Abstract
How the aryl hydrocarbon receptor (AhR) regulates dendritic-cell (DC) differentiation is unknown. We show that activation of AhR by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) caused enhanced differentiation from immature DCs (IDCs) to mature DCs (MDCs) in the bone-marrow-derived DCs (BMDC) from B6 wild-type mice but not in the BMDCs from AhR-null mice as indicated by the expression of CD11c and class II major histocompatibility complex (MHC). Enhanced maturation of BMDCs was associated with elevated levels of CD86 and an increased AhR-dependent nuclear accumulation of nuclear factor-kappa-light-chain enhancer of activated B cell (NF-κB) member RelB in BMDCs. The expression of interleukin (IL) 10 and chemokine DC-CK1 was suppressed, whereas that of CXCL2, CXCL3 and IL-22 was significantly increased in AhR-activated BMDCs. Furthermore, TCDD induced expression of the regulatory enzymes indoleamine 2,3-dioxygenase (IDO1) and indoleamine 2,3-dioxygenase-like 1 (IDO2). Increased expression of IDO2 was associated with coexpression of the cell-surface marker CCR6. Interestingly, mRNA expression of the chemokine receptor CCR6 was drastically decreased in AhR-null IDCs and MDCs. Overall, these data demonstrate that AhR modifies the maturation of BMDCs associated with the induction of the regulatory enzyme IDO and altered expression of cytokine, chemokines and DC-specific surface markers and receptors.
Keywords: AhR, chemokines, DC, IDO, NF-κB RelB, TCDD
Numerous studies have shown that activation of the aryl hydrocarbon receptor (AhR) through binding of a variety of ligands has immunomodulatory effects. For instance, the prototype of AhR agonists 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) has been reported to be among the most immunosuppressive chemicals known.1 Early studies showed that both humoral and cell-mediated immunities are affected after chronic or acute exposure to TCDD.2, 3 Studies with AhR-null mice have shown clearly the requirement of AhR to mediate the immunosuppressive effects of TCDD.4 Despite extensive studies, the precise mechanism by which activation of AhR affects immunity is unknown. Studies have revealed that dendritic cells (DCs), similar to lymphocytes, are also sensitive cellular targets of TCDD within the immune system.1 Alterations of DC functions can lead to immunological disorders, such as immunodeficiency, autoimmune disease and allergy, or can affect the ability of the immune system to detect malignant transformed cells. Several reports showed that exposure to chemicals that activate AhR promotes immune disorders.5 The central role of DCs as regulators of immune responses6 has stimulated investigations into which intracellular signaling pathways regulate gene expression that controls the differentiation of precursors into DCs; especially the nuclear factor-kappa-light-chain enhancer of activated B cells (NF-κB) family of transcription factors is critically involved in this process.7 AhR is a transcription factor that can interact with NF-κB RelA and RelB signaling and modulate immune responses.8, 9 The interaction of AhR with NF-κB RelB to regulate chemokine expression (interleukin (IL)-8) was first described in human macrophages.10 AhR was also shown to interact with NF-κB RelA to suppress acute-phase gene SAA, IgH, or induce Pai-2.11, 12, 13
Recent findings suggest that AhR activation changes the function of inflammatory DCs and that immunoregulatory genes within DC are critical targets of AhR.14, 15, 16 Several studies show that exposure to AhR agonists such as TCDD leads to an increase in regulatory FoxP3+ T cells (Treg)1, 17 and promotes the development of IL-10-producing Tr1 cells in synergy with cMaf.18 Initially, we showed that activation of AhR induces the expression of immunoregulatory enzymes IDO1 and IDO2 in various tissues of mice as well as in human DC,16 which could be responsible for an increased number of FoxP3+ Tregs. The AhR-dependent induction of IDO has been confirmed in other cell types including Langerhans cells (LC).14, 19, 20 On the other hand, exposure to AhR ligands such as the tryptophan metabolite 6-formylindolo[3,2-b]carbazole may promote the differentiation of inflammatory T helper (Th)17 cells.21, 22 A recent study shows that the AhR is involved in the development of IL-22-producing Th17 cells coexpressing the chemokine receptor CCR6.23 Most recent studies show that the AhR is a key regulator for the development and function of IL-22-secreting innate lymphoid cells (ILCs) in the lamina propria,24 and activation of AhR by dietary substances such as indole-3-carbinol is required in maintaining intestinal immune function and host-defense mechanisms.25
The current study addresses the mechanisms by which AhR regulates the differentiation of DC and regulation of DC-specific markers. The endogenous role of AhR in DCs and the exact genesis and development of the different types and subsets of DCs and their inter-relationship are only marginally understood at the moment. AhR is a critical regulator of T-cell immunity, and AhR activation may generate tolerogenic DCs. Therefore, a better understanding of AhR function in DCs is of interest for immunotherapy studies. Here we show that AhR has a critical role in the expression of DC-specific genes and in the process of maturation of DCs, which is important in understanding the development of the different types and subsets of DCs.
Results
AhR activation promotes the differentiation of CD11c+ DCs from GM-CSF-expanded murine bone marrow cultures
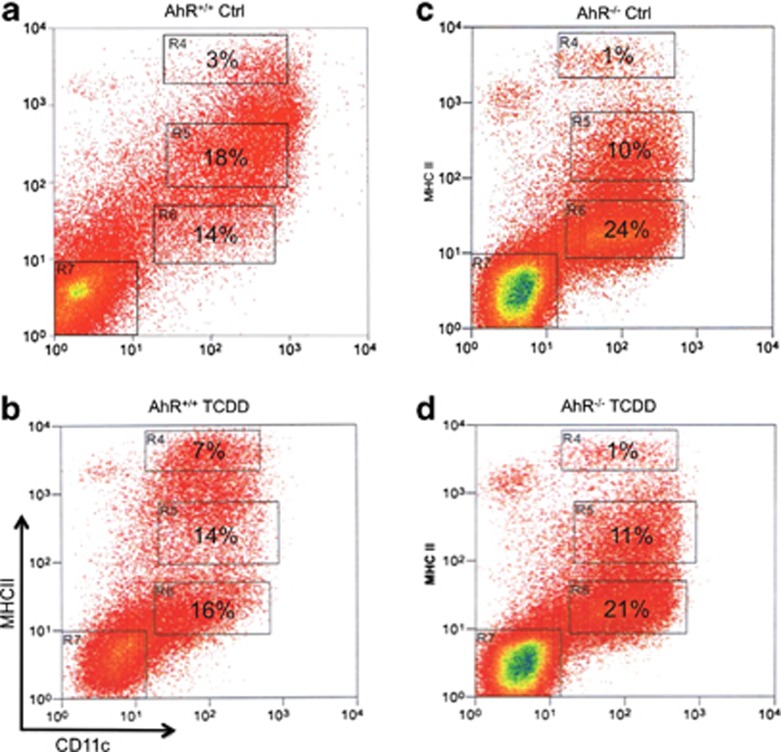
Pooled bone marrow cells from C57BL/6 female mice (AhR+/+) and AhR-null mice (AhR−/−) were plated in GM-CSF (granulocyte–monocyte colony-stimulating factor)-containing medium. TCDD was added to the culture medium to 10 nM on day 1. Six days later, nonadherent cells were harvested, counted, antibody-stained and analyzed using flow cytometry; MHC II and CD11c expression levels for the different culture conditions are shown as dot plots (Figure 1). Figure 1 shows that the culture conditions generated four distinct cell subpopulations based on antibody epitope expression: double-negative (DN) cells (region R7), CD11c+ class II MHCNEG (region 6), CD11c+ class II MHCINT (region 5) and CD11c+ class II MHCHI (region 4). DC populations were identified by CD11c expression and DC maturation status by their class II MHC expression. Thus, CD11c+ class II MHCNEG, CD11c+ class II MHCINT and CD11c+ class II MHCHI were classed as precursor DCs, immature DCs (IDCs) and mature DCs (MDCs), respectively. The percentage of class II MHCHI DCs (MDCs) was increased by TCDD, whereas that of CD11c+ class II MHCINT (IDCs) was decreased in bone-marrow-derived DC (BMDC) from AhR+/+ mice (Figures 1a and b). The number of class II MHCNEG precursor DCs remained unchanged under TCDD in the culture of BMDC from AhR+/+ mice (Figure 1b). The percentages of CD11c+ class II MHCINT and CD11c+ class II MHCHI were consistently higher for control cells from AhR+/+ compared with control cells from AhR−/−, which was because of the increased proportion of precursor DCs and DNs in the cultures of AhR−/− (Figures 1c and d). TCDD did not significantly change the percentage of DC subpopulations derived from AhR−/− compared with that from the vehicle-treated control group (Figures 1c and d).
