Abstract
Atherosclerosis is a chronic inflammatory disease characterized by T lymphocyte infiltration into the atherosclerotic plaque. Assessments of T cell subtypes have demonstrated a predominance of CD4+ T helper (Th) cells, implicated Th1 and Th17 immunity in both human and mouse atherogenesis, and provided some evidence suggesting protective roles of Th2 and T regulatory cells. Observations that certain inbred mouse strains have an inherent T helper bias suggests a genetic predisposition toward developing a particular T helper phenotype. This review summarizes our current understanding of mechanisms of antigen processing for major histocompatibility complex (MHC) molecules, describes the different T helper cell subsets and their roles in atherosclerosis, and discusses mechanisms of genetic predisposition toward Th1/Th2 bias in mice. We also present data from our laboratory demonstrating inherent Th1/Th2 phenotypes in apparently healthy human volunteers that are stable over time, and discuss the potential implications for cardiovascular disease.
Keywords: Atherosclerosis, cardiovascular disease, CD4+ lymphocyte, T helper cell, immunology, inflammation, immunoglobulin
Introduction
T helper (Th) cell polarization arises from programmed differentiation following T cell receptor (TCR) engagement by peptide antigen-major histocompatibility (MHC) complex. In response to antigen-MHC stimulation, naive, antigen inexperienced, T helper cells (Th0) can differentiate into several functional subclasses, including Th1, Th2, Th17, regulatory T cells (Treg), and follicular T helper cells (Tfh), which are characterized by the expression of distinct transcription factors, and the secretion of unique signature cytokine profiles. The function and regulation of these respective Th subsets are critical to both normal physiological host responses and pathophysiological responses such as occur in asthma, autoimmune diseases, and atherosclerosis. Despite this, little is known about the distribution of Th cell polarization in healthy people. In this report we first provide a detailed review of the innate and adaptive immune responses, emphasizing their connection to atherosclerosis. We then describe Th cell polarization in healthy adults, identifying for the first time that this is a consistent variable, suitable for clinical and epidemiological research, and discuss implications for cardiovascular disease (CVD).
Detailed Review of Innate and Adaptive Immunity with an Emphasis on Atherosclerosis
Inflammation in Atherosclerosis: Innate and Adaptive Immune Activation
Atherosclerosis is a chronic inflammatory disease characterized by accumulations of lipid laden macrophage (foam cells) in the walls of large and medium sized arteries. There are distinct populations of monocytes/macrophage and they play different and dynamic roles in atherogenesis. In the mouse, a common monocyte-dendritic cell precursor (MDP) in the bone marrow [1] will ultimately give rise to all monocytes, macrophage, and dendritic cells. The healthy arterial wall contains large numbers of resident macrophage and dendritic cells especially at sites which are disposed to develop atherosclerotic plaques [2]. There are at least two major subpopulations of monocytes based on surface expression of granulocyte receptor-1 antigen (Gr1) and lymphocyte antigen 6 complex locus C (Ly6C) antigens. Gr1+Ly6Chigh cells are usually associated with pro-inflammatory responses and produce tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-12, and inducible nitric oxide synthase (iNOS; NOS2) while Gr1-Ly6Clow cells may be involved with wound healing, tissue repair, and phagocytosis [3–5]. While both LyC6high and LyC6low cells may accumulate lipid and become foam cells, based on cytokine expression patterns and expression of major MHC class II and CD80/CD86 accessory molecules, the pro-inflammatory Gr1+Ly6Chigh monocyte/dendritic cells are considered to have the major impact on adaptive immunity.
In the shoulder region of the atherosclerotic plaque, there are infiltrations of T lymphocytes including both CD4+ and CD8+ cells with a predominance of CD4+ cells in both human and mouse atheromas [6–8]. Evaluations of the TCR repertoire of the infiltrating cells in mouse lesions suggest oligoclonal activation indicative of expansion of a limited number of antigen-specific T cell clones [9]. Consistent with this, T cells in human plaques express the CD45RO effector/memory activation marker indicative of previous stimulation [10]. Various lines of evidence implicate adaptive immunity in atherogenesis, especially in mouse models. Severe combined immunodeficient (Scid) mice develop accelerated atherosclerosis when reconstituted with CD4+ T cells [11]. Similarly, mice deficient in CD4+ cells develop significantly less atherosclerosis than immunocompetent animals [12].
Major Histocompatibility Complex Antigen Processing
Beyond simply implicating T cells in atherogenesis, the phenotype of the T cell response is also crucial. Classically, naive T cells (Th0; CD45RA+) begin as non-polarized cells which express limited amounts of IL-2 but little or no interferon gamma (IFN-γ) or IL-4. Immature dendritic cells in peripheral tissues are highly phagocytic but have reduced antigen presenting capabilities. However, when the dendritic cells phagocytize antigen, they leave the peripheral tissues for draining lymph node or spleen and mature into highly effective antigen presenting cells with increased MHC-antigen on their cell surface and accessory molecule (CD80/CD86) expression, but severely reduced phagocytic activity. The reduction in phagocytic activity restricts the ability to take up, process, or present new antigens.
T helper cells are generally classified as T cells that are CD4+, and these cells primarily recognize antigens presented by MHC class II molecules. This is in contrast to cytotoxic CD8+ T cells which usually react to antigen presented by MHC class I molecules. Both the structure and antigen processing for MHC class I and class II molecules differ. Class I molecules consist of a single polypeptide chain coded for by the HLA-A, -B or -C genes in humans, or the H-2K, H-2L or H-2D genes in mice, in association with an invariant β2 microglobulin (β2M) chain. Endogenously synthesized proteins are digested in proteasomes to peptides which are transported to the endoplasmic reticulum (ER) using the transporter associated with antigen presentation (TAP) pathway. Inside the ER, MHC class I molecules, as they are synthesized, fold around the peptide so that the formed MHC-peptide-β2M complex is transported to the cell surface for presentation to CD8+ T cells. In contrast, MHC class II molecules consist of an a and β chain, both of which are coded by MHC genes in HLA-DR, -DQ, -DP in humans and H-2IA and H-2IE in mouse.
Class II molecules are synthesized in the ER but the groove region is filled with an invariant chain known as class II-associated invariant chain peptide (CLIP). The class II molecule moves from the ER through the golgi into the endosome pathway. Antigens entering through phagocytosis, whether they are, e.g., microbial products or oxidized low density lipoproteins (oxLDL), will enter phagosomes which will fuse with the endosomes. The very low pH of the phagosome causes degradation of the CLIP which opens the groove of the MHC class II molecule and allows binding of peptides in the phagosome to the groove of the MHC molecule. The MHC-peptide finishes its journey to the cell surface for presentation to CD4+ T cells. There are exceptions, and cross-presentation does occur, but in general, CD4+ T cells respond to exogenous or phagocytized antigen while CD8+ T cells respond to endogenous antigen or proteins synthesized within the antigen presenting cell. The reason for this dichotomy is probably that CD8+ T cells are usually highly cytolytic and often the major effector eliminating viral or intracellular pathogens through killing of the infected cell. If an infected host cell, such as an epithelial or endothelial cell, can present virus antigens quickly on its own MHC class I molecules, a virus-specific CD8+ T cell would be able to kill the infected cell before substantial amounts of progeny virus were made, thus aborting the infection. As each infected cell could produce over a million new virus particles if the replication cycle were allowed to progress to completion, rapid death of one infected cell would still be of benefit to the organism as a whole. In human atherosclerotic lesions, MHC class II molecules have been specifically implicated [13], with one study reporting that 10% of all T cells in atheromas recognize oxLDL presented by MHC class II molecules [14].
CD4+ T Helper Cell Subsets
Once in the lymphoid tissue, the dendritic cells migrate to T cell rich areas where they interact with T cell clones expressing the correct TCR and accessory molecule (CD4 for MHC class II or CD8 for MHC class I) for the peptide-MHC of the dendritic cell. At this point various factors determine whether the naive T cells will differentiate into an activated effector cell with a specific Th phenotype [15, 16]. Most notable of the controlling factors is the cytokine milieu in which the activation phase occurs. T cells are frequently characterized as having a specific T helper phenotype based on the cytokines they produce and cell surface molecules and transcription factors they express. The initial paradigm was the discovery of Th1 and Th2 cells by Mosmann and Coffman [17] and has been confirmed by many investigators in multiple species including humans [15]. Since this initial report, many other apparent subtypes of T helper cells have been identified. Brief descriptions of several of the more extensively investigated subtypes are discussed here; their roles in atherosclerosis are introduced here and are discussed in further detail subsequently:
Th1 cells: Classically, Th1 cells are defined by expression of IFN-γ, but also produce lymphotoxin, IL-2, and TNF-α. These cells are pro-inflammatory and mediate cellular immunity through activation of pro-inflammatory macrophage, activation of cytolytic CD8+ T cells, and protection from intracellular viral and bacterial infections. They also produce macrophage inflammatory protein-1 alpha (MIP-1α), MIP-1β, and T cell activation-3 (TCA-3) which are chemokines for macrophage. One potential Th1 activation pathway involves signal transducer and activator of transcription 1 (STAT1). IFN-γ binds to the IFN-γ receptor (IFNGR) to phosphorylate STAT1 and this can directly activate T-bet, the transcription factor closely associated with the Th1 phenotype. While this pathway may have some impact on IFN-γ production, its overall role in this cytokine response is controversial. Serum levels of IFN-γ and CD4+ cell expression of IFN-γ are similar in T. gondii infected STAT1−/− and wild-type mice despite somewhat decreased levels of T-bet expression in the STAT1−/− animals [18]. This suggests that STAT1 is not the only transcription factor controlling T-bet activation. More important is the role of IL-12, a cytokine produced by pro-inflammatory macrophage. STAT4 is primarily activated by IL-12, but might also be activated by type 1 interferons and IFN-γ. Activated STAT4 stimulates T-bet activation and transcription of IFN-γ and IL-12 receptor beta 2 subunit (IL-12Rβ2) which increases cellular responsiveness to IL-12 making a positive feedback loop to stabilize the Th1 phenotype. Recently, Schultz and colleagues suggested a sequential mechanism for Th1 polarization and maintenance where IFN-γ induces initial T-bet expression and starts polarizing naive T cells into a Th1 lineage. IL-12 signaling and T-bet will then upregulate IL-12Rβ2 and maintain T-bet expression resulting in imprinting of Th1 cells to ensure IFN-γ re-expression [19]. Th1 cells predominate among the T cell populations in both human and mouse atherosclerotic plaques, and numerous studies investigating both human samples and mouse models have implicated Th1 immunity in atherosclerosis.
