (7–9 wk old) were sensitized and challenged with CRA according to guidelines established by previous studies (19, 23, 24). CRA was from Holister Steir Laboratories, WA. For the experiment in Fig. 1, age-matched control (compound heterozygotes, abr+/−, bcr+/−, phenotypically wild type (wt, hereafter referred to as wt) and abr−/− mice were immunized by intraperitoneal (i.p., 50 µl) and subcutaneous (s.c., 50 µl) injections of a 1:1 dilution of CRA (120,000 protein nitrogen units (PNU)/ml) in Freund’s incomplete adjuvant (Sigma) followed by intranasal (i.n., 20 µl) instillation of a 1:2 dilution of CRA in PBS on days 7, 14, 20 and 22. For all other experiments, mice were immunized i.p. and s.c. with 50 µl of emulsion, consisting of a mixture of CRA (20,000 PNU/ml) with incomplete Freund’s adjuvant at a ratio of 1:1. On day 14, mice were anesthetized i.p. with a cocktail of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight), and given an intranasal (i.n.) instillation of 20 µl of CRA (10,000 PNU/ml) to localize the systemic response to the airways of the lung. After 6 additional days (on day 20 from initial sensitization), mice were challenged with an intratracheal administration of 40 µl CRA (10,000 PNU/ml) followed by a second administration 48 h later (day 22). All measurements were performed (or samples were taken) 24 h following the second challenge (day 23).
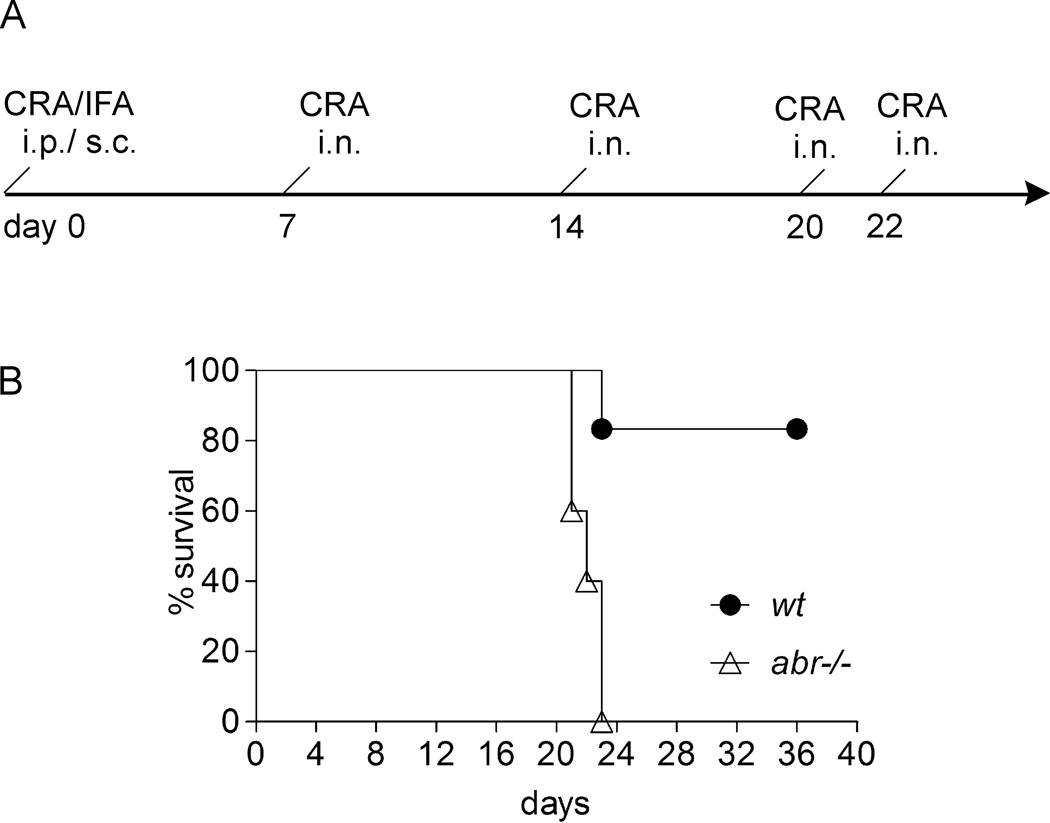
FIGURE 1. Increased asthma-associated mortality in CRA-challenged abr−/− mice.
(A) Schematic CRA challenge protocol and (B) survival in wt (n=6) and abr−/− (n=5) mice upon CRA challenges. Kaplan-Meier survival curves were analyzed by the log-rank test (p<0.01). IFA, incomplete Freund’s adjuvant; i.p., intraperitoneal; s.c., subcutaneous; i.n., intranasal. Data representative of two independently performed experiments.
Measurement of airway hyper-responsiveness
Airway hyper-responsiveness (AHR) was measured using a mouse plethysmograph (FlexiVent, SCIREQ USA INC., AZ). Briefly, mice were weighed and anesthetized with sodium pentobarbital (90 mg/kg body weight) by intraperitoneal injection to achieve deep anesthesia. Trachea were then exposed and intubated with an 18-gauge metal tube, through which mice were connected to the pre-calibrated plethysmograph and quasi-sinusoidally ventilated with a computer-controlled ventilator with a tidal volume of 10 ml/kg at a frequency of 150 breaths/minute to achieve a mean lung volume close to that of normal breathing. Once the anesthesia and ventilation were stabilized (no spontaneous breathing, because it interferes with the measurement by the FlexiVent system), a “snapshot” perturbation, which is a sinusoidal wave of inspiration and expiration controlled by the ventilator, was applied to measure airway resistance by the computer software that comes with the FlexiVent system. After the baseline levels of airway resistance were measured, mice were challenged with methacholine via an in-line nebulizer. The optimal methacholine dose (10 mg/ml, via the FlexiVent nebulizer) for the airway hyper-responsiveness in our asthma model was determined by a dose-response curve (1 to 160 mg/ml) and used throughout our experiments. After the methacholine challenge via the nebulizer, the airway resistance was measured by the FlexiVent software following the “snapshot” perturbation. Airway resistance levels (following methacholine challenges), as indices for airway hyper-activity, were plotted and compared among different groups of mice to indicate the severity of airway hyper-responsiveness in our asthma model.
