Abstract
Aims
The misfolding and the aggregation of specific proteins are key features of neurodegenerative diseases, specifically Transmissible Spongiform Encephalopathies (TSEs). In TSEs, neuronal loss and inflammation are associated with the accumulation of the misfolded isoform (PrPsc) of the cellular prion protein (PrPc). Therefore we tested the hypothesis that augmenting a natural anti-inflammatory pathway mediated by epoxygenated fatty acids (EpFAs) will delay lethality. EpFAs are highly potent but enzymatically labile molecules produced by the actions of a number of cytochrome P450 enzymes. Stabilization of these bioactive lipids by inhibiting their degradation mediated by the soluble epoxide hydrolase (sEH) results in potent anti-inflammatory effects in multiple disease models.
Main methods
Mice were infected with the mouse-adapted RML strain of scrapie by intracerebral or intraperitoneal routes. Animals received the sEH inhibitor, by oral route, administrated in drinking water or vehicle (PEG400). Infected mice were euthanized at a standard clinical end point. Histopathological, immunohistochemical and Western blot analyses of brain tissue confirmed the presence of pathology related to prion infection.
Key findings
Oral administration of the sEHI did not affect the very short survival time of the intracerebral prion infection group. However, mice infected by intraperitoneal route and treated with t-AUCB survived significantly longer than the control group mice (p < 0.001).
Significance
These findings support the idea that inhibition of sEH or augmentation of the natural EpFA signaling in the brain offers a potential and different route to understand prion diseases and may become a therapeutic strategy for diseases involving neuroinflammation.
Keywords: Soluble epoxide hydrolase, Inhibitor, Prion, Inflammation, Neuroinflammation, Epoxy fatty acid, EET
Introduction
The cytochrome P450 generated epoxy-fatty acids (EpFAs) are natural bioactive lipids that mediate a number of physiological events (Imig and Hammock, 2009; Ingraham et al., 2011; Wagner et al., 2011). Free fatty acids of linoleic, arachidonic (ARA), eicosapentaenoic and docosahexaenoic acid origin can be oxygenated by several different cytochrome P450 isozymes to form numerous EpFAs with multiple regioisomers that act selectively and in a context dependent manner (Spector, 2009). Although EpFAs have pleiotropic effects a receptor for these molecules has not been identified, thus it is not known how exactly EpFAs lead to numerous biological effects. A major effect ascribed to the EpFAs and more specifically to the arachidonic acid metabolites epoxyeicosatrienoic acids (EETs) is attenuation of inflammation and pain associated with inflammation (Inceoglu et al., 2006, 2007). The EpFAs are hypothesized to be homeostasis mediating molecules (Shen and Hammock, 2012). They are rapidly hydrated and inactivated by soluble epoxide hydrolase (sEH, EC 3.3.2.10). The predicted half-life of EpFAs in the presence of metabolic enzymes is on the order of seconds (Spector, 2009). However, their degradation can be blocked by inhibiting the sEH. The availability of highly potent and orally bioavailable inhibitors for sEH enabled our group and other laboratories to test numerous hypotheses about the functions of EpFAs. Specifically, the anti-inflammatory effects of EETs that were initially observed in in vitro studies have been corroborated by a number of in vivo studies in which EETs, other EpFAs and inhibitors of sEH all display strong anti-inflammatory effects in animal models of inflammation (Inceoglu et al., 2008; Liu et al., 2010). However recently, we demonstrated that the anti-inflammatory effects of EpFAs extend beyond decreasing the transcriptional upregulation of cox-2 through the NfκB pathway, and inhibition of sEH directly blocks the effects of PGE2 without binding to the E-prostanoid receptors (Inceoglu et al., 2011).
The sEH is well expressed throughout the body though certain organs and tissues including regions of the brain selectively express higher levels of this enzyme (Sura et al., 2008). To investigate if inhibition of sEH will limit the progression of neurodegenerative diseases in the brain we used a model of prion infection (Poli et al., 2012). Prion diseases (Transmissible Spongiform Encephalopathies, TSEs), are a group of rare neurodegenerative diseases affecting animals and man (Prusiner, 1982). The ensuing loss of neurons in the brain is invariably fatal (Prusiner, 1998). Most inherited forms of prion diseases are associated with mutations within the prion protein gene (PRNP) though they may be acquired by transmission of an infectious agent (Prusiner, 1982, 1998).
Prion diseases are initiated by the post-translational modification of the native prion protein (PrPc) that results in the formation of the pathogenic isoform called prion (proteinaceous infectious only —PrPsc) where a conformational change of the protein leads to a different secondary structure in which the soluble and insoluble PrPs have higher α-helical and β-sheet content respectively (PrPc and PrPsc) (Griffith, 1967; Prusiner, 1998). This process leads to the formation of aggregates of the highly insoluble and partially protease resistant PrPsc in the brain, which underlies the neurotoxicity that occurs in prion diseases. Neuronal vacuolation, progressive neuronal loss and activation of microglia accompanied by alteration of the brain structure where amyloid fibrils are deposited lead to a spongiform appearance. Though these changes do not induce major inflammatory or immune responses in the host, accumulating evidence points to microglial activation contributing to the maintenance of inflammatory environment and neurological damage (Heikenwalder et al., 2007; Prusiner, 1998).
