Abstract
LncRNAs (long non-coding RNAs) are thought to play a significant role in cellular homeostasis during development and disease by interacting with CMPs (chromatin-modifying proteins). We recently showed that following transient focal ischemia, the expression of many lncRNAs was altered significantly in rat brain. We currently analyzed whether focal ischemia also alters the association of lncRNAs with the CMPs Sin3A and coREST (corepressors of the RE-1 silencing transcription factor). RIP (RNA immunoprecipitation) combined with lncRNA microarray analysis showed that 177 of the 2497 lncRNAs expressed in rat cerebral cortex showed significantly increased binding to either Sin3A or coREST following ischemia compared with sham. Of these, 26 lncRNAs enriched with Sin3A and 11 lncRNAs enriched with coREST were also up-regulated in their expressions after ischemia. A majority of the lncRNAs enriched with these CMPs were intergenic in origin. Evaluation of the expression profiles of corresponding protein-coding genes showed that their expression levels correlate with those of the lncRNAs with which they shared a common locus. This is the first study to show that stroke-induced lncRNAs might associate with CMPs to modulate the post-ischemic epigenetic landscape.
Keywords: cerebral ischemia, corepressor, genomics, long non-coding RNA, REST, Sin3A, transcription factor
Abbreviations: ChIP, chromatin immunoprecipitation; CMP, chromatin-modifying protein; CNS, central nervous system; Dclk1, doublecortin-like kinase 1; Fmr1, fragile X mental redardation 1; Fos, FBJ osteosarcoma oncogene; Galntrl6, UPD-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-like 6; GFAP, glial fibrillary acidic protein; lncRNA, long non-coding RNA; MCAO, middle cerebral artery occlusion; REST, RE-1 silencing transcription factor; RIP, RNA immunoprecipitation
INTRODUCTION
LncRNAs (long non-coding RNAs) are a unique class of RNAs that are >200 bp long and show specific spatiotemporal expression profiles (Batista and Chang, 2013). Perturbations in the cerebral lncRNAome were shown to exacerbate the pathophysiology of a variety of CNS (central nervous system) disorders, drug addiction and cancer (Michelhaugh et al., 2011; Pastori and Wahlestedt, 2012; Qiu et al., 2013). However, very little is known about the significance of lncRNAs after acute injuries to the CNS. We recently showed that the expression of many lncRNAs altered rapidly in rat brain following transient focal cerebral ischemia (Dharap et al., 2012). Recent studies have shown evidence of physical associations between lncRNAs and CMPs (chromatin modifying proteins) such as polycomb repressive complex 2, lysine (K)-specific demethylase, euchromatic histone-lysine N-methyltransferase 2 and heterogeneous nuclear ribonucleoprotein K (Nagano et al., 2008; Pandey et al., 2008; Khalil et al., 2009; Huarte et al., 2010; Nagano and Fraser, 2011; Rinn and Chang, 2012a). These interactions were shown to be crucial for global processes such as chromosome inactivation and lineage-specific gene repression (Nagano and Fraser, 2009; Pandey and Kanduri, 2011), as well as local events such as the p53 response to environmental insults (Huarte et al., 2010). These studies suggest that lncRNAs play an essential role in epigenetic silencing exerted by CMPs.
Recent studies showed that the neuronal REST (RE-1 silencing transcription factor) and its corepressors Sin3A and coREST were robustly activated in the rodent brain and together mediated the epigenetic silencing of several neuronal genes resulting in neuronal death after cerebral ischemia (Noh et al., 2012). REST is also known to control the expression of non-coding RNAs in brain pathologies such as Huntington's disease (Johnson et al., 2010). We currently evaluated whether stroke-responsive lncRNAs interact with Sin3A and coREST, the CMPs associated with REST (Andres et al., 1999; Grimes et al., 2000; Ballas and Mandel, 2005).