Cytokine and serum IgE measurements
Blood was collected through retro-orbital bleeding and centrifuged at 2500 g for 10 min. Serum was separated and stored at −80°C until analysis. Total serum IgE levels were measured using an ELISA kit (BioLegend, San Diego, CA) according to the manufacturer’s instructions. Lungs were collected on day 23 following the sensitization protocol. Samples were homogenized and an aliquot of lung homogenates was centrifuged at 2000 rpm for 5 min. Supernatants were collected for IL-4 and IL-5 level measurement using mouse ELISA kits (BioLegend) according to the manufacturer’s protocol. CCL21 levels in total lung and BALF IL-4 and IL-5 levels were determined using murine ELISA kits for 6Ckine/CCL21 (Sigma-Aldrich, St Louis, MO) and for IL-4 and IL-5 (BioLegend).
Bronchoalveolar lavage cells
To prepare bronchoalveolar lavage (BAL), tracheas were exposed and cannulated with an 18-gauge angiocath. Lungs were lavaged five times with 0.8 ml of cold sterile PBS containing 25 nM EDTA. For studies of cytokines, the first wash was collected separately, clarified by centrifugation and the supernatant was stored at −80°C. Total BAL cells were pooled from the five washes. Total cell counts were recorded. BAL cells were analyzed by flow cytometry or cytospin. For cytospins, 80–100,000 cells were dispersed using a cytospin centrifuge (Shandon Scientific) and differentially stained with Kwik-Diff stain (Fisher Scientific, similar to Wright-Giemsa). The cell types (alveolar macrophages, monocytes, lymphocytes, neutrophils, and eosinophils) were differentially counted and expressed as a percentage of the total leukocytes. Eosinophils were identified morphologically, based on their distinct nuclei and cytoplasmic granules. To confirm alveolar macrophage quantification, we also used flow cytometry analysis. BAL cells were stained with FITC-Ly6G, PerCP-CD45 and APC-CD11b. Due to the autofluorescence (into the FITC channel) of alveolar macrophages, they form a unique population Ly6GmediumCD11bmedium, which distinguishes them from neutrophils (Ly6GhiCD11bhi) and from monocytes/inflammatory macrophages (Ly6GlowCD11bhi).
Flow cytometry and intracellular staining
Cells were obtained from blood, spleen and lung. To prepare lung single cell-suspensions, tissues were cut into small pieces (1–2 mm) and digested with collagenase B (2 mg/ml, Roche) and DNase I (0.5 mg/ml, Roche) at 37°C for 40 min. The suspension was ground through a 70 µm cell strainer (Fisher Scientific) using a syringe plunger to disrupt residual tissue. The cells were pelleted and resuspended in Red Blood Cell Lysis Buffer (PharmLyse, BD). Cells were collected after a PBS wash. Cells were stained with fluorochrome-labeled monoclonal antibodies. Data were acquired on an Accuri flow cytometer and analyzed using Accuri software (BD Bioscience). FITC-Ly6G, FITC-CD4, PerCP-CD45, APC-CD11b, PE-IL-4, and PE-CD8 were from BioLegend. For intracellular staining of IL-4, spleen cells from naive mice were isolated and cultured in complete DMEM medium at 2×106 cells/ml/well in a 24-well plate precoated with anti-CD3 and anti-CD28 (2 µg/ml) for 48 h. For Th2 polarization, cells were primed in the presence of IL-4 (10 µg/ml), with anti-IFNγ (10 µg/ml), and anti-IL-12 (10 µg/ml) blocking antibodies added. For Th0 cells, no cytokines or antibodies were added. Cells were washed and analyzed after 2–3 days of priming. Aliquots of 106 cells/ml were incubated for 4–6 h with brefeldin A (1 µg) (BD Biosciences, GolgiPlug), PMA (50 ng/ml) and ionomycin (0.5 µg/ml) for analysis of cytokine production. Cells were pelleted, and resuspended in PBS with 2% FCS, stained with surface marker antibodies, fixed and permeabilized with BD Cytofix/Cytoperm and stained intracellularly with antibodies to IL-4.
Measurement of peroxidase
Cell-free BAL supernatants were collected and frozen at −80°C. A total of 50 µl of each sample was mixed with 100 µl of substrate (0.2 mg/ml o-phenylenediamine in Tris, pH 8, containing 0.1% Triton and 0.02% H2O2) in 96-well plates. The reaction was allowed to progress for 30 min before quenching with 50 µl of 2 M sulfuric acid. ODs were read at 490 nm using an ELISA plate reader. The cellular components of the BAL preparation contained 1.23 × 106 eosinophils/0.06 × 106 neutrophils (for wt) and 1.82 ×106 eosinophils/0.092 × 106 neutrophils (for abr−/−). Thus, neutrophil numbers were 5% of that of eosinophil numbers and the difference observed in these assays represents mainly eosinophil peroxidase release with a small contribution of neutrophil myeloperoxidase in BAL.
Lung histopathology and peribronchial eosinophil quantification
Twenty-four hours following the final CRA challenge, lungs were fixed with 10% buffered formalin for 10 h (or overnight), then transferred to 70% ethanol. The fixed lungs were embedded in paraffin and multiple 5 µm sections were cut. Sections were stained with hematoxylin and eosin for light microscopic analysis. Eosinophils in the peribronchial region were counted in 50 high-power fields under ×400 magnification. Other sections were stained with periodic acid Schiff’s to examine mucin production.
Measurements of T cell activation
To measure CD4+ T cell activation, we purified T cells from the spleens of naive mice (n=3/genotpye) using a MACS column (Miltenyi Biotech). T cells were stimulated for 48 hours by plating at 2×106 cells/ml on plates coated with 2 µg/ml anti-CD3/CD28 antibodies. Controls were incubated on non-coated plates. Cell surface expression of the T-cell activation markers CD25 and CD69 (BioLegend) was compared using FACS. Supernatants of CD4+ T cells from two wt and abr−/− mice per genotype that had been activated with anti-CD3/CD28 were compared for cytokine secretion using a mouse cytokine antibody array (Cat. ARY006, R&D Systems, Minneapolis, MN) according to the supplier’s protocols.
Adoptive transfer
CD4+ T cells were isolated from spleen using a MACS column (Miltenyi Biotec.), whereas eosinophils (SSChiCD11b+Ly6G−) were flow-sorted from single lung cell suspensions using a BD FACSaria sorter on day 21 following the protocol in Fig. 2A. Post-sort purity of eosinophil preparations was around 90%, as assessed by characteristic morphology after examination of stained cytospin preparations. FACS analysis of post-purification T cells showed that both isolates consisted for more than 95% of CD4+ T cells (not shown). 5×106 CD4+ T cells (or 0.5×106 eosinophils) in 0.1 ml PBS were injected via the tail vein into naïve wt mice followed by intratracheal CRA challenge at 24 h and 72 h after adoptive transfer (0.1 ml PBS alone was injected as procedure control). Airway hyper-responsiveness was measured 24 h after the final CRA challenge.