Th2 cells: Th2 cells secrete IL-4, IL-5, IL-9, IL-13, and IL-23; promote humoral immunity; and protect against extracellular microbial infections. For Th2 cell differentiation, naive T cells stimulated with antigen in the presence of IL-4 phosphorylate STAT6. This leads to activation of GATA3, the signature transcription factor of Th2 cells, and the factor responsible for expression of IL-4, IL-5, and IL-13. While IL-4 is a powerful positive regulator of STAT6, it is an equally negative regulator of STAT4, just as IFN-γ is a negative regulator of STAT6. Thus, immune response polarization has a tendency to be self-perpetuating and extend to new antigenic stimulations occurring in an existing cytokine environment. The role of Th2 cells in atherosclerosis is less certain than for Th1 cells. In contrast to IFN-γ, there is a paucity of Th2 cytokines in atherosclerotic plaques, and collectively their role in atherogenesis remains incompletely understood.
Th17 cells: A third population of Th cells which may be important in atherosclerosis are Th17 cells which make IL-17A and IL-17F. Stimulation of naive T cells in the presence of transforming growth factor beta (TGF-β), and either IL-6 or IL-21, leads to phosphorylation of STAT3. Downstream of STAT3 activation, the transcription factor retinoic acid receptor (RAR)-related orphan receptor gamma T (RORγt) is activated which is essential for Th17 cell differentiation and expression of IL-17A, IL-17F, and IL-23 receptor (IL-23R). IL-23 is a cytokine produced by macrophage and while not required for Th17 cell differentiation, its binding to IL-23R on differentiating Th17 cells further activates STAT3 and makes the Th17 cell less sensitive to IL-12, thus stabilizing the Th17 phenotype. Both Th1 and Th2 cells inhibit Th17 cell responses [15, 20, 21]. Th17 cells, and the IL-17A that they produce, are usually considered highly pro-inflammatory as this cytokine induces expression of TNF-α, IL-1β, and monocyte chemoattractant protein-1 (MCP-1), and upregulates the expression of intercellular adhesion molecule-1 (ICAM-1) [22, 23]. Th17 cells have both protective and pathogenic roles in immunity. These effectors are protective against specific pathogens including C. albicans and S. aureus. However, in a number of autoimmune disease models which had been associated with Th1 cell pathogenesis, including experimental autoimmune encephalomyelitis (EAE), rheumatoid arthritis, psoriasis, inflammatory bowel disease, allergic reactions, and diabetes, it is now believed that Th17 cells play a major role in tissue damage and inflammation [24]. Th17 cells are present in both human and mouse atherosclerotic plaques, and various lines of evidence provide support for a pathologic role for this subpopulation.
Regulatory T (Treg) cells: Regulatory T cells function as negative immune effectors. These cells constitute up to 10% of peripheral CD4+ T cells in naive mice and humans, and express CD25 (IL-2 receptor α chain; IL-2rα) [25–27]. Treg cells can be defined as natural (nTreg) or inducible (iTreg) populations; nTreg cells are generated in the thymus during normal T cell ontogeny as CD4+CD25+ cells; iTregs include type 1 regulatory T cells (Tr1) and Th3 cells. It is likely that T cells with high affinity TCRs for self antigen become committed to the T regulatory cell line. Unlike other cells leaving the thymus, nTreg cells are functionally mature and do not require antigen exposure in the periphery to gain immunosuppressive activity. These cells express the forkhead box P3 (FoxP3) transcription factor which is essential to their immunosuppressive activity. In fact, transducing exogenous FoxP3 into CD4+CD25- cells converts these cells into CD4+CD25+ Treg-like cells that are able to suppress T cell proliferation in vitro and inhibit autoimmune disease in vivo [26, 28]. Although expression of FoxP3 is essential for suppressive activity in Treg cells, IL-2 is probably important in Treg cell maintenance and survival since mice which lack either CD25 (IL-2rα) or IL-2 have decreased numbers of Treg cells and develop lymphoproliferative and autoimmune diseases [29]. In addition to nTreg cells, iTreg cells can be converted from effector T cell populations in the periphery subsequent to antigen challenge. These iTreg cells are CD4+CD25+, but may be either positive or negative for FoxP3 [30]. Both nTreg and iTreg cells can secret either IL-10 (Tr1) or TGFβ (Th3) cytokines [30]. Treg cells express similar chemokine receptor patterns as effector T cells and can migrate to peripheral lymphoid tissues and inflammatory sites similarly to the effector population [26]. There are three general hypothesized mechanisms for Treg cell suppression of immunity [26]. First, Treg cells may bind to and out-compete effector T cells for MHC-antigen complexes on dendritic cells and effectively block antigen presentation to the effector T cell populations. Secondly, direct Treg-dendritic cell interactions through cytotoxic T-lymphocyte antigen 4 (CTLA4) can downregulate accessory molecule expression (CD80/CD86) on the dendritic cells making them less effective in antigen presentation. Third, Treg cells can either kill or inhibit T cell differentiation. TGF-β produced by Treg cells will activate NOTCH and its downstream target gene, hairy and enhancer of split-1 (Hes1), which suppresses gene expression in T cells [30]. IL-10 blocks CD2, CD28, and inducible T-cell costimulator (ICOS) signaling in T cells and suppressor of cytokine signaling 3 (SOCS3) signaling in monocytes, resulting in reduced T cell proliferation and cytokine response [31]. There is significant evidence that Treg cells are protective in mouse models of atherosclerosis, however, associations with human CVD remains unclear.
Follicular T helper lymphocytes (Tfh cells): A new subset of CD4+ T cells has been suggested to manifest the importance of temporal and spatial regulation during T cells differentiation. Follicular helper T cells comprise a specialized subset that was reported recently to be phenotypically distinct from other T helper lymphocytes both in mice and humans, and to be functionally essential to provide selection signals to germinal center B cells. These signals are required to generate long-lived antibody responses [32–36]. Tfh cells are generated from naive CD4+ T cells upon activation by peptide MHC class II complexes on antigen presenting cells, resulting in upregulation of the costimulatory molecule ICOS, the coinhibitory molecule programmed cell death protein 1 (PD-1), the signature transcriptional factor B-cell lymphoma 6 protein Bcl-6, and the chemokine receptor CXCR5. This is believed to take place in the first 2–3 days of exposure to antigen. By day 4, dynamic changes in the form of lymphocyte migration are suggested to occur within the secondary lymphoid tissue to enhance the chance of encounter between these activated CD4+ T cells and rare antigen-experienced B cells [37]. Although the evidence for lymphocyte migration is still controversial, cognate interaction with B cells is thought to play a role in sustaining Tfh cells phenotypes, and it is the homing of Tfh cells in germinal centers that distinguishes them from other differentiated T helper cells. As the principle T helper cell in germinal centers associated with promotion of humoral immunity and B cell responses, Tfh cells should gain future prominence in atherosclerosis research as the roles of B cells and humoral immunity in this disease become better understood [38].