MATERIALS AND METHODS
Transient focal ischemia
A 1 h transient MCAO (middle cerebral artery occlusion) was induced in adult, male, spontaneously hypertensive rats (280–320 g; Charles River) under isoflurane anesthesia by the intraluminal suture method as described earlier (Dharap et al., 2012; Pandi et al., 2013). All surgical procedures were approved by the Research Animal Resources and Care Committee of the University of Wisconsin-Madison and animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services Publication number 86–23 (revised). After suturing the wound, 0.5% bupivacaine (0.25 ml) was injected along the incision to provide short duration local anesthesia. The animals were allowed to recover from anesthesia and returned to the cage with ad libitum access to food and water. During the surgery, rats were under spontaneous respiration. Rats were killed at 6 h of reperfusion and the ipsilateral cortex was dissected. Sham-operated rats served as control.
RIP (RNA immunoprecipitation)
Cortical tissue was homogenized in 1:1 phosphate-buffered saline and nuclear isolation buffer [1.28 M sucrose; 40 mM Tris–HCl pH 7.5; 20 mM MgCl2; 4% (v/v) Triton X-100], centrifuged at 2750 g for 15 min and the nuclear pellet was resuspended in RIP buffer (Millipore). Resuspended nuclear fraction was mechanically sheared using a homogenizer, centrifuged at 13000 g for 10 min and the lysate was collected. Antibodies against Sin3A [polyclonal ChIP (chromatin immunoprecipitation grade) ab3479; Abcam] and coREST (polyclonal ChIP grade; 07–455; Millipore) were incubated with magnetic agarose A/G beads (Invitrogen) for 1 h and the nuclear lysates were incubated with these antibody-beads complex overnight with gentle rotation. The beads were then collected using a magnetic stand, resuspended and washed four times in RIP buffer. 20 μl of this extract was used for Western blotting to confirm Sin3A and coREST pull-down. Briefly, 20 μl of RIP lysate was electrophoresed on a denaturing PAGE, transferred onto a nitrocellulose membrane and probed using Sin3A, coREST and β-actin antibodies and the corresponding HRP (horseradish peroxidase)-tagged secondary antibodies (Cell Signaling Technology) followed by chemiluminescence detection and visualization of the bands.
lncRNA microarray
RNA was extracted from the RIP nuclear lysate preparation using the Magna RIP kit (Millipore), linearly amplified, labeled with Cy3-dCTP, purified by RNAeasy Mini Kit (Qiagen), fragmented and hybridized to an Arraystar lncRNA expression microarray containing 9300 lncRNA probes as described previously (Dharap et al., 2012). Differentially expressed transcripts were identified by fold-change screening with a threshold of ≥2-fold. Statistically significant differences between the groups were identified by the statistical measures built in the GeneSpring based on the t test P value method with a high stringency (fold change cutoff of >2 and a probability value of <0.001 to decrease false-positives).
Real-time PCR
The mRNA expression of Dclk1 (doublecortin-like kinase 1), GFAP (glial fibrillary acidic protein), Fmr1 (fragile X mental retardation 1); Galntrl6 (UPD-N-acetyl-alphaD-galactosamine:polypeptide N-acetylgalactosaminyltransferase-like 6); Fos (FBJ osteosarcoma oncogene) was evaluated with real-time PCR using the SYBR-Green method as described earlier using 18s rRNA as an internal control (Dharap et al., 2009). The following primer sequences (5′ to 3′) designed with Primer Express Software (Applied Biosystems) based on the GenBank numbers given in parenthesis were used for real-time PCR: Dclk1 (NM_053343): CGGCAAGTCACCAAGTCCAT and ACATCGCTCCACTGTGTCTTT; GFAP (NM_017009): GCCTCTCCCTGTCTCGAATG and CGCCTTGTTTTGCTGTTCCA; Fmr1 (NM_052804): TTGCCACCAAGTTCCCTA and AGTGGCATTAGCGATGCTGT; Galntrl6 (NM_001135756): TTGTGCGCACCAAGAAAAGG and GTGCCTCGTACCCAAAGTGA; and Fos (NM_022197): TACTACCATTCCCCAGCCGA and GCTGTCACCGTGGGGATAAA.