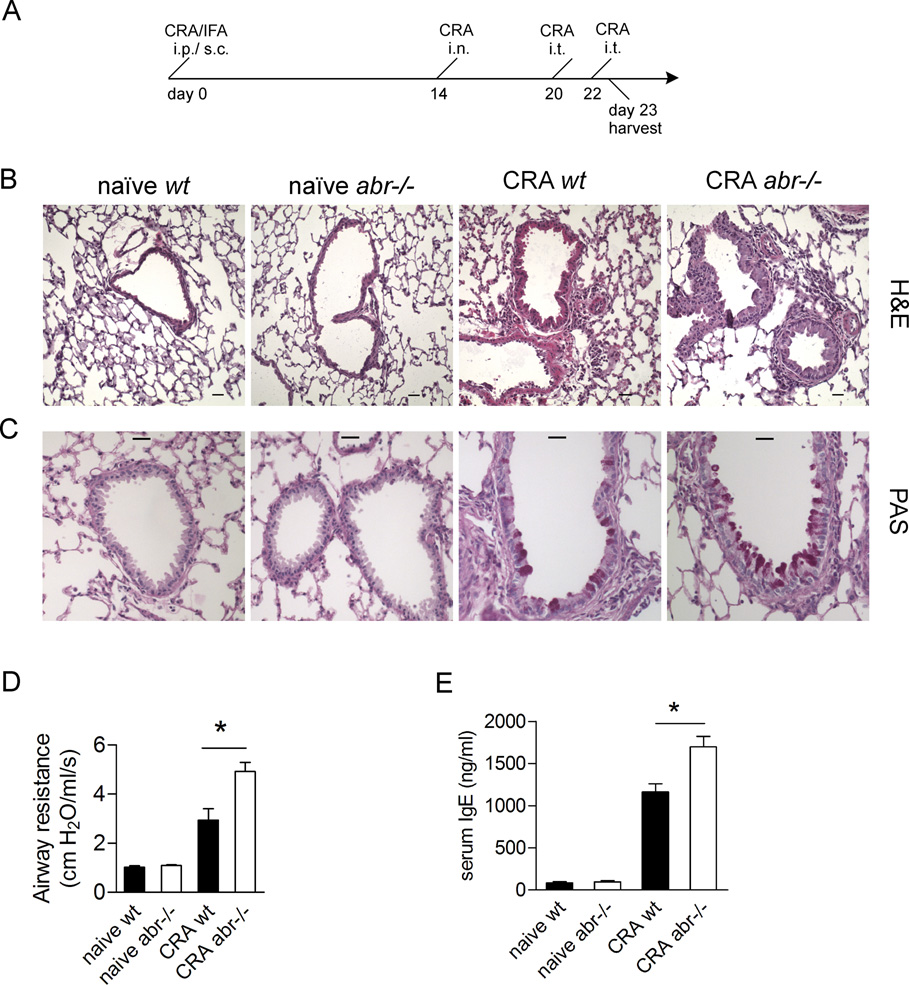
FIGURE 2. Lack of Abr leads to exacerbated asthma pathology.
(A) Schematic timeline for the CRA sensitization and challenge. (B and C) Representative histology of lung sections stained by (B) H&E and (C) periodic acid Schiff’s reagent. Mucin stains as a purple-red color. Sections were made at comparable depth on lungs oriented in a similar fashion. Scale bars, 25 µm. (D) Airway resistance (cm H2O/ml/sec) measurements on day 23. (E) Serum IgE levels on day 23 assayed by ELISA. Blood (for serum samples in E) was collected from the same mice of which lungs were subsequently harvested for histology staining (in B and C). Data (D and E) are expressed as mean ±SEM and analyzed by one-way ANOVA (*, p<0.05). n=3–4 mice/group. B–E, results representative of two independent experiments.
T cell migration assay
CD4+ T cells were isolated from spleen on day 21 following the protocol in Fig. 2A. 1×105 CD4+ T cells were seeded in the upper chamber of 5-µm pore size Transwells (Corning). CCL21 (200 ng/ml) was added in the lower chamber. The Transwell plates were incubated for 2 hr at 37°C. Cells in the lower chambers were counted and expressed as % of the total cells.
Rac activation assay and Western blotting
CD4+ T cells were isolated using a MACS column (Miltenyi Biotec.) from spleens of CRA-challenged mice on day 23 following the protocol in Fig 2A. Cells were lyzed in Mg lysis/washing buffer (11) on ice. Lysates were incubated with recombinant GST-Pak1-binding domain that had been pre-coupled with glutathione-agarose beads at 4°C for 1.5 hr with constant rotation. Beads were washed with Mg lysis/washing buffer and resuspended in SDS-sample buffer. Lysates for total Rac1 measurement were collected prior to the incubation with pull-down tool. Western blots were incubated with anti-Rac1 antibodies (Cytoskeleton). We used previously described rabbit polyclonal antibodies (22) to investigate Abr expression in different cell types. Mouse embryonic endothelial cells were isolated and immortalized using polyoma middle-T as described (25). Human pulmonary artery endothelial cells were from Invitrogen (Carlsbad, CA). Mouse alveolar type II cells were isolated according to the procedure described in (26).
Statistical analysis
Data are expressed as mean ± SEM and analyzed by the unpaired Student's t test or one-way ANOVA using Prism software (GraphPad Software, CA) unless indicated otherwise. p< 0.05 was considered statistically significant.
Results
Increased asthma-associated mortality in CRA-challenged abr−/− mice
To examine if abr−/− mice differ from control wild type (wt) mice in their reaction to CRA, age-matched control wt and abr−/− mice were immunized with CRA followed by intranasal CRA challenges (Fig. 1A; also see Materials and Methods). As shown in Fig. 1B, 5 of 6 wt mice survived sensitization and re-challenge with this dose of CRA. However, none of the mice lacking Abr survived after the 4th CRA challenge on day 22. This indicates that Abr function provides protection to mice against a fatal asthma attack subsequent to repeated exposure to high-dose CRA.
Lack of Abr leads to exacerbated asthma pathology
To generate a milder outcome, we reduced the intensity of CRA sensitization and challenge (Fig. 2A; Materials and Methods). Previous studies have demonstrated that this procedure elicits a strong Th2-mediated asthmatic response (19, 23, 24). Mice challenged with this protocol were then analyzed for pathophysiology.