The etiopathology of prion diseases shares a number of characteristics with Alzheimer’s Disease (AD) in which protein misfolding and the deposition of amorphous aggregates or amyloid fibrils in the central nervous system (CNS) lead to microglial activation, neuroinflammation and neurotoxicity (Checler and Vincent, 2002; Dickson, 1997; Ronga et al., 2006). While other authors have approached therapeutic intervention by using various antiprionic drugs here we targeted neuroinflammation as a strategy to limit the progression of the disease (Bareggi et al., 2009; Magri et al., 2005; Poli et al., 2004). Large scale clinical trials demonstrated no benefit in inhibiting the major pro-inflammatory proteins cox-1 (cyclooxygenase-1) or cox-2 (cyclooxygenase-2). Nonetheless arachidonic acid is not an exclusive substrate for the cox isozymes but is also a substrate for lipoxygenases and cytochrome P450 isozymes which produce pro and anti-inflammatory bioactive lipid mediators (Spector, 2009). Specifically the cytochrome P450 generated EETs not only have anti-inflammatory effects but also reduce cox-2 gene transcription by a feedback mechanism (Inceoglu et al., 2008). Furthermore administration of inhibitors of cox and sEH resulted in a synergistic reduction of acute inflammation in a murine model of sepsis (Schmelzer et al., 2006). Here we studied the potential role of EETs and sEH in prion disease.
Materials and methods
Animals and tissue collection
This study was approved by the Ethical Committee of the University of Milan. Female mice of CD1 strain aged 3–4 weeks were housed under standard conditions at 22 ± 1 °C, 55 ± 5% relative humidity, and 12 h light/dark cycle. Food and water were supplied ad libitum. Mice were randomized into treatment groups. A total of 91 mice were used. Animals were infected with the mouse-adapted Rocky Mountains Laboratories (RML) strain of scrapie a) by intracerebral (i.c.) or b) by intraperitoneal (i.p.) route using a 10% (weight/volume) homogenate of RML-infected CD1 brain extract. This solution was prepared in sterile saline and diluted to a final concentration of 1%. Injections were done as described previously by administering 50 μl for the i.p route and 25 μl for the i.c. route (Spilman et al., 2008). Scrapie inoculation day was considered the first day of the study.
Mice from the prion infected group were randomized into treatment groups (n = 15 per group) consisting of i.c. infected + sEHI treatment, i.c. infected + vehicle, i.p. infected + sEHI treatment and i.p. infected + vehicle. Another group of mice received an injection of 1% homogenate (same volume as above) of uninfected CD1 mice brain and randomized into groups (n = 8 per group) that consisted of i.c. mock inoculation + sEHI and i.p. mock inoculation + sEHI. In addition a negative control group (n = 15) was also used (Table 1).
Table 1.
Treatment groups, histological and immunohistochemical assessment of pathology and prion presence.
| Group | Infection | Treatment | n | Median survival time (days) | Histologya (brain) | Immunohistochemistryb (brain PrPsc/spleen) |
|---|---|---|---|---|---|---|
| 1 | None | Vehiclec | 15 | N/A | Negative | Negative |
| 2 | i.c. scrapied | Vehiclec | 15 | 163 | Positive | Positive |
| 3 | i.c. scrapied | t-AUCB | 15 | 165 | Positive | Positive |
| 4 | i.c. healthye | t-AUCB | 8 | N/A | Negative | Negative |
| 5 | i.p. scrapied | Vehiclec | 15 | 207 | Positive | Positive |
| 6 | i.p. scrapied | t-AUCB | 14f | 216 | Positive | Positive |
| 7 | i.p. healthye | t-AUCB | 8 | N/A | Negative | Negative |
N/A, not applicable.
Semi quantitative lesion scores from brainstem, cerebellum, diencephalon, telencephalon and cortex.
PrPsc deposition.
PEG400, 1% in drinking water.
Infected brain homogenate.
Healthy brain homogenate.
One mouse out of 15 mice in this group did not display behavioral signs of infection and was negative for the presence of PrPsc in the brain.
The sEH inhibitor, t-AUCB was delivered in drinking water following formulation in 1% v/v PEG400 to a final concentration of 10 mg/l sEHI in drinking water. The drug treatment was initiated 14 days prior to scrapie inoculation in order to test the prophylactic efficacy. The vehicle treated animals received drinking water that contained the same amount of PEG400. Following inoculation, mice were observed daily, monitoring the appearance and development of signs of neurological defects. The test panel included kyphotic posture, ataxia, proprioceptive deficits, lethargy and frozen posture (Thackray et al., 2002). All surviving mice were euthanized at a standard clinical end point, based on symptoms established previously for terminally ill animals (Meeker et al., 2005; Thackray et al., 2002).
During the necropsy, the cerebral hemispheres, the brain stem and the cerebellum were carefully removed and each brain was divided into two hemispheres with a sagittal cut. One of the hemispheres was fixed in 10% formalin for histopathological and immunohistochemical analyses and the other stored at −20 °C for Western blot analysis.