Figure 1.
Activation of AhR changes maturation of precursor DCs. Activation of AhR increases maturation of precursor DCs derived from B6 wt (AhR+/+) but not in BMDCs from AhR-null (AhR−/−) mice. Bone marrow cells were cultured for 7 days in RPMI containing 20 ng ml−1 GM-CSF with 0.1% DMSO (vehicle control, a and c) or 1 nM TCDD (b and d). Generation of BMDCs was carried out as described earlier.27 Representative flow cytometric analysis dot plots of CD11c and class II MHC coexpression on propidium iodide-negative cells are shown. Dot plots are representative of three independent experiments. Cells within region R7 indicate DC subset of double-negative cells (DN), R6 precursor DC, R5 IDC and R4 indicate MDC. The mean of the percentage of each DC subset is shown within each region from three independent experiments. The total yield of nonadherent cells was 2.6±0.5 × 106 (AhR+/+ Control), 2.2±0.4 × 106 (AhR+/+ TCDD), 2.3±0.7 × 106 (AhR−/− Control) and 2.4±0.3 × 106 (AhR−/− TCDD). Data are presented as means±s.e.
Activation of AhR alters the expression of DC-specific markers
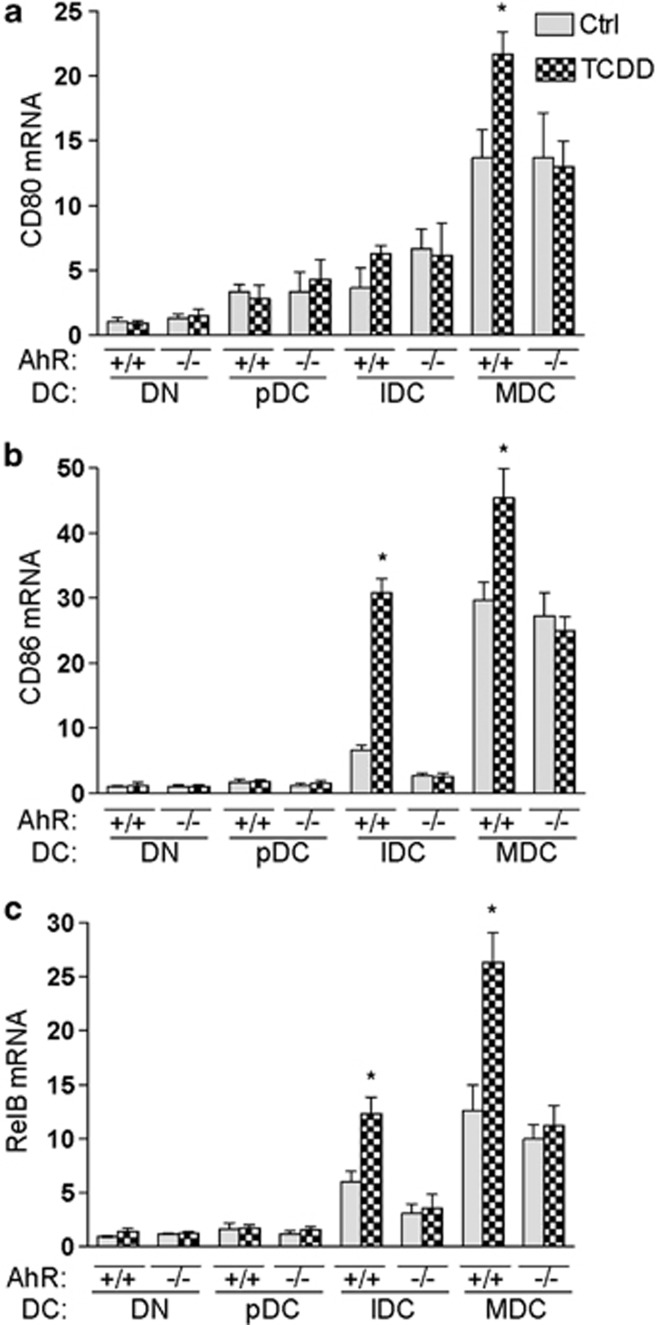
To identify the mechanisms by which AhR influences the differentiation of BMDCs, bone-marrow cells derived from B6 wt and AhR-null mice were cultured in the absence or presence of 10-nM TCDD for 6 days. To examine DC-differentiation status, nonadherent cells were harvested and prepared for separation into DC populations according to their maturation status by their MHC II and CD11c expression levels, as shown in Figure 1. Cells were then harvested for RNA extraction and analyzed for expression of DC-maturation markers CD80, CD86 and RelB via real-time polymerase chain reaction (PCR) as shown (Figures 2a–c). The basal expression levels of the co-stimulatory and adhesion molecules CD80 and CD86 as well as that of the maturation marker RelB were significantly increased in subpopulations of MDCs compared with precursor DCs or DNs derived from B6 wt. The expression levels of CD86 and RelB were significantly increased by TCDD in IDC and MDC in wt mice but not in AhR-null mice. CD80 mRNA was induced by TCDD only in wt MDC.
Figure 2.
