Abstract
The Murphy Roths Large (MRL/MpJ) mice provide unique insights into wound repair and regeneration. These mice and the closely related MRL/MpJ-Faslpr/J and Large strains heal wounds made in multiple tissues without production of a fibrotic scar. The precise mechanism of this remarkable ability still eludes researchers, but some data has been generated and insights are being revealed. For example, MRL cells reepithelialize over dermal wound sites faster than cells of other mouse strains. This allows a blastema to develop beneath the protective layer. The MRL mice also have an altered basal immune system and an altered immune response to injury. In addition, MRL mice have differences in their tissue resident progenitor cells and certain cell cycle regulatory proteins. The difficulty often lies in separating the causative differences from the corollary differences. Remarkably, not every tissue in these mice heals scarlessly, and the specific type of wound and priming affect regeneration ability as well. The MRL/MpJ, MRL/MpJ-Faslpr/J, and Large mouse strains are also being investigated for their autoimmune characteristic. Whether the two phenotypes of regeneration and autoimmunity are related remains an enigma.
Keywords: MRL, wound healing, regeneration
Introduction
Biologic knowledge of various disease states, traumas, and development has been greatly enriched by animal models. Among the studied mouse models are genetically engineered knockouts, and, more recently, tissue specific knockouts and temporal knockouts. Transgenic mice overexpressing a wild type or dominant-negative gene, miRNA or shRNA have also been produced in vast numbers. These mouse models span the physiologic range from disease models to improving our understanding of development and the role of individual molecules in these complex processes. Animal models also provide irreplaceably useful tools to assess therapeutics.
A bonus group of mouse models have arisen spontaneously. In the muscular dystrophy field, the well-used mdx mouse arose spontaneously in The MRC Laboratory Animal Centre in the laboratory of M. Festing (Bulfield et al., 1984). This mouse approximates Duchenne Muscular Dystrophy (Cullen and Jaros, 1988), the most prevalent genetic mutation in humans. The muscular dystrophy field has also benefited from the spontaneous merosin deficient dy/dy mouse (Xu et al., 1994). The dy/dy mouse is a model of a severe congenital muscular dystrophy.
Additional mouse models have been produced through breeding. This is the case for the autoimmune prone and super-healing Murphy Roths Large/lymphoproliferative (lpr) mouse strain (MRL/MpJ-Faslpr/J). These mice were established by selective interbreeding of the B6 (0.3%), C3H (12.1%), AKR (12.6%) and Large (75%) strains. A significant portion of the autoimmune phenotypes were attributed to a mutant Fas gene, which arose spontaneously during the selective breeding at generation F12 (Adachi et al., 1993). The MRL/MpJ mice have the wild type Fas gene and were maintained as a control strain for the MRL/MpJ-Faslpr/J mice. However, the autoimmune phenotypes remain, although presenting later in the mouse’s life span (Theofilopoulos and Dixon, 1985; Watson et al., 1992). In addition, both mouse strains and the closely related Large strain display the enhanced healing ability (Clark et al., 1998; Kench et al., 1999).
The first report of enhanced healing in the MRL/MpJ and MRL/MpJ-Faslpr/J mouse lines was a closing of ear holes used for mouse identification (Clark et al., 1998). The ear hole healing from these mice approximates embryonic epidermal healing (Hopkinson-Woolley et al., 1994), or the healing observed in some amphibians (Stocum, 1984). The authors made the concise and very important distinction between regeneration (healing) and wound repair (Clark et al., 1998). Regeneration is defined as “the gross replacement and restoration of adult tissue mass with normal architecture and function.” Regeneration is also associated with the formation of a blastema, basement membrane breakdown, and often, the functional reestablishment of multiple tissue types. The blastema cells are dedifferentiated and can, therefore, re-differentiate into multiple lineages. Alternatively, wound repair “involves the migration of fibroblasts to the wound site, formation of granulation tissue, and the laying down of collagen in a disorganized fashion with formation of scar tissue” (Clark et al., 1998). Certain tissues, including the liver (Michalopoulos and DeFrances, 1997) and skeletal muscle (Sacco et al., 2008), regenerate throughout an animal’s lifetime. Alternatively, there are other adult tissues, such as cardiac muscle (Anversa et al., 2005), that demonstrate little evidence of regeneration. Most tissue healing abilities lie between these two extremes. The ability of the regenerating tissues to regenerate has exceptions, such as certain disease situations, including hepatitis and muscular dystrophy or advanced age.
This review will focus upon the exceptionally unique and interesting super-healing characteristic of the MRL mouse strain, including a discussion of the possible molecular and cellular mechanisms behind this characteristic. In the end, it will be obvious that multiple questions remain and further research is necessary to fully extract all translational possibilities from this exceptional mouse strain.
Key features of wound repair are listed below. Though written out sequentially, there is much overlap between the phases (Peled et al., 2002; Harty et al., 2003; Midwood et al., 2004; Metcalfe et al., 2006), MRL references are cited in the text). For a recent review of the differences between fetal and adult dermis wound healing, I refer the reader to Namazi et al. (2011).
Multiple MRL tissues have healing ability
Ear punch wounds of 2 mm, made by Clark et al., regenerated in the MRL mice within 4 weeks, without fibrosis or scarring (Clark et al., 1998). The regenerated ear contained properly aligned and functional cartilage, epidermis, hair follicles and glands. These structures were not present in the C57BL/6 control animals. The authors also demonstrated that the healing trait was genetically inherited and that multiple loci are associated with the healing trait. Many others have repeated the ear hole wounds with very similar results [(Kench et al., 1999; Li et al., 2001; Masinde et al., 2001; Rajnoch et al., 2003; Gourevitch et al., 2003; Bedelbaeva et al., 2004; Davis et al., 2005; Beare et al., 2006; Bedelbaeva et al., 2010) and Heydemann (unpublished)]. A particular earwounding model provides very interesting results. Here, it was demonstrated that a previous ear hole accelerates healing of secondary wounds made 30 days later either proximal or distal to the first wound (Davis et al., 2005). These results provide evidence that circulating cytokines and cells likely play a role in the MRL regenerative response. Since the initial discovery of ear hole healing (Clark et al., 1998), other wound sites on the MRL mouse have been evaluated for healing ability. A list of wounds, regenerated and non-healed, appears in Table 2.
Table 2.
MRL/MpJ tissue healing summary
| Heal/regenerate | References | Wound with scar/do not regenerate | References |
|---|---|---|---|
| 2mm ear-punch wounds | (Clark et al., 1998) | Cortical stab lesion | (Hampton et al., 2004) |
| 2mm ear-punch wounds | (Kench et al., 1999) | Dopaminergic neuron lesions | (Hampton et al., 2004) |
| 2mm ear-punch wounds | (Li et al., 2001) | Skin wounds | (Beare et al., 2006) |
| 2mm ear-punch wounds | (Masinde et al., 2001) | Skin wounds | (Colwell et al., 2006) |
| 2mm ear-punch wounds | (Beare et al., 2006) | Skin wounds | (Metcalfe et al., 2006) |
| 2mm ear-punch wounds | (Gourevitch et al., 2003) | Skin wounds | (Buckley et al., 2011) |
| 2mm ear-punch wounds | (Rajnoch et al., 2003) | Thermal skin injury | (Davis et al., 2007) |
| 2mm ear-punch wounds | (Bedelbaeva et al., 2004) | Partial thickness cartilage lesion | (Fitzgerald et al., 2008) |
| 2mm ear-punch wounds | (Davis et al., 2005) | Adult digit amputations^ | (Gourevitch et al., 2009) |
| 2mm ear-punch wounds | (Metcalfe et al., 2006) | ||
| 2mm ear-punch wounds | (Colwell et al., 2006) | ||
| 2mm ear-punch wounds | (Naseem et al., 2007) | ||
| 2mm ear-punch wounds | (Fitzgerald et al., 2008) | ||
| 2mm ear-punch wounds | Heydemann unpublished | ||
| Alkali-burned corneas | (Ueno et al., 2005) | ||
| Neonatal digit amputations | (Chadwick et al., 2007) | ||
| Intraarticular fracture healed without arthritis | (Ward et al., 2008) | ||
| Full-thickness articular cartilage | (Fitzgerald et al., 2008) | ||
| Skin transplantation | (Tolba et al., 2010) |
Important differences were noted between the MRL/MpJ and control mice, although regeneration was not achieved.
MRL/MpJ (with the intact Fas gene) mice also heal alkali-burned corneas faster and more completely then C57BL/6J mice (Ueno et al., 2005). Normally, this specific wounding model has a robust inflammatory cell infiltration, extensive scarring, continued epithelial erosion, ulceration, stromal edema, neovascularization and results in loss of transparency caused by non-remodeled scar tissue (Saika, 2007). Successful cornea healing is defined as no loss of corneal transparency (Saika, 2007). The super-healing of corneas by the MRL/MpJ mice is characterized by rapid reepithelialization which mitigates chances of infection and reduces inflammation (Ueno et al., 2005). As soon as 3 days after wounding significantly more MRL/MpJ mouse corneas were completely reepithelialized across the wounds [MRL/MpJ 22 of 30 mice; B6 mice 3 of 28 mice, p < 0.01 (Ueno et al., 2005)]. The authors provide data that supports a decreased immune response in the MRL/MpJ mice as being beneficial for this type of wound healing (please see below for mechanistic discussions). It also appears from their data that the MRL/MpJ mice are capable of fully remodeling fibrosis, thereby healing the eye to its full functional capacity, without any loss of corneal transparency.
