Abstract
The diagnosis of primary Sjögren’s syndrome (pSS) is difficult due to the lack of specific laboratory and clinical tests. As an initial step for the global discovery of changes in the abundance of parotid salivary proteins in pSS, a pooled sample was compared to that from healthy control (HC) subjects by multidimensional protein identification technology (MudPIT). A total of 1246 proteins were identified by MudPIT. The abundance of 477 of these proteins did not change, 529 were only detected in either the pSS or HC sample, while 206 of these proteins were significantly up-regulated ≥ 2-fold and 34 were down-regulated ≤ 0.5. Ingenuity Pathway Analyses of differentially expressed proteins identified by MudPIT resulted in the identification of 100 significant pathways. The same samples were quantified in parallel using reversed phase (RP) mass spectrometry. Fifty eight of 71 proteins identified by RP overlapped with MudPIT results. Five proteins were further analyzed by targeted label-free quantification to confirm the similar relative differential expression observed by RP and MudPIT approaches. The present study supports the use of mass spectrometry for global discovery and validation of marker proteins for improved and early diagnosis of pSS.
Keywords: Autoimmune, Biomarkers, Label-Free Quantitation, Parotid Saliva, Sjögren’s Syndrome
1 Introduction
Sjögren’s syndrome (SS) is a chronic autoimmune disease of exocrine glands (e.g. salivary and lacrimal glands) with lymphocytic infiltration and epithelial cell destruction [1]. SS is the second most common autoimmune disease in the U.S. affecting 2–4 million people [2]. Although men and children are affected with SS, it is most commonly seen in perimenopausal women in their 4th–5th decades [3]. The disease occurs alone (primary SS) in about half of the cases [4] or in common with another autoimmune disorder (secondary SS) such as rheumatoid arthritis [5] or systemic lupus erythmatosus [6].
The current American-European classification criteria (AECG) for diagnosis of SS patients require a positive minor gland biopsy showing lymphocytic infiltration, with oral and ocular dryness, and autoantibodies [7]. However, symptoms typically precede by several months to many years the establishment of a diagnosis of pSS. A provisional diagnosis is often based on serological markers such as anti-Ro/SSA [8] and anti-La/SSB [9], but these autoantibody markers lack the specificity and sensitivity for a confirmatory diagnosis [4]. A lymphocytic infiltration score of >1 in a labial salivary gland biopsy is the most widely accepted test to confirm SS. Nevertheless, a labial salivary gland biopsy has its limitation, e.g. causing in rare cases an abnormal labial sense of touch [10], and there is no observable lymphocytic infiltration in some subjects who otherwise fulfill the diagnostic criteria for SS [7]. Thus, saliva pSS biomarkers might prove superior to assess disease progression and therapeutic response.
Saliva has recently gained considerable interest as a diagnostic medium for diseases such as type-2 diabetes and oral malignant lesions [11]. Salivary proteins have been reported to change in pSS [4, 10, 12–14] and between pSS and sSS [15], but such changes in protein expression have not been confirmed as relevant diagnostic biomarkers. Some have argued that changes in the abundance of locally expressed salivary proteins would be the best indicators of salivary gland disease and for monitoring disease status after therapeutic intervention [4, 16].
The aim of the present study was to identify differentially expressed parotid salivary proteins that might ultimately prove to be biomarkers for early disease detection and surveillance in pSS subjects. To maximize protein identifications, MudPIT analysis was performed as an initial global discovery step [17]. Qualitative and quantitative spectral count analyses of the resulting data identified 1246 proteins. Of these proteins, >62% (769) changed in abundance (pSS/HC, cut-off ≤ 0.5 and ≥ 2) including 529 proteins only identified in pSS or HC. These samples were also analyzed by an RP separation approach to quantify proteins in a relatively short time (2 hrs compared to approximately 21 hrs for MudPIT). The relative abundance of selected proteins detected by both the MudPIT and RP approaches (e.g. β-2-microglobulin, lactotransferrin and α-amylase) was confirmed by targeted label-free quantification.
2 Materials and methods
2.1 SS and control subjects
The clinical research protocol for the collection of human parotid saliva by written informed consent was approved by the University of Rochester Institutional Review Board. Recruitment of pSS subjects was based on strict criteria established by the AECG for SS [7]. Clinical details of the pSS and HC subjects are provided in Supplementary Table 1.
2.2 Sample collection
Parotid saliva samples were collected between 8.30 a.m. and 2 p.m. from subjects who had refrained from eating or drinking for 2 hr prior to saliva collection. Saliva was collected on ice as parotid ductal secretions under stimulation (0.4% citric acid for 30 min) into sterile tubes using a Lashley cup-like device [18]. Immediately after collection, 1/20 volume of protease cocktail inhibitor was added to the saliva and frozen at −80°C until further analysis [19]. Equal amount of protein was aliquoted from each subject within a group and pooled to normalize the difference between subjects and reduce individual variation.