AhR-dependent increase in DC-maturation-specific genes CD80, CD86 and RelB. Effect of TCDD on CD80 (a), CD86 (b) and RelB (c) mRNA expression in BMDC subsets from B6 wt (AhR+/+) and AhR−/− (AhR-null) mice as determined by real-time qRT-PCR 7 days after treatment with 0.1% DMSO (Ctrl) or 1 nM TCDD. Results are expressed as Relative Expression, which indicates the expression of each gene relative to its expression in control of double-negative cells (DN). Shown are the mean and s.d. of four independent experiments (*P<0.05).
TCDD increases nuclear accumulation and DNA-binding activity of RelB in BMDC
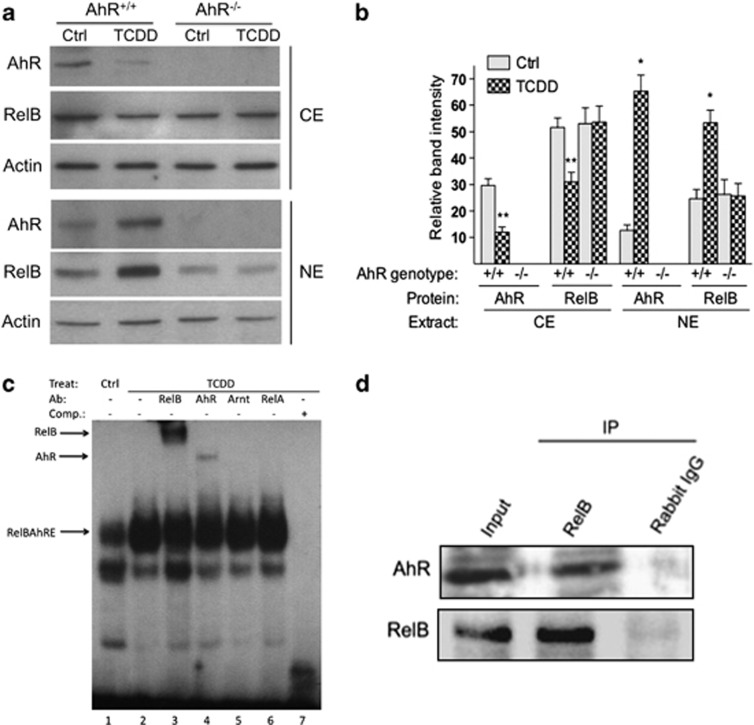
Results from western blot analysis showed that TCDD increases the AhR level in the nuclei of BMDC from B6 wt mice but not in the BMDCs from AhR-null mice (Figure 3a). We detected an increased accumulation of RelB by TCDD only in B6 wt BMDCs but not in AhR-null BMDCs (Figure 3a), suggesting that the TCDD-mediated translocation of RelB into the nucleus involved a functional AhR. The TCDD-stimulated increased accumulation of AhR and RelB in the nuclei correlated with a decreased level in the cytosolic extract of BMDC. The increased accumulation of RelB in the nucleus was associated with increased DNA-binding activity of RelB and AhR on a RelBAhRE element (Figure 3b) previously described on the promoter of IL-8.10 In order to test whether the AhR physically interacted with RelB, we performed co-immunoprecipitation studies with nuclear proteins extracted from BMDC. The results shown in Figure 3c suggest that AhR and RelB are associated proteins in BMDC from B6 wt mice, confirming previous data showing the interaction of AhR and RelB in a human macrophage cell line.10
Figure 3.
AhR controls TCDD-stimulated nuclear accumulation and DNA-binding activity of RelB in BMDC. (a) Levels of AhR and RelB proteins in cytosolic extract (CE) and nuclear extract (NE) of BMDCs from B6 wt (AhR+/+) or AhR-null mice (AhR−/−) are shown. Bone marrow cells were cultured for 7 days in RPMI with GM-CSF in presence or absence of 1 nM TCDD. AhR, RelB and Actin proteins were analyzed using the western blot analysis. One representative experiment out of three is presented. (b) Densitometric evaluation of band intensities of the AhR and RelB protein bands. Results of three independent experiments are shown as mean values±s.d. (*, significantly higher than control P<0.05; **, significantly lower than control P<0.05). (c) TCDD-enhanced binding of AhR and RelB to a RelBAhRE-binding element during DC differentiation. Electromobility-shift assay (EMSA) was performed with nuclear extracts derived from BMDCs incubated with32P-end-labeled double-stranded oligonucleotide coding for AhR/RelB binding as described earlier.10 Lanes 3 and 4 show bandshift reaction mixtures that were incubated additionally with antibodies against AhR and the NF-κB family member RelB. Addition of antibodies against Arnt (lane 5) and RelA (lane 6) did not cause a supershift or decrease in binding activity. A 200-M excess of wild-type cold-competition oligonucleotide (lane 5) has also been included as control. An arrow indicates a binding reaction (supershift and/or decrease in bandshift intensity). Ab, Antibody; Comp, competition; Ctrl, control; Treat, treatment. One representative experiment out of three independently performed experiments is shown. (d) Detection of the AhR/RelB complex in nuclear extracts of BMDC. Bone marrow cells were cultured in RPMI containing 20 ng ml−1 GM-CSF for 7 days. RelB and AhR immunoprecipitates were prepared with nuclear extracts collected from wt BMDC. The antigen–antibody complexes were visualized via chemoluminescence. Polyclonal NF-κB member RelB (Active Motif, Carlsbad, CA, USA) and polyclonal AhR (Novus Biologicals, Littleton, CO, USA) antibodies were used for western blot, Supershift in EMSA and co-immunoprecipitation. Data are representative of three independent experiments.