A prominent feature of asthma development is the overproduction of mucin, which leads to narrowing of the lumen of the airways and obstruction of breathing (27). As shown in Fig. 2B, upon CRA challenges, infiltration of leukocytes was markedly elevated in the lungs of abr−/− mice, particularly around the peribronchial and perivascular regions. Moreover, the airway lumen of abr−/− mice were reduced due to epithelial hyperplasia. Mucin production was also increased in the lungs of abr−/− mice (Fig. 2C). Clinically, airway constriction is assessed by measurement of airway hyper-responsiveness after administration of methacholine (24). As shown in Fig. 2D, the airway resistance was significantly greater in CRA-sensitized and challenged abr−/− mice than in control mice. Serum IgE levels, another hallmark of asthma, were also substantially elevated in abr−/− mice (Fig. 2E). Non-immunized abr−/− and wt mice had similar airway resistance and IgE levels (Fig. 2D and E). This shows that Abr function reduces typical pathological characteristics associated with asthma in the non-lethal CRA model.
Increased eosinophil recruitment in CRA-challenged abr−/− mice
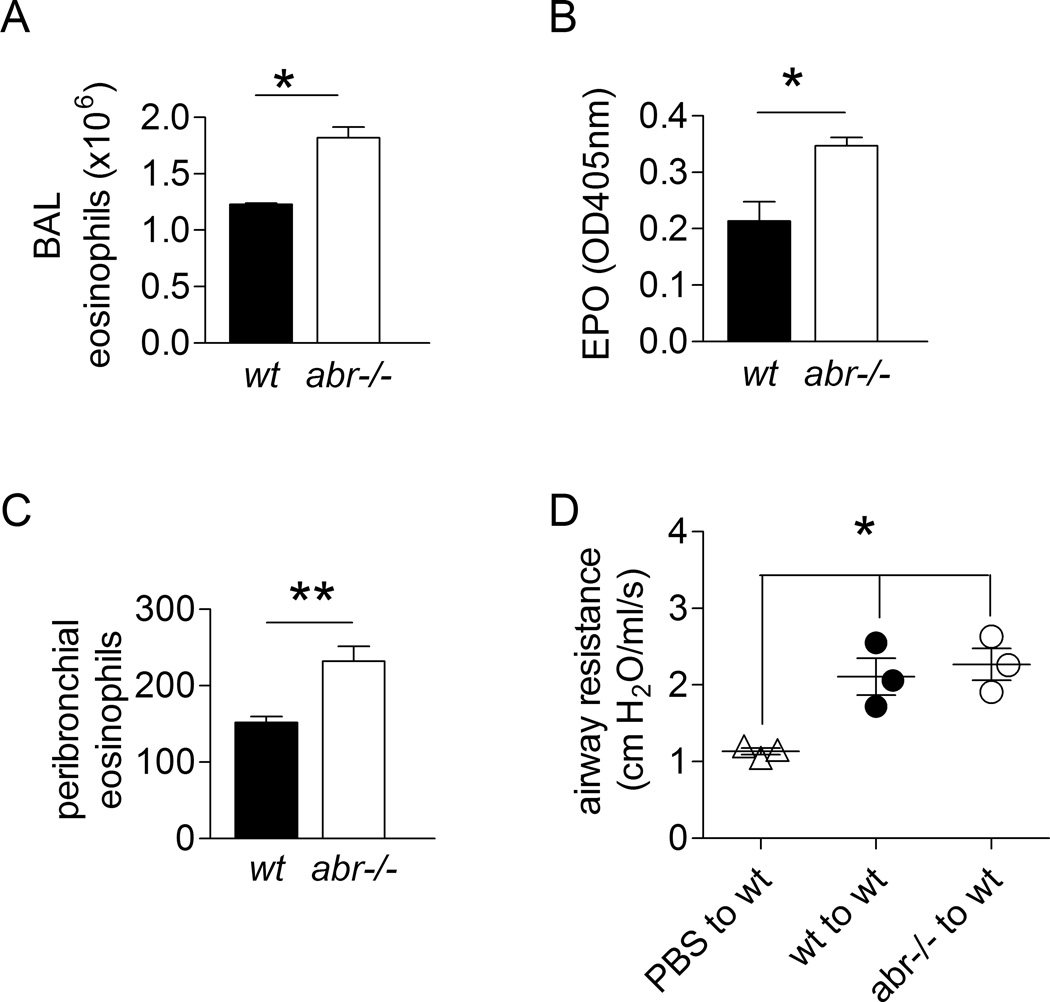
The inflammation in chronic asthma is associated with leukocyte infiltration and accumulation in the airways (28). Non-immunized abr−/− and wt mice had comparable numbers of CD45+ lymphocyte and CD19+ B cells in lung and spleen (not shown). As expected, upon CRA challenge, the airways of both wt and abr−/− mice were infiltrated by eosinophils and lymphocytes. Interestingly, the absolute number of eosinophils was markedly higher in the airways of abr−/− mice (Fig. 3A). Absolute neutrophil numbers, comprising about 5% of that of the eosinophils, were comparable between genotypes. Moreover, peroxidase activity, of which eosinophil peroxidase (EPO) most probably constituted the major fraction, was elevated in the cell-free bronchoalveolar lavage fluid (BALF) of the abr−/− mice (Fig. 3B). Since EPO is a marker of eosinophil degranulation, this further supports the concept that absence of Abr leads to an increased accumulation of eosinophils in the airways. Quantitation of eosinophils in peribronchial regions also revealed a significant increase of their infiltration in lungs of CRA-challenged abr−/− mice compared to controls (Fig. 3C). Since eosinophil degranulation is directly correlated with airway function (29), this result is consistent with the increased airway resistance in the abr−/− mice as reported in Fig. 2D.
FIGURE 3. Increased eosinophil recruitment in CRA-challenged abr−/− mice.
(A) Eosinophil counts in BAL. (B) Eosinophil peroxidase (EPO) levels in cell-free BAL fluid. A–B, data of one experiment were shown. (C) Total peribronchial eosinophils counts at 50 high-power fields (under ×400 magnification) of H&E stained sections. Results representative of two independent experiments. For A–C, data are expressed as mean ±SEM and analyzed by unpaired Student’s t test (*, p<0.05; **, p<0.01). n=3–4 mice per genotype. (D) Adoptive transfer of eosinophils. Eosinophils (SSChiCD11b+Ly6G−) flow-sorted from single cell suspensions of lungs on day 21 following the immunization/challenge protocol in Fig. 2A were transferred into naïve wt mice, followed by intratracheal CRA challenge at 24 h and 72 h after adoptive transfer. Airway hyper-responsiveness was measured 24 h after the final CRA challenge. Data of one experiment were analyzed by one-way ANOVA (*, p<0.05). n = 3 mice per group.