2.3 Reduction, alkylation, and trypsin digestion
For MudPIT analysis, protein concentration was determined by micro-BCA assay (Thermo Fisher Pierce Rockford, IL), according to the manufacturer’s instructions. Twenty five µg of protein for each group (pSS and HC) was precipitated with tricholoro acetic acid at 4°C overnight. After centrifugation, the protein pellet was washed with acetone and air-dried pellets were dissolved in 8M urea/100 mM Tris pH 8.5. The samples were then reduced by adding 5 mM of Tris (2-carboxyethyl) phosphine hydrochloride and alkylated by adding iodoacetamide. Subsequently, the samples were digested overnight at 37°C with 2 µg of trypsin (Promega, Madison, WI). The reaction was stopped by addition of 90% formic acid to a final concentration of 4%.
For RP analysis, 100 µg of pooled saliva from each group (pSS & HC) was processed and digested with trypsin as above, except for desalting of peptides using Oasis HLB-1 (1 mg) reversed phase cartridge (Waters, Milford, MA) and vacuum concentrated to dryness. Subsequently, digests were divided into two aliquots, with one aliquot used for peptide/protein identification and relative protein quantification by spectral counting, and the second aliquot for label-free “targeted” quantification of select proteins.
2.4 Multidimensional protein identification technology
Digested proteins were analyzed by MudPIT as previously described [17]. In brief, 25 µg of digested protein was pressure loaded onto an in-house biphasic microcapillary column (250 µm ID/360 µm OD capillary of 30 cm length) packed with a strong cation exchanger (SCX Luna, Phenomenex, Torrance, CA) and RP resin (Aqua C18, Phenomenex, Ventura, CA). The MudPIT column was equilibrated with 60% buffer A (5% acetonitrile/0.1% formic acid), 40% buffer B (80% acetonitrile/0.1% formic acid) for 5 min followed by 100% buffer A for 15 min. Subsequently, an analytical microcapillary column packed with RP resin was attached to the biphasic column with a filter union and placed in line with an Agilent 1200 HPLC pump (Agilent, Palo Alto, CA) on an LTQ-Orbitrap Velos (Thermo Fisher Scientific, San Jose, CA). Samples were analyzed using a modified 8 or 12 step separation as previously described [17], with the first step corresponding to running buffer C (500 mM ammonium acetate) for 5 min followed by 2 hr gradient of buffer B. Peptides eluted from the microcapillary column (100 µm ID/360 µm OD capillary with 12 cm length) were electrosprayed directly into the mass spectrometer with the application of a distal 2.5 kV spray voltage with at an inlet capillary temperature of 250 °C. From one full-scan of mass spectrum (300–2000m/z), 20 most intense ions were sequentially isolated and fragmented by collision induced dissociation (CID) with 35% normalized collision energy repeating continuously through each step of the multidimensional separation. The m/z ratios selected for MS/MS were dynamically excluded for 75s.
2.5 Ingenuity pathway analysis (IPA)
The data set containing up- or down-regulated proteins (≥2 and ≤0.5) including proteins identified only in pSS or HC samples were analyzed by Ingenuity Pathway Analysis version 9.0 (Ingenuity ® Systems, www.ingenuity.com, Mountain View, CA). A right-tailed Fisher’s Exact Test was used to calculate the p-value of the probability that the association between each protein in the dataset and the canonical pathway is random [20]. Pathways with p value < 0.05 were selected.
2.6 Fast protein identification and targeted label-free quantification
Tryptic peptide mixtures from pSS and HC subjects were loaded onto Zorbax C18 trap column (Agilent Tech., Santa Clara, CA) for further desalting of the peptide mixture with 0.1% formic acid. The peptides were then separated on a 10 cm Picofrit Biobasic C18 analytical column (100 µm ID/360 µm OD, New Objective, Woburn, MA) using an on-line Eksigent (Dublin, CA) nano-LC ultra HPLC system. The peptides were eluted using a 120 min acetonitrile gradient (5–35%) of 100 % acetonitrile with 0.1% formic acid at flow rate of 250 nL/min. Peptides were ionized using electrospray ionization (ESI) in positive ion mode and detected on an LTQ-Orbitrap Velos. The six most intense ions were selected for MS/MS from the MS1 precursor scan. All precursor ions were measured in the Orbitrap with a resolution of 30,000 (m/z 400). Precursor ions were fragmented by CID with normalized collision energy of 35%, and all fragment ions were measured in the LTQ.
For targeted analysis, all nano-LC parameters and experimental set up were the same as described above. However, MS parameters were adjusted to target only a specific set of peptides. An inclusion list was prepared, consisting of the accurate m/z values of tryptic peptides from a select group of proteins showing the same trends in expression by MudPIT and RP discovery methods. Peptides were selected for the inclusion list based on 3 criteria: 1) contained no missed cleavages, 2) had a charge state of +2, +3, or +4, and 3) contained no methionine residues. Fifteen µg of peptides were injected into the MS in technical duplicates. Once a precursor m/z from the inclusion list was detected in the MS1 scan, a subsequent MS/MS spectrum was acquired.