Thus far, two papers examined the ability of MRL/MpJ mice to regrow digits. The results of the papers cannot be compared directly due to the age differences and the wound site differences. The first analyzed digit tip regrowth, as the amputations performed at neonatal day 1 were at the midpoint of the third phalanges (Chadwick et al., 2007). These wounds are known to regenerate in mice and reports of this are also found in humans, possibly by nail bed stem cell contributions (Han et al., 2005). It was shown that the MRL digits regrew faster than either DBA/2 or C57BL/6 control strains at days 7, 14 and 21 days post amputation, but lost their advantage by day 28. The authors were extremely careful to normalize for the faster overall growth rates of the larger MRL/MpJ pups. At day 28, the degree of nail regeneration was analyzed. The MRL mice had completely or partially regrown the nail statistically more frequently than either of the control strains. Just as in the ear wounds, MRL/MpJ mice rapidly reepithelialized over the wound site, allowing blastema cells to dedifferentiate in the protected niche created below the epithelium. The authors did not observe this rapid reepithelialization in the other strains and, perhaps, therefore the blastema could not form. The second paper analyzed a more severe wound by amputating midway in the second phalangeal bone (Gourevitch et al., 2009). This paper wounded adult female mice. Although important differences were noted between the MRL/MpJ, control C57BL/6, and Swiss Webster, none of the three strains regrew their digits. Striking differences include the large blastema developing distally from the wound site and the many cells positive for BrdU and phospho-histone 3 in the MRL/MpJ wounds. The MRL/MpJ wounds were, again, quicker to reepithelialize, completing this layer within the week after wounding. C57BL/6 mice completed this layer by 2 weeks and the Swiss-Webster mice by 3 weeks. In addition, as part of the important remodeling step, the MRL wounds had a greatly reduced basement membrane layer. Importantly, this paper demonstrated that fibrosis is not detrimental. The MRL/MpJ mice had larger trichrome positive areas 2 weeks after injury, and, by week 3, there was a reduction in trichrome area. Therefore, it is understood that correct remodeling of the collagens is critical. This work also documented significant differences in immediate immune response. The Swiss Webster mice had a large infiltrate; the MRL/MpJmice displayed a moderate infiltrate; and the C57BL/6 mice had almost no infiltrate. Therefore, the presence or absence of an immune response is too simplistic of a distinction. If, as is suspected, the immune system has a role in the super-healing phenotype of the MRL animals, it is the presence and absence of specific subsets of immune cells in a proper temporal pattern which are required.
A further set of experiments demonstrated that the MRL/MpJ mice could heal full thickness articular cartilage wounds (Fitzgerald et al., 2008). The authors also demonstrated that male MRL/MpJ mice healed articular cartilage more completely then the females. The authors cleverly subjected half of their animals to 2 mm ear hole wounds to analyze possible correlations between the healing abilities of the different wounds. There was only a very weak correlation, r2 = 0.32, when MRL/MpJ males and C57Bl/6 males and females were considered (Fitzgerald et al., 2008). This is highly interesting and suggests that the factors governing one healing phenotype may not affect healing in a different tissue. Given that these animals are inbred and largely true-breeding homozygous at every locus, the disparate healing phenotypes may involve the heteroplasmic mitochondria (Sachadyn et al., 2008). This possibility will be discussed below. Another cohort of mice was subjected to partial thickness articular cartilage lesions, which did not heal in either mouse strain (Fitzgerald et al., 2008).
The MRL/MpJ mice were also analyzed for their ability to heal without arthritis after an intraarticular fracture (Ward et al., 2008). The control C57BL/6 mice had a decrease in bone density and increase in subchondral bone thickness. They also displayed an increase in cartilage degeneration. Alternatively, the wounded knee of the MRL mice was not statistically different than the contralateral uninjured control leg for these three characteristics.
Thus far, four studies were conducted that analyzed the ability of MRL/MpJ mice to heal surgically induced skin wounds. One study made 5 mm full-thickness incisional wounds and 2 mm diameter excisional wounds on the dorsum of adult mice (Colwell et al., 2006). No differences were observed between the MRL/MpJ and the C57BL/6 and Balb/c control mice. None of the mice achieved the rapid reepithelialization or a blastema structure, and all three strains healed both types of wound with a scar. The second study made two 4 mm excisional wounds on adult dorsal skin (Beare et al., 2006). Minimal differences in skin healing were identified between the MRL/MpJand C57BL/6 control mice. Both groups healed with minimal reepithelialization, granulation tissue and a lasting scar. In both of these skin wounding studies, the MRL/MpJ mice did not produce hair follicles or sebaceous glands, as they do with their ear wounds (Beare et al., 2006; Colwell et al., 2006). Two further dorsal skin wound experiments also demonstrated that the MRL/MpJ mice do not heal these wounds differently than the C57BL/6 control strain (Metcalfe et al., 2006; Buckley et al., 2011).
Another study subjected mice to burn injury (Davis et al., 2007). Both the MRL/MpJ and BALB/c control strain showed evidence of healing with a scar. The MRL/MpJ wounds had increased angiogenesis, collagen, scarring and decreased granulation tissue, α-smooth muscle actin-positive cells, myofibroblasts, dermal neutrophils, and contraction. Surprisingly, the BALB/c mice lost their scabs sooner and appeared to finish their healing more quickly; but as stated before, neither strain regenerated intact, functional, scar-free skin.
A clinically relevant manuscript investigates the healing of skin transplants in MRL/MpJ and B10.BR cross transplanted mice (Tolba et al., 2010). The authors identified that the recipient animal specifies the grafts health. B10.BR grafts onto MRL animals were characterized by less collagen, less inflammation, less immune infiltration across the entire wound area, less apoptosis, more neovascularization, increased VEGF-α mRNA expression, and more, local stem cell recruitment then the reciprocal grafts. To identify the initial differences between the mouse strains the author’s identified that the MRL recipient wounds had increased STAT3 phosphorylation, and therefore increased STAT3 activity which can explain the increased VEGF and had decreased Smad7 and decreased phosphorylated STAT1 which can explain the reduced immune response.
Scientists have also tested the MRL/MpJ response to central nervous system injury. The dopaminergic projection was subjected to a stab lesion, which usually does not allow axon regeneration due to the excessive scar tissue deposited by glial cells (Fawcett and Asher, 1999). Although some early differences were noted between the MRL/MpJ and Swiss Webster mice, these differences were transient and neither mouse displayed axonal regeneration (Hampton et al., 2004). Early differences include greater loss of astrocytes, increased microglial inflammatory response, increased blood-brain barrier compromise, increased mRNAs for matrix metalloproteinase (MMP)-2, and MMP-9 in the MRL/MpJ wounds (Hampton et al., 2004).
Another study characterized the subventricular zones (SVZ) of MRL/MpJ, C57BL/6 and CD-1 mice without wounding (Baker et al., 2006). The SVZ is responsible for producing neurons which will continually migrate into the olfactory bulbs. The MRL/MpJ SVZs were identified to contain more neuronal progenitors, which were also dividing and migrating to the bulbs in greater numbers. It was noted that the neurogenesis process in the MRL/MpJ mice appeared normal except for the increase in cell numbers. This increase in new neurons is countered by an increase of caspase-3 staining and apoptosis in the olfactory bulbs. In a different portion of the brain; the dentate gyrus of the hippocampus, it was demonstrated that MRL/MpJ mice had reduced numbers of neurons at baseline compared to C57BL/6 mice (Thuret et al., 2009). The decrease is attributed to reduced neurogenesis and survival of neurons. The reduction in neurons is associated with reduced scores on the Morris water maze test and the visual-paired comparison task. Very interestingly, voluntary wheel running improved the MRL/MpJ performance on both learning tests. Alternatively, the control C57BL/6 mouse scores did not improve with exercise. The exercise also increased the number of neurons in both strains of mice, but the increase was larger in the MRL/MpJ mice such that after exercise there was no difference between the numbers of neurons in the two strains. The MRL/MpJ brains also behaved differently when subjected to chronic antidepressant treatments (Balu et al., 2009). In this work hippocampal cell proliferation was induced in the MRL/MpJ mice but not in the C57BL/6 control mice with 21 days of antidepressant treatment. In addition, only the treated MRL/MpJ mice had shorter latencies to consume food when placed in a novel environment.
Peripheral nerve regeneration was also tested in the ear hole wounding model. In uninjured tissue the MRL/MpJ and C57BL/6 mice displayed the same nerve density. At 14 and 21 days post injury, the MRL/MpJ mice demonstrated increased nerve area in the proximal wound, but not in the peripheral wound (Buckley et al., 2011). Interestingly, the non-regenerative dorsal dermal wounds on the MRL/MpJ animals did not display re-innervation. This manuscript reported no difference in revascularization between the two strains.
We have recently shown that the MRL/MpJ genotype benefits mice with γ-sarcoglycan null muscular dystrophy (Heydemann, manuscript in preparation). The F2 dystrophic mice of MRL/MpJ and DBA/2J intercrosses (50% MRL/MpJ genome and 50% DBA/2J genome) do not display any of the debilitating fibrosis that the pure DBA/2J dystrophic mice do. Lack of fibrosis in the F2 mice was evident in all muscle groups tested; diaphragm, quadriceps, abdominals and cardiac. This complete lack of fibrosis was evident in all 60 F2 mice analyzed, indicating that multiple genes are acting additively for the animal’s benefit. We also noted increased regenerative characteristics in the MRL/MpJ mice with muscular dystrophy (Heydemann, manuscript in preparation). Because of this drastically reduced phenotype, we wished to investigate the MRL/MpJ skeletal muscles in more depth. Preliminary data indicates that wild type MRL/MpJ mice regenerate cardiotoxin induced skeletal muscle injury differently than wild type DBA/2J mice. Late (at day 21 after cardiotoxin injection) in the healing process, the MRL/MpJ mice have increased central nuclei (48%± 4% versus 13%± 0.2%, p = 0.047)indicating, at this time point, the MRL/MpJ gastrocnemius muscles are regenerating more than the DBA/2J muscle. Further characterization of these wounds across a time course may assist in illuminating common beneficial mechanisms in the various MRL/MpJ wound models.
Many laboratories have investigated the cardiac healing ability of the MRL mice with surprisingly variable results (Table 3). Beneath the table some mechanisms to explain the cardiac disparities are listed, although no clear elucidations are available.
Table 3.