T Helper Cell Plasticity
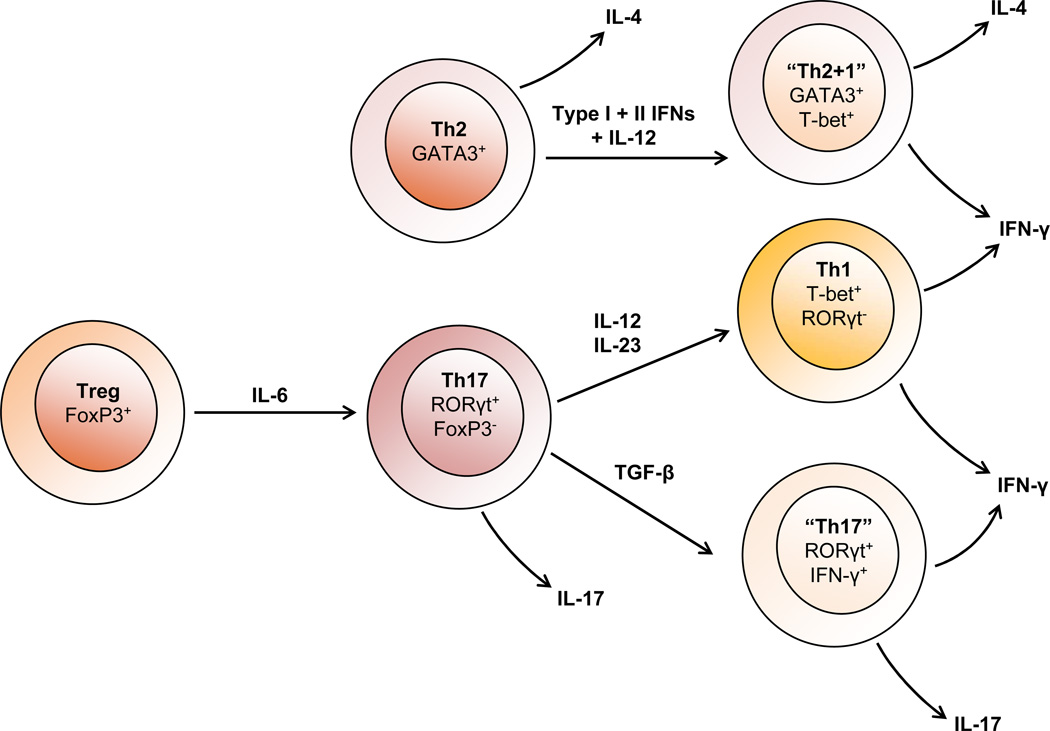
One problem with determining specific subsets involved with disease processes is that while there is cross-regulation among the subpopulations, there is also plasticity among the populations [39]. It is now clear that most T cell populations are highly malleable and capable of converting to different phenotypes (Figure 1). For example, culturing FoxP3+ Treg cells with IL-6 downregulates FoxP3 expression and abrogates suppressive activity while increasing expression of IL-17, indicating conversion of Treg cells to classical Th17 cells [40]. There is also excellent evidence for conversion between Th17 and Th1 cells both in vitro and in vivo. Transfer of Th17 cells from IL-17 reporter mice (which clearly identify the cells as IL-17 producing) show that these cells will secrete substantial amounts of INF-γ in immunodeficient recipient animals [41]. Additionally, cells can be found coexpressing IFN-γ and IL-17 suggesting an intermediate phenotype [41] [20]. Plasticity in cytokine expression also correlates with expression of signature transcription factors. Thus, Th2 cells have been induced to make IFN-γ and such cells express both GATA3 and T-bet [42]. However, while expression of hybrid phenotypes are possible, expression may not be equal and one phenotype may still dominate in the hybrid cell type. Plasticity of the T helper cells may also depend upon epigenetic alterations mediated by histone methylation [43].
Fig. 1.
CD4+ T helper cell plasticity. Various lines of evidence in vitro and in vivo demonstrate plasticity between T helper subsets. Among the best characterized is the conversion of Tregs to IL-17 cells. In the presence of IL-6, Treg cells downregulate FoxP3 expression and convert to a Th17 phenotype. In the absence of TGF-β, both IL-23 and IL-12 promote differentiation of Th17 cells to interferon-γ (IFN-γ) producing Th1 cells. While TGF-β promotes the maintenance of Th17 cells, culturing these cells in the presence of TGF-β can also give rise to an “intermediate” subset that secrete both IFN-γ and IL-17. There is also evidence for the reprogramming of Th2 cells in response to type I and II interferons (IFNs) and interleukin-12 (IL-12), resulting in hybrid “Th2+1” cells that stably coexpress GATA-3 and T-bet and secrete both IL-4 and IFN-γ.
Role of CD4+ T Helper Cells in Atherosclerosis
Evidence from both humans and mouse models implicate Th1 immunity in atherosclerosis. oxLDL is one of the leading antigens implicated in immunity in atherosclerosis, and T cells have been isolated from human plaques which are reactive to this antigen and which have a Th1 phenotype [14]. There is also a bias toward Th1 cytokines (IFN-γ, IL-12, IL-15, IL-18) in human plaques but a paucity of Th2 cytokines [8, 14, 44]. Our laboratory recently identified cross-sectional associations of Th1 cells with subclinical atherosclerosis in 917 participants of the Multi-Ethnic Study of Atherosclerosis (MESA) [45]. In this study, Th1 cells were associated with two different measures of subclinical atherosclerosis, common carotid artery intimal media thickness (IMT) and coronary artery calcification (CAC), independent of known CVD risk factors [45]. Another recent prospective study by Engelbertsen et al. failed to identify associations of Th1 cells with IMT or incident CVD, however this study utilized cells stored at −140°C for several years, which may not have been representative of the original cell population [46].
The evidence from animal models is even more convincing. Transgenic mice deficient in the Th1 transcription factor T-bet [47], signature cytokine IFN-γ [48], or the Th1 polarizing factors IL-12 [49] or IL-18 [50] developed significantly less atherosclerosis than animals having competent Th1 responses. Exogenous administration of IL-6 [51], IL-12 [52], IL-18 [53], or IFN-γ [54] exacerbates atherosclerosis in mice. In particular, IFN-γ has been implicated as pro-atherogenic through multiple mechanisms including: activation of macrophages, endothelial, and smooth muscle cells; inhibition of cholesterol efflux; and weakening of the fibrous cap [55].
The role of Th2 cells in atherosclerosis is less certain. Th2 cells can inhibit Th1 development [56] and suppress pro-inflammatory cytokine release [57], and have been suggested to be anti-atherogenic in murine atherosclerosis, as evidenced by the finding that STAT6−/− mice, which lack Th2 cells, developed increased atherosclerosis compared to wild-type animals [12]. Th2 cells, however, are pro-inflammatory in conditions such as asthma [58], and have also been reported in some experimental models as pro-atherogenic [49, 59]. In contrast to IFN-γ, the Th2 signature cytokine IL-4 has more controversial effects on atherogenesis. In one study, no effect on lesion size was observed with IL-4 deficiency, or by exogenous administration of IL-4 [60], while another study showed a significant reduction in lesion size in IL-4 deficient mice [49]. In this regard, site-specific roles of IL-4 have been proposed as one possible explanation for these discrepancies [49, 59]. Recent results from a prospective population-based epidemiology study with 15 years of follow-up reported that high numbers of circulating Th2 cells at baseline were independently associated with decreased IMT [46]. High numbers of Th2 cells were also independently associated with a reduced risk of acute myocardial infarction in women in this same study [46]. Similarly, Th2 cells were negatively associated with common carotid IMT in the MESA [45]. These results from population-based studies are consistent with an anti-atherogenic role of Th2 cells.
While Th1 cells predominate among the T cell populations in both human and mouse atherosclerotic plaque infiltrating T cells, Th17 cells are present in both species and various lines of evidence provide support for a pathologic role for this subpopulation [61]. Evidence in the human is more circumstantial and depends predominantly on the demonstration that levels of IL-17A or Th17 cells are increased in CVD patients compared to controls [62]. However, plasma levels of IL-17A also showed no correlations to carotid artery IMT in a separate study [63]. In the mouse where genetic manipulations are possible, there is more direct and concrete evidence for a part for Th17 in atherogenesis. Use of IL-17a−/−or IL-17 receptor a−/− (IL-17ra−/−) bone marrow chimeras, or blocking of IL-17A significantly reduced aortic lesion size by approximately 50% in most, but not all, studies [63–69], with other reports indicating an IL-17-dependent reduction in lesion development and vascular inflammation [70]. Reduced lesion size in the former studies correlated with decreased numbers of macrophage in the plaque and reduced expression of adhesion molecules, suggesting that monocyte recruitment is inhibited when Th17 cells are blocked. IL-6 and granulocyte colony-stimulating factor (G-CSF) levels were decreased which could also contribute to protection. Further investigations are required to determine the role of Th17 cells in atherosclerosis in humans.
There is significant evidence that Treg cells are protective in atherosclerosis in mouse models [71]. Evidence in human CVD, however, remains circumstantial and minimal. A recent study reported that patients with vulnerable coronary plaques have lower circulating levels of Treg cells and IL-10 compared with stable controls [72]. Another study found that low circulating levels of Treg cells at baseline (identified as CD4+FoxP3+) were associated with an increased risk for the development of myocardial infarction [73]. In contrast, no differences in Treg cell levels (identified as CD4+CD25highCD127low) were observed in a subgroup of coronary artery disease patients with rapid versus slow common carotid artery IMT progression over 6 year of follow-up [74]. Treg cell levels were also found to be similar in chronic stable angina patients compared to controls in a study by this same group [74]. Experiments in mice demonstrated that Treg numbers were reduced, and functional properties suppressed, in atherosclerotic animals [75]. Adoptive transfer of Treg cells or induction of tolerance reduced atherogenesis through either IL-10 or TGF-β [75–77]. Furthermore, studies have shown that statins significantly increase Treg cell numbers, TGF-β, and IL-10 in atheromas in mice [78], which may partially explain the atheroprotective effects of these drugs. Finally, as indicated above, IL-2 is essential to the maintenance and survival of Treg cells. Studies have been performed using fusion IL-2 protein in mouse models which preferentially localized to the atheroma, and this approach resulted in enhanced Treg cell localization to the plaque, and was associated with reduced macrophage localization and an overall reduction in plaque size [79]. In humans, elevated levels of soluble IL-2 receptor (sIL-2r) were associated with progression of CAC [80], and sIL-2r and IL-2 have been proposed as atherosclerosis risk markers [80–82]. Collectively, the animal model data implicate a protective role of Treg cells in atherosclerosis; whether these cells play a similar role in atherosclerosis in humans, and which specific Treg subsets, needs to be clarified.
Genetic Predisposition to Th1/Th2 Bias
Early in the study of T helper cell differentiation, it became clear that certain inbred mouse strains have an inherent bias towards either a Th1 or Th2 cell phenotype. This was first apparent with Leishmania major infections where pathogen control is highly dependent upon Th1 cells and IFN-γ activation of macrophage. C57Bl/6 mice naturally give a strong Th1 cytokine response and are inherently resistant to Leishmania. In contrast, BALB/c mice give a natural Th2 cytokine response and are inherently susceptible to infection [83]. This Th1/Th2 bias is also seen between C57Bl/6 and BALB/c mice fed high fat diets, with C57Bl/6 mice showing a predominant Th1 phenotype and BALB/c mice showing a predominant Th2 phenotype based on IFN-γ or IL-4 expression by CD4+ cells in peripheral blood and spleen [12].