2.7 Data analysis
For MudPIT analysis, tandem MS/MS spectra were extracted with RawExtract 1.9.9 [21] and searched against an NCBInr human database (version 37.2) with reversed sequences using ProLuCID [22, 23]. Candidate peptides could be fully, or half-tryptic and carbamidomethylation of cysteine was considered as a static modification. DTA Select was used to filter peptide candidates and assemble into proteins and protein groups with at least two unique peptide hits per protein with a false positive rate of 0.05 at the protein level [24].
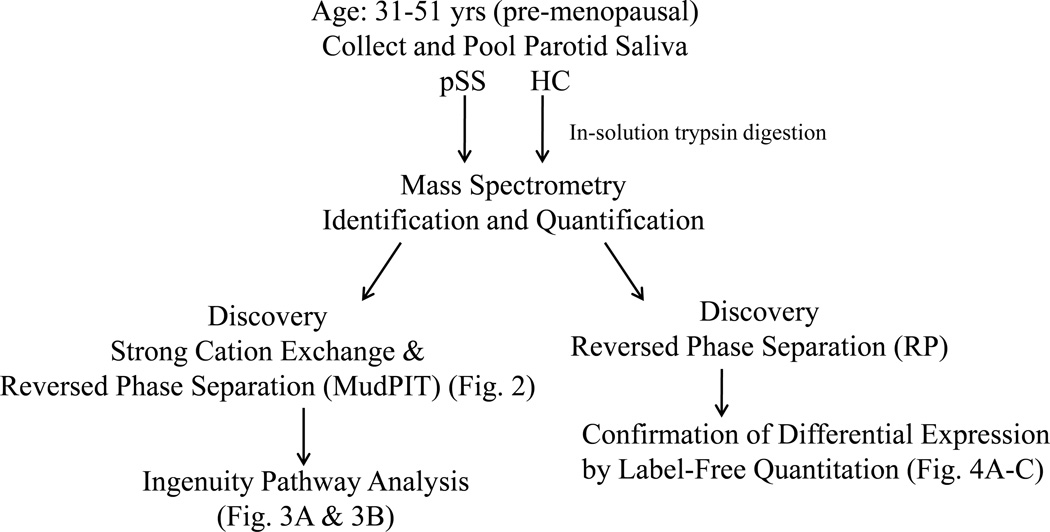
For RP analysis, all LC-MS/MS data were searched using the MASCOT algorithm within Proteome Discoverer 1.3 (Thermo Electron Corp, San Jose, CA) against human Swissprot protein database (Sprot_101911) to obtain peptide and protein identifications. For all searches, trypsin was specified as the enzyme for protein cleavage allowing up to 2 missed cleavages. Oxidation (M) and carbamidomethylation (C) were set as dynamic and fixed modifications, respectively. Mass tolerance of 20 ppm and 0.8 Da were set for precursor and fragment ions, respectively. For confirmation of peptides, automated label-free quantification was carried out using in-house developed software, QUOIL [25]. For MS/MS data visualization, MASCOT results were imported into Scaffold 3Q+ (Proteome Software, Portland, OR). Figure 1 shows the schematic of work flow of the study. User-specified false positive rate was set to 0.05 at the protein level.
Figure 1.
Study Workflow. An overview of the procedures used for the identification and quantification of proteins in Primary Sjögren’s syndrome (pSS) and Healthy control (HC) subjects.
3 Results
3.1 Demographic and clinical characteristics of study subjects
Pre-menopausal female subjects were diagnosed with primary Sjögren’s Syndrome (pSS) using the AECG classification criteria. Age- and gender-matched healthy controls (HC) were screened for good health. Supplementary Table 1 summarizes the clinical evaluation of pSS and HC subjects. All five pSS subjects had a salivary gland biopsy score > 1, tested positive for anti-SSA, while anti-SSB was detected in 4 patients. In contrast, all HC subjects were negative for anti-SSA and anti-SSB. All female subjects had normal monthly menstrual cycles.