Western blot analysis
Brain homogenates containing 10% (w/v) brain matter were prepared in a lysis buffer consisting 10% N-lauroylsarcosine diluted in Tris buffered saline and adjusted to a pH of 7.4. These homogenates were incubated for 20–30 min at room temperature and centrifuged at 22,000 ×g for 20 min at 10 °C (Ultracentrifuge Optima TLX, Rotor TLA 55, Beckman Coulter, Fullerton, CA, USA) to obtain clear protein extracts. A 1 ml portion of this protein extract was removed from each supernatant and digested with proteinase K (40 μg/ml) for 1 h at 37 °C with continuous shaking. Following the protease reaction, 10 μl of a protease inhibitor phenylmethyl sulfonyl fluoride (PMSF 100 mM) was added into the reaction. The samples were then centrifuged again at 215,000 ×g for 1 h at 10 °C (Optima TLX ultracentrifuge, rotor TLA 110, Beckman Coulter, Fullerton, CA, USA). The obtained pellets were dissolved in 50 μl of Laemmli sample buffer and boiled for 5–10 min. A 10 μl portion of each extract corresponding to 10 mg of tissue were separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis on a 12% gel (acrylamide/ bisacrylamide ratio of 37.5:1) and transferred onto PVDF membranes (Immobilion P; Millipore, Billerica, MA). Membranes were blocked with 5% BSA in TBS buffer for 1 h and incubated at 4 °C overnight with the monoclonal antibody SAF 70 (0.5 μg/ml; Spi Bio, Cayman Chemical, Ann Arbor, MI), which reacts with human PrP residues 142–160. The immunodetection was carried out with an alkaline phosphatase-conjugated goat anti-mouse IgG, and signal was detected using the chemiluminescent substrate (Immunostar, Bio-Rad) visualized on Hyperfilm ECL (GE-Healthcare Ltd., St. Giles, UK) or by a UVI Prochemi (Uvitec, Cambridge, UK) analysis system. Following visualization, signals from PrPsc bands were quantified using UVI Bandmap software suite (Uvitec, Cambridge, UK). Quantitative analysis was conducted on gels which contained the same signal intensity for the standard ovine scrapie sample (R) which was used as an internal standard. An equal area of region of interest was selected in each different gel. To obtain an intensity score, sample signals were divided by the intensity of the standard scrapie sample and significance was analyzed by Student’s t test.
Histopathological and immunohistochemical analyses
Brains were coronally separated to five sections (medulla, pons and cerebellum, mid-brain, diencephalon, telencephalon) following fixation (Fraser and Dickinson, 1968). These samples were then embedded in paraffin wax according to standard histopathological procedures. Sections of 3 μm thickness were taken from each hemisphere and placed on slides with positive electrostatic charge and incubated for 24 h at 37 °C. Hematoxylin–eosin staining was performed for each brain section.
Slides for immunohistochemical analysis were dewaxed and rehydrated and immersed in 98% formic acid for 15 min. To expose antigenic sites the sections were autoclaved for 30 min at 121 °C in citrate buffer (pH 6.1) following a water wash. Hydrogen peroxide (3%) diluted in methanol was used to block endogenous peroxidase activity for 20 min at room temperature and samples were incubated overnight in distilled water at 2–8 °C. The sections were incubated with 2% horse blocking serum in TBST, pH 7.4 for 20 min at room temperature to block non-specific tissue antigens. This was followed by 1 h incubation at room temperature with monoclonal antibody ICSM35 diluted 1:1000, which recognizes the amino acid sequence 93–102 of human PrP (D-Gen, London, UK). Following rinsing in TBST, a biotinylated goat anti-mouse secondary antibody (1:200 dilution, Vector Laboratories, Burlingame, CA) was added to the tissue sections for 30 min at room temperature. This was followed by incubation with the avidin–biotin–peroxidase complex (Vectastain ABC kit; Vector Laboratories, Burlingame, CA), according to the manufacturer’s instructions. After another rinse in TBST, PrPsc immunoreactivity was visualized using 3,3′-diaminobenzidine (Dakocytomation, Carpinteria, CA) as a chromogen. The reaction was stopped with distilled water. The sections were then counterstained with Mayer’s hematoxylin. Spongiosis and PrPsc deposition in different encephalic areas (medulla, cerebellum, mid-brain, hypothalamus, thalamus, hippocampus, para terminal body, frontal cortex and parietal cortex) were subjectively evaluated by light microscopy by two independent trained and blinded pathologists and an intensity grade was assigned to vacuolation and to the different patterns detected. The semi-quantitative intensity grade was composed of absent (0), slight (1), moderate (2), marked (3) and very marked (4).