Cardiac healing? green indicates regeneration, red indicates fibrotic wound
| Injury | Assay | References |
|---|---|---|
| Cryoinjury of the right ventricle | Increased healing vs. C57BL/6; | (Leferovich et al., 2001) |
| 60 days post injury | ||
| >Reduced hydroxyproline | ||
| >Increased mitosis | ||
| >Increased function by echo | ||
| Cryoinjury of the right ventricle | Increased healing vs. C57BL/6; | (Bedelbaeva et al., 2004) |
| > MT | ||
| > less collagen | ||
| Permanent occlusion | No control strain | (Oh et al., 2004) |
| 27 and 104 days post-surgery | ||
| >MRI demonstrated transmural infarction with fibrosis | ||
| > MT demonstrated fibrosis | ||
| Transient 45′ IR | No difference vs. C57BL/6; | (Abdullah et al., 2005) |
| 24 hours and 10 weeks post injury | ||
| >Calculated infarct area with TTC and MT staining | ||
| Cryoinjury, superficial | Increased healing vs. C57BL/6; | (Naseem et al., 2007) |
| Restoration of cardiac function in MRL hearts | ||
| Less collagen deposition | ||
| Cryoinjury, severe | Increased survival | |
| Reduced scarring | ||
| Decreased apoptosis | ||
| Decreased ventricular remodeling | ||
| Not complete regeneration | ||
| Transmural myocardial injury, severe | Increased survival | |
| Increased cellular proliferation | ||
| Reduced scarring | ||
| Decreased ventricular remodeling | ||
| Not complete regeneration | ||
| Permanent occlusion | No difference vs. C57BL/6; | (Robey and Murry, 2008) |
| 1 – 90 days post injury | ||
| >Calculated infarct area with H&E and Sirius red staining | ||
| >Mitosis | ||
| Cryoinjury | No difference vs. C57BL/6; | |
| 7 and 28 days post injury | ||
| >H&E and Sirius red staining | ||
| >Mitosis | ||
| Permanent occlusion | No difference vs. C57BL/6; | (Grisel et al., 2008) |
| 60 days post injury | ||
| >Calculated infarct area with H&E and Sirius red staining | ||
| >Mitosis | ||
| Cryoinjury | No difference vs. C57BL/6; | |
| 60 days post injury | ||
| >H&E and Sirius red staining | ||
| >Mitosis | ||
| Permanent occlusion | No difference vs. C57BL/6; | (Cimini et al., 2008) |
| 0, 5, 15, 60 days post injury | ||
| >Heart function by echo and Millar catheter | ||
| > MT, H&E | ||
| > Mitosis | ||
| Permanent occlusion | No difference vs. C57BL/6; | (Moseley et al., 2011) |
| 4 weeks post injury | ||
| >Calculated infarct area with MT staining | ||
| >Heart function; contractility, pressure and volume | ||
| Muscular dystrophy | Reduced fibrosis and reduced functional decline vs. DBA/2J; | Heydemann manuscript in preparation |
| >Hydroxyproline | ||
| >Echocardiography |
TTC-(2,3,5)-triphenyltetrazolium: MT- Masson’s trichrome; H&E- hematoxylin and eosin staining; IR- ischemia reperfusion injury
Possible explanations for the cardiac healing differences
Altered immune function in the different mouse colonies. Some colonies may also be receiving antibiotics. Despite some of the animals being freshly bought from Jackson Laboratories the pathogens at the different institutions are variable.
Differences in mouse chow and bedding may affect the general health of the mice.
Gender and ages of animals may be different.
Genetic drift. Regular breeding pair replenishment assures that all laboratories are working with the same genome.
Maternal inheritance of mitochondrial heteroplasmies could affect the outcome of these injury models.
Differences in wound severity. The work by Naseem et al. (2007) illustrates that mild superficial injuries heal while severe injuries do not.
Autoimmune phenotype
Despite the early identification of a mutant FAS gene in the original MRL/MpJ-Faslpr/J mice, it has become increasingly clear that autoimmune disease, like everything concerned with the immune system, is a truly complex process. For systemic lupus erythematosus (SLE), a disease seen in both of the MRL mouse strains (Theofilopoulos and Dixon, 1985; Watson et al., 1992), 12 susceptibility loci have thus far been identified in the mouse (Wandstrat and Wakeland, 2001). The closely related Large mouse strain is also susceptible to autoimmune diseases (Peng et al., 1996). When considering the autoimmune and super-healing phenotypes of these three mouse strains, it is natural to suppose that there might be a common genetic predisposition to these phenotypes. Although the genetics of either phenotype remain to be identified, a multi strain wound healing survey gives preliminary results that the two phenotypes are not obviously genetically linked (Kench et al., 1999). The authors surveyed 22 mouse strains for their abilities to heal ear wounds. Only the two MRL strains and their ancestral Large strain displayed super-healing. These 3 strains and 3 others that are not super-healing have been reported to be autoimmune susceptible, indicating that the two phenotypes are not genetically overlapping. Corroborating this lack of mechanistic or genetic overlap; hematopoietic cells can transfer the autoimmune phenotype (Ito et al., 2003), but not the ear hole healing phenotype (Kench et al., 1999).
There is however counter evidence that indicates a correlation between autoimmune phenotype and super healing mice. When the cell cycle checkpoint gene p21 is mutated in mice, they develop a lupus like phenotype (Balomenos et al., 2000; Santiago-Raber et al., 2001). This knockout mouse strain also displays a super-healing phenotype for ear hole wounds (Bedelbaeva et al., 2010). As with many other MRL/MpJ characteristics, the final answer requires additional research.
Potential healing mechanisms
As you have read the above descriptions, I am sure multiple mechanisms come to mind that could account for the super-healing ability demonstrated by the MRL/MpJ, MRL/MpJ-Faslpr/J mice and Large mice. Of the many possible mechanisms to account for this healing the following will be discussed in detail: (1) Altered cell cycle, enhanced proliferation; (2) Altered stem cells; (3) Altered immune response; (4) Altered remodeling later stage blastema> fibrosis transition; (5) Altered mitochondria and metabolism.
The evidence supporting all of these proposed mechanisms is consistent with multiple mechanisms acting additively in the MRL/MpJ mice.
Cell cycle/proliferative differences
Classic regeneration models — axolotl, newt, liver and stem cells — display a propensity for cell cycle arrest at the G2/M boundary (Michalopoulos and DeFrances, 1997; Rao et al., 2009; Tassava, 1983; Hong et al., 2007), respectively. Recently, such an arrest was also demonstrated in the blastema cells and uninjured MRL fibroblasts (Bedelbaeva et al., 2010). This alteration in cell cycle supports the idea that the blastema will proliferate until the wound is healed without a scar. Therefore, it is proposed that, in the ear wound model, all of the cell types will continue to proliferate until fully functional tissue is reestablished. The authors also demonstrated that this arrest was correlated with decreased p21 protein in the MRL fibroblasts. Fibroblasts from the Large mouse strain also demonstrate reduced p21 protein levels (Bedelbaeva et al., 2010). p21 is a target of the cell-cycle master regulator p53. Without p21, the cells pass a usual cell cycle check point at G1 and add to the constriction of cells at the G2/M cycle checkpoint. To further validate the role of this cell cycle regulator, the authors analyzed the ear hole healing response in p21−/− mice. These mice healed the wounds without scarring to the same extent as the MRL mice (Bedelbaeva et al., 2010). It is not entirely clear what beneficial roles G2/M arrest bestows upon healing tissue. Further work by the same group demonstrated that the p21−/− healing occurred independently of p53. Alternatively, the authors presented preliminary data that p21−/− could increase healing by diminishing the TGF-β1 signaling cascade (Arthur et al., 2010). In corroboration with this theory, the authors showed that TGF-β1−/− Rag2−/− mice healed better than Rag2−/− mice (Arthur). Furthermore, others have shown that Smad3−/− mice also heal more quickly (Ashcroft et al., 1999). Because the majority of TGF-β1 signaling is Smad3 dependent, these data are consistent with TGF-β1 being central to scar formation and impaired healing. Changes in the TGF-β1 levels of the MRL/MpJ mice will be discussed in further detail below in the immune section.
Other evidence in support of cell cycle differences comes from the cryoinjured hearts. Seven and 15 days after injury, the MRL cardiomyocytes had increased Ki-67 staining, a marker associated with active cell-cycling (Heber-Katz et al., 2004b). The authors also demonstrated increased proliferation in the MRL hearts by BrdU labeling. Increased proliferation is often balanced by cell death to avoid hyperplasia. Increased apoptosis was found in the cryoinjured hearts (Heber-Katz et al., 2006). Interestingly, these increases in cell cycle progression and apoptosis were also identified in uninjured hearts (Heber-Katz et al., 2006). Increased turnover would help to explain regeneration. Summation of this data indicates increased cell cycle progression, balanced by apoptosis.
The digit amputation work also indicates that MRL/MpJ wounded cells tend to proliferate more. Within the wounds, the MRL cells had increased BrdU and phospho-histone-3, both indicators of proliferation, cumulative and short-term, respectively (Fitzgerald et al., 2008). In many of the ear hole wounding experiments and the digit amputation experiments, the epithelium was also demonstrated to proliferate faster and cover the wound earlier then the control strains, providing a niche for the blastema cells. The data from the SVZ analysis is also consistent with increased proliferation and increased apoptosis as a balance (Baker et al., 2006).
In contrast, other MRL/MpJ mouse studies have demonstrated reduced apoptosis. This is the case for the severe myocardial cryoinjury. The MRL/MpJ animals have reduced remodeling and increased preservation of cardiac function, but they have not fully regenerated (Naseem et al., 2007). This maybe a reflection of the severity of injury and the different tissues analyzed.
Beyond the proliferative increases in the structural cells, increased proliferation will also affect specific cell types, which directly benefit regeneration. A common characteristic of the MRL/MpJ ear hole wounding model is rapid reepithelialization. The rapid proliferation of the epithelial cells provides the underlying blastema cells a proper niche to de-and re-differentiate into the multiple cell types required for full regeneration (Darby et al., 2002; Heber-Katz, 1999). Increased proliferation in epithelial cells would also help explain the increased angiogenesis seen in the MRL/MpJ ear hole and cryoinjury models (Heber-Katz, 1999). Increased proliferation may also benefit stem cells in their basal self-renewal capacity and their response to wounding.