3.2 Differentially expressed proteins
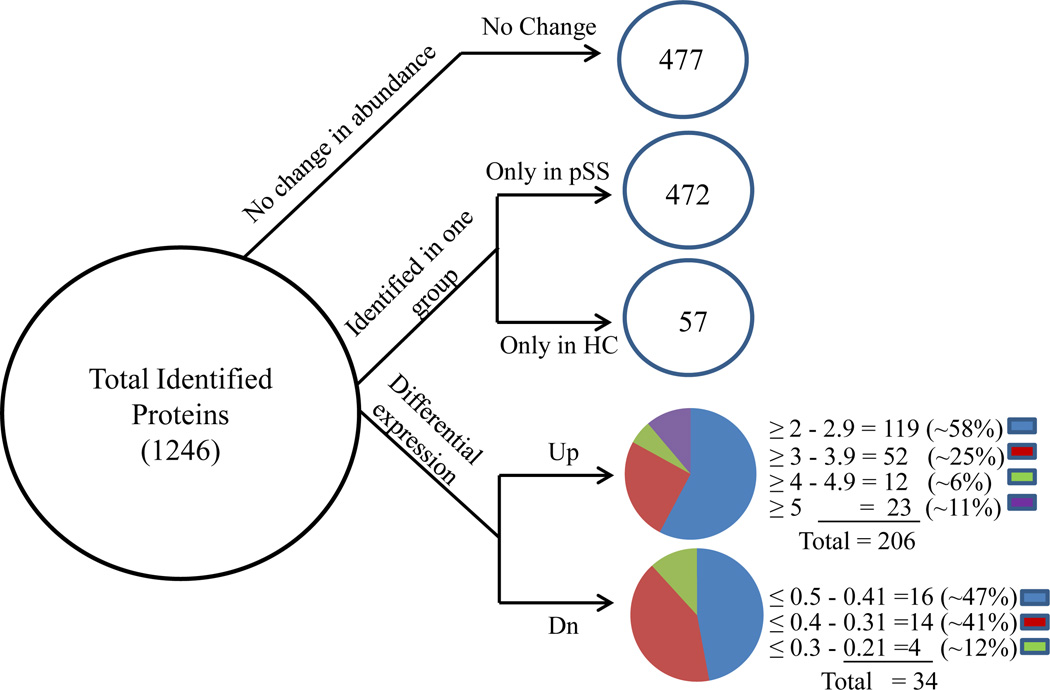
A protein identified by MudPIT analysis was deemed a confident match if at least two unique peptides were detected for that protein; such analysis lead to the identification of 1246 proteins. Spectral count was used for quantification and when an arbitrary fold ratio change cut-off (≤ 0.5 or ≥ 2) was applied to the above 1246 proteins, ~38% (n=477) showed little or no change in abundance, and ~62% (n=769) were considered differentially expressed, including 529 proteins identified only in either pSS or HC parotid saliva. Caution should be taken in interpreting the observation that some proteins were only seen in one group as there may have been under-sampling of low abundance proteins by MS. The differentially expressed proteins are listed in Supplementary Table 2. Of the 240 differentially expressed proteins found in pSS and HC parotid saliva, 206 proteins were up-regulated (≥ 2) while 34 proteins were down-regulated (≤ 0.5) (Figure 2).
Figure 2.
Distribution of differentially expressed proteins. Proteins detected by MudPIT (n=1246) were categorized using an arbitrary fold ratio change cut-off of ≤ 0.5 or ≥ 2 into proteins that did not change in abundance (n=477), proteins identified only in pSS (n=472), proteins identified only in HC (n=57), and proteins up-regulated (n=206) or down-regulated (n=34) in pSS.
3.3 Ingenuity pathways analysis
Differentially expressed proteins identified by MudPIT, including proteins only in pSS or HC, were mapped using Ingenuity Pathway Analyses (IPA) software (9.0) to determine statistically significant (p < 0.05) canonical pathways. The differentially expressed proteins (n=769) were divided into (1) up-regulated in pSS (n=206), (2) down-regulated in pSS (n=34), (3) identified only in pSS (n=472), and (4) identified only in HC (n=57). Based on published literature to date, 132 of the 206 up-regulated proteins mapped to 32 different pathways (Supplementary Fig. 1A), while 410 proteins identified only in pSS were involved in 68 different pathways (Supplementary Fig. 1B). Down-regulated proteins (n=5) were mapped to 5 different pathways (e.g. fatty acid elongation in mitochondria and N-glycan degradation) while 13 proteins identified only in HC were mapped to 7 different pathways (e.g. methane metabolism and amino sugar metabolism).