Analysis of inhibitor levels
The sEH inhibitor t-AUCB was quantified from the drinking water used to treat the mice and plasma and brain samples of i.p. infected + sEHI group mice. An aliquot of 10–50 μl of mouse plasma was placed in a tube containing 10 μl of internal standard (500 nM final concentration). The internal standard was 1-(1-methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea, (TUPS), synthesized in our laboratory as described earlier (Tsai et al., 2010). Four hundred microliter of ethyl acetate was added, samples were vortex mixed and centrifuged at 15,000 ×g for 5 min. The organic phase was recovered and transferred into a fresh tube. These steps were repeated twice. The collected organic phase was then dried under nitrogen and the samples were reconstituted with 60 μl of mobile phase and 30 μl used for LC/MS/MS analysis. For brain samples each mouse brain was sterilized for 1 h at 132 °C. The tissues were then transferred into 5 ml tubes and the internal standard solution (10 μl, 500 nM final concentration in ACN) was added. The samples were homogenized following the addition of 1 ml of ethyl acetate and vortexed for 1 min, followed by centrifugation at 15,000 ×g for 5 min. The supernatants were then transferred into fresh tubes and the extractions were repeated two more times. The combined organic phase from three extractions was evaporated to dryness under nitrogen. The samples were reconstituted with 150 μl of mobile phase and 30 μl aliquot was used for LC/MS/MS analysis. The separation was carried out using a Hypersil Gold aQ C18 column (50 × 4.6 mm I.D., 3 μm, Thermo Scientific). A linear solvent gradient from 0 to 80% ACN in 10 mM ammonium acetate at a flow rate of 300 μl/min, at a temperature of 40 °C for 10 min was applied. The separation module was connected to a LC 1100-MS Trap XCT (Agilent) instrument. The LC-ESI–MS/MS instrument was operated in positive electrospray ionization mode with selected reaction monitoring (SRM). The following transitions were monitored: for t-AUCB m/z 413 [M + H]+ > 135 and for internal standard (TUPS), m/z 382 [M + H]+ to m/z 178. The results were converted to and expressed as nanomolar using 1050 g/l for the average density of brain tissue.
Statistical analysis
The survival analysis was performed using the non-parametric log-rank test using the MedCalc Software package (Mariakerke, Belgium). To evaluate the differences in the PrPsc among the groups, the results of quantification performed by Western blot analysis were analyzed by Student’s t test using the SigmaPlot analysis package (Systat Software, Inc., Chicago, IL).
Results
Disease model validation
We used clinical symptoms, Western-blotting, histopathological and immunohistochemical analyses to confirm and validate the generation of the model. Typically, in this model, the progression of neurological disease is fairly slow, with clinical symptoms beginning to appear 4–6 months following inoculation (Salmona et al., 2005; Spilman et al., 2008; Thackray et al., 2002). As expected, in the infected + vehicle treated mice, early clinical symptoms induced by RML strain of scrapie began at approximately 130 and ~180 days post-inoculation for i.c. or i.p. route, respectively. None of the uninfected + vehicle or uninfected + sEHI treated animals showed any clinical symptoms of prion disease.
Early symptoms included ruffled coats, kyphotic posture and Straub tail. These symptoms were then followed by ragged or wobbly gait, ataxia and proprioceptive deficits, as observed by clasped feet when raised by the tail. Later, infected animals became limp, lethargic, cachectic and appeared to adopt a frozen posture for prolonged periods.
Following the appearance of clinical signs all animals, with the exception of one mouse, in the infected groups reached terminal stage of the disease at a very similar time. One mouse that did not display typical infected phenotype was later confirmed to be not infected by Western blotting and immunohistochemistry. All mice were euthanized when they reached the standard clinical end points as described previously (Meeker et al., 2005; Thackray et al., 2002). For groups that received i.c. prion inoculation the clinical end points were reached starting day 145 post-inoculation. We continued to euthanize mice as they reached the standard clinical end points, until day 179 for i.c. infected + vehicle and day 180 post-inoculation for i.c. infected + sEHI group. The group i.p. infected + vehicle, reached clinical end points between days 193 and 222 post-inoculation. The group i.p. infected + sEHI reached clinical end points between days 199 and 233 post-inoculation.
The one i.p. infected + sEHI treated mouse that did not display classical symptoms of the disease survived and continued to receive sEHI in drinking water until day 330 of the experiment. At this time the treatment was discontinued and the mouse was euthanized 31 days later, while it still lacked any clinical signs of disease. The lack of infection was later confirmed in this mouse by Western blot and immunohistochemistry.
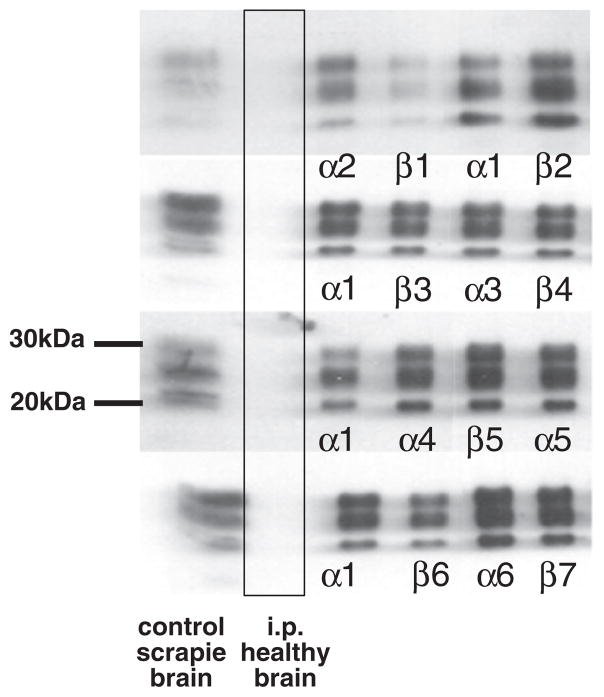
All the mice euthanized at the clinical end stage of disease and analyzed by Western blot analysis displayed the presence of PrPsc, characterized by three bands corresponding to the di-, mono- and un-glycosylated forms with a molecular weight between 30 and 20 kDa (Fig. 2). In parallel to the results obtained by Western blotting, histological and immunohistochemical analyses confirmed the presence of PrPsc in all the samples from symptomatic animals (Table 1).