TCDD alters the expression levels of CCR6, IDO1 and IDO2 in DC subpopulations
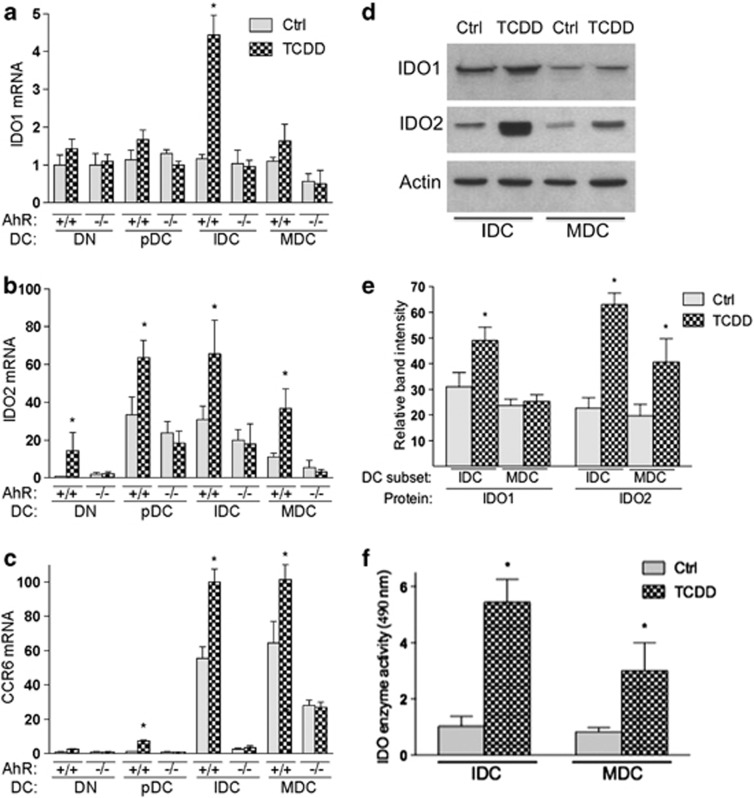
The tryptophan-catabolizing and -tolerogenic enzymes IDO1 and IDO2 were determined intracellularly via real-time PCR in BMDC subcultures. IDO1 and IDO2 were induced by AhR activation14, 16 and may have a role in TCDD's effect to increase Treg; however, IDO was also shown to exert efficient antimicrobial activity26 that might be important for the role of AhR in mucosal immunity. The expression levels of IDO1 and IDO2 mRNA were induced by TCDD in IDC by four- and two-fold, respectively (Figures 4a and b). In contrast to IDO1, IDO2 was also significantly induced in precursor DC and MDC subpopulations of AhR+/+ (wt) mice. In order to test an associated expression of the chemokine receptor CCR6 with IDO as described earlier,27 we analyzed the expression of CCR6 in DC subpopulations. Similar to IDO2, we found significantly increased level of CCR6 in precursor DCs, IDCs and MDCs of wt BMDCs; the basal expression of CCR6 was lower in IDCs and MDCs from AhR-null mice compared with wt mice (Figure 4c). IDO1 protein level was not increased by TCDD in MDCs of wt mice, whereas IDO2 protein increased in IDCs and MDCs of wt mice (Figure 4d), which was in line with the mRNA expression profiles of IDO1 and IDO2. The induced expression of IDO1 and IDO2 was associated with increased IDO enzymatic activity in BMDC subsets of mice (Figure 4e).
Figure 4.
AhR-dependent increase in IDO2 is associated with CCR6 expression in DC subsets. (a–c) Effect of TCDD on IDO1, IDO2 and CCR6 mRNA expression in BMDC subsets from B6 wt (AhR+/+) and AhR−/− mice as determined using real-time qRT-PCR 7 days after treatment with 0.1% DMSO (Ctrl) or 1 nM TCDD. Results are expressed as Relative Expression, which indicates the expression of each gene relative to its expression in control of double-negative cells (DN). (d) Detection of IDO1 and IDO2 protein expressions. Cell lysates were prepared from BMDC subsets IDC and MDC using RIPA buffer. Twenty microgram of the total protein lysate was separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to polyvinylidene difluoride (PVDF) membranes, protein loading was monitored using Actin. IDO1 and IDO2 proteins were detected using a rabbit polyclonal antibody (IDO1, sc-25809, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and IDO2 preparation (kindly provided by Dr Richard Metz from NewLink Genetics, Wynnewood, PA, USA) and anti-rabbit IgG-horseradish peroxidase (HRP; Santa Cruz Biotechnology), and visualized by chemiluminescence (ECL; Amersham, Piscataway, NJ, USA). One representative experiment out of three independently performed experiments is shown. (e) Densitometric evaluation of band intensities of the IDO1 and IDO2 protein bands. Results of three independent experiments are shown as mean values±s.d. (f) Determination of IDO enzymatic activity. After 7 days of exposure to TCDD, BMDC subsets were harvested. Cells were washed twice with phosphate-buffered saline (PBS) and frozen at −70 °C. Cell pellets were lyophilized overnight. The powdered cell residues were resuspended in PBS and, after centrifugation, the supernatants were assayed for IDO activity as described.16 Optic density was measured at 490 nm using a Mithras LB 940 (Berthold Technologies, Germany) microplate reader. Shown are the mean±s.d. of four independent experiments (*, significantly higher than control P<0.05).
TCDD alters the expression of cytokines, chemokines and receptors in unstimulated and TLR4-activated BMDC
The function of DCs and their immunoregulatory role in T-cell differentiation depend on the regulation and expression of cytokines and chemokines. Here we analyzed the mRNA expression of cytokines IL-6, IL-10, IL-12, IL-22 and IL-23, as well as of chemokines DC-CK1 (CCL18), CXCL2 and CXCL3, in unstimulated and lipopolysaccharide (LPS)-stimulated BMDCs (Table 1). The expression of IL-6 was unchanged by TCDD in unstimulated BMDCs; however, TCDD increased IL-6 mRNA expression in LPS-stimulated BMDCs. The level of IL-10 mRNA was significantly decreased by TCDD in the absence of LPS; however, TCDD led to a three-fold increase in IL-10 in LPS-stimulated BMDCs. No significant effect was found by TCDD on the expression of IL-12 in BMDCs. The most significant effect by TCDD was found on the expression of IL-22, which was increased to 212-fold in unstimulated BMDCs and increased up to 6845-fold in LPS-stimulated BMDCs. LPS alone increased the expression of IL-22 about 60-fold compared with vehicle-treated controls. TCDD did not significantly increase the expression of IL-23 in unstimulated BMDCs but led to a two-fold increase in IL-23 in LPS-stimulated BMDCs. Expression of TNFα was not changed by TCDD. The DC-specific chemokine DC-CK1 (CCL18) was >70% suppressed by TCDD in unstimulated BMDCs, and in contrast to IL-10 TCDD also suppressed the increased expression of DC-CK1 in LPS-activated BMDCs (Table 1).
Table 1. AhR activation by TCDD results in altered expression of cytokines, chemokines and receptors in unstimulated and TLR4-activated BMDC.
|
Treatment |
|||
|---|---|---|---|
| Gene | TCDD | LPS | LPS+TCDD |
| IL-6 | 1.2±0.2 | 8.5±0.7* | 13.3±1.2** |
| IL-10 | 0.3± 0.1*** | 2.5±0.1* | 8.2±0.8** |
| IL-12 | 1.1±0.2 | 56.5±3.2* | 62.6±5.0* |
| IL-22 | 212.6±20.5* | 59.5±6.1* | 6,845±71.2** |
| IL-23 | 1.8±0.7 | 14.5±3.2* | 25.4** |
| DC-CK1 | 0.2±0.3*** | 6.5±0.6* | 1.3±0.5*** |
| CXCL2 | 3.5±0.3* | 13.2±1.7* | 45.2±7.5** |
| CXCL3 | 6.5±0.4* | 25.6±3.1* | 135.9±16.8** |
| TNFα | 0.8±0.5 | 4.3±0.5* | 3.7±0.2* |
| CCR3 | 3.6±0.9* | 0.2±0.2*** | 1.5±0.9 |
| C3aR1 | 2.6±0.2* | 1.1±0.8 | 2.9±0.2* |
| SRA1 | 4.6±0.8* | 8.3±0.4* | 86.4±5.2** |
Abbreviations: BMDC, bone-marrow-derived dendritic cell; C3aR1, Complement C3a receptor 1; IL, interleukin; LPS, lipopolysaccharide; TLR, toll-like receptor; TTCD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TNF, tumor necrosis factor; SRA, scavenger receptor A.