3.4 Fast protein identification and targeted label-free quantification
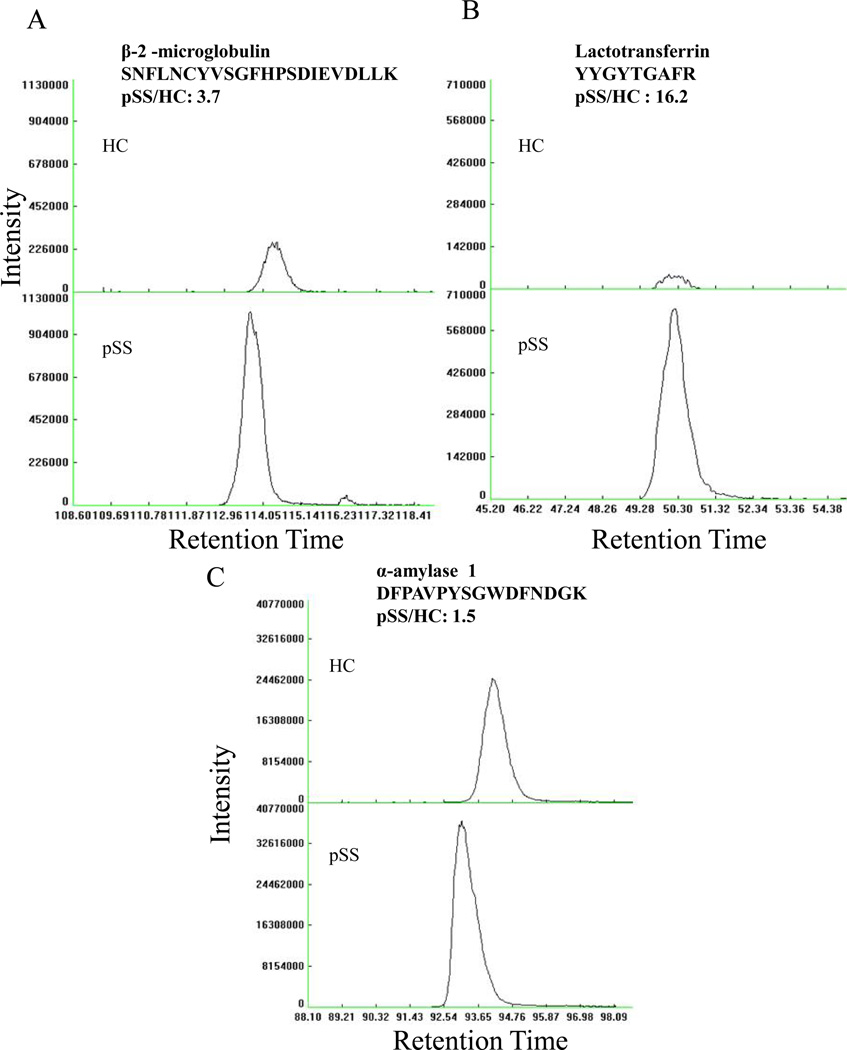
A total of 71 proteins were detected by the label-free ‘fast’ discovery approach as shown in Supplementary Table 3. Of these proteins, 58 overlapped with MudPIT results. Twelve proteins detected by RP were not detected by the MudPIT approach (e.g. Immunoglobulin - α-1 chain C), while one protein that did not change in abundance by RP was only detected in pSS by MudPIT (Histatin-3). Of the proteins detected by both MudPIT and RP approaches, five proteins were selected to confirm their expression levels by targeted label-free approach (3 proteins shown in Figure 3). These five specific proteins were chosen because they are secreted by the parotid gland (e.g. lactotransferrin) and/or were previously reported to change abundance in pSS patients (e.g. β-2-microglobulin, α-amylase, lysozyme C and cystatin C). The protein name, peptide sequences and precursor mass of the 5 selected proteins are given in Supplementary Table 4.
Figure 3.
Label-free quantification of selected differentially expressed proteins between pSS and HC. (A) Up-regulation of β-2-microglobulin, (B) Up-regulation of lactotransferrin, and (C) Modest change in abundance of α-amylase. Primary Sjögren’s syndrome = pSS and Healthy control = HC subjects.
Figure 3 shows the confirmation of differential expression by label-free approach of three representative proteins (lactotransferrin, β-2-microglobulin and α-amylase). For each protein, quantification was deemed confident if at least two different peptides showed similar differential expression. In addition, an MS/MS spectrum was acquired to confirm the identification of each protein by database search. Abundance is calculated from the height of the peak as shown on the y-axis, while the x-axis shows the time at which the peptides eluted. The top chromatograms in Figure 3 panels A-C represent HC, while the bottom panels of A-C are pSS chromatograms. Consistent with the MudPIT and RP approaches, there are large differences in peptide abundance for β-2-microglobulin and lactoferrin (Figs. 3A and 3B, respectively) as shown by fold change of 3.7 and 16.2, respectively, while there was a very modest increase (1.5) for α-amylase (Fig. 3C). Differential expression of lysozyme C and cystatin C is shown in Supplementary Figures 2A & 2B.
4 Discussion
Sjögren’s syndrome is an autoimmune disease that primarily affects women in their 40’s and 50’s [3]. SS typically goes undiagnosed for many years with a long delay from the onset of symptoms to the final diagnosis [4]. To date, diagnosis is based on relatively non-specific factors [7] and thus it is critical to identify specific diagnostic markers to aid early diagnosis, assess prognosis, and monitor therapies.