Fig. 2.
Analysis of brain of mice by Western-blotting demonstrates equal prion load in t-AUCB and vehicle treated mice. All samples were treated with proteinase K prior to SDS–PAGE separation. All animals were subjected to Western blot analysis with positive results (n = 15 for vehicle and n = 14 for t-AUCB group). Representative samples are shown in the figure. In the first column from the left brain homogenates of con-firmed positive control prion infected mice are displayed. The second column displays brain samples of negative control mice inoculated (i.p.) with brain homogenates of healthy mice. In subsequent columns α1–6 denote i.p. infected + vehicle animals and β1–7 denote i.p. infected + t-AUCB treated animals.
Disease progression and effects of sEH inhibition
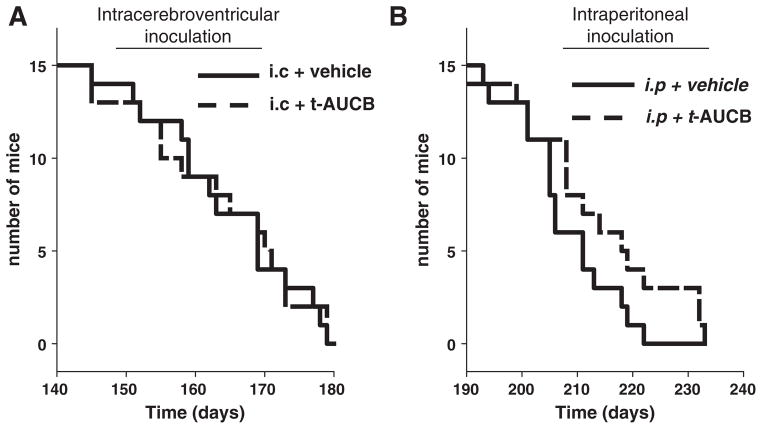
Mice from both of the i.c. inoculation groups (i.c. infected + vehicle and i.c. infected + sEHI) died equally rapidly with median survival times of 163 and 165 days respectively (n = 15 per group, log-rank p = 0.86) (Fig. 1A). On the contrary, the sEHI treatment led to a highly significant increase in survival of the i.p. infected + sEHI group with a mean survival of 216 days compared to the i.p. infected + vehicle group with a mean survival of 207 days (n = 14 for sEHI and n = 15 for vehicle, log-rank p = 0.025) (Fig. 1B).
Fig. 1.
Inhibition of sEH by orally administered inhibitor delays lethality of prion infection. A) Mice inoculated by intracerebroventricular (i.c.) route and administered vehicle (PEG400, 1% in drinking water) displayed median survival time of 163 days which was not significantly different than the group receiving 1 mg/kg/day t-AUCB which had a median survival time of 165 days (log-rank test p = 0.86). B) Mice inoculated by intraperitoneal (i.p.) route and administered vehicle (PEG400, 1% in drinking water) displayed a delayed median survival time of 207 days compared to i.c. inoculation. This survival time was further delayed in the t-AUCB (1 mg/kg/day in drinking water) treatment groups which had a median survival time of 216 days (log-rank test p = 0.025).
The densitometric analysis of Western blots of brains showed no significant decrease in PrPsc levels in the sEHI treated mice compared to the vehicle group (n = 7 per group, t test, p = 0.1) (Fig. 2). In contrast, Western blot analysis performed on the brains of mice euthanized without clinical signs of disease demonstrated the lack of PrPsc, suggesting that these mice did not develop the prion infection. Histological and immunohistochemical analyses performed on the brains of the non-symptomatic animals were also negative, demonstrating neither the presence of PrPsc nor the neuropathological lesions associated to prion diseases.
To determine the extent of neurologic damage, spongiotic lesion profiles of the different encephalic areas were evaluated using the hematoxylin–eosin staining for the detection of spongiform degeneration and immunostaining of PrPsc. The average lesion scores obtained by either method in the i.p. infected + vehicle and i.p. infected + sEHI groups were not significantly different from each other across the brain regions examined.
Administration of t-AUCB orally in drinking water over the duration of the study resulted in significant levels of the inhibitor in the brain of treated mice (23 nM or 9.05 ± 3.11 pg/mg of brain tissue, n = 6 mice). The in vitro potency (IC50, concentration which will inhibit half of the enzymatic activity) of t-AUCB on purified recombinant mouse sEH using the fluorescent substrate, α-cyanocarbonate is 8 nM (Hwang et al., 2006). Thus brain levels of t-AUCB well exceeded the in vitro IC50 value of t-AUCB at the end of the study.