Significantly increased compared with control P<0.05.
Significantly increased compared with LPS P<0.05.
Significantly lower than control or LPS P<0.05.
We extended the expression analysis to genes critical for the function of DCs using the Profiler PCR array system for DCs and APC (QiagenSA Biosciences, Valencia, CA, USA). After verification by real-time PCR, we found that TCDD increases the expression of chemokines CXCL2 (3.5-fold) and CXCL3 (6.5-fold) in unstimulated BMDCs, and TCDD led to a further increase in LPS-stimulated BMDCs. The chemokine receptor CCR3 was induced 3.6-fold by TCDD, whereas LPS led to an 80% decrease in CCR3 mRNA levels. TCDD did not lead to a significant induction of CCR3 in LPS-activated BMDCs. We also found a significant increase in the Complement C3a receptor 1 (C3aR1) mRNA by TCDD. LPS did not alter the expression of C3aR1. Furthermore, we identified the immunosuppressive scavenger receptor A (SRA1) as a target for TCDD. SRA1 was 4.6-fold increased in unstimulated BMDCs and over 80-fold increased by TCDD in LPS-activated BMDCs.
Discussion
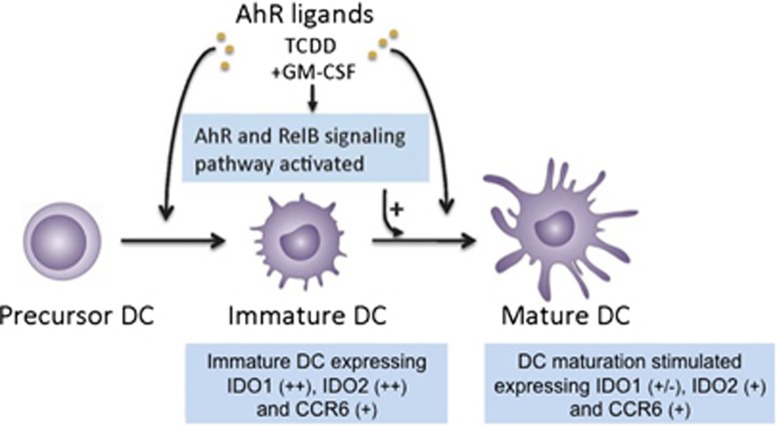
The results of the current study provide new insight into the mechanism of AhR modifying the differentiation of BMDCs and promoting the differentiation of MDCs recruited from IDCs (Figure 5). The results extend previous studies showing a suppressed maturation of BMDCs in the presence of an AhR antagonist28 as well as TCDD-enhanced maturation of BMDCs expressing elevated levels of CD86 and MHC II.14 Another study using LCs also indicate the involvement of AhR in the maturation of LCs, as AhR-null LCs showed less capacity to mature compared with LCs derived from B6 mice.19 Besides DCs and LCs, the AhR may also control the differentiation and homeostasis of mast cells.29, 30 On the other hand, studies have shown that activation of AhR may inhibit the function and maturation of DCs or LCs.15, 31, 32, 33, 34 The different effects of AhR on DC differentiation and function may depend on the type of ligand-activating AhR, the cellular context and the DC-differentiation model used. The results using TCDD to activate AhR are likely to extend to polycyclic aromatic hydrocarbons and other persistent organic pollutants with a high AhR affinity; however, natural or endogenous ligands such as indole-3-carbinol or 6-formylindolo[3,2-b]carbazole may have different effects on DCs.22 The findings of this study were obtained from GM-CSF-stimulated BMDCs, and a different response from different subsets of steady-state DCs in the intact animal must be considered, which indicates the limitation of extrapolating data from a DC model to the more complex role of DCs in vivo. As reported earlier, AhR may exhibit anti-inflammatory and immunosuppressive roles in several settings, and AhR agonists can repress NF-κB-responsive genes.12 However, AhR ligands can also transactivate NF-κB-responsive genes and promote Th17 polarization.10, 21
Figure 5.
Activation of AhR induces RelB and stimulates the maturation of DC. The AhR, also known as dioxin receptor, is expressed at high levels in DCs. Addition of an AhR agonist such as TCDD affects the normal DC differentiation of bone marrow cells. This abnormal differentiation has at least three main effects: induction and accumulation of RelB in the nucleus, increased production of MDCs and induction of IDO1 and IDO2 associated with expression of CCR6 in immature and MDCs, respectively. Together with a decreased production of DC-CK1 and increased expression of SRA1, activation of AhR by TCDD in DC might result in the induction of tolerance.
DC maturation is regulated by the NF-κB-signaling pathway, and the NF-κB subunit RelB is the transcription factor that has been associated most directly with DC differentiation and function.7, 35 Results from the current study show that the AhR-dependent and TCDD-stimulated maturation of BMDCs is associated with an increased level and DNA-binding activity of RelB in the nucleus of BMDCs. Furthermore, co-immunoprecipitation studies show that RelB physically interacts with AhR in the nucleus of BMDCs, confirming data from macrophages.10 Nuclear expression of RelB is one of the hallmarks of DC differentiation and correlates with the degree of maturation.36 Maturation of DCs is induced, for instance, through interaction with the CD40 ligand (CD40L), which is well known to mediate nuclear localization and activation of a RelB.37 It is noteworthy that CD40L clearly caused constitutive AhR activation and induction of Cytochrome P4501a1 (CYP1a1) in B cells,38 which supports the results from the current study that not only RelB but also AhR activation has a physiological role in DC differentiation and maturation.
Here we have shown that TCDD induces the regulatory enzyme IDO1 in IDCs of wt mice but not in IDCs of AhR-null mice. A recent report has shown that the expression and induction of IDO1 by LPS can also be observed in RelB-deficient DCs.39 However, both the activity of IDO and expression of RelB in DCs were required to achieve long-term tolerance in an autoimmune model, indicating the important role of RelB in tolerance. In contrast to IDO1, IDO2 expression level increased from double-negative cells differentiating into precursor DCs and IDCs and slightly decreased in MDCs, indicating an association of IDO2 expression with the maturation of DC subsets of wt mice. The AhR-dependent induction of IDO2 in DC subsets was associated with the increased expression of CCR6. Interestingly, a discrete subset of IDO-positive APCs has been identified by coexpression of the cell-surface marker CCR6.27 Coexpression of CCR6 has also been found for IL-22-producing Th17 cells,23 and the AhR seems to be required for the expression of IL-22.25 In the current study, the most significant effect by TCDD was found on the expression of IL-22 in unstimulated as well as LPS-activated BMDCs. The results confirm recent findings showing an AhR-dependent activation of IL-22 in CD4(+) T cells by TCDD in mice,40 which is in line with a long-lasting effect on human CD4+ T cells to produce IL-22 and not other T-cell cytokines, with no effect on T regulatory cells after exposure to dioxins in humans.41 IL-22 exerts both proinflammatory and tissue-protective functions, and various cell types may produce IL-22, such as ILCs, NK, NKT and γδ T cells, and the cell types that produce IL-22 are all of hematopoietic origin.42 IL-22-producing ILCs have been found to be an important component of the mucosal antimicrobial host defense. It is interesting to note that the AhR-dependent expression of IDO may also support an efficient antimicrobial function as recently shown in mice and macrophages.26, 43 As most recent studies show that AhR is a key regulator in the development and function of IL-22-secreting ILCs in the lamina propria,24, 44 and activation of AhR by dietary substances such as indole-3-carbinol is obviously required in maintaining intestinal immune function and host-defense mechanisms,24, 25 these data suggest an important role for AhR in regulating not only IL-22 but also IDO in maintaining mucosal immunity.