RESULTS
Association of lncRNAs with Sin3A and coREST
Cortical nuclear lysates from rats subjected to focal ischemia were IP (immunoprecipitated) for Sin3A and coREST (confirmed by Western blotting; Supplementary Figure; available at http://www.asnneuro.org/an/005/an005e124add.htm) and the precipitated RNA was subjected to lncRNA microarray analysis (RIP-chip). The RIP-chiP showed a significant increase in enrichment of 99 lncRNAs with Sin3A and 78 lncRNAs with coREST in the ischemia group compared with the sham group. Three lncRNAs showed increased binding to both Sin3A and coREST, as well as increased expression after focal ischemia compared with sham (Table 1). Twelve lncRNAs showed increased binding to both Sin3A and coREST after focal ischemia, but were not induced in their expression after stroke (Table 1). A further 23 lncRNAs that showed increased binding to Sin3A (Table 2) and eight lncRNAs that showed increased binding to coREST (Table 3) were also significantly up-regulated after focal ischemia compared with sham. A further 61 lncRNAs showed increased binding to Sin3A and 55 lncRNAs showed increased binding to coREST after focal ischemia, but none of them were induced in their expression after stroke (Supplementary Tables S1 and S2; available at http://www.asnneuro.org/an/005/an005e124add.htm).
Table 1. LncRNAs that showed increased binding to both Sin3A and coREST after focal ischemia.
All lncRNAs are confirmed to be the annotated non-coding transcripts from NCBI, ENSEMBL and UCSC genome browser. Δ fold over sham is mean fold chance (<20% S.D. in each case) in comparison with the sham group (n=3/group). Intragenica represents sense_exon overlap. RIP, RNA immunoprecipitation. NC, no change. Fos, FBJ osteosarcoma oncogene; Slc2a3, solute carrier family 2 (facilitated glucose transporter), member 3; Lgi3, leucine-rich repeat LGI family, member 3; Prpf4b, PRP4 pre-mRNA processing factor 4 homolog B (yeast).
| Δ fold over sham | |||||
|---|---|---|---|---|---|
| LncRNA | Expression | RIP Sin3A | RIP coREST | Location | Associated protein-coding gene |
| MRAK154943 | 12.91 | 3.42 | 2.56 | Intergenic | |
| XR_005513 | 14.50 | 2.81 | 2.25 | Intergenic | |
| MRAK159688 | 12.96 | 2.73 | 3.15 | Intragenica | Fos (NM_022197) |
| BC158675 | NC | 6.04 | 3.10 | Intergenic | |
| MRAK013532 | NC | 5.59 | 2.55 | Intergenic | |
| MRBC030402 | NC | 4.36 | 2.22 | Intergenic | |
| BC094214 | NC | 3.98 | 4.28 | Intergenic | |
| MRBC019134 | NC | 3.02 | 2.31 | Intergenic | |
| XR_007454 | NC | 2.73 | 2.15 | Intergenic | |
| MRBC052873 | NC | 2.14 | 2.55 | Intergenic | |
| XR_008939 | NC | 2.07 | 2.45 | Intergenic | |
| MRuc007jsx | NC | 2.00 | 2.18 | Intergenic | |
| BC063168 | NC | 3.14 | 2.93 | Intragenica | Slc2a3 (NM_017102) |
| BC158671 | NC | 2.48 | 2.10 | Intragenica | Lgi3 (NM_001107277) |
| MRAK047212 | NC | 2.43 | 2.22 | Intragenica | Prpf4b (NM_001011923) |
Table 2. Stroke-induced lncRNAs that showed increased binding to Sin3A, but not to coREST.