Because eosinophils can drive asthma development, their abnormal reactivity could explain the more severe phenotype in abr−/− mice challenged with CRA. To investigate this, we flow-sorted eosinophils from lungs of CRA-challenged wt and abr−/− mice on day 21 following the CRA challenge protocol in Fig. 2A and injected them via the tail vein into naïve wt mice. As shown in Fig. 3D, upon challenge of the recipient mice with CRA, airway hyperreactivity was significantly increased in comparison to control mice that received only PBS (no eosinophils), confirming that transfer of eosinophils conferred airway hypersensitivity. However, the responses of mice transplanted with wt or abr−/− eosinophils were not significantly different, indicating eosinophils lacking Abr are not responsible for the increased reaction of these animals to CRA compared to eosinophils with Abr function.
Increased T cell involvement in CRA-challenged abr−/− mice
To examine whether naïve (non-CRA-challenged) mice lacking Abr differ from their wt counterparts in T cell populations, we compared these using FACS on spleen and lung cell suspensions. As shown in Fig. 4A, the numbers of CD4+ T cells and CD8+ T cells in lung were comparable between genotypes in the absence of CRA sensitization. A similar result was obtained with populations from spleen (not shown).
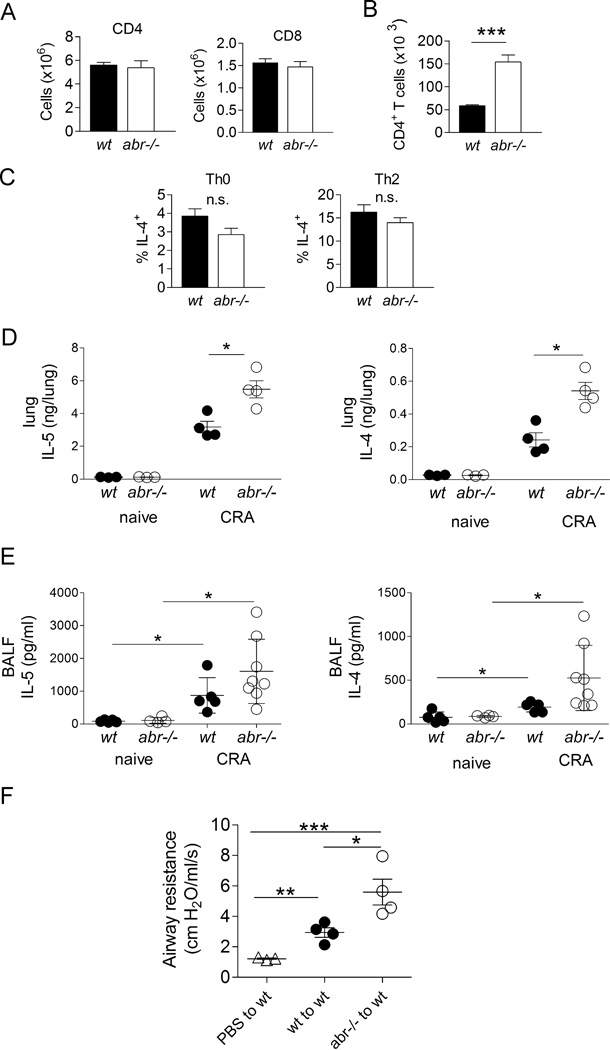
FIGURE 4. Increased CD4+ T cell involvement in asthmatic response of CRA-challenged abr−/− mice.
(A) Single cell suspensions of total lung of naive abr−/− and control mice evaluated by FACS for numbers of CD4+ or CD8+ T cells. (B) CD4+ T cell counts in BAL of CRA-immunized and challenged mice. For A–B, data of one experiment were shown, n = 3–4 mice/genotype. (C) Percentage of IL-4-producing CD4+ T cells in naïve wt and abr−/− mice. Splenic T cells cultured under Th0 or Th2 conditions for 2 days were incubated with PMA and ionomycin for 4 hr in the presence of brefeldin A. IL-4 was measured by intracellular staining. n = 4 mice/genotype. n.s., not significant. For A–C, data (mean ±SEM) were collected from 3–4 mice/genotype and analyzed by unpaired Student’s t test (***, p<0.001). (D) IL-4 and IL-5 in lung homogenates of naïve and CRA-immunized/challenged wt and abr−/− mice. (E) IL-4 and IL-5 in BALF of naïve control and CRA-immunized and challenged mice. (F) Airway resistance measurements after adoptive transfer of CD4+ T cells from CRA-challenged abr−/− mice into naïve wt mice. Splenic CD4+ T cells on day 21 (protocol in Fig. 2A) were transferred to naïve wt mice followed by intratracheal CRA challenge at 24 h and 72 h after adoptive transfer. Airway hyper-responsiveness was measured 24 h after the final CRA challenge. For D–E, data were analyzed by one-way ANOVA (*, p<0.05; **, p<0.01; ***, p<0.001). E, pooled data of two experiments with 4–5 control naïve mice per group and 5 wt and 8 abr−/− mice per treatment group. For C–D, one of two experiments with similar results. n = 3–4 mice per group.
However, upon CRA challenge, the airways of abr−/− mice showed significantly elevated levels of T cells, with CD4+ T cell numbers around 10-fold higher than that of CD8+ T cells (Fig. 4B and not shown). CD4+ T cells produce IL-4 and IL-5, cytokines that are critical to the development of asthma. IL-4 is crucial for the class switch of B cells to produce IgE, whereas IL-5 promotes the production and maturation of eosinophils (30, 31). In naïve mice, IL-4 production, as indicated by the percentage of splenic Th0- or Th2-polarized CD4+ T cells that were IL-4-positive, was not significantly different between abr−/− and wt controls (Fig. 4C). However, consistent with a role for increased numbers of CD4+ T cell in CRA-challenged abr−/− mice, we measured significantly elevated levels of both cytokines in lung homogenates of these abr−/− mice compared to wt controls (Fig. 4D). Although levels of these cytokines were also higher in the BALF of the abr−/− mice, these differences were not statistically significant (Fig. 4E).