Furthermore, the results show that TCDD suppresses the expression of DC-CK1 in unstimulated and LPS-activated BMDCs, which agrees with findings in human DCs.16 DC-CK1 primarily targets lymphocytes and IDCs and is mainly expressed by a broad range of monocytes/macrophages and DCs. It has been shown that IL-10 may increase the expression of DC-CK1 in DCs.45 Therefore, it is possible that suppression of IL-10 by TCDD consequently leads to a decreased expression of DC-CK1; however, the TCDD-induced expression of IL-10 in LPS-activated BMDCs does not support this hypothesis. The specific expression of DC-CK1 by DCs at the site of initiation of an immune response, combined with its chemotactic activity for naive T cells, suggests that the AhR-mediated suppression of DC-CK1 may contribute to the immunosuppressive effect TCDD.
The current study shows that AhR activation modulates not only the expression of cytokines and chemokines but also that of critical surface markers and receptors such as C3aR1 and SRA1. An increased expression of C3aR1 mRNA was found in TCDD-treated BMDCs. Complement activation has been shown to modulate DC-mediated T-cell activation, but whether complement affects the capability of DCs to enhance their function in T-cell stimulation has been unclear. A recent report showed that engagement of C3aR1 on the surface of DCs leads to a potent negative regulation of inflammatory cytokines.46 Furthermore, we identified the SRA1 as a target of TCDD in BMDCs and detected a significant increase in SRA1 in unstimulated and LPS-activated BMDCs. The class A SR SRA1 is expressed on most macrophages and on DCs, where they act as phagocytic receptors mediating non-opsonic phagocytosis of pathogenic microbes. Another important function of some SR is to act as co-receptors to TLR, modulating the inflammatory response to TLR agonists.47 The maturation of DCs and their capacity to capture Ag from live cells have been shown to be associated with a switch in expression from type II SRA to the immunosuppressive scavenger receptor SRA1 splice variant.48 SRA1 silencing in DC enhanced antitumor immunity and increased the number of tumor-infiltrating CD8(+) cells,49 underlining the immunosuppressive function of SRA1 in DCs.
One open question is how critical the contribution of RelB is in mediating the induction of DC-specific genes by AhR ligands. RelB is a critical factor in the development and maturation of DCs including myeloid and splenic DCs.50, 51, 52 Studies with mice and DCs deficient in RelB have shown that the upregulation of MHC class II and CD80/86 and co-stimulatory capacity depend on RelB.39 Furthermore, RelB seems to be critical in promoting IDO activity.39, 53 Thus, it is possible that enhanced DC maturation and expression of CD86 as well as induction of IDO promoted by AhR activation involve the integrity of RelB.52, 53
As described above, AhR signaling controls the differentiation of DCs, which involves activation of the NF-κB subunit RelB, and the absence of AhR in DCs may change the control of RelB in DC differentiation and function. Activation of AhR also leads to altered expression of the key factors critical for the function of DCs. The AhR mediates induction of the immunosuppressive enzymes IDO1 and IDO2 as well as of anti-inflammatory IL-10 and SRA1, which may negatively regulate the immunogenicity of DCs.
Methods
Materials
Recombinant murine GM-CSF (rmGM-CSF) was purchased from Sigma (St Louis, MO, USA) or R&D Systems (Minneapolis, MN, USA). Antibodies (all purchased from BD Pharmingen, San Diego, CA, USA) to the following murine marker epitopes are as follows, with the clone names in parentheses: MHC II (2G9 or M5/114.15.2), CD11c-APC and -PE (HL3), CD86-PE (GL1), biotinylated anti-CD8α (53-6.7), CD45R/B220-PE (RA3-6B2), CD11b-FITC (M1/70) and unconjugated anti-invariant chain (In-1) and anti-CD16/32 (2.4G2). Fluorophore-conjugated isotype control rat IgG2a (R35–95), IgG2b (A95-1), monoclonal antibodies and hamster IgG (G235—2356) were purchased from BD Pharmingen, and isotype control mouse IgG2a (5–205) monoclonal antibody was purchased from Accurate Chemical (Westbury, NY, USA). Dimethylsulfoxide (DMSO) and LPS were obtained from Sigma. [γ-32P]ATP (6000 Ci mmol−1) was purchased from ICN Biochemicals, Inc (Costa Mesa, CA, USA). TCDD (>99% purity) was obtained from Dow Chemical Co (Midland, MI, USA).
Animals and cell culture
Female C57BL/6 mice aged 6–8 weeks were obtained from JAX West Inc (Davis, CA, USA) and were euthanized in accordance with a protocol approved by the UC Davis Animal Resources Service. AhR-null (AhR−/−) mice were generated and kindly provided by Christopher Bradfield and coworkers from the McArdle laboratory for Cancer Research at the University of Wisconsin. Extracted bone marrow was depleted of red blood cells by ammonium chloride lysis. The R10 medium used to culture bone marrow and sorted DCs was RPMI 1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA), 2 mM l-glutamine, 2 mM sodium pyruvate and 1000 IU penicillin and 1000 μg streptomycin (Invitrogen) per 500 ml of medium. 2-Mercaptoethanol (Sigma; 50 μM final) was added to R10 for cells cultured at 20% O2, 37 °C and 5% CO2 (standard culture conditions). Generation of BMDCs was then carried out according to the protocol published by Goth et al.54 using either 10-cm diameter or six-well uncoated sterile plastic Petri dishes for culture. For every 78 cm2 of culture area, 2 × 106 red cell-depleted marrow cells were plated in 10 ml of R10 medium supplemented with 20 ng ml−1 rmGM-CSF. After 3 days, an equal volume of fresh R10 with 20 ng ml−1 rmGM-CSF was added per plate. Vehicle, LPS or TCDD was added to the culture medium at the indicated time points. Nonadherent cells were harvested and prepared for flow cytometry and RNA analysis between days 6 and 7. BMDCs were purified (⩾85–90%) with anti-CD11c-APC and anti-APC beads (Miltenyi Biotec, Auburn, CA, USA) using MACS separation columns as per the manufacturer's instructions.