All lncRNAs are confirmed to be the annotated non-coding transcripts from NCBI, ENSEMBL and UCSC genome browser. Δ fold over sham is mean fold chance (<20% S.D. in each case) in comparison with the sham group (n=3/group). RIP, RNA immunoprecipitation. Intragenica represents sense_exon overlap and Intragenicb represents sense_intron overlap. Dclk1, doublecortin-like kinase 1; CDC91, cell division cycle 91-like 1; GFAP, glial fibrillary acidic protein.
| ΔFold over sham | ||||
|---|---|---|---|---|
| LncRNA | Expression | RIP | Location | Associated protein-coding gene |
| XR_008555 | 2.86 | 3.60 | Intergenic | |
| XR_007499 | 6.49 | 3.08 | Intergenic | |
| XR_007404 | 5.28 | 3.04 | Intergenic | |
| XR_006148 | 11.70 | 3.02 | Intergenic | |
| XR_007321 | 8.66 | 2.56 | Intergenic | |
| XR_005733 | 8.32 | 2.22 | Intergenic | |
| XR_007247 | 18.03 | 2.21 | Intergenic | |
| XR_009083 | 22.07 | 2.13 | Intergenic | |
| XR_009151 | 14.91 | 2.12 | Intergenic | |
| XR_005800 | 15.80 | 2.12 | Intergenic | |
| NR_027324 | 5.81 | 2.05 | Intergenic | |
| XR_006778 | 4.96 | 2.02 | Intergenic | |
| DQ266361 | 8.33 | 3.87 | Intergenic | |
| MRAK166199 | 3.70 | 3.60 | Intergenic | |
| XR_008295 | 7.93 | 2.88 | Itergenic | |
| XR_008876 | 6.66 | 2.62 | Intergenic | |
| MRAK049735 | 18.43 | 2.13 | Intergenic | |
| XR_007101 | 5.74 | 2.08 | Intergenic | |
| XR_007384 | 12.11 | 2.02 | Intergenic | |
| AF030089 | 3.85 | 2.90 | Intragenica | Dclk1 (NM_053343) |
| MRAK135044 | 3.74 | 2.83 | Intragenicb | Dclk1 (NM_053343) |
| AY383714 | 10.61 | 2.62 | Intragenica | CDC9111 (NM_181637) |
| EF094477 | 6.83 | 2.42 | Intragenica | GFAP (NM_017009) |
Table 3. Stroke-induced lncRNAs that showed increased binding to coREST, but not to Sin3A.
All lncRNAs are confirmed to be the annotated noncoding transcripts from NCBI, ENSEMBL and UCSC genome browser. Δ fold over sham is mean fold chance (<20% S.D. in each case) in comparison to the sham group (n=3/group). RIP, RNA immunoprecipitation. Intragenica represents sense_exon overlap and Intragenicb represents sense_intron overlap. Fmr1, fragile X mental redardation 1; Sh3bgrl1, SH3 domain-binding glutamic acid-rich protein like 2; Galntl1, UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase-like 6.
| ΔFold over sham | ||||
|---|---|---|---|---|
| LncRNA | Expression | RIP | Location | Associated protein-coding gene |
| MRAK163011 | 4.19 | 2.15 | Intergenic | |
| XR_008508 | 2.75 | 2.08 | Intergenic | |
| MRAK143109 | 2.51 | 6.55 | Intergenic | |
| XR_008791 | 2.81 | 2.22 | Intergenic | |
| MRuc008ymd | 10.45 | 2.19 | Intergenic | |
| MRAK053211 | 6.57 | 6.26 | Intragenica | Fmr1 (NM_052804) |
| MRAK080604 | 2.21 | 4.46 | Intragenica | Sh3bgrl1 (NM_001137647) |
| XR_007355 | 7.66 | 3.02 | Intragenicb | Galntl1 (NM_001135756) |
Importantly, of 537 lncRNAs significantly induced (≥ 2-fold) at 6 h of reperfusion after transient MCAO, the top 17 stroke-induced lncRNAs (up-regulated from 22- to 93-fold over sham) (Dharap et al., 2012) were not enriched with either protein (Sin3A or coREST) evaluated by RIP. For the top 50 stroke-induced lncRNAs (up-regulated from 7- to 93-fold over sham), only seven showed increased enrichment with Sin3A and three showed increased enrichment with coREST. Together, this demonstrates that the enrichment of lncRNAs with Sin3A and coREST is not an artifact of an overall increase in lncRNA gene expression after stroke, but instead selective recruitment by these proteins. Interestingly, 74 and 85% of the lncRNAs enriched with Sin3A and coREST, respectively, were not significantly up-regulated following stroke suggesting that they may be present as dormant reserve pools in the healthy cortex that are subsequently targeted for recruitment by activated Sin3A and coREST following an ischemic insult.