Increased stem cell quantity and/or quality
A change in stem cell quantity is supported by the characterization of the subventricular zone cells. These cells demonstrated an increase in neuronal progenitors in the SVZ, which functionally progress to functional neurons in the olfactory bulbs (Baker et al., 2006). When qRT-PCR experiments were conducted upon injured cardiac tissue, an increase of the stem cell marker Nanog was observed. Immunohistochemistry identified increases in the stem cell markers Islet-1 and Sox2 compared to C57BL/6 mice (Naviaux et al., 2009). The differences were present preinjury but were amplified post-injury, perhaps indicating both an increase of basal stem cell numbers and an increased response to damage.
Additional stem cell data are lacking due to the minimal number of cells to investigate and the paucity of indicative antibodies.
Immune differences
Regeneration is often described as embryonic healing (Whitby and Ferguson, 1991). Mouse embryos younger than 16 days heal without a scar (Hopkinson-Woolley et al., 1994). Embryonic healing is equated with lower numbers of less differentiated inflammatory cells (Cowin et al., 1998). The embryonic wounds also have reduced TGF-β1, TGF-β2 and PDGF and higher levels of TGF-β3 (Whitby and Ferguson, 1991). In further experiments the cytokine profile has been manipulated and the expected opposite phenotype has been observed. For example, the reduction of TGF-β1 and β2 or the addition of exogenous TGF-β3 results in reduced scarring in adult wounds (Shah et al., 1995). We may, thus, expect that the MRL/MpJ mice have reduced TGF-β1 and β2 and increased TGF-β3 expression. This is not the case; MRL splenocytes have 8-fold more TGF-β1 than control cells (Kench et al., 1999). However, LPS-induced peritoneal macrophages had reduced IL-1β and TNF-α levels (Kench et al., 1999).
The immune system’s involvement in the apparent differences between regenerative healing and wounding is also indicated by the timing of the embryonic transition to a non-healing phenotype. In mice, this transition is at embryonic day 16 (Hopkinson-Woolley et al., 1994). This is the same period when T cell rearrangements begin in the thymus and when inflammation begins to appear in wounds (Havran and Allison, 1988; Hopkinson-Woolley et al., 1994). Further support that T cells mediate healing with a scar comes from athymic, nude mice, which regenerate their ear holes scarlessly (Gawronska-Kozak, 2004). Of future interest will be whether T cell transplants are capable of transferring the healing to C57BL/6 mice or transferring the non-healing response to MRL/MpJ mice.
In response to LPS MRL/MpJ, macrophages have reduced IL-1 expression (Donnelly et al., 1990). The MRL/MpJ mice also have reduced IL-6 and TNF-α levels after LPS challenge (Alleva et al., 1997). Whether these changes are important to healing remains unknown as the autoimmune prone NZB mouse strain is not reported to have enhanced healing, and also has reduced inflammatory macrophage cytokines IL-1, IL-6 and TNF-α levels post LPS challenge (Donnelly et al., 1990; Alleva et al., 1997). Many other cytokine differences between embryos and adults can be listed. However, it would be difficult to identify and reject those irrelevant to the scar-free healing mechanism (Ferguson and O’Kane, 2004).
The cornea wounds also demonstrate decreased immune response in the MRL/MpJ animals (Ueno et al., 2005). The control C57BL/6J mice showed robust inflammation, especially increased neutrophil quantities, early in their attempted repair processes. The authors hypothesized that the neutrophils impede reepithelialization. In agreement with this, neutrophil depletion in the C57BL/6 mice enhanced their healing ability (Ueno et al., 2005). Gene expression analysis also supports that the C57BL/6 mice have a higher immune response than the MRL/MpJ mice (Ueno et al., 2005).
Cytokine differences between MRL/MpJ and the control C57BL/6 mice were also assessed in the articular fracture model (Ward et al., 2008). Baseline differences were identified in the pro-inflammatory IL-1α (MRL/MpJ mice had less p = 0.001) and the anti-inflammatory IL-4 and IL-10 (for which the MRL/MpJ mice had more p < 0.045). Of real interest is that the anti-inflammatory cytokine response to injury was different in the two mouse strains. The C57BL/6 mice had significantly decreased levels of IL-4 and IL-10, while the MRL/MpJ mice trended an increase in these cytokines. Therefore, not only is the baseline cytokine profile different, but the cytokine response to this injury model is in opposite directions. These authors did not find a baseline difference in TGF-β1 in these 22 to 30 week old male mice.
Immune response differences have also been illuminated with microarray techniques. 24 h after ear hole wounding – during the inflammation phase — C57BL/6 mice had a greater number of inflammation-regulator-genes increase in expression than the MRL/MpJ-Faslpr/J mice (Li et al., 2001). Either the MRL/MpJ-Faslpr/J mice have less inflammatory response, or they progress through that stage faster and were already increasing expression of structural genes. In keeping with a completion of healing phases, MRL/MpJear hole wounds analyzed at 7, 14 and 28 days after injury had fewer differences of expression in inflammation-linked genes (Masinde et al., 2005).
The differences in immune response in the MRL/MpJ mice are not simply a, more or less, characterization. But specific cells and cytokines are coordinately increased and others are decreased. The timings of certain increases and decreases are also altered (Heber-Katz et al., 2004b). The different TGF-β levels in the MRL/MpJ animals are an example of the importance of timing and cells producing the cytokines. It is worth repeating that many differences are not causative, but could merely be a portion of the effect. For example, MRL/MpJ mice have ~30% fewer macrophages than C57BL/6 after isothioglycate mobilization (Davis et al., 2005; Potter et al., 2003). However, depletion of macrophages from C57BL/6 or MRL/MpJ mice had no effect upon healing kinetics (Davis et al., 2005). This difference is, therefore, not easily argued as the causative factor of regeneration in the MRL mice although it could be a portion of the factors causing regeneration.
Alterations in the extra cellular matrix (ECM)
A number of years ago Dr. Heber-Katz, the true pioneer of the MRL super healing field, proposed that an “enhanced breakdown of scar-like tissue may be” the critical difference in healing versus non-healing mouse strains (Heber-Katz et al., 2004b). At the time, the authors had provocative data to this effect and, now, the intervening years have provided much more evidence for this hypothesis.
In normal mouse wound healing, a basement membrane is formed by day 5 for the ear hole wounds, between the epidermis and dermal layers (Heber-Katz et al., 2004b). This layer persists throughout wound healing. The layer is also found in the MRL wounds, but is resolved very quickly and is only detected for a single day (Gourevitch et al., 2003; Heber-Katz, 1999). By correlation, when a basement membrane is introduced into an amphibian limb amputation wound model, healing was halted and the wound produced a scar (Stocum and Crawford, 1987). This illustrates the importance of remodeling and resolving physical barriers to cell movement for wound regeneration.
Neutrophils and macrophages of MRL/MpJ wounds secrete significantly increased amounts of active MMP-2 and MMP-9 and decreased amounts of TIMPs (Gourevitch et al., 2003). These alterations in MMP and TIMP content were identified through both mRNA, by microarray, and active protein, by zymograms (Gourevitch et al., 2003). The MRL wounds are, therefore, rapidly remodeling their ECMs. This allows for the eventual production of a normal matrix with normal collagen architecture as opposed to disorganized and excessively cross-linked collagen, as is usually left in the scar filled wound. It is also plausible that the increased MMPs, decreased TIMPs and subsequent increases in remodeling are also responsible for the accelerated basement membrane removal which allows the blastema to form in the regenerative animals (Heber-Katz et al., 2004b). A similar increase in MMP-2 and MMP-9 mRNA levels was also seen in the CNS injury model (Hampton et al., 2004).
Of importance in ECM construction and ECM remodeling are the immune cells, fibroblasts and myofibroblast cells that are largely responsible for ECM characteristics. The immune cells at work here are the neutrophils and macrophages of the 2a type (M2a). Neutrophils excrete MMP-2 and MMP-9, which are known to enhance remodeling. M2a macrophages are considered anti-inflammatory and, therefore, appear later in the normal scar type wound process. M2a cells secrete TGF-β1 and fibronectin. TGF-β1 not only causes fibroblasts to produce collagen, but also causes fibroblasts to transform into myofibroblasts, which secrete further collagens and cause wound contraction (Mann et al., 2011). Contraction is thought to limit remodeling, thus, leading to an unresolved scar (Desmouliere et al., 2005).
Because fibrosis is clearly necessary for sealing a wound, total absence of fibrosis is not desired. For example, myocardial infarction healing with a scar prevents cardiac rupture. The key step in the MRL/MpJ regenerating myocardium is the remodeling of this scar tissue to cardiomyocytes. The quality — for example, stiffness — of the ECM is, also, now being appreciated as modifying nearby cells, particularly, stem cells (Alexakis et al., 2007). A rigid ECM inhibits myoblast differentiation (Alexakis et al., 2007). Additional work in tissue culture has also revealed the significance of the ECM stiffness for specifying cell differentiation into particular lineages. Neurons prefer 0.5kPa; striated muscle prefers 10–20kPa; and fibroblasts grow best on acrylamide matrices of 50kPa (Chaudhuri et al., 2010).
An additional difference that MRL/MpJ mice might present is the source of the fibroblasts found in the wounds, as this source is still unclear. The fibroblasts could 1) be invading from the circulation; 2) be resident cells; 3) arise from transdifferentiation of epithelial cells; 4) dedifferentiation from myoblasts, or most likely, they may arise from multiple sources (Mann et al., 2011). Therefore, the MRL/MpJ mice may not only have different numbers of fibroblasts and myofibroblasts, but they may also have cells of different characteristics. As conjecture, the MRL/MpJ fibroblasts and myofibroblasts may be prone to apoptosis and, thereby, accelerate the remodeling phase.
Mitochondria, metabolism
Very interesting recent work has illuminated mitochondrial differences in the MRL/MpJ strain (Sachadyn et al., 2008; Naviaux et al., 2009). The initial work identified two heteroplasmies (cells which contain multiple mitochondrial genomes) in the MRL mitochondria (Sachadyn et al., 2008). These heteroplasmies are in the tRNAs for methionine and arginine. Although it is difficult to surmise the functional implications of these heteroplasmies, the tRNA polymorphisms likely affect many mitochondrial-translated genes, and may, therefore, globally alter the metabolism and other functions of the MRL/MpJ cells. In keeping with this hypothesis, the latter manuscript identifies metabolism differences in cardiomyocytes, liver cells, and fibroblasts between the MRL/MpJ and C57BL/6 mice (Naviaux et al., 2009). In short, the authors identified increased mitochondrial mass, reliance upon glycolysis, decreased membrane potential, reactive oxygen species, and oxidative phosphorylation. If these differences arise because of the mitochondrial genome’s heteroplasmies and how these differences affect regeneration or autoimmune phenotypes are yet to be determined.