Flow cytometric cell sorting and analysis
For flow sorting, bulk or gradient fractionated nonadherent cells were preblocked with 2 μl anti-CD16/32 and 0.5 μl hamster IgG per 1 × 106 for 10 min on ice. Then saturating anti-MHC II (2G9) and anti-CD11c monoclonal antibodies were added to bind for 15 min. The stained cells were washed, passed through a sterile 35-μm nylon mesh and propidium iodide added (0.5 μg ml−1) immediately before aseptic sorting on a MoFlo cytometer (Cytometric, Fort Collins, CO, USA). Single stained and unstained controls were used to define sorting gates and adjust fluorescence compensation. CD11c-positive cells were considered DCs, which were then further graded as precursor DCs, IDCs or MDCs depending on staining intensity (negative, intermediate or high) for MHC II. Sorted cells of DC subsets were collected for RNA analysis using a Moflow cytometer (Cytometric).
Electromobility-shift assay
Nuclear extracts were isolated from BMDCs, as described previously.55 DNA–protein-binding reactions were performed at room temperature for 20 min in a total volume of 20 μl containing 10 μg nuclear protein, 60 000 cpm of double-stranded DNA RelBAhRE oligonucleotide (5′–AGATGAGGGTGCATAAGTTC–3′), 5% glycerol and 1 μg poly (dI-dC), and in (mM) 25 Tris buffer (pH 7.5), 50 NaCl, 1 EDTA and 0.5 dithiothreitol. The samples were incubated at room temperature for 20 min. Competition experiments were performed in the presence of a 100-fold molar excess of unlabeled DNA fragments. Protein–DNA complexes were resolved by 4% nondenaturating polyacrylamide gel electrophoresis and visualized by exposure of the dehydrated gels to X-ray films. For quantitative analysis, the respective bands were quantified using a ChemiImager4400 (Alpha Innotech Corporation, San Leandro, CA, USA).
Nuclear complex co-immunoprecipitation assay and western blot analyses
Preparation of nuclear extracts and co-immunoprecipitation were performed according to the manufacturer's protocol (Active Motif, Carlsbad, CA, USA). To analyze the levels of AhR and RelB proteins in the cytosol and nuclei, protein extracts (15 μg) were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto a polyvinylidinedifluoride membrane (Immuno-Blot; Bio-Rad Laboratories, Hercules, CA, USA). The antigen–antibody complexes were visualized using the chemoluminescence substrate SuperSignal, West Pico (Pierce Chemical Co, Rockford, IL, USA), as recommended by the manufacturer.
Quantitative real-time reverse transcription-PCR
Total RNA was isolated from BMDCs using a Quick-RNA Mini prep isolation kit (Zymo Research, Irvine, CA, USA), and complementary DNA synthesis was performed as previously described.55 Quantitative detection of rps13 and differentially expressed genes was performed with a LightCycler Real-Time PCR System LC480 (Roche Molecular Diagnostics, Pleasanton, CA, USA) using Fast SYBR Green Master Mix (Life Technologies, Grand Island, NY, USA) according to the manufacturer's instructions. The primers for each gene (Supplementary Table 1) were designed on the basis of the respective complementary DNA or mRNA sequences so that the targets were 100–200 bp in length. Amplification specificity of the PCR products was confirmed by melting curve analysis.
Statistics
All data were obtained from more than three independent experiments performed in duplicate, with results reported as mean±s.d. Statistical significance was determined with one-sided Student's t-tests at P<0.05.
Acknowledgments
This work was supported by NIEHS and USA grants R01ES019898 and 5R21ES015846. RD, AL and AG were supported by the DAAD RISE program. Additional support was from P01ES011269 from the National Institute of Environmental Health Sciences and Award Numbers R833292 and R829388 from the Environmental Protection Agency (INP and SG).
The authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int Immunopharmacol. 2002;2:277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Morris DL, Wood SC, Snyder NK. 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced changes in immunocompetence: possible mechanisms. Annu Rev Pharmacol Toxicol. 1991;31:73–100. doi: 10.1146/annurev.pa.31.040191.000445. [DOI] [PubMed] [Google Scholar]
- Vogel C, Donat S, Dohr O, Kremer J, Esser C, Roller M, et al. Effect of subchronic 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on immune system and target gene responses in mice: calculation of benchmark doses for CYP1 A1 and CYP1A2 related enzyme activities. Arch Toxicol. 1997;71:372–382. doi: 10.1007/s002040050401. [DOI] [PubMed] [Google Scholar]
- Vorderstrasse BA, Steppan LB, Silverstone AE, Kerkvliet NI. Aryl hydrocarbon receptor-deficient mice generate normal immune responses to model antigens and are resistant to TCDD-induced immune suppression. Toxicol Appl Pharmacol. 2001;171:157–164. doi: 10.1006/taap.2000.9122. [DOI] [PubMed] [Google Scholar]
- Marshall NB, Kerkvliet NI.Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells Ann N Y Acad Sci 2010118325–37.20146706 [Google Scholar]
- Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- Shih VF, Davis-Turak J, Macal M, Huang JQ, Ponomarenko J, Kearns JD, et al. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-kappaB pathways. Nat Immunol. 2012;13:1162–1170. doi: 10.1038/ni.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y. Ah receptor and NF-kappaB interplay on the stage of epigenome. Biochem Pharmacol. 2009;77:670–680. doi: 10.1016/j.bcp.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Matsumura F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem Pharmacol. 2009;77:734–745. doi: 10.1016/j.bcp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol. 2007;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RD, Murray IA, Flaveny CA, Kusnadi A, Perdew GH. Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab Invest. 2009;89:695–707. doi: 10.1038/labinvest.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulentic CE, Kang JS, Na YJ, Kaminski NE. Interactions at a dioxin responsive element (DRE) and an overlapping kappaB site within the hs4 domain of the 3'alpha immunoglobulin heavy chain enhancer. Toxicology. 2004;200:235–246. doi: 10.1016/j.tox.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K, et al. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol. 2009;29:6391–6400. doi: 10.1128/MCB.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankoti J, Rase B, Simones T, Shepherd DM. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol Appl Pharmacol. 2010;246:18–28. doi: 10.1016/j.taap.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin GB, Moore AJ, Head JL, Neumiller JJ, Lawrence BP. Aryl hydrocarbon receptor activation reduces dendritic cell function during influenza virus infection. Toxicol Sci. 2010;116:514–522. doi: 10.1093/toxsci/kfq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KA, Lawrence BP. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) renders influenza virus-specific CD8+ T cells hyporesponsive to antigen. Toxicol Sci. 2003;74:74–84. doi: 10.1093/toxsci/kfg110. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J Immunol. 2009;182:6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Nguyen LP, Kennedy G, Nukaya M, Fechner JH, Zhang X, et al. SU5416, a VEGF receptor inhibitor and ligand of the AHR, represents a new alternative for immunomodulation. PLoS One. 2012;7:e44547. doi: 10.1371/journal.pone.0044547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- Lawrence BP, Sherr DH. You AhR what you eat. Nat Immunol. 2012;13:117–119. doi: 10.1038/ni.2213. [DOI] [PubMed] [Google Scholar]