The overall levels of PrPsc quantified by Western blotting suggested no decrease in animals treated with sEH inhibitor. The lack of changes in specific areas of the brain argues that inhibition of sEH improved survival duration largely without altering the progression of the disease state.
Discussion
The soluble epoxide hydrolase (sEH) regulates the activity of a major group of bioactive lipids, the epoxygenated fatty acids by degrading these to their less active and sometimes pro-inflammatory corresponding diols. The epoxygenated fatty acids are produced by the actions of several cytochrome P450 isozymes which oxidatively introduce epoxide groups to the parent fatty acids. The cytochrome P450 branch is also a major branch of the arachidonic acid (AA) cascade, which leads to the production of AA derived epoxyeicosatrienoic acids (EETs). The EETs and other epoxygenated fatty acids have important roles in a number of physiological and pathological processes. In peripheral tissues the EETs are considered to be endothelium-derived hyperpolarizing factors mediating physiological responses (i.e., relaxation to various stimuli) and regulate the vascular tone. The two most prominent effects of EETs in the CNS involve attenuation of inflammatory responses and regulation of vascular tone both of which may be relevant to observations reported in this study. The cytochrome P450 products, EETs, have broad anti-inflammatory functions in contrast to the largely proinflammatory functions of the products of the cyclooxygenase and lipoxygenase branches of the AA cascade. These effects are hypothesized to occur in part through modulation of Nf B mediated gene expression where EETs inhibit I k and maintain the inactive Nf B. Specifically, stabilization of EETs with a CNS permeant sEH inhibitor effectively suppressed the spinal upregulation of cox-2 mRNA, a gene responsive to Nf B activation in response to peripheral inflammation induced by LPS. In the CNS, the EETs and possibly other epoxygenated fatty acids regulate cerebral blood flow auto regulation and neurovascular coupling; activity based local regulation of blood flow to neuronal demand. Under a number of pathological conditions stabilization of EETs by inhibiting the sEH yields desirable activities including suppression of altered nociceptive signaling and decreasing damage due to ischemic stroke (Dorrance et al., 2005; Inceoglu et al., 2012). Thus sEH is a candidate therapeutic target for inflammatory conditions and CNS disorders.
Based on the effects of sEH inhibitors on suppressing inflammation in the spinal cord, we hypothesized that inhibition of sEH would be neuroprotective in diseases involving neuronal inflammation (Inceoglu, Jinks, 2008). This hypothesis is addressed here using a prion infection model. There was improvement in survival of mice infected with the mouse-adapted Rocky Mountain Laboratory (RML) strain of scrapie by intraperitoneal route and treated with sEH inhibitor. Consistent with this observation the brain inhibitor levels quantified were sufficiently high to stabilize EETs in the brain. Given that healthy mice consume 3–5 ml of water per day, the medicated water containing 10 μg/ml of t-AUCB is expected to result in an oral dose of 30–50 μg/day/mouse or about 0.9 to 1.5 mg/kg/day. Previously we demonstrated that per oral dose of 1 mg/kg of t-AUCB formulated in trans-free trioleate resulted in a Cmax of 150 ± 23 nM in plasma (Hwang et al., 2007). In the rat, brain level of t-AUCB is approximately 10 fold lower than plasma concentrations (Inceoglu et al. unpublished). This indicates that in this study the sEH inhibitor was successfully delivered into the brain to effectively stabilize brain levels of epoxygenated fatty acids. However, by using a new generation of sEH inhibitors with longer plasma half life, higher bioavailability, increased water solubility, and decreased logP, in future studies it will be possible to better examine the inhibition of sEH as a therapeutic approach to treat prion or other neuroinflammatory diseases.
It is possible that neuroinflammation in the brain of mice elicited by prion injection was decreased by inhibition of sEH. The current mechanism of anti-inflammatory action of EpFAs is thought to occur through blocking I K and consequently nuclear translocation of NfκB. In prion diseases both in vivo and in vitro studies demonstrate an increase in NfκB mediated signaling, though genetic ablation of NfκB signaling through CNS selective I Kβ, and I Kγ knockouts or non-phosphorylatable I Kα knockin provides no protection(Julius et al., 2008). Blockage of NfκB signaling by EETs may have provided a minor contribution to the overall effect here and more detailed studies are required to support the main hypothesis that anti-inflammatory effects of EETs are responsible for the observed delay in lethality. Although there may be a number of reasons for the inability of sEH inhibitor to counteract the high infection load achieved via intracerebral scrapie inoculation, one possibility is that the prion propagation from the periphery to the brain may have been affected by sEH inhibition. The profile and intensity of neurological damage overall was highly similar in sEH inhibitor and vehicle treated animals. Given that once seeding of PrPsc aggregates is initiated in the brain these aggregates are expected to convert more PrPc to the protease-resistant and altered isoform, PrPsc. Thus it is difficult to correlate sEH inhibition mediated delay in lethality to delayed prion propagation from the periphery to the brain. However this possibility is an interesting hypothesis for future studies.