Consistent with the reported roles of Th1 and Th2 cells in atherosclerosis described above, C57Bl/6 mice developed significantly larger atherosclerotic lesions at the aortic root while BALB/c mice had few or no lesions despite equivalent hypercholesterolemia in both mouse strains [12]. The protection in the BALB/c mice was clearly the result of a Th2 cell bias as BALB/c STAT6−/− mice, which could not develop Th2 cells due to the absence of this crucial transcription factor, generated excellent Th1 cell responses and atherosclerosis when placed on a high fat diet [12].
These data suggest that with a diverse set of antigens, both infectious and non-infectious, there is a genetic predisposition that can modulate a developing adaptive immune response toward a particular T helper phenotype, and that this predisposition may confer enhanced susceptibility towards different Th-biased diseases. Congenic mapping has determined genetic trait loci on mouse chromosomes 5, 11, 12, 14, 15, 16, 17, 18, and 19 which impact Th2 bias, but the specific underlying determinants defined by the genetic loci are not known in most instances [84–87]. On chromosome 16, Dicer1.2, also known as Mina, acts as an IL-4 repressor. Mina is a member of the jumonji (JmjC) protein family and inversely correlates to Th2 bias in inbred mouse strains [88].
Genetic variations in other transcription factors, cytokines or cytokine receptors involved in T helper cell differentiation pathways could equally impact genetic bias in individuals resulting in a sustained predilection toward a specific adaptive immune response. For example, single nucleotide polymorphism (SNP) analysis of polymorphisms in T-bet (TBX21) and STAT4, transcription factors involved in Th1 responses, showed specific genotypes associated with increased risk for systemic sclerosis, and with other genotypes associated with elevated Th2 cytokine levels [89]. Emerging evidence has also identified important consequences of epigenetic histone modification on the regulation of chromatin states at loci of genes for Th-differentiating transcription factors and signature Th cytokines [43, 90]. These data suggest that changes in epigenetic profiles, as well as polymorphisms or altered regulation of genes involved in epigenetic regulation [91], may influence inherent Th bias. On an individual basis, the predisposition towards a particular Th bias could have a major impact on health outcomes as individuals with a predilection toward Th1 bias might be more susceptible to Th1 dependent diseases including atherosclerosis, rheumatoid arthritis, Type 1 diabetes, or multiple sclerosis, whereas individuals with a predilection toward a Th2 bias would likely be more susceptible to Th2 dependent diseases such as asthma or systemic lupus erythematosus (SLE).
Stable Th1/Th2 Phenotypes in Healthy Human Volunteers
If inbred mouse strains have an inherent Th1/Th2 bias which is genetically determined and which can have a major impact in susceptibility or resistance to various diseases, the question arises whether humans also show natural genetic variation in T helper cell bias in the absence of known disease. It is well known that patients with specific diseases such as asthma, SLE, or multiple sclerosis have an increase in Th2 or Th1 cells relative to controls without these inflammatory/autoimmune diseases [89, 92, 93], but as these individuals have established pathologic conditions, it is not clear whether the T helper cell imbalance occurred first or is a later consequence of the disease process.
To evaluate whether humans have inherent stable Th1/Th2 biases, our laboratory performed a prospective study on thirty apparently healthy Caucasian individuals (14 men and 16 women) ranging in age from 17 to 53 years (average 38 ± 10 years). Peripheral blood mononuclear cells (PBMC) were obtained multiple times over an 18 month period (average=6 blood collections/individual in twenty-eight individuals). T helper indices were measured by activating PBMCs in vitro with PMA, ionomycin, and brefeldin A, which is a standard method for activating T cells for determining intracellular cytokine expression [94]. The activated cells were stained with antibodies to CD4, IFN-γ, and IL-4 and analyzed by flow cytometry to measure the percentage of CD4+ cells that were Th1 cells (%Th1; CD4+IFN-γ+), and Th2 cells (%Th2; CD4+IL-4+), and to calculate %Th1/%Th2 ratios. Detailed materials and methods are available in the Online Resource. The T helper indices of the study population are shown in Table 1.
Table 1.
T Helper Indices and Immunoglobulin Subclass Measures in Apparently Healthy Men and Women
| Phenotype | Mean (SD) | Median | Range |
|---|---|---|---|
| T helper Indices (n=30) | |||
| % Th1 cells (% of CD4+ cells) | 20.9 (7.4) | 19.0 | 9.4–40.2 |
| Secreted IFN-γ (pg/ml) | 535 (212) | 212 | 503–789 |
| % Th2 cells (% of CD4+ cells) | 0.70 (0.33) | 0.66 | 0.18–1.6 |
| Secreted IL-4 (pg/ml) | 96.9 (56.6) | 94.9 | 22.5–270 |
| %Th1/%Th2 | 46.4 (40.0) | 33.3 | 10.9–223 |
| Immunoglobulin Subclasses (n=28) | |||
| IgE (serum) (IU/mL) | 93.2 (122.8) | 26.5 | 2.3–404 |
| IgG1 (plasma) (g/L) | 6.9 (2.3) | 6.4 | 4.2–15.1 |
| IgG2 (plasma) (g/L) | 3.2 (1.3) | 3.5 | 1.3–6.1 |
| IgG3 (plasma) (g/L) | 0.39 (0.19) | 0.34 | 0.12–1.0 |
| IgG4 (plasma) (g/L) | 0.43 (0.31) | 0.34 | 0.14–1.28 |
Data in this table originally appeared in Sallam R, Bernstein I, Tracy RP. The Cellular Epidemiology of Inflammation: The Biochemical Indices of T Helper Cells are Stable Phenotypes Related to Plasma Markers of Inflammation, Adiposity, and Hormonal Status. EJBMB 26 (Supplement): 453–472 (2008).
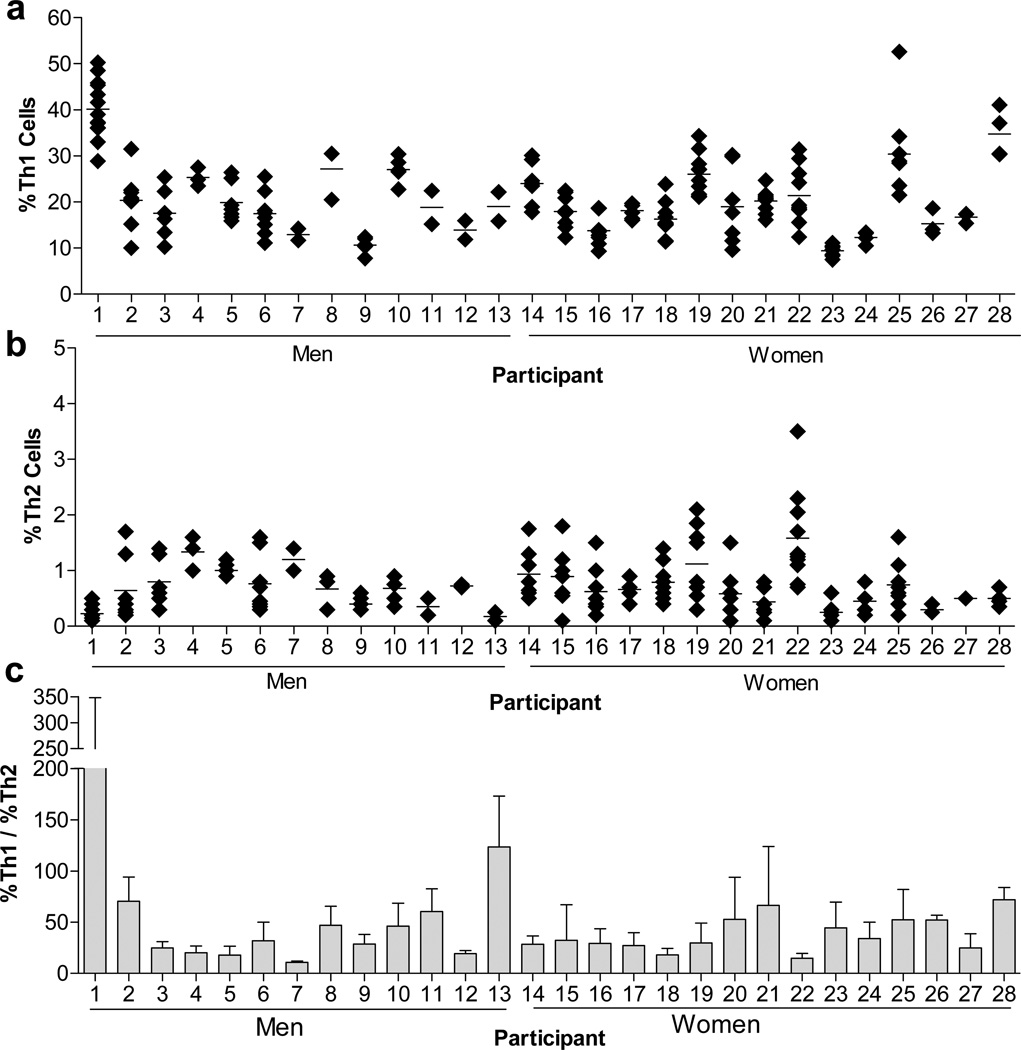
As shown in Figures 2a&b, we found that Th1 and Th2 cell phenotypes were generally stable over time; the calculated coefficients of variation (CVs) are shown in Table 2. We observed analytical variability comparable with well established biomarkers, such as cholesterol and C-reactive protein, routinely used in clinical and cardiovascular epidemiological research [95]. Less stability was observed with the Th2 phenotype, likely due to the analytical challenges associated with the much smaller number of circulating Th2 cells (mean (SD)=%0.70 (%0.33)). The %Th1/%Th2 ratio was also generally consistent over time (Figure 2c).