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- Divanovic S, Sawtell NM, Trompette A, Warning JI, Dias A, Cooper AM, et al. Opposing biological functions of tryptophan catabolizing enzymes during intracellular infection. J Infect Dis. 2012;205:152–161. doi: 10.1093/infdis/jir621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- Ilchmann A, Krause M, Heilmann M, Burgdorf S, Vieths S, Toda M. Impact of culture medium on maturation of bone marrow-derived murine dendritic cells via the aryl hydrocarbon receptor. Mol Immunol. 2012;51:42–50. doi: 10.1016/j.molimm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Sibilano R, Frossi B, Calvaruso M, Danelli L, Betto E, Dall'Agnese A, et al. The aryl hydrocarbon receptor modulates acute and late mast cell responses. J Immunol. 2012;189:120–127. doi: 10.4049/jimmunol.1200009. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Tung HY, Tsai YM, Hsu SC, Chang HW, Kawasaki H, et al. Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood. 2013;121:3195–3204. doi: 10.1182/blood-2012-08-453597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera-Montilla N, Chamorro S, Nieto C, Sanchez-Cabo F, Dopazo A, Fernandez-Salguero PM, et al. Aryl hydrocarbon receptor contributes to the MEK/ERK-dependent maintenance of the immature state of human dendritic cells. Blood. 2013;121:e108–e117. doi: 10.1182/blood-2012-07-445106. [DOI] [PubMed] [Google Scholar]
- Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz VJ, van Roest M, Bol-Schoenmakers M, van Duursen MB, van den Berg M, Pieters RH, et al. Aryl hydrocarbon receptor activation affects the dendritic cell phenotype and function during allergic sensitization. Immunobiology. 2013;218:1055–1062. doi: 10.1016/j.imbio.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Platzer B, Richter S, Kneidinger D, Waltenberger D, Woisetschlager M, Strobl H. Aryl hydrocarbon receptor activation inhibits in vitro differentiation of human monocytes and Langerhans dendritic cells. J Immunol. 2009;183:66–74. doi: 10.4049/jimmunol.0802997. [DOI] [PubMed] [Google Scholar]
- Clark GJ, Gunningham S, Troy A, Vuckovic S, Hart DN. Expression of the RelB transcription factor correlates with the activation of human dendritic cells. Immunology. 1999;98:189–196. doi: 10.1046/j.1365-2567.1999.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan BJ, MacDonald KP, Pettit AR, Thomas R. RelB nuclear translocation regulates B cell MHC molecule, CD40 expression, and antigen-presenting cell function. Proc Natl Acad Sci USA. 2000;97:11421–11426. doi: 10.1073/pnas.97.21.11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejardin E. The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem Pharmacol. 2006;72:1161–1179. doi: 10.1016/j.bcp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Allan LL, Sherr DH. Constitutive activation and environmental chemical induction of the aryl hydrocarbon receptor/transcription factor in activated human B lymphocytes. Mol Pharmacol. 2005;67:1740–1750. doi: 10.1124/mol.104.009100. [DOI] [PubMed] [Google Scholar]
- O'Sullivan BJ, Pai S, Street S, An X, MacDonald KP, Wong M, et al. Immunotherapy with costimulatory dendritic cells to control autoimmune inflammation. J Immunol. 2011;187:4018. doi: 10.4049/jimmunol.1101727. [DOI] [PubMed] [Google Scholar]
- Rohlman D, Pham D, Yu Z, Steppan LB, Kerkvliet NI. Aryl hydrocarbon receptor-mediated perturbations in gene expression during early stages of CD4(+) T-cell differentiation. Front Immunol. 2012;3:223. doi: 10.3389/fimmu.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembilla NC, Ramirez JM, Chicheportiche R, Sorg O, Saurat JH, Chizzolini C. In vivo dioxin favors interleukin-22 production by human CD4+ T cells in an aryl hydrocarbon receptor (AhR)-dependent manner. PLoS One. 2011;6:e18741. doi: 10.1371/journal.pone.0018741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonian PL, Wehrmann F, Roark CL, Born WK, O'Brien RL, Fontenot AP. Gammadelta T cells protect against lung fibrosis via IL-22. J Exp Med. 2010;207:2239–2253. doi: 10.1084/jem.20100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani R, Jordan MB, Divanovic S, Herbert DR. IFN-gamma-driven IDO production from macrophages protects IL-4Ralpha-deficient mice against lethality during Schistosoma mansoni infection. Am J Pathol. 2012;180:2001–2008. doi: 10.1016/j.ajpath.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Voort R, Kramer M, Lindhout E, Torensma R, Eleveld D, van Lieshout AW, et al. Novel monoclonal antibodies detect elevated levels of the chemokine CCL18/DC-CK1 in serum and body fluids in pathological conditions. J Leukoc Biol. 2005;77:739–747. doi: 10.1189/jlb.0804435. [DOI] [PubMed] [Google Scholar]
- Li K, Anderson KJ, Peng Q, Noble A, Lu B, Kelly AP, et al. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood. 2008;112:5084–5094. doi: 10.1182/blood-2008-05-156646. [DOI] [PubMed] [Google Scholar]
- Areschoug T, Gordon S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell Microbiol. 2009;11:1160–1169. doi: 10.1111/j.1462-5822.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J Immunol. 2003;170:2302–2309. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- Guo C, Yi H, Yu X, Zuo D, Qian J, Yang G, et al. In situ vaccination with CD204 gene-silenced dendritic cell, not unmodified dendritic cell, enhances radiation therapy of prostate cancer. Mol Cancer Ther. 2012;11:2331–2341. doi: 10.1158/1535-7163.MCT-12-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, et al. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- Wu L, D'Amico A, Winkel KD, Suter M, Lo D, Shortman K. RelB is essential for the development of myeloid-related CD8alpha- dendritic cells but not of lymphoid-related CD8alpha+ dendritic cells. Immunity. 1998;6:839–847. doi: 10.1016/s1074-7613(00)80649-4. [DOI] [PubMed] [Google Scholar]
- Castiglioni P, Lu C, Lo D, Croft M, Langlade-Demoyen P, Zanetti M, et al. CD4 T cell priming in dendritic cell-deficient mice. Int Immunol. 2003;1:127–136. doi: 10.1093/intimm/dxg015. [DOI] [PubMed] [Google Scholar]
- Tas SW, Vervoordeldonk MJ, Hajji N, Schuitemaker JH, van der Sluijs KF, May MJ, et al. Noncanonical NF-kappaB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood. 2007;110:1540–1549. doi: 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- Goth SR, Chu RA, Pessah IN. Oxygen tension regulates the in vitro maturation of GM-CSF expanded murine bone marrow dendritic cells by modulating class II MHC expression. J Immunol Methods. 2006;308:179–191. doi: 10.1016/j.jim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Park S, Liedtke C, Trautwein C, Matsumura F. Dioxin increases C/EBPbeta transcription by activating cAMP/protein kinase A. J Biol Chem. 2004;279:8886–8894. doi: 10.1074/jbc.M310190200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.