We next examined if CD4+ T cells could be responsible for the more severe asthma development in mice lacking Abr function. Splenic CD4+ T cells were isolated from CRA-sensitized wt and abr−/− mice. FACS analysis of post-purification T cells showed that both isolates consisted of more than 95% CD4+ T cells (not shown). Upon adoptive transfer of these CD4+ T cells into naïve wt mice, followed by CRA challenge, CD4+ T cells from abr−/− mice were clearly significantly more active in causing airway resistance than their wt counterparts (Fig. 4F), showing that lack of Abr function in CD4+ T cells is a major cause of the exacerbated asthma development in abr−/− mice.
To address the possibility that T cells lacking Abr are intrinsically more sensitive to activation, we activated splenic CD4+ T cells from naïve abr−/− and wt mice for 48 hours with antibodies against CD3 and CD28. However, FACS analysis for CD25 and CD69 activation markers yielded similar results, and there were no statistically significant differences in cytokine secretion between the activated wt and abr−/− cells (Fig. S1).
CD4+ T cells from CRA-challenged abr−/− mice show increased migration and elevated GTP-Rac1 levels
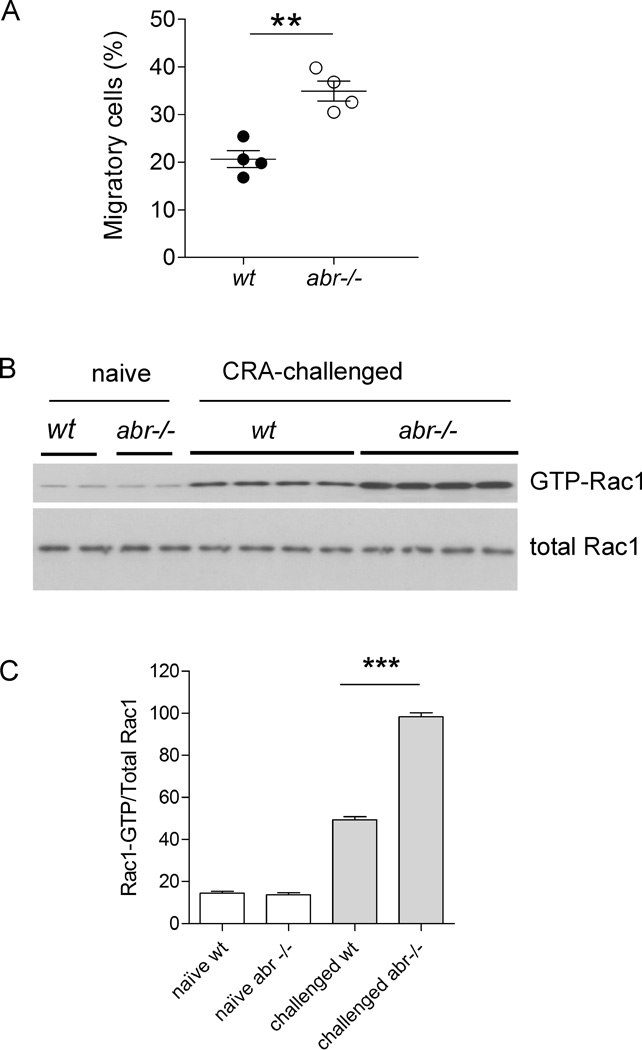
Our data showed that absolute CD4+ T cell numbers were increased in CRA-challenged abr−/− mice. Since Abr can regulate actin cytoskeletal reorganization needed for motility (11), increased cell numbers could be caused by increased migration of these cells to the lung. To investigate this, we compared chemotaxis of activated CD4+ T cells isolated from the spleens of wt and abr−/− CRA-sensitized mice towards CCL21, a chemokine that promotes chemotaxis of activated T cells to lymph nodes (32). Interestingly, in vivo CRA-activated abr−/− CD4+ T cells exhibited significantly enhanced chemotaxis compared to wt CD4+ T cells (Fig. 5A). Because Rac activity mediates T cell migration and Rac-deficient T cells exhibit defective chemokine-induced chemotaxis (33), we examined the activation state of Rac1 protein in CD4+ T cells from CRA-challenged wt and abr−/− mice. As shown in Fig. 5B and C, the levels of GTP-Rac1 in CD4+ T cells of CRA-sensitized abr−/− mice were substantially higher than those of wt control mice, consistent with a role for Abr in negatively regulating the pool of activated, GTP-bound Rac in CD4+ T cells that have been sensitized by CRA.
FIGURE 5. CD4+ T cells from CRA-challenged abr−/− mice show increased migration and elevated GTP-Rac1 levels compared to wt controls.
(A) CD4+ T cells were isolated from spleen on day 21 following the protocol in Fig. 2A. 1×105 CD4+ T cells were seeded in the upper chamber of Transwells. Cells migrated to CCL21 in the lower chamber for 2 hr at 37°C. Migrating cells in the lower chambers were counted and expressed as % of the total cells. Data (mean±SEM) were analyzed by the Student’s t test (**, p<0.01). n = 4 mice per group. (B) Detection of activated Rac by Western blot in CD4+ T cells isolated from spleen on day 21 following the protocol in Fig. 2A. (C) Quantification of activated Rac1. The relative levels of activated Rac1 were calculated by normalizing the ratio of GTP-Rac1/Total Rac1. For A–C, results were similar in two independent experiments.
Discussion
Asthma is an economically significant health problem of which the underlying causes are incompletely understood. The identification of molecular mechanisms that promote asthma development may be an important component in the search for more effective therapies. In the current study we have used a mouse model to investigate the development of asthma caused by cockroach exposure. This allergen has been not studied extensively as allergen in mouse models although it is directly relevant to human asthma. We identify Abr as the first Rac-specific GTPase-activating protein that mitigates the development of lethal, CRA-evoked allergic asthma.
In the model used here, as in human asthma, inflammation is an essential component. Fatal asthma in humans is associated with luminal obstruction caused by exudate composed of mucus and cells (34). The finding that our non-lethal model was characterized by increased mucus production and infiltrates of immune cells suggests that obstruction caused by similar masses may have contributed to the death in the lethal model.
Asthma pathogenesis is a complicated process that involves multiple cell types of the immune system. Eosinophils and CD4+ T cells are known to play major roles in the pathology of antigen-induced allergic asthma (35). Eosinophils are the central effector cells for asthma symptoms, because they produce proteases that degrade and remodel tissue extracellular matrix, promote mucus production and airway constriction, and secrete proinflammatory factors that propagate inflammation and recruit other immune cells (36). CD4+ T cells, particularly Th2-polarized CD4+ T cells, produce IL-4 that stimulates B cell IgE class switch, and IL-5 that causes eosinophil maturation and recruitment. Thus intrinsic abnormalities of either eosinophils or CD4+ T cells in the absence of Abr could have resulted in the exacerbated asthma pathology observed in CRA-challenged abr−/− mice. We used an adoptive transfer approach to clearly establish that abr−/− CD4+ T cells, not abr−/− eosinophils, are responsible for the worsened asthma pathology. This indicates that CD4+ T cells have intrinsic abnormalities in the absence of Abr.