Preliminary data (n = 2 for each strain) indicates a similar change in MRL/MpJ skeletal muscle metabolism. Electron micrographs of the quadriceps muscles were compared between the MRL/MpJ and DBA/2J strains (Fig. 1). Here we show that the MRL/MpJ muscles have significantly more inter-myofibril percentage mitochondrial area than the control mice (29.7%±18% versus 9.3%±3%, p = 0.01). Although not quantified, the mitochondria also appear to have increased variations in shape and size (arrows, Fig. 1).
Figure 1.
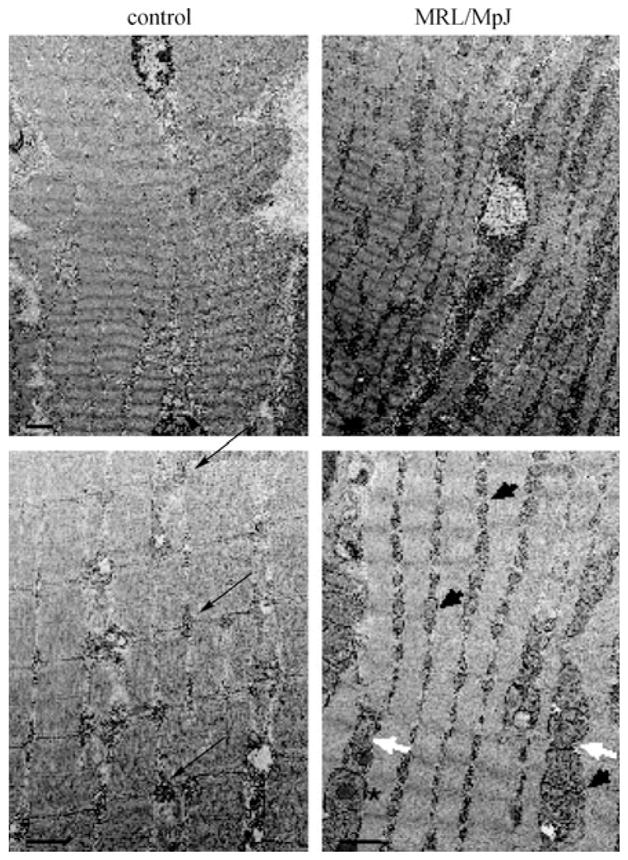
Electron micrographs demonstrating aberrant mitochondria in the MRL/MpJ mouse strain quadriceps, compared to the DBA/2J control strain. Note the standard arrangement of interfibril mitochondrial pairs in the control quadriceps (black arrows) and the increased number of mitochondria at this anatomical position in the MRL/MpJ strain (black arrowheads). Some of the MRL/MpJ mitochondria also demonstrate abnormal shapes (white arrows) and lipid-like inclusion bodies (asterisk). Top panels’ bar is 2 μm, bottom panels’ bar is 1 μm.
Transfer of healing
To assess which cell populations are required for healing, various cell transfer experiments were conducted. In one case, fetal liver cells were transferred between MRL/MpJ and C57BL/6 irradiated mice (Bedelbaeva et al., 2004). The cryoinjury cardiac healing was transferred by the fetal liver cells, while the ear hole closing phenotype could not be transferred and was recipient dependent (Bedelbaeva et al., 2004). At first glance that different cell populations would reconstitute different types of healing is remarkable. However, it is fully probable that the stem cells regenerating cardiac tissue are included in the fetal liver cells, while at least one of the many cell types required for ear hole closing may reside elsewhere. At its most basic; the heart wound would require cardiomyocytes, fibroblasts and endothelial cells for the vessels. The regenerating ear would require muscle cells, fibroblasts, endothelial and epithelial cells, and the full complement of hair follicle cells, glands, and chondrocytes. It may be that lack of a single cell type would disrupt the ear hole healing cascade. A further source for the difference may relate to the degree of wounding. The cryoinjury to the heart exterior may involve injury to fewer cells than the ear hole wound. In addition, in the ear wound, large numbers of cells are eliminated from the animal with the biopsy punch while in the heart cells are killed and remain; these necrotic cells would secrete many cytokines. Clearly these differences and conjectures require further investigation.
In another experiment, bone marrow was transferred into irradiated hosts. This interesting work demonstrates that bone marrow does not transfer the ear hole healing phenotype (Kench et al., 1999). The strain of the irradiated recipient determined the animal’s ability to heal the ear wound. In another bone marrow transfer experiment, it was shown that most dividing cells in the cryoinjured heart are not from the donated bone marrow (Heber-Katz et al., 2004b). Due to this and other data, the authors reason that MRL/MpJ hearts mounted a heart-wide response to injury while the control mice did not respond in any detectable manor (Heber-Katz et al., 2004b). Similarly, the recipient’s phenotype was apparent in the cryoinjured heart (Heber-Katz et al., 2004b). That is, MRL/MpJ bone marrow transferred to a C57BL/6 irradiated recipient does not transfer the healing phenotype. The authors also demonstrated that bone marrow did not transfer the ear hole healing phenotype either. In the cornea wound model, the authors also performed MRL/MpJ bone marrow transfer into C57BL/6 mice. The recipient mice were irradiated prior to transfer and received 3 injections of anti-mouse CD-154 to eliminate the CD-154 costimulatory pathway. The authors achieved high levels of chimerism (> 96%), but no increase in reepithelialization or reduced inflammation was observed (Ueno et al., 2005). Therefore, the healing phenotype apparently does not reside exclusively within bone marrow cells.
A further work injected purified macrophages from MRL or C57BL/6 mice into C57BL/6-SCID mice within one hour of ear wounding (Davis et al., 2005). The transfer of either macrophage population did not alter the ear wound healing kinetics. The previously discussed skin transplant study indicates that the recipient mouse is the determining factor in graft health (Tolba et al., 2010).
A clever study involved the harvesting of cell-free blastema-derived extra cellular matrix (ECM) from the MRL/MpJ ear wounds (Vorotnikova et al., 2010). A single application of the cell-free ECM into a C57BL/6 dorsal wound caused greater regeneration in the form of hair follicles, dermis and epidermis, and lack of scar tissue. This indicates that factors within the ECM such as cytokines and matrix components are responsible for the enhanced healing, and that cells, including stem cells, may not be required.
As pointed-out by Dr. Heber-Katz (Heber-Katz et al., 2004b), irradiation may introduce an unanticipated and hitherto uninvestigated injury. Irradiation may also introduce artificial cell niches by depletion of endogenous cells, which, only in this artificial situation, can be repopulated by transplanted cells (Heber-Katz et al., 2004b).
Gene expression analysis
To understand the mechanism of enhanced regeneration in the MRL/MpJ mice, multiple gene expression analyzes have been conducted. As with the cytokine discussion earlier, it is difficult to separate the causative gene expression changes from downstream effects of previous phenotypic and irrelevant differences.
One such study compared the 2 mm donut of tissue surrounding the 2mm punched out ear wound between MRL/MpJ-Faslpr and C57BL/6 mice 24 h after injury (Li et al., 2001). Interestingly, the majority of genes whose expression only increased in the MRL/MpJ tissue were of the tissue rebuilding functional class. Only three of the identified genes were possibly involved in inflammatory responses. Alternatively, of the 18 genes that are upregulated in C57BL/6 ear holes, 10 are involved in inflammation. Overall, the data of Li et al. (2001) indicates that MRL/MpJ mice have reduced inflammatory response and increased proliferation profile soon after injury.
Transcriptosome results from the alkali-burned cornea model focused upon immunological processes (Ueno et al., 2005). Largely, these results demonstrate less abundant transcripts for inflammation and increased expression of anti-inflammatory genes in the MRL/MpJ mice. Specifically, MRL/MpJ tissue showed increased MMP-1a, tumor necrosis factor receptor member 13c, small chemokine ligand 11, chemokine ligand 28 and suppressor of cytokine signaling 1. Alternatively, glycoprotein 49A and PU.1 were underrepresented in the MRL/MpJ mRNA pool.
A well designed study analyzed three time points after ear hole wounding at 7, 14, and 28 days to cover the inflammatory, fast-healing, and remodeling stages respectively (Masinde et al., 2005). Examples of genes that had elevated expression in MRL/MpJ than C57BL/6 are vimentin, elongation factor alpha 1, and cytochrome b, while genes more highly expressed in C57BL/6 include decorin, cytochrome C oxidase, and cytochrome C oxidase 5.
Microarray analyzes were performed on MRL/MpJ and C57BL/6 control mice 30 days after coronary artery ligation (Naseem et al., 2007). Generally, the authors saw increases in transcripts for cytoprotection, vasculogenesis, growth factors, cell cycle regulators, and oxidative stress reducers (Naseem et al., 2007).
During neonatal digit tip regrowth, it was determined that the LRP6 and FMN2 genes were differentially expressed in MRL/MpJ digits compared to C57BL/6 and DBA/2 digits (Chadwick et al., 2007). LRP6 is known to have a role in limb morphogenesis as part of the WNT signaling pathway (Williams and Insogna, 2009). FMN2 is also involved in WNTsignaling and is known to be important for development of the central nervous system (Leader and Leder, 2000).
A parallel method of discovery using proteomics was applied to ear hole healing (Li et al., 2000). Surrounding ear tissues were collected 1, 5, 10, and 20 days post-injury and compared to uninjured MRL/MpJ-Faslpr and C57BL/6 injured ear tissues. These time points were chosen to cover the three wound healing phases; day 1 for inflammation, days 5 and 10 for proliferation, and day 20 for the remodeling components. Five protein sizes had more than twofold changes in abundance (Li et al., 2000). Based upon protein size, the possible differentially present proteins are; neurnatin, calgranulin A, Lymphocyte antigen 6, Growth differentiation factor 3, ras-related protein (RAB-8), Mad4, ras-related protein 7. There are more possible proteins than protein size values as a few proteins have identical sizes. RAB-8 is of particular interest because it resides within heal1 in the chromosome 8 locus identified by QTL (see below (McBrearty et al., 1998). Calgranulin A was confirmed by antibody to be upregulated on day 1 of MRL/MpJ-Faslpr wound healing.