Inhibition of sEH has demonstrated anti-inflammatory effects and sEH is well expressed in rodent and human brain cortical neurons while more cytochrome P450 epoxygenase activity is prevalent in glia (Sarkar et al., 2011; Sura et al., 2008). The sEH degrades anti-inflammatory epoxyeicosatrienoic acids. Inhibitors of sEH are neuroprotective following ischemia-reperfusion injury and reduce infarct size following stroke (Dorrance et al., 2005). The mechanisms of these effects involve regulation of blood flow and oxygenation. Interestingly, while inhibition of EET production render neuronal cells more susceptible to oxidative damage, inhibition of sEH prevent ischemic damage. Thus a possible mechanism of delay of prion induced lethality by the sEH inhibitor may be due to the effects of epoxygenated fatty acids to increase blood flow and re-oxygenation of the brain tissue.
Recently, Sarkar et al. demonstrated that the cytochrome P450 epoxygenase activity responsible for the production of sEH substrate EETs is significantly reduced by exposure to amyloid beta protein, Aβ (Sarkar et al., 2011). Thus it is possible that inhibition of sEH as would be expected prevents a drastic decrease in the levels of epoxygenated fatty acid levels which are essential for survival of neurons. Given the parallel mechanisms of cytotoxicity mediated by PrPsc and Aβ by activating oxidative stress and pro-apoptotic pathways a plausible hypothesis is that inhibition of sEH led to augmented levels of the cytochrome P450 epoxygenase products (EETs) and prolonged survival. The EETs have several distinct positive effects that may have contributed to longer survival times in prion infected mice. One possibility is that propagation of inflammation is suppressed and delayed in the brain of sEH inhibitor treated animals much like in other models of inflammation. Another possibility is that sEH inhibitor mediated increases in EETs and other epoxygenated fatty acids in the brain may have led to augmented nerve regeneration that was sufficient to delay lethality. We demonstrated that inhibitors of sEH and EET concentration dependently enhance axon outgrowth in cortical neurons and sEH may be a factor that prevents EET-induced axon outgrowth (Abdu et al., 2011). Thus in the presence of an sEH inhibitor prion infected mice may have experienced slower loss of neurons.
Conclusion
Overall, we demonstrate that elevation of the levels of epoxygenated fatty acids by inhibition of sEH may have utility in the treatment of neurodegenerative pathological conditions including prion and Alzheimer’s disease. However the mechanism of effect of sEH inhibition is not clear. Prion diseases are driven by highly complex pathogenic processes that result in 100% lethality. The complexity is highlighted by a lack of drugs and by the existence of very few potential therapeutic approaches. Once clinical symptoms are apparent lethality occurs in a relatively short period of time. Compounds that lead to a delay in lethality in rodent models may have significant impact on human health as leads for better drugs or as pharmacological tools that extend life. While this study provides a framework to expand from, future studies investigating markers of neuroinflammation and neuronal regeneration and target engagement by more brain permeable sEH inhibitors will enhance our understanding of the pathophysiology of prion diseases and the functions of epoxygenated fatty acids in the brain.
Acknowledgments
We are grateful to Mr. Massimo Dagrada, Mr. Antonio Longo, Dr. Joel Filipe, Dr. Luana Dell’Atti and Dr. Paola Gazzuola for their technical support. This work was partially supported by the US National Institute of Environmental Health Sciences (NIEHS) Grant R01 ES002710 (to B.D.H.), NIEHS Superfund Basic Research Program P42 ES004699, CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institutes of Neurological Disorders and Stroke (NINDS), Grant Number U54 NS079202. B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
Footnotes
Conflict of interest statement
SH Hwang, B. D. Hammock and B. Inceoglu are co-authors on multiple patents filed by the University of California related to soluble epoxide hydrolase inhibitors.