Changes in salivary constituents have been reported in pSS [4, 10, 12–14] and between pSS and secondary SS [15] suggesting that the salivary proteome holds great potential as a non-invasive method to evaluate SS and other disease conditions [4, 11, 15]. However, little progress has been made related to SS diagnosis [26]. Previous SS studies have typically used two dimensional gel electrophoresis (2DE) followed by protein identification by mass spectrometry [4, 10, 12, 13, 15]. Although 2DE/MS can be an effective tool, it has limitations such as restricted dynamic range and poor reproducibility [17].
To overcome these limitations, in-solution trypsin digestion was performed and analyzed by MudPIT as an initial global discovery step. Spectral count was used to identify and determine fold change of proteins between pSS and HC saliva samples [27]. MudPIT significantly increased the number of differentially expressed protein identifications; however, its feasibility in a clinical setting is debatable due to the need for longer sample run times (21 hrs) and greater computing power. Consequently, MudPIT was performed as an initial global discovery step.
RP was performed to rapidly (2 hrs) quantify medium and high copy proteins. Amongst the overlapping proteins between MudPIT and RP, proteins showing similar trends in differential expression were selected for verification. To date, confirmation of differential expression of potential biomarkers studies has been performed using Western blots, ELISA and luciferase immunoprecipitation systems (LIPS) [4, 14, 28]. Although these techniques can be informative, there are limitations, such as the availability, sensitivity and specificity of antibodies, and the time and cost for development of multiplexing immunoassays [29]. Furthermore, it is critical to identify signature, diagnostic peptides for each protein. Consequently, selected proteins (e.g. lactotransferrin and β-2-microglobulin) were verified using a targeted label-free quantification strategy in a relatively short time (2 hrs) based on ion intensity [25]. Furthermore, by this MS-based approach it would be more practical to target a reasonable number of candidate marker proteins in a relatively short time to quantify differences in protein abundance between two samples in a clinical laboratory setting for disease diagnosis. Similarly, MS-based techniques such as multiple reaction monitoring (MRM) or accurate inclusion mass screening (AIMS) can be used to confirm differential expression of candidate marker proteins in a large patient cohort [29].
In this study, a number of differentially expressed proteins overlapped with proteins previously catalogued in salivary exosomes, small membrane-bound vesicles released into saliva [30]. Exosomes have been shown to stimulate or inactivate T cells, as well as to mediate antigen transfer to antigen presentation cells as a normal physiological mechanism or in disease condition such as SS [31].
Examples of previously reported exosomal proteins also found to be expressed in this study include antigen presenting molecules (e.g. HLA-DR), lipid binding proteins (e.g. apolipoprotein I, II) and cell adhesion proteins (e.g. CD9) [30]. Two notable exosomal proteins up-regulated in the present study were the B cell activation marker β-2-microglobulin precursor and α-enolase, a protein associated with systemic and local inflammation was elevated, as reported previously in pSS [4, 15, 32]. Glypican, neutrophil defensin-1 and mucin-5AC were down-regulated proteins in pSS samples. It may prove informative to elucidate their biological and pathophysiological role.
Several members of cysteine proteinase inhibitors family were also identified in this study. Cystatins inhibit proteases, thus secretion of these proteins limits proteolysis and tissue damage [33]. High levels of these proteins may be indicative of both the activation of apoptosis as well as the processing of pro-inflammatory cytokine precursors [34]. Cystatin-SA, A, C and D were up-regulated in pSS, while cystatin-S, B and SN did not change in abundance. Of the up-regulated cystatins, elevated expression of cystatin-C and D in pSS subjects is consistent with previous observations [10, 12], but contrary to Hu et. al. [4]. Although the abundance of cystatin-S and cystatin-SN did not change much in our study, they were previously reported as down-regulated [10, 12, 13, 15].
Network analysis of differentially expressed proteins using IPA indicated a striking level of upregulation/engagement of immunological proteins involved in cross talk and activation between innate immune cells and adaptive B cell and T cells. Of note, acute phase response signaling proteins were not detected by the MS possibly due to their low levels and/or below the limits of detection by the MS as observed by low spectral counts for IL-6 (HC: 4, pSS: 2). Nevertheless, over-expression of IL-6, a pro-inflammatory cytokine previously found in serum and salivary gland biopsies of pSS [35] which among multiple other activities may govern the differentiation of TH17 cells, likely plays an important pathogenic role in pSS [36]. Of significant interest, enhanced involvement of the ICOS (inducible costimulator) pathway would be consistent with the involvement of follicular T helper cells which may be central to the pathogenesis of multiple autoimmune diseases including pSS and SLE [37]. Importantly, the recognition of multiple inflammatory and immunological pathways may also be invaluable for the rationale design of biological interventions targeted to specifically interrupt the activated cells/pathways. This promising potential of salivary proteomics should be next tested in larger validation patient cohorts for use as noninvasive tests for screening, early detection, and monitoring of pSS.