Quantitative trait loci investigations
Despite the MRL super healing phenotype being recognized in 1998 and multiple attempts to do so, no genes have been identified as being responsible for the phenotype. In fact, almost every mouse chromosome contains at least one loci associated with the MRL regeneration phenotype (Table 4). Some candidate genes that lie within the 95% confidence intervals on various chromosomes have previously been listed (Heber-Katz et al., 2004b, 2006; Yu et al., 2005). Some of these loci have been identified more than once and may, therefore, indicate the most interesting loci to pursue. Various breeding strategies have also indicated that the phenotype is inherited in a complex manner, with multiple genes working additively. From our own work, in which every MRL/MpJ x DBA/2J F2 animal generated (we analyzed 60 F2 animals) was free of muscular dystrophy mediated fibrosis (Heydemann, manuscript in preparation), we know the phenotype is multi-genic.
Table 4.
Ear hole healing associated quantitative trail loci
| References | (McBrearty et al., 1998) | (Masinde et al., 2001) | (Blankenhorn et al., 2003) | (Heber-Katz et al., 2004a) | (Yu et al., 2005) | (Blankenhorn et al., 2009) | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Strains
|
MRL/MpJ-Faslpr/J x C57BL/6 | MRL/MpJ x SJL/J | MRL/MpJ-Faslpr/J x C57BL/6 | MRL/MpJ x CAST/Ei | MRL/MpJx CAST/Ei | LG/J x SM/J | ||
| chr | Marker | ~CM | ||||||
| 1 | d1mit334 | 49.2 | Sth 1 | |||||
| 3 | d3mit217 | 43.7 | Sth2 | |||||
| 4 | d4mit214 | 21.9 | Sth3 | |||||
| 4 | d4mit31 | 50.3 | Sth4 | |||||
| 4 | d4mit13/170 | 66–71 | Heal8 | Heal8 | ||||
| 6 | d6mit261 | 29.5 | Sth5 | |||||
| 7 | d7mit220/85 | 38–52 | Sth6 | Heal6 | ||||
| 7 | d7mit12/98 | 62.3 | Sth7 | Sth7 | ||||
| 8 | d8mit211 | 49–68 | Heal1 | Heal1 | ||||
| 9 | d9mit207/129 | 26–32 | Sth8 | Sth8 | Sth8 | |||
| 9 | d9mit270/198/355/347 | 42–56 | Sth9 | Heal 14 | Sth9 | Sth9 | ||
| 10 | d10mit170 | 29 | Heal16 | |||||
| 10 | rs8258353 | 88 | Chr10b | |||||
| 11 | d11mit213/61 | 55–61 | Heal10 | Heal 10 | ||||
| 11 | d11mit100/203 | 68–75 | Heal6 | |||||
| 12 | d12mit132/233 | 13–52 | Heal5 | Heal5 | ||||
| 13 | d13mit115/117/16 | 9–19 | Heal2 | Heal2 | Heal2 | |||
| 13 | d13mit245/139/13 | 30–35 | Heal7 | Heal7 | ||||
| 13 | d13mit129/144/288 | 46–60 | Heal3 | Sth10 | Heal3 | |||
| 14 | d14mit233/201 | 12–20 | Heal12 | |||||
| 15 | d15mit244/189 | 48–57 | Heal4 | Heal4 | Heal4 | |||
| 16 | d16mit122/110 | 4–20 | Heal11 | |||||
| 17 | d17mit93/51 | 23–44 | Heal13 | Heal13 | ||||
| 18 | d18mit33 | 30–44 | Heal9 | |||||
To further complicate the matter, it has been demonstrated that MRL/MpJ healing is sexually dimorphic in some injury models. In the ear hole model, between Large mice (majority genetic contribution to the MRL/MpJ mouse strain) and Small mice, females healed more quickly (Blankenhorn et al., 2009).
Genetic dimorphism was also seen in the ear hole wound model between MRL/MpJ-Faslpr and C57BL/6 mice and between MRL/MpJ and CAST/Ei mice, again, the females healing more quickly (Blankenhorn et al., 2003; Heber-Katz et al., 2004a), respectively. Both of these investigations also demonstrated that genetic loci were unequally distributed between the genders. Alternatively, the articular cartilage wound model demonstrated that the males healed faster (Fitzgerald et al., 2008). These same authors also performed ear hole closing experiments and saw no gender difference in wound healing.
The locus on chromosome 9 was chosen for fine mapping, using congenic mouse strains (Yu et al., 2007). At generation N8 (produced by repeated backcrosses to SJL mice) the animals were homozygous for SJL genes at 99.2% at all of their loci except at the chromosome 9 QTL region, which was variably MRL/MpJ derived. By following the ear hole regeneration phenotype with chromosome 9MRL/MpJ markers, the scientists minimized the sth8 locus to 61–64 Mb and sth9 between 85 and 90 Mb (Yu). Thereby, making the region manageable for candidate gene approaches (Heydemann et al., 2009).
Future directions
Of primary importance is to identify which of the possible healing mechanisms is at work in the various MRL wounds (Fig. 2). Likely there will be a combination of mechanisms for proper regeneration to occur. It may also be likely that different wounds and different tissues rely more heavily upon certain mechanisms than other wounds and tissues do. The ultimate goal will be the ability to manipulate these mechanisms for therapeutic benefit in human healing.
Figure 2.
Schematic of possible mechanisms altering the wound healing properties of MRL/MpJ animals.
An interesting question that arises when discussing the MRL/MpJ mice is whether they have an increased life expectancy (Heber-Katz et al., 2006). Many age-related conditions are linked to increased fibrosis, at least in humans (Torres and Leof, 2011). Therefore, we could expect that the MRL mice, which have reduced fibrosis, would then have increased life spans. However, this does not appear to be the case. In a paper comparing the serum IGF-1 levels and lifespans, the MRL mice were actually found to have decreased median lifespans. MRL female mice lived to 557 days and males to 645 days while the median lifespans for the other 30 strains analyzed were 703 days for females and 701 for the males (Yuan et al., 2009). The authors also demonstrated a strong inverse correlation between IGF-1 levels and longevity p = 0.01. The male MRL/MpJ mice had the highest mean circulating IGF1 levels of 423±13 ng/mL compared to the male average of 320±54. The female MRL/MpJ mice also had above average levels of IGF1 at 395±10 compared to the female average of 329±76. The inverse correlation corroborates previous data regarding the IGF signaling pathway and longevity (Ziv and Hu, 2011). As with other phenotypes, the MRL/MpJ mice are likely balancing between their super-healing phenotype and autoimmune disease.
Another interesting topic regarding the MRL/MpJ mice would be their response to solid tumors. On the one hand, the mice might be expected to succumb to injected tumors more easily because of their altered immune response and their increased expression of MMP2 and MMP9, allowing for metastases. Alternatively, their immune system may be skewed to successfully combat an injected or implanted tumor. Perhaps, just as with their wound healing, some tumors may be eradicated while others would successfully invade the MRL/MpJ mouse. I look forward to the results of such experiments. Some pertinent data from humans with systemic lupus erythematosus demonstrates that these patients are at an increased risk of developing cervical cancer (Liu et al., 2011). As no super-healing humans have been identified, this only illuminates a portion of what the MRL/MpJ response to tumors may be.
I also eagerly anticipate the results of breeding the MRL/MpJ mice to other models of fibrotic diseases. I expect the fibrosis aspects of these diseases to be inhibited in the intercrossed mice, but it remains to be seen if this is true for all tissue types and the functional significance of fibrosis inhibition will need to be determined.
Table 1.
Wound repair, embryonic differences, MRL, differences
1. Hemostasis
|
2. Inflammation
, reduced in embryonicand
altered in MRL wounds
|
3. Proliferation
|
4. Remodeling
|
Acknowledgments
I thank Susan T. Varghese, Jenan Holley-Cuthrell, Ann F. Kuenster, and Nathan W. Roberts for their help with the writing of this review and preliminary data. This work was supported by a National Institutes of Health Grant (RO1 HL 102322-01A1).