Fig. 2.
Evaluation of T helper phenotypes in 28 apparently healthy men and women over an 18 month period. PBMCs were collected, activated in vitro with PMA, ionomycin, and brefeldin A, and measured by flow cytometry for (A) the % of CD4+ T cells positive for IFN-γ (CD4+IFN-γ+; Th1 cells), and (B) the % of CD4+ T cells positive for IL-4 (CD4+IL-4+; Th2 cells). (C) The ratio of %Th1/%Th2 cells was also evaluated. Each data point represents a single measurement in apparently healthy individuals collected over a period of 18 months. Data in this figure originally appeared in Sallam R, Bernstein I, Tracy RP. The Cellular Epidemiology of Inflammation: The Biochemical Indices of T Helper Cells are Stable Phenotypes Related to Plasma Markers of Inflammation, Adiposity, and Hormonal Status. EJBMB 26 (Supplement): 453–472 (2008).
Table 2.
Components of Variability for T Helper Indices in 28 Healthy Men and Women
| Phenotype | CVa (%) | CVi (%) | CVg (%) | II |
|---|---|---|---|---|
| % CD4+ cells | 3.7 | 8.7 | 11.4 | 0.76 |
| % Th1 cells | 8.3 | 23.0 | 37.6 | 0.61 |
| Secreted IFN-γ | 6.6 | 23.1 | 39.5 | 0.58 |
| % Th2 cells | 35.9 | 61.0 | 37.1 | 1.64 |
| Secreted IL-4 | 9.6 | 37.1 | 50.0 | 0.74 |
CVa = analytical coefficient of variation; CVi = within subject coefficient of variation; CVg = between subject coefficient of variation; II = Index of Individuality (CVi/CVg). Data in this table originally appeared in Sallam R, Bernstein I, Tracy RP. The Cellular Epidemiology of Inflammation: The Biochemical Indices of T Helper Cells are Stable Phenotypes Related to Plasma Markers of Inflammation, Adiposity, and Hormonal Status. EJBMB 26 (Supplement): 453–472 (2008).
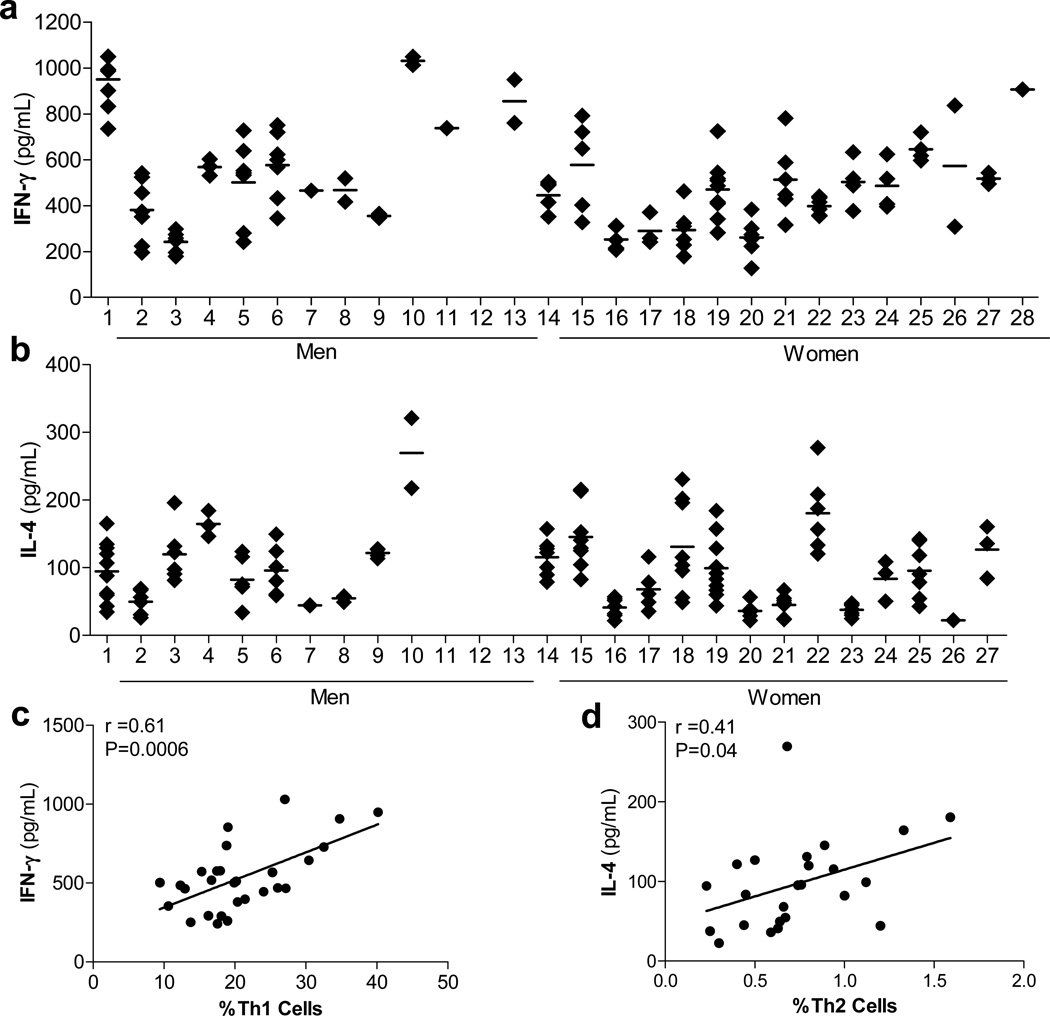
Evaluation of signature Th1 and Th2 cytokines from the supernatants of stimulated PBMCs, shown in Figures 3a&b, demonstrated that %Th1 cells were correlated with secreted IFN-γ (r=0.61; P=0.0006), while %Th2 cells were correlated with secreted IL-4 (r=0.41; P=0.04) (Figures 3c & d). The natural logarithm (ln) transformed %Th1/%Th2 ratio was correlated with IFN-γ (r=0.60; p=0.0008) and not IL-4 (r=−0.20; P=0.34).
Fig. 3.
Associations of Th1 and Th2 phenotypes with secretion of IFN-γ and IL-4. (A) PBMCs were activated in vitro and measured by ELISA and chemiluminescence immunoassay for the secretion of (A) IFN-γ and (B) IL-4; the analytical coefficients of variation (CVs) were 6.0% and 8.2%, respectively. Each point represents a single measurement in apparently healthy volunteers over an 18 month period. The number of participants with IL-4 measurements varies to a small degree. The averaged analyte value of each volunteer was used to calculate Pearson correlation coefficients (r) of (C) %Th1 and IFN-γ and (D) %Th2 and IL-4. Data in this figure originally appeared in Sallam R, Bernstein I, Tracy RP. The Cellular Epidemiology of Inflammation: The Biochemical Indices of T Helper Cells are Stable Phenotypes Related to Plasma Markers of Inflammation, Adiposity, and Hormonal Status. EJBMB 26 (Supplement): 453–472 (2008).
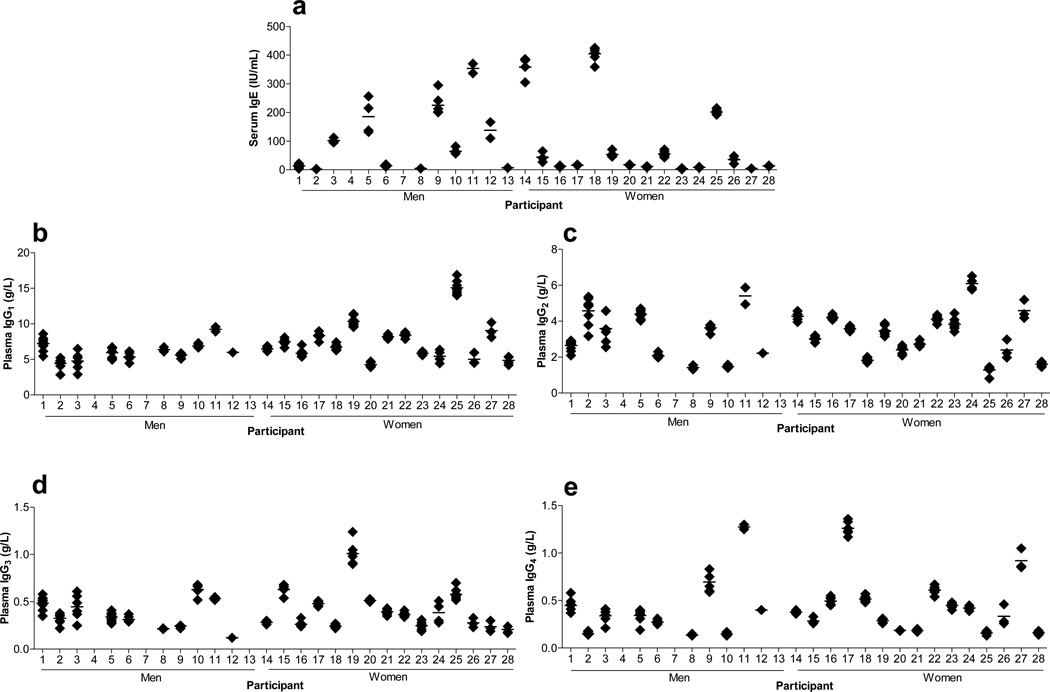
T helper cells are critical regulators of immunoglobulin production including control of immunoglobulin isotype switching [96]. We measured the immunoglobulin subclass molecules, IgE, IgG1, IgG2, IgG3, and IgG4, and examined their associations with the T helper indices; the individual data are presented in Figures 4a–e. As shown in Table 3, %Th1 cells were negatively correlated with IgG2 and lnIgG4, and showed a positive, but not statistically significant, correlation with IgG1 and IgG3. Despite moderate correlation coefficients (r=0.32 and 0.31), the lack of statistical significance with IgG1 and IgG3 likely reflected the small sample size of our study population. %Th2 cells were positively correlated with lnIgE, while the ln%Th1/%Th2 ratio was negatively correlated with lnIgE (Table 3). Associations with Th2 measures were likely biased towards the null due to the increased variability in the measurements of a much smaller population of circulating cells. These results were generally consistent with data from other lines of research [97–100], and suggest the value of studying these associations in larger cohorts of healthy participants.