Supplementary Material
Acknowledgments
We gratefully acknowledge Mireya Gonzalez-Begne, Gene Watson, Bingwen Lu, Robin Park and Charlene Chung for assistance with samples and discussions during the course of this study. This work was supported in part by Intramural NIH programs, NIH grants DE017585 (I.S.) U19-AI56390 (I.S.) and P41 RR011823 (J.R.Y.).
Footnotes
The authors have declared no conflict of interest.
References
- 1.Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 2.Kassan SS, Thomas TL, Moutsopoulos HM, Hoover R, Kimberly RP, Budman DR, Costa J, Decker JL, Chused TM. Increased risk of lymphoma in sicca syndrome. Ann. Intern. Med. 1978;89:888–892. doi: 10.7326/0003-4819-89-6-888. [DOI] [PubMed] [Google Scholar]
- 3.Pink R, Simek J, Vondrakova J, Faber E, Michl P, Pazdera J, Indrak K. Saliva as a diagnostic medium. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2009;153:103–110. doi: 10.5507/bp.2009.017. [DOI] [PubMed] [Google Scholar]
- 4.Hu S, Wang J, Meijer J, Ieong S, Xie Y, Yu T, Zhou H, Henry S, Vissink A, Pijpe J, Kallenberg C, Elashoff D, Loo JA, Wong DT. Salivary proteomics and genomic biomarkers for primary Sjögren’s syndrome. Arthritis Rheum. 2007;56:3588–3600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andonopoulos AP, Drosos AA, Skopouli FN, Moutsopoulos HM. Sjögren’s syndrome in rheumatoid arthritis and progressive systemic sclerosis. A comparative study. Clin. Exp. Rheumatol. 1989;7:203–205. [PubMed] [Google Scholar]
- 6.Salliot C, Mouthon L, Ardizzone M, Sibilia J, Guillevin L, Gottenberg J-E, Mariette X. Sjögren’s syndrome is associated with and not secondary to systemic sclerosis. Rheumatol. 2007;46:321–326. doi: 10.1093/rheumatology/kel252. [DOI] [PubMed] [Google Scholar]
- 7.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH. Classification criteria for Sjögrens syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harley JB, Scofield RH. Systemic lupus erythematosus: RNA protein autoantigens, models of disease heterogeneity, and theories of etiology. J. Clin. Immunol. 2005;11:297–316. doi: 10.1007/BF00918796. [DOI] [PubMed] [Google Scholar]
- 9.Hendrick JP, Wolin SL, Rinke J, Lerner MR, Steitz JA. Ro small cytoplasmic ribonuclearproteins are a subclass of La ribonuclearproteins: further characterization of the Ro and La small ribonuclearproteins from uninfected mammalian cells. Mol. Cell Biol. 1981;1:1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu OH, Atkinson JC, Hoehn GT, Illei GG, Hart TC. Identification of parotid salivary biomarkers in Sjögren's syndrome by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry and two-dimensional difference gel electrophoresis. Rheumatol. 2006;45:1077–1086. doi: 10.1093/rheumatology/kei212. [DOI] [PubMed] [Google Scholar]
- 11.Rao VP, Reddy AP, Lu X, Dasari S, Krishnaprasad A, Biggs E, Roberts CT, Jr, Nagalla SR. Proteomic identification of salivary biomarkers of type-2 diabetes. J. Proteome Res. 2009;8:239–245. doi: 10.1021/pr8003776. [DOI] [PubMed] [Google Scholar]
- 12.Giusti L, Baldini C, Bazzichi L, Ciregia F, Tonazzini I, Mascia G, Giannacini G, Bombardieri S, Luccacchini A. Proteome analysis of whole saliva: A new tool for rheumatic diseases- the example of Sjögren’s syndrome. Proteomics. 2007;7:1634–1643. doi: 10.1002/pmic.200600783. [DOI] [PubMed] [Google Scholar]
- 13.Peluso G, De Santis M, Inzitari R, Fanali C, Cabras T, Messana I, Castagnola M, Ferraccioli GF. Proteomic study of salivary peptides and proteins in patients with Sjögrens syndrome before and after pilocarpine treatment. Arthritis Rheum. 2007;56:2216–2222. doi: 10.1002/art.22738. [DOI] [PubMed] [Google Scholar]
- 14.Ching KH, Burbelo PD, Gonzalez-Begne M, Roberts MEP, Coca A, Sanz I, Iadarola MJ. Salivary anti-Ro60 and anti-Ro52 antibody profiles to diagnose Sjögrens syndrome. J. Dent. Res. 2011;90:445–449. doi: 10.1177/0022034510390811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldini C, Giusti L, Ciregia F, Da Valle Y, Giacomelli C, Donadio E, Sernissi F, Bazzichi L, Giannaccini G, Bombardieri S, Lucacchini A. Proteomic analysis of saliva: a unique tool to distinguish primary Sjögren's syndrome from secondary Sjögren's syndrome and other sicca syndromes. Arthritis Res. Ther. 2011;13:R194. doi: 10.1186/ar3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Streckfus CF, Dubinsky WP. Proteomics analysis of saliva for cancer diagnosis. Expert Rev. Proteomics. 2007;4:329–332. doi: 10.1586/14789450.4.3.329. [DOI] [PubMed] [Google Scholar]