Genomic correlates of the Sin3A and coREST-associated lncRNAs
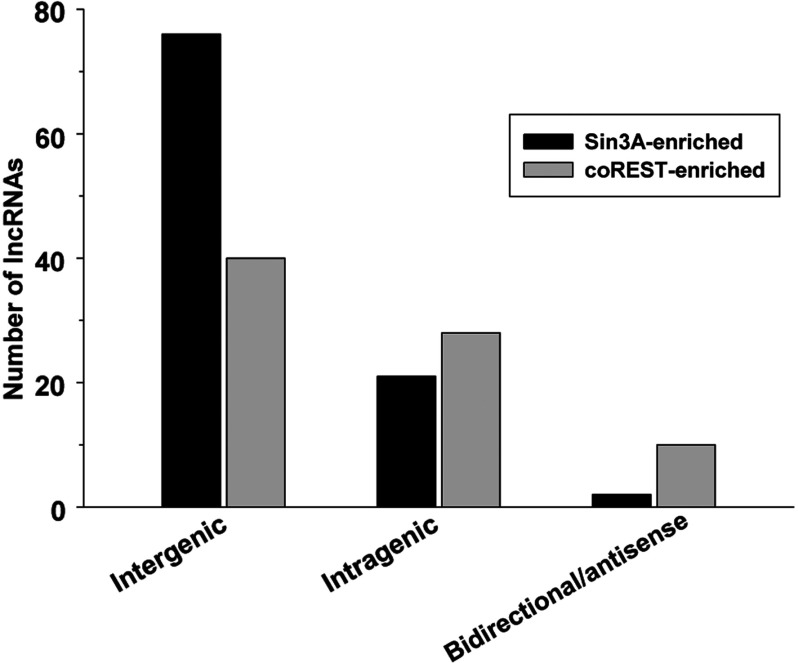
LncRNAs are transcribed from various loci including introns of protein-coding genes (sense), antisense to protein-coding genes and intergenic stretches (Rinn and Chang, 2012b). Majority of the lncRNAs that showed significantly increased binding to Sin3A and coREST after stroke are intergenic (65%) or intragenic (sense exon or intron; 28%), while 7% are either bidirectional or antisense in origin (Figure 1).
Figure 1. Genomic loci of the numbers of lncRNAs that showed increased binding to Sin3A and coREST after transient focal ischemia.
Of the 15 lncRNAs co-enriched with both Sin3A and coREST after stroke, 11 are intergenic and 4 are intragenic (Table 1). Of the three lncRNAs that showed increased enrichment with both Sin3A and coREST as well as induced in expression after stroke, two are intergenic and one is intragenic (Table 1). The Fos locus produced an lncRNA that showed increased binding to both Sin3A and coREST (Table 1). Some Sin3A-enriched lncRNAs transcribed from intragenic loci that are particularly relevant to stroke, such as the transcription factor Fos, Dclk1 and the astrocytic activation marker GFAP (Table 2). Interestingly, the Dclk1 locus produced two lncRNAs, both of which were significantly induced after stroke and showed increased binding to Sin3A compared with sham (Table 2).