Fig. 4.
Quantitative immunoassay analyses of IgE and IgG1–4 immunoglobulin subclasses. (A) Serum was assayed for IgE by chemiluminescence assay; the analytical CV was 5%. (B–E) IgG1–4 subclasses were measured in plasma by immunochemical assay; analytical CVs were all <5%. Each point represents a single measurement collected over 18 months. Data in this figure originally appeared in Sallam R, Bernstein I, Tracy RP. The Cellular Epidemiology of Inflammation: The Biochemical Indices of T Helper Cells are Stable Phenotypes Related to Plasma Markers of Inflammation, Adiposity, and Hormonal Status. EJBMB 26 (Supplement): 453–472 (2008).
Table 3.
Associations of T Helper Phenotypes with IgE and IgG1–4 Immunoglobulin Subclasses in 28 Healthy Men and Women
| Th Phenotype | lnIgE | IgG1 | IgG2 | IgG3 | lnIgG4 |
|---|---|---|---|---|---|
| %Th1 | –0.02 (0.92) | 0.32 (0.12) | –0.44 (0.03) | 0.31 (0.12) | –0.42 (0.03) |
| %Th2 | 0.39 (0.04) | 0.22 (0.30) | 0.01 (0.95) | 0.26 (0.21) | –0.06 (0.74) |
| ln%Th1/%Th2 | –0.43 (0.02) | 0.06 (0.76) | –0.23 (0.26) | 0.18 (0.40) | –0.27 (0.19) |
Data are Pearson correlation coefficients (r) (p-value). ln=natural logarithm transformed. Data in this table originally appeared in Sallam R, Bernstein I, Tracy RP. The Cellular Epidemiology of Inflammation: The Biochemical Indices of T Helper Cells are Stable Phenotypes Related to Plasma Markers of Inflammation, Adiposity, and Hormonal Status. EJBMB 26 (Supplement): 453–472 (2008).
Collectively, these data demonstrated that individuals display inherent T helper phenotypes that are consistent over time, which distinguishes one person from another, in the absence of inflammatory/autoimmune diseases. These data suggest that Th bias may precede clinical disease, and potentially increase risk to pathological conditions such as CVD.
Summary and Future Perspectives: Implications of T Helper Cell Polarization as a Stable Phenotype for Cardiovascular Disease
The presence of various T lymphocyte subsets in atherosclerotic lesions have been well established in human samples, and genetic manipulations in mouse model systems have added considerable information regarding their functional roles. Th1 cells in particular have been the focus of extensive study, revealing that subjects with coronary syndromes and advanced atherosclerosis have a bias towards Th1 [8, 44, 101–103]. Although less firmly established, Th2 cells are considered anti-atherogenic [45, 46]. Collectively, these findings suggest that individuals with an inherent disposition towards a particular Th bias may be at increased risk of disease. While not providing direct evidence, the results from our laboratory demonstrating that healthy individuals express a consistent Th phenotype over time, in the absence of overt disease, strongly suggests that such bias precedes clinical disease. Current animal model and human genetic association data support this position, and provides support for longitudinal epidemiological studies to address this hypothesis.
In addition to genetic determinants, the demonstrated plasticity between T helper populations illustrates the importance of environmental influences on Th polarization. For example, due to plasticity of T helper cell phenotypes, an individual’s inherent Th1 or Th2 polarization may be switched in response to diet, stress, or other factors [104, 105]. As such, if we are to assume an individual born with a protective Th phenotype, and assume a change in their immune status to an injurious subtype due to lifestyle choices and environmental exposures, this leads to general unanswered questions: 1) if the damaging lifestyle exposure were removed, would an individual with a genetic predisposition towards a protective phenotype return to that phenotype faster, and be at a lower disease risk, compared to someone with a genetic predisposition toward a injurious phenotype; and 2) under conditions of damaging lifestyle exposures, do individuals with genetic predisposition to protective immunity ever convert to as strong of a pro-atherosclerotic immune phenotype compared to someone with a genetic predisposition towards that pro-inflammatory phenotype (e.g., Th1, Th17). The studies in the literature suggest that plasticity exists, but our understanding of the extent to which this plasticity influences Th polarization, and disease status, remains unknown.
Given the potential important consequences of inherent Th bias on health outcomes, the need remains to understand the genetic, molecular, and environmental determinants of Th polarization. Despite substantial efforts in identifying loci in mouse models, the specific underlying genetic determinants of Th bias remain largely unknown. Cellular phenotyping, genetic associations studies, and serological analysis in large population-based epidemiology settings is a logical next step in a system-based biological approach to understanding these determinants and their relationships with disease states. Measurements of biomarkers in population-based studies have previously yielded critical new insights regarding key mechanisms related to the pathophysiology of chronic inflammatory diseases, and should continue to be developed in order to allow opportunities for pathway-based analyses, expression patterning, and dynamic inquisition of cells ex vivo through standardized challenges.
At present, little information concerning adaptive immune responses, T helper polarization, and T helper plasticity is available from healthy, free-living population-based studies. Continued improvements in biomarkers and risk factors, along with newer genomic and proteomic approaches, will require an evolution from molecular to cellular epidemiology, and will allow new important insight into Th biasing and plasticity, and the progression of inflammatory disease states, such as atherosclerosis. While the clinical significance and predictive value of inherent T helper bias remains to be examined, the current clinical data indicate the importance of prospective epidemiological studies aimed at determining the role of T helper subpopulations and adaptive immune responses in atherosclerosis.
Evidence from animal models suggests the importance of research into strategies to modify T helper cell biasing as a way to reduce atherosclerotic burden. Data from inbred mouse models delineating a genetic Th1 bias in C57Bl/6 mice which promotes atherosclerotic burden, but also confers a natural resistance to Leishmania major infection [83], suggests immunization strategies that preferentially prime or inhibit specific Th subsets could redirect the subsequent immune response and inhibit the progression of inflammatory diseases such as atherosclerosis.
Of considerable therapeutic interest are the anti-atherogenic, immunomodulatory properties of statins [106], the cholesterol lowering 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Statins inhibit T cell activation by decreasing IFN-γ-dependent MHC class II protein expression through mechanisms that involve isoprenoid intermediates [106, 107]. Statin treatment has been demonstrated to reduce atherosclerosis and autoimmune disease in mouse models by promoting Th2 bias and lowering Th1/Th2 ratios [108–110]. Similarly, human clinical studies have reported modulation of Th1/Th2 ratios in CVD, including unstable angina and MI [110–112], and in patients with chronic heart failure [113]. Collectively, these data suggest that some of the beneficial effects of statins may be conferred through the modulation of the Th1/Th2 balance, which would further indicate the importance of Th bias in atherosclerosis and CVD. Other medical interventions, e.g., weight loss and gastric banding, have also been reported to modulate Th1/Th2 ratios and attenuate inflammation [105].
Importantly, the success of weight loss and other therapeutic interventions may depend upon the plasticity of Th polarization. While global genetic knockout of specific T helper activation pathways have proven successful in reducing atherosclerosis in mouse models, it will be important to continue to refine and develop strategies that selectively modulate appropriate and specific Th subsets in a manner that reduces risk of disease, without compromising their beneficial, normal physiological host responses.
Supplementary Material
Statement of Clinical Relevance.
Atherosclerosis is characterized by the accumulation of T helper lymphocytes in the atherosclerotic plaque, and is a leading cause of mortality globally. Evidence from animal models suggests the importance of research into strategies to modify T helper cell biasing as a way to reduce atherosclerotic burden.
Acknowledgments
The authors wish to thank the Egyptian Journal of Biochemistry and Molecular Biology for permission to re-publish the data in Figures 2–4 and in Tables 1–3 which originally appeared in Sallam R, Bernstein I, Tracy RP. The Cellular Epidemiology of Inflammation: The Biochemical Indices of T Helper Cells are Stable Phenotypes Related to Plasma Markers of Inflammation, Adiposity, and Hormonal Status. EJBMB 26 (Supplement): 453–472 (2008). This work was supported in part by the National Heart, Lung, and Blood Institute (NHLBI) Training Grant 5T32HL007594-27 (NCO), HL108371 (SAH), and R01 HL-46696, R01 HL58329 (RPT).
Footnotes
Ethical standards
All experiments comply with the current laws of the country in which they were performed.