Abbreviations
- ECM
extra cellular matrix
- MMP
matrix metalloproteinase
- MRL/MpJ-Faslpr/J
Murphy Roths Large mouse strain containing the mutant Fas gene and therefore lymphoproliferative disorder
- MRL/MpJ
Murphys Roths Large mouse strain with a wild type Fas gene
- TGF-β
transforming growth factor β
- TIMP
tissue inhibitor of metalloproteinases
References
- Abdullah I, Lepore JJ, Epstein JA, Parmacek MS, Gruber PJ. MRL mice fail to heal the heart in response to ischemia-reperfusion injury. Wound Repair and Regeneration. 2005;13:205–208. doi: 10.1111/j.1067-1927.2005.130212.x. [DOI] [PubMed] [Google Scholar]
- Adachi M, Watanabe-Fukunaga R, Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci USA. 1993;90(5):1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293(2):C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- Alleva DG, Kaser SB, Beller DI. Aberrant cytokine expression and autocrine regulation characterize macrophages from young MRL+/+ and NZB/W F1 lupus-prone mice. J Immunol. 1997;159:5610–5619. [PubMed] [Google Scholar]
- Anversa P, Rota M, Urbanek K, Hosoda T, Sonnenblick EH, Leri A, Kajstura J, Bolli R. Myocardial aging–a stem cell problem. Basic Res Cardiol. 2005;100(6):482–493. doi: 10.1007/s00395-005-0554-3. [DOI] [PubMed] [Google Scholar]
- Arthur LM, Demarest RM, Clark L, Gourevitch D, Bedelbaeva K, Anderson R, Snyder A, Capobianco AJ, Lieberman P, Feigenbaum L, Heber-Katz E. Epimorphic regeneration in mice is p53-independent. Cell Cycle. 2010;9(18):3667–3673. doi: 10.4161/cc.9.18.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1(5):260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- Baker KL, Daniels SB, Lennington JB, Lardaro T, Czap A, Notti RQ, Cooper O, Isacson O, Frasca S, Jr, Conover JC. Neuroblast protuberances in the subventricular zone of the regenerative MRL/MpJ mouse. J Comp Neurol. 2006;498(6):747–761. doi: 10.1002/cne.21090. [DOI] [PubMed] [Google Scholar]
- Balomenos D, Martin-Caballero J, Garcia MI, Prieto I, Flores JM, Serrano M, Martinez AC. The cell cycle inhibitor p21 controls T-cell proliferation and sex-linked lupus development. Nat Med. 2000;6(2):171–176. doi: 10.1038/72272. [DOI] [PubMed] [Google Scholar]
- Balu DT, Hodes GE, Anderson BT, Lucki I. Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments. Neuropsychopharmacology. 2009;34(7):1764–1773. doi: 10.1038/npp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare AH, Metcalfe AD, Ferguson MW. Location of injury influences the mechanisms of both regeneration and repair within the MRL/MpJ mouse. J Anat. 2006;209(4):547–559. doi: 10.1111/j.1469-7580.2006.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedelbaeva K, Gourevitch D, Clark L, Chen P, Leferovich JM, Heber-Katz E. The MRL mouse heart healing response shows donor dominance in allogeneic fetal liver chimeric mice. Cloning Stem Cells. 2004;6(4):352–363. doi: 10.1089/clo.2004.6.352. [DOI] [PubMed] [Google Scholar]
- Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J, Cheverud JM, Lieberman P, Heber-Katz E. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc Natl Acad Sci USA. 2010;107(13):5845–5850. doi: 10.1073/pnas.1000830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenhorn EP, Bryan G, Kossenkov AV, Clark LD, Zhang XM, Chang C, Horng W, Pletscher LS, Cheverud JM, Showe LC. Genetic loci that regulate healing and regeneration in LG/J and SM/J mice. Mammalian Genome. 2009;20:720–733. doi: 10.1007/s00335-009-9216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenhorn EP, Troutman S, Clark LD, Zhang XM, Chen P, Heber-Katz E. Sexually dimorphic genes regulate healing and regeneration in MRL mice. Mamm Genome. 2003;14(4):250–260. doi: 10.1007/s00335-002-2222-3. [DOI] [PubMed] [Google Scholar]
- Buckley G, Metcalfe AD, Ferguson MW. Peripheral nerve regeneration in the MRL/MpJ ear wound model. J Anat. 2011;218(2):163–172. doi: 10.1111/j.1469-7580.2010.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81(4):1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick RB, Bu L, Yu H, Hu Y, Wergedal JE, Mohan S, Baylink DJ. Digit tip regrowth and differential gene expression in MRL/Mpj, DBA/2, and C57BL/6 mice. Wound Repair and Regeneration. 2007;15:275–284. doi: 10.1111/j.1524-475X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- Chaudhuri T, Rehfeldt F, Sweeney HL, Discher DE. Preparation of collagen-coated gels that maximize in vitro myogenesis of stem cells by matching the lateral elasticity of in vivo muscle. Methods Mol Biol. 2010;621:185–202. doi: 10.1007/978-1-60761-063-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini M, Fazel S, Fujii H, Zhou S, Tang G, Weisel RD, Li RK. The MRL mouse heart does not recover ventricular function after a myocardial infarction. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 2008;17:32–39. doi: 10.1016/j.carpath.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88(1):35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- Colwell AS, Krummel TM, Kong W, Longaker MT, Lorenz HP. Skin wounds in the MRL/MPJ mouse heal with scar. Wound Repair and Regeneration. 2006;14:81–90. doi: 10.1111/j.1524-475X.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- Cowin AJ, Brosnan MP, Holmes TM, Ferguson MW. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Developmental Dynamics. 1998;212:385–393. doi: 10.1002/(SICI)1097-0177(199807)212:3<385::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Cullen MJ, Jaros E. Ultrastructure of the skeletal muscle in the X chromosome-linked dystrophic (mdx) mouse. Comparison with Duchenne muscular dystrophy. Acta Neuropathol. 1988;77(1):69–81. doi: 10.1007/BF00688245. [DOI] [PubMed] [Google Scholar]
- Darby IA, Bisucci T, Pittet B, Garbin S, Gabbiani G, Desmouliere A. Skin flap-induced regression of granulation tissue correlates with reduced growth factor and increased metalloproteinase expression. J Pathol. 2002;197:117–127. doi: 10.1002/path.1074. [DOI] [PubMed] [Google Scholar]
- Davis TA, Amare M, Naik S, Kovalchuk AL, Tadaki D. Differential cutaneous wound healing in thermally injured MRL/MPJ mice. Wound Repair and Regeneration. 2007;15:577–588. doi: 10.1111/j.1524-475X.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- Davis TA, Longcor JD, Hicok KC, Lennon GG. Prior injury accelerates subsequent wound closure in a mouse model of regeneration. Cell Tissue Res. 2005;320(3):417–426. doi: 10.1007/s00441-005-1107-7. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair and Regeneration. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- Donnelly RP, Levine J, Hartwell DQ, Frendl G, Fenton MJ, Beller DI. Aberrant regulation of IL-1 expression in macrophages from young autoimmune-prone mice. J Immunol. 1990;145:3231–3239. [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49(6):377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Ferguson MW, O’Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359(1445):839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis and cartilage/OARS. Osteoarthritis Research Society. 2008;16(11):1319–1326. doi: 10.1016/j.joca.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gawronska-Kozak B. Regeneration in the ears of immunodeficient mice: identification and lineage analysis of mesenchymal stem cells. Tissue Eng. 2004;10:1251–1265. doi: 10.1089/ten.2004.10.1251. [DOI] [PubMed] [Google Scholar]
- Goss RJ. Prospects of regeneration in man. Clin Orthop Relat Res. 1980:270–282. [PubMed] [Google Scholar]
- Gourevitch D, Clark L, Chen P, Seitz A, Samulewicz SJ, Heber-Katz E. Matrix metalloproteinase activity correlates with blastema formation in the regenerating MRL mouse ear hole model. Developmental Dynamics. 2003;226:377–387. doi: 10.1002/dvdy.10243. [DOI] [PubMed] [Google Scholar]
- Gourevitch DL, Clark L, Bedelbaeva K, Leferovich J, Heber-Katz E. Dynamic changes after murine digit amputation: the MRL mouse digit shows waves of tissue remodeling, growth, and apoptosis. Wound Repair and Regeneration. 2009;17:447–455. doi: 10.1111/j.1524-475X.2009.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel P, Meinhardt A, Lehr HA, Kappenberger L, Barrandon Y, Vassalli G. The MRL mouse repairs both cryogenic and ischemic myocardial infarcts with scar. Cardiovascular Pathology. 2008;17:14–22. doi: 10.1016/j.carpath.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Hampton DW, Seitz A, Chen P, Heber-Katz E, Fawcett JW. Altered CNS response to injury in the MRL/MpJ mouse. Neuroscience. 2004;127(4):821–832. doi: 10.1016/j.neuroscience.2004.05.057. [DOI] [PubMed] [Google Scholar]
- Han M, Yang X, Taylor G, Burdsal CA, Anderson RA, Muneoka K. Limb regeneration in higher vertebrates: developing a roadmap. Anat Rec B New Anat. 2005;287B(1):14–24. doi: 10.1002/ar.b.20082. [DOI] [PubMed] [Google Scholar]
- Harty M, Neff AW, King MW, Mescher AL. Regeneration or scarring: an immunologic perspective. Developmental Dynamics. 2003;226:268–279. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335(6189):443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E. The regenerating mouse ear. Semin Cell Dev Biol. 1999;10(4):415–419. doi: 10.1006/scdb.1999.0328. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E, Chen P, Clark L, Zhang XM, Troutman S, Blankenhorn EP. Regeneration in MRL mice: further genetic loci controlling the ear hole closure trait using MRL and M.m. Castaneus mice. Wound Repair and Regeneration. 2004a;12:384–392. doi: 10.1111/j.1067-1927.2004.012308.x. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E, Leferovich J, Bedelbaeva K, Gourevitch D, Clark L. The scarless heart and the MRL mouse. Philos Trans R Soc Lond B Biol Sci. 2004b;359(1445):785–793. doi: 10.1098/rstb.2004.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber-Katz E, Leferovich J, Bedelbaeva K, Gourevitch D, Clark L. Conjecture: Can continuous regeneration lead to immortality?. Studies in the MRL mouse. Rejuvenation Res. 2006;9(1):3–9. doi: 10.1089/rej.2006.9.3. [DOI] [PubMed] [Google Scholar]
- Heydemann A, Ceco E, Lim JE, Hadhazy M, Ryder P, Moran JL, Beier DR, Palmer AA, McNally EM. Latent TGF-beta-binding protein 4 modifies muscular dystrophy in mice. J Clin Invest. 2009;119(12):3703–3712. doi: 10.1172/JCI39845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Cervantes RB, Tichy E, Tischfield JA, Stambrook PJ. Protecting genomic integrity in somatic cells and embryonic stem cells. Mutat Res. 2007;614(1–2):48–55. doi: 10.1016/j.mrfmmm.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994;107(Pt 5):1159–1167. doi: 10.1242/jcs.107.5.1159. [DOI] [PubMed] [Google Scholar]