Expression of protein-coding genes associated with the intragenic lncRNAs
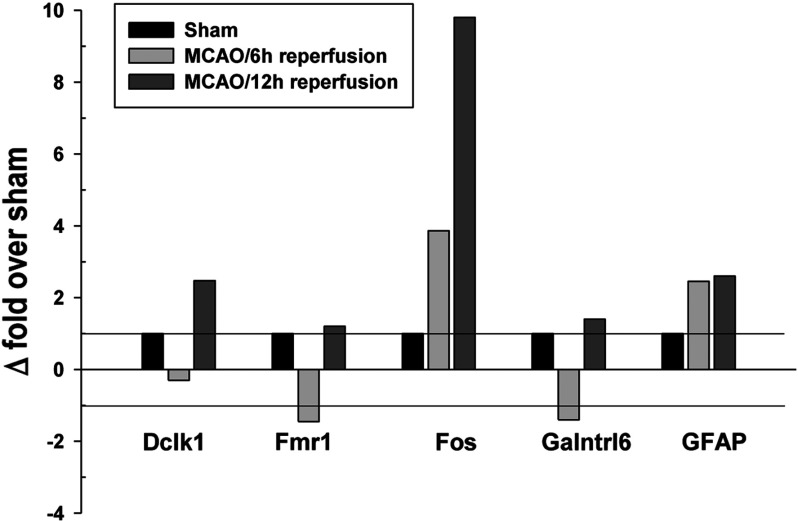
The observation that several Sin3A and coREST associated lncRNAs originated from intragenic loci suggested that their expression might be associated with that of the corresponding protein-coding transcripts. Hence, we analyzed the post-ischemic expression of the protein-coding genes Dclk1, GFAP, Fmr1, Galntrl1 and Fos associated with the intragenic lncRNAs that were up-regulated in their expression as well as association to Sin3A and/or coREST. Of these, Fos was up-regulated by 3.8-fold at 6 h reperfusion and 9.8-fold at 12 h reperfusion compared with sham (Figure 2). GFAP was up-regulated by 2.5-fold at 6 h and 2.6-fold at 12 h reperfusion compared with sham (Figure 2). Dclk1 showed no significant change at 6 h but was up-regulated by 2.5-fold at 12 h reperfusion compared with sham (Figure 2). Whereas, Fmr1 and Galntrl6 showed no significant change over sham at any reperfusion time after transient MCAO (Figure 2).
Figure 2. Expression levels of protein-coding RNAs originating from the same loci as intragenic lncRNAs that showed induced expression as well as increased binding to Sin3A or coREST after ischemia.
DISCUSSION
The mammalian genome transcribes numerous lncRNAs that show evolutionary conservation and are expressed in a stage and cell specific manner (Mercer and Mattick, 2013; Sabin et al., 2013). Recent studies suggested that altered levels and/or functionality of lncRNAs play a role in various pathologies including cancer, Alzheimer's disease and myocardial infarction (Pastori and Wahlestedt, 2012; Ounzain et al., 2013; Qiu et al., 2013).
A major function attributed to lncRNAs is to bind with CMPs and modulate their transcriptional activity (Rinn and Chang, 2012a). Hence, a change in the expression profiles of lncRNAs under pathological conditions might significantly influence the functionality of CMPs. We recently showed that stroke alters the expression profiles of lncRNAs in a rapid and significant manner in rodent brain (Dharap et al., 2012). To understand the implications of the altered lncRNAome in post-stroke pathophysiology, we currently evaluate whether ischemia promotes the association of lncRNAs with the CMPs that act as corepressors of the transcription factor REST. Recent studies showed that REST up-regulation promotes post-ischemic neuronal death (Noh et al., 2012; Pandi et al., 2013). REST is a transcription factor that plays a significant role in the phenotypic maturation of neurons by silencing neural genes in the non-neural cells of CNS during development. REST expression in the normal, adult brain is known to be low. Sin3A and coREST are essential corepressors of REST that bind to its opposite terminals and recruit other silencing factors to the target loci (Ballas and Mandel, 2005). Our results show that the physical association of many lncRNAs with Sin3A and coREST increase significantly following stroke. Although lncRNA expression and binding to CMPs are independent events, several lncRNAs that showed increased binding to Sin3A and coREST also showed increased expression following focal ischemia. This suggests that the Sin3A- and coREST-enriched lncRNAs induced after stroke might play important roles in modifying the post-ischemic epigenetic landscape by modulating the REST-mediated gene silencing.
Previous work exploring the binding of various CMPs to lncRNAs showed that each protein has a distinct lncRNA signature with marginal overlap with other related CMPs (Khalil et al., 2009). Our data support this by showing that a majority of the stroke-induced lncRNAs were bound to Sin3A or coREST with only three common lncRNAs that bound to both. Since Sin3A and coREST bind opposite termini of REST, these overlapping lncRNAs might serve as a scaffold to recruit Sin3A and coREST to the REST complex and/or facilitate the recruitment of other proteins such as histone deacetylases that are common to both corepressors (Ballas and Mandel, 2005; Roopra et al., 2012).