References
- 1.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311(5757):83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 2.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203(9):2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koltsova EK, Ley K. How dendritic cells shape atherosclerosis. Trends Immunol. 2011;32(11):540–547. doi: 10.1016/j.it.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woollard KJ, Geissmann F. Monocytes in atherosclerosis subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106(2):383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 6.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 7.Hansson GK, Libby P. The immune response in atherosclerosis a double-edged sword. Nat Rev Immunol. 2006;6(7):508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 8.Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145(1):33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 9.Paulsson G, Zhou X, Tornquist E, Hansson GK. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20(1):10–17. doi: 10.1161/01.atv.20.1.10. [DOI] [PubMed] [Google Scholar]
- 10.Stemme S, Holm J, Hansson GK. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992;12(2):206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102(24):2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 12.Huber S, Sakkinen M, David C, Newell M, Tracy R. T helper cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103:2610–2616. doi: 10.1161/01.cir.103.21.2610. [DOI] [PubMed] [Google Scholar]
- 13.Hansson GK, Jonasson L, Holm J, Claesson-Welsh L. Class II MHCantigen expression in the atherosclerotic plaque smooth muscle cells express HLA-DR, HLA-DQ and the invariant gamma chain. Clin Exp Immunol. 1986;64(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- 14.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92(9):3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanno Y, Vahedi G, Hirahara K, Singleton K, O'Shea JJ. Transcriptional and epigenetic control of T helper cell specification molecular mechanisms underlying commitment and plasticity. Annu Rev Immunol. 2012;30:707–731. doi: 10.1146/annurev-immunol-020711-075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosmann T, Coffman R. Th1 Th2 cells different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman LA, Banica M, Reiner SL, Hunter CA. STAT1 plays a critical role in the regulation of antimicrobial effector mechanisms, but not in the development of Th1-type responses during toxoplasmosis. J Immunol. 2004;172(1):457–463. doi: 10.4049/jimmunol.172.1.457. [DOI] [PubMed] [Google Scholar]
- 19.Schulz EG, Mariani L, Radbruch A, Hofer T. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-gamma and interleukin-12. Immunity. 2009;30(5):673–683. doi: 10.1016/j.immuni.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Wan YY. Multi-tasking of helper T cells. Immunology. 2010;130(2):166–171. doi: 10.1111/j.1365-2567.2010.03289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jager A, Kuchroo VK. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol. 2010;72(3):173–184. doi: 10.1111/j.1365-3083.2010.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal S, Gurney AL. IL-17 prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71(1):1–8. [PubMed] [Google Scholar]
- 23.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160(7):3513–3521. [PubMed] [Google Scholar]
- 24.Marwaha AK, Leung NJ, McMurchy AN, Levings MK. TH17 Cells in Autoimmunity and Immunodeficiency: Protective or Pathogenic? Front Immunol. 2012;3:129. doi: 10.3389/fimmu.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torgerson TR. Regulatory T cells in human autoimmune diseases. Springer Semin Immunopathol. 2006;28(1):63–76. doi: 10.1007/s00281-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 28.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 29.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4(9):665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 30.Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 2010;3(3):216–229. doi: 10.1038/mi.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozdemir C, Akdis M, Akdis CA. T regulatory cells their counterparts masters of immune regulation. Clin Exp Allergy. 2009;39(5):626–639. doi: 10.1111/j.1365-2222.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- 32.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173(1):68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 33.Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104(7):1952–1960. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- 34.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 35.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5(11):853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 36.Haynes NM. Follicular associated T cells and their B-cell helper qualities. Tissue Antigens. 2008;71(2):97–104. doi: 10.1111/j.1399-0039.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 37.King C. A fine romance: T follicular helper cells and B cells. Immunity. 2011;34(6):827–829. doi: 10.1016/j.immuni.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 38.van Leeuwen M, Damoiseaux J, Duijvestijn A, Tervaert JW. The therapeutic potential of targeting B cells and anti-oxLDL antibodies in atherosclerosis. Autoimmun Rev. 2009;9(1):53–57. doi: 10.1016/j.autrev.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105(47):18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegazy AN, Peine M, Helmstetter C, Panse I, Frohlich A, Bergthaler A, Flatz L, Pinschewer DD, Radbruch A, Lohning M. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 2010;32(1):116–128. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30(1):155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benagiano M, Azzurri A, Ciervo A, Amedei A, Tamburini C, Ferrari M, Telford JL, Baldari CT, Romagnani S, Cassone A, et al. T helper type 1 lymphocytes drive inflammation in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 2003;100(11):6658–6663. doi: 10.1073/pnas.1135726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tracy RP, Doyle MF, Olson NC, Huber SA, Jenny NS, Sallam R, Psaty BM, Kronmal RA. T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2(3):e000117. doi: 10.1161/JAHA.113.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engelbertsen D, Andersson L, Ljungcrantz I, Wigren M, Hedblad B, Nilsson J, Bjorkbacka H. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler Thromb Vasc Biol. 2013;33(3):637–644. doi: 10.1161/ATVBAHA.112.300871. [DOI] [PubMed] [Google Scholar]
- 47.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102(5):1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99(11):2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163(3):1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59(1):234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 51.Huber S, Sakkinen P, Conce D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arteriosclerosis, Thrombosis and Vascular Biology. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 52.Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19(3):734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 53.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ Res. 2002;90(2):E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 54.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157(6):1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou X. CD4+ T cells in atherosclerosis. Biomed Pharmacother. 2003;57(7):287–291. doi: 10.1016/s0753-3322(03)00082-9. [DOI] [PubMed] [Google Scholar]
- 56.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9(5):745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 57.Hart PH, Vitti GF, Burgess DR, Whitty GA, Piccoli DS, Hamilton JA. Potential antiinflammatory effects of interleukin 4 suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989;86(10):3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111(3):450–463. doi: 10.1067/mai.2003.169. quiz 464. [DOI] [PubMed] [Google Scholar]
- 59.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol. 2002;22(3):456–461. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- 60.King VL, Cassis LA, Daugherty A. Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am J Pathol. 2007;171(6):2040–2047. doi: 10.2353/ajpath.2007.060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butcher M, Galkina E. Current views on the functions of interleukin-17A–producing cells in atherosclerosis. Thromb Haemost. 2011;106(5):787–795. doi: 10.1160/TH11-05-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Z, Lu F, Pan H, Zhao Y, Wang S, Sun S, Li J, Hu X, Wang L. Correlation of peripheral Th17 cells and Th17-associated cytokines to the severity of carotid artery plaque and its clinical implication. Atherosclerosis. 2012;221(1):232–241. doi: 10.1016/j.atherosclerosis.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 63.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, et al. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31(7):1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Bockler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183(12):8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 65.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121(15):1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185(10):5820–5827. doi: 10.4049/jimmunol.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Es T, van Puijvelde GH, Ramos OH, Segers FM, Joosten LA, van den Berg WB, Michon IM, de Vos P, van Berkel TJ, Kuiper J. Attenuated atherosclerosis upon IL-17R signaling disruption in LDLr deficient mice. Biochem Biophys Res Commun. 2009;388(2):261–265. doi: 10.1016/j.bbrc.2009.07.152. [DOI] [PubMed] [Google Scholar]
- 68.Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, Yao R, Xie JJ, Yu X, Xia N, et al. Inhibition of IL-17A in atherosclerosis. Atherosclerosis. 2011;215(2):471–474. doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 69.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110(5):675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206(10):2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sasaki N, Yamashita T, Takeda M, Hirata K. Regulatory T cells in atherogenesis. J Atheroscler Thromb. 2012;19(6):503–515. doi: 10.5551/jat.10934. [DOI] [PubMed] [Google Scholar]
- 72.George J, Schwartzenberg S, Medvedovsky D, Jonas M, Charach G, Afek A, Shamiss A. Regulatory T cells and IL-10 levels are reduced in patients with vulnerable coronary plaques. Atherosclerosis. 2012;222(2):519–523. doi: 10.1016/j.atherosclerosis.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Wigren M, Bjorkbacka H, Andersson L, Ljungcrantz I, Fredrikson GN, Persson M, Bryngelsson C, Hedblad B, Nilsson J. Low levels of circulating CD4+FoxP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler Thromb Vasc Biol. 2012;32(8):2000–2004. doi: 10.1161/ATVBAHA.112.251579. [DOI] [PubMed] [Google Scholar]