- 17.Washburn MP, Wolters D, Yates JR., III Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 18.Lashley K. Reflex secretion of the human parotid gland. J. Exp. Psychol. 1916;1:461–493. [Google Scholar]
- 19.Ambatipudi KS, Lu B, Hagen FK, Melvin JE, Yates JR. Quantitative analysis of age specific variation in the abundance of human female parotid salivary proteins. J. Proteome Res. 2009;8:5093–5102. doi: 10.1021/pr900478h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan X, Desiderio DM. Signaling pathway networks mined from human pituitary adenoma proteomics data. BMC Med. Genomics. 2010;3:13. doi: 10.1186/1755-8794-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald WH, Tabb DL, Sadygov RG, MacCoss MJ, Venable J, Graumann J, Johnson JR, Cociorva D, Yates JR., 3rd MS1, MS2, and SQT-three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications. Rapid Commun. Mass Spectrom. 2004;18:2162–2168. doi: 10.1002/rcm.1603. [DOI] [PubMed] [Google Scholar]
- 22.Peng J, Elias JE, Thoreen CC, Licklider LJ, Gygi SP. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J. Proteome Res. 2003;2:43–50. doi: 10.1021/pr025556v. [DOI] [PubMed] [Google Scholar]
- 23.Xu T, Venable JD, Park SK, Cociorva D, Lu B, Liao L, Wohlschlegel J, Hewel J, Yates JRI. ProLuCID; a fast and sensitive tandem mass spectra-based protein identification program. Mol. Cell Proteomics. 2006;5:S174. [Google Scholar]
- 24.Tabb DL, McDonald WH, Yates JR., III DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 2002;1:21–26. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang G, Wu WW, Zeng X, Chou C-L, Shen R-F. Label-free protein Quantification using LC-coupled ion trap or FT mass spectrometry: Reproducibility, linearity, and application with complex proteome. J. Proteome Res. 2006;5:1214–1223. doi: 10.1021/pr050406g. [DOI] [PubMed] [Google Scholar]
- 26.Beeley JA, Khoo KS. Salivary proteins in rheumatoid arthritis and Sjögren’s syndrome: one dimensional and two-dimensional electrophoretic studies. Electrophoresis. 1999;20:1652–1660. doi: 10.1002/(SICI)1522-2683(19990601)20:7<1652::AID-ELPS1652>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 2004;76:4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 28.Hu S, Gao K, Pollard R, Arellano-Garcia M, Zhou H, Zhang L, Elashoff D, Kallenberg CG, Vissink A, Wong DT. Preclinical validation of salivary biomarkers for primary Sjögren’s syndrome. Arthritis Care and Res. 2010;62:1633–1638. doi: 10.1002/acr.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr SA, Anderson L. Protein quantitation through targeted mass spectrometry: The way out for biomarker purgatory? Clin. Chem. 2008;54:1749–1752. doi: 10.1373/clinchem.2008.114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR., III Proteomic analysis of human parotid gland exosome by multidimensional protein identification technology (MudPIT) J. Proteome Res. 2009;8:1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 32.Sfriso P, Ostuni P, Botosios C, Andretta M, Oliviero F, Punzi L, Todesco S. Serum and salivary neopterin and interferon-gamma in primary Sjögren’s syndrome. Correlation with clinical, laboratory and histopathologic features. Scan. J. Rheumatol. 2003;32:74–78. doi: 10.1080/03009740310000067. [DOI] [PubMed] [Google Scholar]
- 33.Al-Hashimi I, Dickinson DP, Levine MJ. Purification, molecular cloning, and sequencing of salivary cystatin SA-1. J. Biol. Chem. 1998;263:9381–9987. [PubMed] [Google Scholar]
- 34.Robinson CP, Yamachika S, Alford CE, Cooper C, Pichardo EL, Shah N, Peck AB, Humphreys-Beher MG. Elevated levels of cysteine protease activity in saliva and salivary glands of the nonobese diabetic (NOD) mouse model for Sjögren’s syndrome. Proc. Natl. Acad. Sci. 1997;94:5767–5771. doi: 10.1073/pnas.94.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjögrens syndrome. J. Immunol. 1994;152:5532–5539. [PubMed] [Google Scholar]
- 36.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2010;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 37.Yu D, Vinuesa CG. Multiple checkpoints keep follicular helper T cells under control to prevent autoimmunity. Cell Mol. Immunol. 2010;7:198–203. doi: 10.1038/cmi.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.