Most of the lncRNAs that showed increased binding to Sin3A and coREST are intergenic. However, some are also intragenic and were mapped to the loci of protein-coding genes such as Fos, Dclk1, GFAP and Fmr1. The impact of the intragenic lncRNAs on the expression of the corresponding protein-coding transcripts is unclear. LncRNAs can silence or activate the expression of the associated genes cis or in trans (Batista and Chang, 2013). The expression of lncRNA MRAK159688 that originates from an exon of Fos was observed to be up-regulated by ~13-fold after focal ischemia. RIP data showed that the enrichment of MRAK159688 with both Sin3A and coREST also increased after focal ischemia by ~3-fold. We observed that Fos expression was also induced significantly after ischemia. Although MRAK159688 is not transcribed by Fos per se, their co-expression from the same locus may position the lncRNA to serve as an upstream regulator or downstream effector of Fos probably via REST and its corepressors. A similar functional association was previously shown for lincRNA-p21 and p53. The lincRNA-p21 induced by p53 was shown to associate with a p53 complex to repress the expression of p53 target genes (Huarte et al., 2010). AF030089 and MRAK135044 are stroke-induced lncRNAs that originate from a sense exon and sense intron, respectively, of the Dclk1 gene. Both lncRNAs showed identical expression levels (distinct probes incorporating the sequence variation were used to detect these two lncRNAs) and were significantly enriched with Sin3A. This suggests that these two lncRNAs might control Dclk1 function via Sin3A in the post-ischemic cortex. The lncRNA EF094477 is also intragenic from GFAP gene locus. We observed that the expression and binding to Sin3A of EF094477 were induced after ischemia by 6.8- and 2.4-fold, respectively, and GFAP expression was also induced concomitantly. Not all lncRNA/protein-coding pairs from the same loci will be co-regulated. For example, expression of Dclk1 and Galntrl1 was not altered although the lncRNAs associated with their gene loci (AF030089, MRAK1335044 and XR007355) were up-regulated in their expression as well as binding to Sin3A/coREST. However, our data indicate that the stroke-induced lncRNAs expressed from intragenic loci does not exhibit a cis inhibitory effect. Instead, these lncRNAs might either induce or serve to maintain the expression of the protein-coding transcripts at a normal level by activating the epigenetic signatures as demonstrated previously (Huang et al., 2010). Furthermore, some lncRNAs showed coexpression with their corresponding protein-coding genes while others did not. Hence, it is possible that intragenic lncRNAs may be transcribed differentially from the same transcription start sites as that of the corresponding protein-coding genes or via novel internal start sites independent of the protein-coding gene unit.
In summary, this is the first study to demonstrate significantly increased association between ischemia- induced lncRNAs with CMPs Sin3A and coREST. While we studied only two CMPs, there might be others that are active in the post-ischemic brain and may bind to ischemia-induced lncRNAs to form unique ribonucleoprotein functional complexes. Such interactions might be functionally significant for the ischemic pathophysiology.
Online data
ACKNOWLEDGEMENT
We thank Kellie Bowen for technical assistance.
AUTHOR CONTRIBUTION
Ashutosh Dharap and Raghu Vemuganti designed the study. Ashutosh Dharap and Courtney Pokrzywa conducted experiments. Ashutosh Dharap analyzed data. Ashutosh Dharap and Raghu Vemuganti wrote the paper.
FUNDING
This work was supported by the National Institutes of Health [grant numbers NS074444 and NS079585].