- 74.Ammirati E, Cianflone D, Banfi M, Vecchio V, Palini A, De Metrio M, Marenzi G, Panciroli C, Tumminello G, Anzuini A, et al. Circulating CD4+CD25hiCD127lo regulatory T-Cell levels do not reflect the extent or severity of carotid and coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(9):1832–1841. doi: 10.1161/ATVBAHA.110.206813. [DOI] [PubMed] [Google Scholar]
- 75.Mor A, Planer D, Luboshits G, Afek A, Metzger S, Chajek-Shaul T, Keren G, George J. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(4):893–900. doi: 10.1161/01.ATV.0000259365.31469.89. [DOI] [PubMed] [Google Scholar]
- 76.Sasaki N, Yamashita T, Takeda M, Shinohara M, Nakajima K, Tawa H, Usui T, Hirata K. Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation. 2009;120(20):1996–2005. doi: 10.1161/CIRCULATIONAHA.109.863431. [DOI] [PubMed] [Google Scholar]
- 77.Mallat Z, Gojova A, Brun V, Esposito B, Fournier N, Cottrez F, Tedgui A, Groux H. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2003;108(10):1232–1237. doi: 10.1161/01.CIR.0000089083.61317.A1. [DOI] [PubMed] [Google Scholar]
- 78.Meng X, Zhang K, Li J, Dong M, Yang J, An G, Qin W, Gao F, Zhang C, Zhang Y. Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol Med. 2012;18:598–605. doi: 10.2119/molmed.2011.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dietrich T, Hucko T, Schneemann C, Neumann M, Menrad A, Willuda J, Atrott K, Stibenz D, Fleck E, Graf K, et al. Local delivery of IL-2 reduces atherosclerosis via expansion of regulatory T cells. Atherosclerosis. 2012;220(2):329–336. doi: 10.1016/j.atherosclerosis.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 80.Wadwa RP, Kinney GL, Ogden L, Snell-Bergeon JK, Maahs DM, Cornell E, Tracy RP, Rewers M. Soluble interleukin-2 receptor as a marker for progression of coronary artery calcification in type 1 diabetes. Int J Biochem Cell Biol. 2006;38(5–6):996–1003. doi: 10.1016/j.biocel.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 81.Sakamoto A, Ishizaka N, Saito K, Imai Y, Morita H, Koike K, Kohro T, Nagai R. Serum levels of IgG4 and soluble interleukin-2 receptor in patients with coronary artery disease. Clin Chim Acta. 2012;413(5–6):577–581. doi: 10.1016/j.cca.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 82.Elkind MS, Rundek T, Sciacca RR, Ramas R, Chen HJ, Boden-Albala B, Rabbani L, Sacco RL. Interleukin-2 levels are associated with carotid artery intima-media thickness. Atherosclerosis. 2005;180(1):181–187. doi: 10.1016/j.atherosclerosis.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 84.Kosarova M, Havelkova H, Krulova M, Demant P, Lipoldova M. The production of two Th2 cytokines, interleukin-4 and interleukin-10, is controlled independently by locus Cypr1 and by loci Cypr2 and Cypr3, respectively. Immunogenetics. 1999;49(2):134–141. doi: 10.1007/s002510050472. [DOI] [PubMed] [Google Scholar]
- 85.Zhang F, Liang Z, Matsuki N, Van Kaer L, Joyce S, Wakeland EK, Aune TM. A murine locus on chromosome 18 controls NKT cell homeostasis and Th cell differentiation. J Immunol. 2003;171(9):4613–4620. doi: 10.4049/jimmunol.171.9.4613. [DOI] [PubMed] [Google Scholar]
- 86.Baguet A, Epler J, Wen KW, Bix M. A Leishmania major response locus identified by interval-specific congenic mapping of a T helper type 2 cell bias-controlling quantitative trait locus. J Exp Med. 2004;200(12):1605–1612. doi: 10.1084/jem.20040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi P, Xanthaki D, Rose SJ, Haywood M, Reiser H, Morley BJ. Linkage analysis of the genetic determinants of T-cell IL-4 secretion, and identification of Flj20274 as a putative candidate gene. Genes Immun. 2005;6(4):290–297. doi: 10.1038/sj.gene.6364192. [DOI] [PubMed] [Google Scholar]
- 88.Okamoto M, Van Stry M, Chung L, Koyanagi M, Sun X, Suzuki Y, Ohara O, Kitamura H, Hijikata A, Kubo M, et al. Mina, an Il4 repressor, controls T helper type 2 bias. Nat Immunol. 2009;10(8):872–879. doi: 10.1038/ni.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gourh P, Agarwal SK, Divecha D, Assassi S, Paz G, Arora-Singh RK, Reveille JD, Shete S, Mayes MD, Arnett FC, et al. Polymorphisms in TBX21 STAT4 increase the risk of systemic sclerosis evidence of possible gene-gene interaction and alterations in Th1/Th2 cytokines. Arthritis Rheum. 2009;60(12):3794–3806. doi: 10.1002/art.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weng NP, Araki Y, Subedi K. The molecular basis of the memory T cell response differential gene expression and its epigenetic regulation. Nat Rev Immunol. 2012;12(4):306–315. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamashita M, Hirahara K, Shinnakasu R, Hosokawa H, Norikane S, Kimura MY, Hasegawa A, Nakayama T. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity. 2006;24(5):611–622. doi: 10.1016/j.immuni.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 92.Misu T, Onodera H, Fujihara K, Matsushima K, Yoshie O, Okita N, Takase S, Itoyama Y. Chemokine receptor expression on T cells in blood and cerebrospinal fluid at relapse and remission of multiple sclerosis imbalance of Th1/Th2-associated chemokine signaling. J Neuroimmunol. 2001;114(1–2):207–212. doi: 10.1016/s0165-5728(00)00456-2. [DOI] [PubMed] [Google Scholar]
- 93.Robinson DS. The role of the T cell in asthma. J Allergy Clin Immunol. 2010;126(6):1081–1091. doi: 10.1016/j.jaci.2010.06.025. quiz 1092–1083. [DOI] [PubMed] [Google Scholar]
- 94.Huber SA. Depletion of gammadelta+ T cells increases CD4+ FoxP3 (T regulatory) cell response in coxsackievirus B3-induced myocarditis. Immunology. 2009;127(4):567–576. doi: 10.1111/j.1365-2567.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sakkinen PA, Macy EM, Callas PW, Cornell ES, Hayes TE, Kuller LH, Tracy RP. Analytical and biologic variability in measures of hemostasis and fibrinolysis inflammation assessment and implications for epidemiology. Am J Epidemiol. 1999;149(3):261–267. doi: 10.1093/oxfordjournals.aje.a009801. [DOI] [PubMed] [Google Scholar]
- 96.Stavnezer J. Immunoglobulin class switching. Curr Opin Immunol. 1996;8(2):199–205. doi: 10.1016/s0952-7915(96)80058-6. [DOI] [PubMed] [Google Scholar]
- 97.Widhe M, Ekerfelt C, Forsberg P, Bergstrom S, Ernerudh J. IgG subclasses in Lyme borreliosis a study of specific IgG subclass distribution in an interferon-gamma-predominated disease. Scand J Immunol. 1998;47(6):575–581. [PubMed] [Google Scholar]
- 98.Greve B, Magnusson CG, Melms A, Weissert R. Immunoglobulin isotypes reveal a predominant role of type 1 immunity in multiple sclerosis. J Neuroimmunol. 2001;121(1–2):120–125. doi: 10.1016/s0165-5728(01)00436-2. [DOI] [PubMed] [Google Scholar]
- 99.Sousa AO, Henry S, Maroja FM, Lee FK, Brum L, Singh M, Lagrange PH, Aucouturier P. IgG subclass distribution of antibody responses to protein and polysaccharide mycobacterial antigens in leprosy and tuberculosis patients. Clin Exp Immunol. 1998;111(1):48–55. doi: 10.1046/j.1365-2249.1998.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lundgren M, Persson U, Larsson P, Magnusson C, Smith CI, Hammarstrom L, Severinson E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol. 1989;19(7):1311–1315. doi: 10.1002/eji.1830190724. [DOI] [PubMed] [Google Scholar]
- 101.Soejima H, Irie A, Miyamoto S, Kajiwara I, Kojima S, Hokamaki J, Sakamoto T, Tanaka T, Yoshimura M, Nishimura Y, et al. Preference toward a T-helper type 1 response in patients with coronary spastic angina. Circulation. 2003;107(17):2196–2200. doi: 10.1161/01.CIR.0000066317.23972.CE. [DOI] [PubMed] [Google Scholar]
- 102.Methe H, Brunner S, Wiegand D, Nabauer M, Koglin J, Edelman ER. Enhanced T-helper-1 lymphocyte activation patterns in acute coronary syndromes. J Am Coll Cardiol. 2005;45(12):1939–1945. doi: 10.1016/j.jacc.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 103.Han SF, Liu P, Zhang W, Bu L, Shen M, Li H, Fan YH, Cheng K, Cheng HX, Li CX, et al. The opposite-direction modulation of CD4+CD25+ Tregs and T helper 1 cells in acute coronary syndromes. Clin Immunol. 2007;124(1):90–97. doi: 10.1016/j.clim.2007.03.546. [DOI] [PubMed] [Google Scholar]
- 104.Sherry CL, Kim SS, Dilger RN, Bauer LL, Moon ML, Tapping RI, Fahey GC, Jr, Tappenden KA, Freund GG. Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization. Brain Behav Immun. 2010;24(4):631–640. doi: 10.1016/j.bbi.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Viardot A, Lord RV, Samaras K. The effects of weight loss and gastric banding on the innate and adaptive immune system in type 2 diabetes and prediabetes. J Clin Endocrinol Metab. 2010;95(6):2845–2850. doi: 10.1210/jc.2009-2371. [DOI] [PubMed] [Google Scholar]
- 106.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6(12):1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 107.Dunn SE, Youssef S, Goldstein MJ, Prod'homme T, Weber MS, Zamvil SS, Steinman L. Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin. J Exp Med. 2006;203(2):401–412. doi: 10.1084/jem.20051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aprahamian T, Bonegio R, Rizzo J, Perlman H, Lefer DJ, Rifkin IR, Walsh K. Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J Immunol. 2006;177(5):3028–3034. doi: 10.4049/jimmunol.177.5.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 110.Hakamada-Taguchi R, Uehara Y, Kuribayashi K, Numabe A, Saito K, Negoro H, Fujita T, Toyooka T, Kato T. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces Th1 development and promotes Th2 development. Circ Res. 2003;93(10):948–956. doi: 10.1161/01.RES.0000101298.76864.14. [DOI] [PubMed] [Google Scholar]
- 111.Kinlay S, Schwartz GG, Olsson AG, Rifai N, Leslie SJ, Sasiela WJ, Szarek M, Libby P, Ganz P. High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the MIRACL study. Circulation. 2003;108(13):1560–1566. doi: 10.1161/01.CIR.0000091404.09558.AF. [DOI] [PubMed] [Google Scholar]
- 112.Link A, Ayadhi T, Bohm M, Nickenig G. Rapid immunomodulation by rosuvastatin in patients with acute coronary syndrome. Eur Heart J. 2006;27(24):2945–2955. doi: 10.1093/eurheartj/ehl277. [DOI] [PubMed] [Google Scholar]
- 113.Cheng X, Ding Y, Xia C, Tang T, Yu X, Xie J, Liao M, Yao R, Chen Y, Wang M, et al. Atorvastatin modulates Th1/Th2 response in patients with chronic heart failure. J Card Fail. 2009;15(2):158–162. doi: 10.1016/j.cardfail.2008.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.