Because the levels of IL-4 and IL-5 were increased in the lungs of abr−/− mice that developed CRA-evoked asthma, it was possible that Abr deficiency would increase the production of these cytokines by individual activated CD4+ T cells. However, upon in vitro activation, CD4+ T cells of naïve abr−/− mice produced similar levels of IL-4 on a per cell basis as their wt counterparts. Thus the increased levels of IL-4 and IL-5 are likely the result of the significantly increased absolute numbers of CD4+ T cells in the lungs of mice deficient for Abr.
De novo migration of T cells into the lung upon allergen challenge contributes significantly to the increased numbers of peribronchial and airway infiltrates. Indeed, imaging of CD4+ T cells in mice showed that this is a very rapid process, and within 6 hours of allergen inhalation, focus formation of CD4+ T cells occurs in the lung, which precedes eosinophil infiltration (37). Thus we examined migration of CD4+ T cells from CRA-sensitized and challenged abr−/− mice towards CCL21. This chemokine promotes CD4+ T cell migration to lymphoid tissue (38). Our results show for the first time that CD4+ T cells activated in vivo by CRA contain increased levels of activated Rac and, moreover, that activated CD4+ abr−/− T cells migrate more rapidly towards CCL21. However, it is unlikely that this cytokine is responsible for CRA-evoked migration of CD4+ T cells into the lung, because CCL21 levels did not increase in lungs of wt or abr−/− CRA-challenged mice compared to controls (Figure S2). These results are in agreement with those of Ploix et al, who did not find evidence for involvement of CCL21 in the pathology of asthma using mouse models (39).
Our overall results are consistent with studies on T cells of mice with conditional ablation of Rac1 and complete knockout of Rac2. These Racs were shown to be critical for T cell migration to and within lymph nodes (33). Because Abr is a GAP specific for Rac, we conclude that Abr is a major GAP in CD4+ T cells that regulates T cell mobility through suppression of Rac activation, thus attenuating the CRA-induced asthma. Interestingly, the concept of targeting Th2 cell migration into lungs as a therapeutic target for asthma has been proposed (40), and inhibition of SDF-1α-CXCR4 mediated T cell migration in a mouse model of ovalbumin-evoked asthma was shown to have beneficial effects (41).
Asthma has a prominent involvement of immune cells but other cell types also contribute to its pathology. The characteristic airway remodeling involves increases in smooth muscle cell mass and angiogenesis (42). We have previously shown that Abr is expressed in pulmonary artery smooth muscle cells (13) and Western blot analysis indicates that Abr is expressed in other cell types including endothelial cells (Figure S3). Thus it is possible that expression of Abr in non-immune cell types contributes to the exacerbated pathology exhibited by abr−/− mice immunized and challenged with CRA. Overall, our study has identified a new component of the regulatory pathway that is in place to prevent lethal asthma attacks in reaction to an allergen that is relevant to human asthma. Abr and Rac represent new potential therapeutic agents that could be explored for asthma treatment.
Supplementary Material
Acknowledgements
We thank Donna Foster for excellent care of the mice. Sun-ju Yi and Urban Deutsch are acknowledged for the endothelial cell lysates, and for procedures and reagents to isolate mouse embryonic endothelial cells, respectively. We acknowledge Gang Chen for the introduction of the CRA asthma model and early contributions to this study and Wei Shi for access to the plethysmograph.
Footnotes
Disclosures
The authors declare no competing interests.
This work was supported in the past by the National Institutes of Health grants HL071945 and HL060231 to JG.
Abbreviations used in this article: ABR, active BCR-related; BCR, breakpoint cluster region; AHR, airway hyper-responsiveness; BAL, bronchoalveolar lavage; BALF, bronchoalveolar lavage fluid; CCL21, CC chemokine ligand 21; Cdc42, cell cycle control protein 42 homolog, CRA, cockroach allergen; EPO, eosinophil peroxidase; GAP, GTPase-activating protein; GEF, G-nucleotide exchange factor; i.n., intranasally; Pak, p21 protein {Cdc42/Rac}-activated kinase; PNU, protein nitrogen units; wt, wild type; Rac, Ras-related C3 botulinum toxin substrate.
References
- 1.Heisterkamp N, Morris C, Groffen J. ABR, an active BCR-related gene. Nucleic Acids Res. 1989;17:8821–8831. doi: 10.1093/nar/17.21.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heisterkamp N, Kaartinen V, van Soest S, Bokoch GM, Groffen J. Human ABR encodes a protein with GAPrac activity and homology to the DBL nucleotide exchange factor domain. J Biol Chem. 1993;268:16903–16906. [PubMed] [Google Scholar]
- 3.Tan EC, Leung T, Manser E, Lim L. The human active breakpoint cluster region-related gene encodes a brain protein with homology to guanine nucleotide exchange proteins and GTPase-activating proteins. J Biol Chem. 1993;268:27291–27298. [PubMed] [Google Scholar]
- 4.Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- 5.Arrizabalaga O, Lacerda HM, Zubiaga AM, Zugaza JL. Rac1 protein regulates glycogen phosphorylase activation and controls interleukin (IL)-2-dependent T cell proliferation. J Biol Chem. 2012;287:11878–11890. doi: 10.1074/jbc.M111.297804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 7.Schiller MR. Coupling receptor tyrosine kinases to Rho GTPases--GEFs what's the link. Cell Signal. 2006;18:1834–1843. doi: 10.1016/j.cellsig.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 9.Gregg D, Rauscher FM, Goldschmidt-Clermont PJ. Rac regulates cardiovascular superoxide through diverse molecular interactions: more than a binary GTP switch. Am J Physiol Cell Physiol. 2003;285:C723–C734. doi: 10.1152/ajpcell.00230.2003. [DOI] [PubMed] [Google Scholar]