References
- Abdu E, Bruun DA, Yang D, Yang J, Inceoglu B, Hammock BD, et al. Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures. J Neurochem. 2011;117:632–42. doi: 10.1111/j.1471-4159.2010.07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareggi SR, Braida D, Pollera C, Bondiolotti G, Formentin E, Puricelli M, et al. Effects of clioquinol on memory impairment and the neurochemical modifications induced by scrapie infection in golden hamsters. Brain Res. 2009;1280:195–200. doi: 10.1016/j.brainres.2009.05.031. [DOI] [PubMed] [Google Scholar]
- Checler F, Vincent B. Alzheimer’s and prion diseases: distinct pathologies, common proteolytic denominators. Trends Neurosci. 2002;25:616–20. doi: 10.1016/s0166-2236(02)02263-4. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Neuropathological diagnosis of Alzheimer’s disease: a perspective from longitudinal clinicopathological studies. Neurobiol Aging. 1997;18:S21–6. doi: 10.1016/s0197-4580(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2005;46:842–8. doi: 10.1097/01.fjc.0000189600.74157.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H, Dickinson AG. The sequential development of the brain lesion of scrapie in three strains of mice. J Comp Pathol. 1968;78:301–11. doi: 10.1016/0021-9975(68)90006-6. [DOI] [PubMed] [Google Scholar]
- Griffith JS. Self-replication and scrapie. Nature. 1967;215:1043–4. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- Heikenwalder M, Julius C, Aguzzi A. Prions and peripheral nerves: a deadly rendezvous. J Neurosci Res. 2007;85:2714–25. doi: 10.1002/jnr.21246. [DOI] [PubMed] [Google Scholar]
- Hwang SH, Morisseau C, Do Z, Hammock BD. Solid-phase combinatorial approach for the optimization of soluble epoxide hydrolase inhibitors. Bioorg Med Chem Lett. 2006;16:5773–7. doi: 10.1016/j.bmcl.2006.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–40. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79:2311–9. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A. 2008;105:18901–6. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins Other Lipid Mediat. 2007;82:42–9. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, Ulu A, et al. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc Natl Acad Sci U S A. 2011;108:5093–7. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Wagner KM, Yang J, Bettaieb A, Schebb NH, Hwang SH, et al. Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model of type I diabetes. Proc Natl Acad Sci U S A. 2012;109:11390–5. doi: 10.1073/pnas.1208708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham RH, Gless RD, Lo HY. Soluble epoxide hydrolase inhibitors and their potential for treatment of multiple pathologic conditions. Curr Med Chem. 2011;18:587–603. doi: 10.2174/092986711794480212. [DOI] [PubMed] [Google Scholar]
- Julius C, Heikenwalder M, Schwarz P, Marcel A, Karin M, Prinz M, et al. Prion propagation in mice lacking central nervous system NF-kappaB signalling. J Gen Virol. 2008;89:1545–50. doi: 10.1099/vir.0.83622-0. [DOI] [PubMed] [Google Scholar]
- Liu JY, Yang J, Inceoglu B, Qiu H, Ulu A, Hwang SH, et al. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2010;79:880–7. doi: 10.1016/j.bcp.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri G, Clerici M, Dall’Ara P, Biasin M, Caramelli M, Casalone C, et al. Decrease in pathology and progression of scrapie after immunisation with synthetic prion protein peptides in hamsters. Vaccine. 2005;23:2862–8. doi: 10.1016/j.vaccine.2004.11.067. [DOI] [PubMed] [Google Scholar]
- Meeker HC, Ye X, Carp RI. The mouse model for scrapie: inoculation, clinical scoring, and histopathological techniques. Methods Mol Biol. 2005;299:309–23. doi: 10.1385/1-59259-874-9:309. [DOI] [PubMed] [Google Scholar]
- Poli G, Corda E, Lucchini B, Puricelli M, Martino PA, Dall’ara P, et al. Therapeutic effect of CHF5074, a new gamma-secretase modulator, in a mouse model of scrapie. Prion. 2012;6 doi: 10.4161/pri.6.1.18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Martino PA, Villa S, Carcassola G, Giannino ML, Dall’Ara P, et al. Evaluation of anti-prion activity of congo red and its derivatives in experimentally infected hamsters. Arzneimittelforschung. 2004;54:406–15. doi: 10.1055/s-0031-1296992. [DOI] [PubMed] [Google Scholar]
- Prusiner S. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–44. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proc Natl Acad Sci. 1998;95:13363–83. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronga L, Tizzano B, Palladino P, Ragone R, Urso E, Maffia M, et al. The prion protein: structural features and related toxic peptides. Chem Biol Drug Des. 2006;68:139–47. doi: 10.1111/j.1747-0285.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Salmona M, Capobianco R, Colombo L, De Luigi A, Rossi G, Mangieri M, et al. Role of plasminogen in propagation of scrapie. J Virol. 2005;79:11225–30. doi: 10.1128/JVI.79.17.11225-11230.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P, Narayanan J, Harder DR. Differential effect of amyloid beta on the cytochrome P450 epoxygenase activity in rat brain. Neuroscience. 2011;194:241–9. doi: 10.1016/j.neuroscience.2011.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:13646–51. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC, Hammock BD. Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications. J Med Chem. 2012;55:1789–808. doi: 10.1021/jm201468j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50:S52–6. doi: 10.1194/jlr.R800038-JLR200. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Lessard P, Sattavat M, Bush C, Tousseyn T, Huang EJ, et al. A gamma-secretase inhibitor and quinacrine reduce prions and prevent dendritic degeneration in murine brains. Proc Natl Acad Sci U S A. 2008;105:10595–600. doi: 10.1073/pnas.0803671105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sura P, Sura R, Enayetallah AE, Grant DF. Distribution and expression of soluble epoxide hydrolase in human brain. J Histochem Cytochem. 2008;56:551–9. doi: 10.1369/jhc.2008.950659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray AM, Klein MA, Aguzzi A, Bujdoso R. Chronic subclinical prion disease induced by low-dose inoculum. J Virol. 2002;76:2510–7. doi: 10.1128/jvi.76.5.2510-2517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HJ, Hwang SH, Morisseau C, Yang J, Jones PD, Kasagami T, et al. Pharmacokinetic screening of soluble epoxide hydrolase inhibitors in dogs. Eur J Pharm Sci. 2010;40:222–38. doi: 10.1016/j.ejps.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition, epoxygenated fatty acids and nociception. Prostaglandins Other Lipid Mediat. 2011;96:76–83. doi: 10.1016/j.prostaglandins.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]