References
- Andres ME, Burger C, Peral-Rubio MJ, Battaglioli E, Anderson ME, Grimes J, Dallman J, Ballas N, Mandel G. CoREST: a functional corepressor required for regulation of neural-specific gene expression. Proc Natl Acad Sci USA. 1999;96:9873–9878. doi: 10.1073/pnas.96.17.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol. 2005;15:500–506. doi: 10.1016/j.conb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Nakka VP, Vemuganti R. Effect of focal ischemia on long noncoding RNAs. Stroke. 2012a;43:2800–2802. doi: 10.1161/STROKEAHA.112.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab. 2009;29:675–687. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes JA, Nielsen SJ, Battaglioli E, Miska EA, Speh JC, Berry DL, Atouf F, Holdener BC, Mandel G, Kouzarides T. The co-repressor mSin3A is a functional component of the REST–CoREST repressor complex. J Biol Chem. 2000;275:9461–9467. doi: 10.1074/jbc.275.13.9461. [DOI] [PubMed] [Google Scholar]
- Huang YC, Chen HT, Teng SC. Intragenic transcription of a noncoding RNA modulates expression of ASP3 in budding yeast. RNA. 2010;16:2085–2093. doi: 10.1261/rna.2177410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, Lander ES, Jacks T, Rinn JL. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Richter N, Jauch R, Gaughwin PM, Zuccato C, Cattaneo E, Stanton LW. The Human Accelerated Region 1 noncoding RNA is repressed by REST in Huntington's disease. Physiol Genomics. 2010;41:267–274. doi: 10.1152/physiolgenomics.00019.2010. [DOI] [PubMed] [Google Scholar]
- Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, Regev A, Lander ES, Rinn JL. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20:300–307. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- Michelhaugh SK, Lipovich L, Blythe J, Jia H, Kapatos G, Bannon MJ. Mining Affymetrix microarray data for long non-coding RNAs: altered expression in the nucleus accumbens of heroin abusers. J Neurochem. 2011;116:459–466. doi: 10.1111/j.1471-4159.2010.07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Fraser P. Emerging similarities in epigenetic gene silencing by long noncoding RNAs. Mammal Genome. 2009;20:557–562. doi: 10.1007/s00335-009-9218-1. [DOI] [PubMed] [Google Scholar]
- Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–181. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, Fraser P. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science. 2008;322:1717–1720. doi: 10.1126/science.1163802. [DOI] [PubMed] [Google Scholar]
- Noh KM, Hwang JY, Follenzi A, Athanasiadou R, Miyawaki T, Greally JM, Bennett MV, Zukin RS. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc Natl Acad Sci USA. 2012;109:E962–E971. doi: 10.1073/pnas.1121568109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounzain S, Crippa S, Pedrazzini T. Small and long non-coding RNAs in cardiac homeostasis and regeneration. Biochim Biophys Acta. 2013;1833:923–933. doi: 10.1016/j.bbamcr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Pandey RR, Kanduri C. Transcriptional and posttranscriptional programming by long noncoding RNAs. Prog Mol Subcell Biol. 2011;51:1–27. doi: 10.1007/978-3-642-16502-3_1. [DOI] [PubMed] [Google Scholar]
- Pandey RR, Mondal T, Mohammad F, Enroth S, Redrup L, Komorowski J, Nagano T, Mancini-Dinardo D, Kanduri C. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell. 2008;32:232–246. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Pandi G, Nakka VP, Dharap A, Roopra A, Vemuganti R. MicroRNA miR-29c Down-Regulation Leading to De-Repression of Its Target DNA Methyltransferase 3a Promotes Ischemic Brain Damage. PLoS ONE. 2013;8:e58039. doi: 10.1371/journal.pone.0058039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori C, Wahlestedt C. Involvement of long noncoding RNAs in diseases affecting the central nervous system. RNA Biol. 2012;9:860–870. doi: 10.4161/rna.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu MT, Hu JW, Yin R, Xu L. Long noncoding RNA: an emerging paradigm of cancer research. Tumour Biol. 2013;34:613–620. doi: 10.1007/s13277-013-0658-6. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012a;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012b;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopra A, Dingledine R, Hsieh J. Epigenetics and epilepsy. Epilepsia. 2012;53(Suppl 9):2–10. doi: 10.1111/epi.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin LR, Delas MJ, Hannon GJ. Dogma derailed: the many influences of RNA on the genome. Mol Cell. 2013;49:783–794. doi: 10.1016/j.molcel.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.