- 10.Chuang TH, Xu X, Kaartinen V, Heisterkamp N, Groffen J, Bokoch GM. Abr and Bcr are multifunctional regulators of the Rho GTP-binding protein family. Proc Natl Acad Sci U S A. 1995;92:10282–10286. doi: 10.1073/pnas.92.22.10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho YJ, Cunnick JM, Yi SJ, Kaartinen V, Groffen J, Heisterkamp N. Abr and Bcr, two homologous Rac GTPase-activating proteins, control multiple cellular functions of murine macrophages. Mol Cell Biol. 2007;27:899–911. doi: 10.1128/MCB.00756-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunnick JM, Schmidhuber S, Chen G, Yu M, Yi SJ, Cho YJ, Kaartinen V, Minoo P, Warburton D, Groffen J, Heisterkamp N. Bcr and Abr cooperate in negatively regulating acute inflammatory responses. Mol Cell Biol. 2009;29:5742–5750. doi: 10.1128/MCB.00357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu M, Gong D, Lim M, Arutyunyan A, Groffen J, Heisterkamp N. Lack of bcr and abr promotes hypoxia-induced pulmonary hypertension in mice. PLoS One. 2012;7:e49756. doi: 10.1371/journal.pone.0049756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011:1–14. [PubMed] [Google Scholar]
- 15.Arruda LK, Chapman MD. The role of cockroach allergens in asthma. Curr Opin Pulm Med. 2001;7:14–19. doi: 10.1097/00063198-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Matsui EC, Wood RA, Rand C, Kanchanaraksa S, Swartz L, Curtin-Brosnan J, Eggleston PA. Cockroach allergen exposure and sensitization in suburban middle-class children with asthma. J Allergy Clin Immunol. 2003;112:87–92. doi: 10.1067/mai.2003.1588. [DOI] [PubMed] [Google Scholar]
- 17.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 18.Katial RK. Cockroach allergy. Immunol Allergy Clin North Am. 2003;23:483–499. doi: 10.1016/s0889-8561(03)00002-x. [DOI] [PubMed] [Google Scholar]
- 19.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–7053. [PubMed] [Google Scholar]
- 20.Sarpong SB, Zhang LY, Kleeberger SR. A novel mouse model of experimental asthma. Int Arch Allergy Immunol. 2003;132:346–354. doi: 10.1159/000074902. [DOI] [PubMed] [Google Scholar]
- 21.Corry DB, Irvin CG. Promise and pitfalls in animal-based asthma research: building a better mousetrap. Immunol Res. 2006;35:279–294. doi: 10.1385/IR:35:3:279. [DOI] [PubMed] [Google Scholar]
- 22.Kaartinen V, Gonzalez-Gomez I, Voncken JW, Haataja L, Faure E, Nagy A, Groffen J, Heisterkamp N. Abnormal function of astroglia lacking Abr and Bcr RacGAPs. Development. 2001;128:4217–4227. doi: 10.1242/dev.128.21.4217. [DOI] [PubMed] [Google Scholar]
- 23.Lundy SK, Berlin AA, Lukacs NW. Interleukin-12-independent down-modulation of cockroach antigen-induced asthma in mice by intranasal exposure to bacterial lipopolysaccharide. Am J Pathol. 2003;163:1961–1968. doi: 10.1016/S0002-9440(10)63554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narala VR, Ranga R, Smith MR, Berlin AA, Standiford TJ, Lukacs NW, Reddy RC. Pioglitazone is as effective as dexamethasone in a cockroach allergen-induced murine model of asthma. Respir Res. 2007;8:90. doi: 10.1186/1465-9921-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May T, Mueller PP, Weich H, Froese N, Deutsch U, Wirth D, Kroger A, Hauser H. Establishment of murine cell lines by constitutive and conditional immortalization. J Biotechnol. 2005;120:99–110. doi: 10.1016/j.jbiotec.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 26.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 27.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, Fahy JV. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 28.Corrigan CJ, Kay AB. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992;13:501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- 29.Gundel RH, Letts LG, Gleich GJ. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest. 1991;87:1470–1473. doi: 10.1172/JCI115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotsimbos AT, Hamid Q. IL-5 and IL-5 receptor in asthma. Mem Inst Oswaldo Cruz. 1997;92(Suppl 2):75–91. doi: 10.1590/s0074-02761997000800012. [DOI] [PubMed] [Google Scholar]
- 31.Stirling RG, van Rensen EL, Barnes PJ, Chung KF. Interleukin-5 induces CD34(+) eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am J Respir Crit Care Med. 2001;164:1403–1409. doi: 10.1164/ajrccm.164.8.2010002. [DOI] [PubMed] [Google Scholar]
- 32.Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 33.Faroudi M, Hons M, Zachacz A, Dumont C, Lyck R, Stein JV, Tybulewicz VL. Critical roles for Rac GTPases in T-cell migration to and within lymph nodes. Blood. 2010;116:5536–5547. doi: 10.1182/blood-2010-08-299438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuyper LM, Pare PD, Hogg JC, Lambert RK, Ionescu D, Woods R, Bai TR. Characterization of airway plugging in fatal asthma. Am J Med. 2003;115:6–11. doi: 10.1016/s0002-9343(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 35.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 36.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa A, Hayashi K, Kishimoto H, Yang M, Tofukuji S, Suzuki K, Nakajima H, Hoffman RM, Shirai M, Nakayama T. Color-coded real-time cellular imaging of lung T-lymphocyte accumulation and focus formation in a mouse asthma model. J Allergy Clin Immunol. 2010;125:461–468. doi: 10.1016/j.jaci.2009.09.016. e466. [DOI] [PubMed] [Google Scholar]
- 38.Gollmer K, Asperti-Boursin F, Tanaka Y, Okkenhaug K, Vanhaesebroeck B, Peterson JR, Fukui Y, Donnadieu E, Stein JV. CCL21 mediates CD4+ T-cell costimulation via a DOCK2/Rac-dependent pathway. Blood. 2009;114:580–588. doi: 10.1182/blood-2009-01-200923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ploix C, Zuberi RI, Liu FT, Carson MJ, Lo DD. Induction and effector phase of allergic lung inflammation is independent of CCL21/CCL19 and LT-beta. Int J Med Sci. 2009;6:85–92. doi: 10.7150/ijms.6.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heijink IH, Van Oosterhout AJ. Targeting T cells for asthma. Curr Opin Pharmacol. 2005;5:227–231. doi: 10.1016/j.coph.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Lukacs NW, Berlin A, Schols D, Skerlj RT, Bridger GJ. AMD3100, a CxCR4 antagonist, attenuates allergic lung inflammation and airway hyperreactivity. Am J Pathol. 2002;160:1353–1360. doi: 10.1016/S0002-9440(10)62562-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girodet PO, Ozier A, Bara I, Tunon de Lara JM, Marthan R, Berger P. Airway remodeling in asthma: new mechanisms and potential for pharmacological intervention. Pharmacol Ther. 2011;130:325–337. doi: 10.1016/j.pharmthera.2011.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.