- Ito MR, Ono M, Itoh J, Nose M. Bone marrow cell transfer of autoimmune diseases in a MRL strain of mice with a deficit in functional Fas ligand: dissociation of arteritis from glomerulonephritis. Pathol Int. 2003;53(8):518–524. doi: 10.1046/j.1440-1827.2003.01516.x. [DOI] [PubMed] [Google Scholar]
- Kench JA, Russell DM, Fadok VA, Young SK, Worthen GS, Jones-Carson J, Henson JE, Henson PM, Nemazee D. Aberrant wound healing and TGF-beta production in the autoimmune-prone MRL/+ mouse. Clin Immunol. 1999;92(3):300–310. doi: 10.1006/clim.1999.4754. [DOI] [PubMed] [Google Scholar]
- Leader B, Leder P. Formin-2, a novel formin homology protein of the cappuccino subfamily, is highly expressed in the developing and adult central nervous system. Mech Dev. 2000;93(1–2):221–231. doi: 10.1016/s0925-4773(00)00276-8. [DOI] [PubMed] [Google Scholar]
- Leferovich JM, Bedelbaeva K, Samulewicz S, Zhang XM, Zwas D, Lankford EB, Heber-Katz E. Heart regeneration in adult MRL mice. Proc Natl Acad Sci USA. 2001;98(17):9830–9835. doi: 10.1073/pnas.181329398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mohan S, Gu W, Baylink DJ. Analysis of gene expression in the wound repair/regeneration process. Mammalian Genome. 2001;12:52–59. doi: 10.1007/s003350010230. [DOI] [PubMed] [Google Scholar]
- Li X, Mohan S, Gu W, Miyakoshi N, Baylink DJ. Differential protein profile in the ear-punched tissue of regeneration and non-regeneration strains of mice: a novel approach to explore the candidate genes for soft-tissue regeneration. Biochim Biophys Acta. 2000;1524(2–3):102–109. doi: 10.1016/s0304-4165(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Liu H, Ding Q, Yang K, Zhang T, Li G, Wu G. Meta-analysis of systemic lupus erythematosus and the risk of cervical neoplasia. Rheumatology. 2011;50(2):343–348. doi: 10.1093/rheumatology/keq304. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skeletal muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masinde G, Li X, Baylink DJ, Nguyen B, Mohan S. Isolation of wound healing/regeneration genes using restrictive fragment differential display-PCR in MRL/MPJ and C57BL/6 mice. Biochem Biophys Res Commun. 2005;330(1):117–122. doi: 10.1016/j.bbrc.2005.02.143. [DOI] [PubMed] [Google Scholar]
- Masinde GL, Li X, Gu W, Davidson H, Mohan S, Baylink DJ. Identification of wound healing/regeneration quantitative trait loci (QTL) at multiple time points that explain seventy percent of variance in (MRL/MpJ and SJL/J) mice F2 population. Genome Res. 2001;11(12):2027–2033. doi: 10.1101/gr.203701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrearty BA, Clark LD, Zhang XM, Blankenhorn EP, Heber-Katz E. Genetic analysis of a mammalian wound-healing trait. Proc Natl Acad Sci USA. 1998;95(20):11792–11797. doi: 10.1073/pnas.95.20.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe AD, Willis H, Beare A, Ferguson MW. Characterizing regeneration in the vertebrate ear. J Anat. 2006;209(4):439–446. doi: 10.1111/j.1469-7580.2006.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36(6):1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Moseley FL, Faircloth ME, Lockwood W, Marber MS, Bicknell KA, Valasek P, Brooks G. Limitations of the MRL mouse as a model for cardiac regeneration. J Pharm Pharmacol. 2011;63(5):648–656. doi: 10.1111/j.2042-7158.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- Namazi MR, Fallahzadeh MK, Schwartz RA. Strategies for prevention of scars: what can we learn from fetal skin? Int J Dermatol. 2011;50(1):85–93. doi: 10.1111/j.1365-4632.2010.04678.x. [DOI] [PubMed] [Google Scholar]
- Naseem RH, Meeson AP, Michael Dimaio J, White MD, Kallhoff J, Humphries C, Goetsch SC, DeWindt LJ, Williams MA, Garry MG. Reparative myocardial mechanisms in adult C57BL/6 and MRL mice following injury. Physiol Genomics. 2007;30(1):44–52. doi: 10.1152/physiolgenomics.00070.2006. [DOI] [PubMed] [Google Scholar]
- Naviaux RK, Le TP, Bedelbaeva K, Leferovich J, Gourevitch D, Sachadyn P, Zhang XM, Clark L, Heber-Katz E. Retained features of embryonic metabolism in the adult MRL mouse. Mol Genet Metab. 2009;96(3):133–144. doi: 10.1016/j.ymgme.2008.11.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YS, Thomson LE, Fishbein MC, Berman DS, Sharifi B, Chen PS. Scar formation after ischemic myocardial injury in MRL mice. Cardiovascular Pathology. 2004;13:203–206. doi: 10.1016/j.carpath.2004.03.610. [DOI] [PubMed] [Google Scholar]
- Peled ZM, Phelps ED, Updike DL, Chang J, Krummel TM, Howard EW, Longaker MT. Matrix metalloproteinases and the ontogeny of scarless repair: the other side of the wound healing balance. Plast Reconstr Surg. 2002;110(3):801–811. doi: 10.1097/00006534-200209010-00013. [DOI] [PubMed] [Google Scholar]
- Peng SL, Madaio MP, Craft J. Systemic autoimmunity in LG/J mice. Immunol Lett. 1996;53(2–3):153–155. doi: 10.1016/s0165-2478(96)02616-8. [DOI] [PubMed] [Google Scholar]
- Potter PK, Cortes-Hernandez J, Quartier P, Botto M, Walport MJ. Lupus-prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. J Immunol. 2003;170:3223–3232. doi: 10.4049/jimmunol.170.6.3223. [DOI] [PubMed] [Google Scholar]
- Rajnoch C, Ferguson S, Metcalfe AD, Herrick SE, Willis HS, Ferguson MW. Regeneration of the ear after wounding in different mouse strains is dependent on the severity of wound trauma. Developmental Dynamics. 2003;226:388–397. doi: 10.1002/dvdy.10242. [DOI] [PubMed] [Google Scholar]
- Rao N, Jhamb D, Milner DJ, Li B, Song F, Wang M, Voss SR, Palakal M, King MW, Saranjami B, Nye HLD, Cameron J, Stocum DL. Proteomic analysis of blastema formation in regenerating axolotl limbs. BMC Biol. 2009;7(1):83. doi: 10.1186/1741-7007-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey TE, Murry CE. Absence of regeneration in the MRL/MpJ mouse heart following infarction or cryoinjury. Cardiovascular Pathology. 2008;17:6–13. doi: 10.1016/j.carpath.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456(7221):502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachadyn P, Zhang XM, Clark LD, Naviaux RK, Heber-Katz E. Naturally occurring mitochondrial DNA heteroplasmy in the MRL mouse. Mitochondrion. 2008;8(5–6):358–366. doi: 10.1016/j.mito.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S. Yin and yang in cytokine regulation of corneal wound healing: roles of TNF-alpha. Cornea. 2007;26(Supplement 1):S70–S74. doi: 10.1097/ICO.0b013e31812f6d14. [DOI] [PubMed] [Google Scholar]
- Santiago-Raber ML, Lawson BR, Dummer W, Barnhouse M, Koundouris S, Wilson CB, Kono DH, Theofilopoulos AN. Role of cyclin kinase inhibitor p21 in systemic autoimmunity. J Immunol. 2001;167:4067–4074. doi: 10.4049/jimmunol.167.7.4067. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Stocum DL. The urodele limb regeneration blastema. Determination and organization of the morphogenetic field. Differentiation. 1984;27(1–3):13–28. doi: 10.1111/j.1432-0436.1984.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Stocum DL, Crawford K. Use of retinoids to analyze the cellular basis of positional memory in regenerating amphibian limbs. Biochemistry and Cell Biology. 1987;65:750–761. doi: 10.1139/o87-098. [DOI] [PubMed] [Google Scholar]
- Tassava RA. Limb regeneration to digit stages occurs in well-fed adult newts after hypophysectomy. J Exp Zool. 1983;225(3):433–441. doi: 10.1002/jez.1402250311. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–290. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Thuret S, Toni N, Aigner S, Yeo GW, Gage FH. Hippocampus-dependent learning is associated with adult neurogenesis in MRL/MpJ mice. Hippocampus. 2009;19(7):658–669. doi: 10.1002/hipo.20550. [DOI] [PubMed] [Google Scholar]
- Tolba RH, Schildberg FA, Decker D, Abdullah Z, Buttner R, Minor T, von Ruecker A. Mechanisms of improved wound healing in Murphy Roths Large (MRL) mice after skin transplantation. Wound Repair Regen. 2010;18(6):662–670. doi: 10.1111/j.1524-475X.2010.00631.x. [DOI] [PubMed] [Google Scholar]
- Torres VE, Leof EB. Fibrosis, regeneration, and aging: playing chess with evolution. J Am Soc Nephrol. 2011;22(8):1393–1396. doi: 10.1681/ASN.2011060603. [DOI] [PubMed] [Google Scholar]
- Ueno M, Lyons BL, Burzenski LM, Gott B, Shaffer DJ, Roopenian DC, Shultz LD. Accelerated wound healing of alkali-burned corneas in MRL mice is associated with a reduced inflammatory signature. Invest Ophthalmol Vis Sci. 2005;46(11):4097–4106. doi: 10.1167/iovs.05-0548. [DOI] [PubMed] [Google Scholar]
- Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, Cordero K, Bedelbaeva K, Gourevitch D, Heber-Katz E. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biology. 2010;29:690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Wandstrat A, Wakeland E. The genetics of complex autoimmune diseases: non-MHC susceptibility genes. Nat Immunol. 2001;2(9):802–809. doi: 10.1038/ni0901-802. [DOI] [PubMed] [Google Scholar]
- Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 2008;58(3):744–753. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]
- Watson ML, Rao JK, Gilkeson GS, Ruiz P, Eicher EM, Pisetsky DS, Matsuzawa A, Rochelle JM, Seldin MF. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med. 1992;176(6):1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby DJ, Ferguson MW. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991;147(1):207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. Journal of Bone and Mineral Research. 2009;24:171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Christmas P, Wu XR, Wewer UM, Engvall E. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc Natl Acad Sci USA. 1994;91(12):5572–5576. doi: 10.1073/pnas.91.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Baylink DJ, Masinde GL, Li R, Nguyen B, Davidson HM, Xu S, Mohan S. Mouse chromosome 9 quantitative trait loci for soft tissue regeneration: congenic analysis and fine mapping. Wound Repair and Regeneration. 2007;15:922–927. doi: 10.1111/j.1524-475X.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- Yu H, Mohan S, Masinde GL, Baylink DJ. Mapping the dominant wound healing and soft tissue regeneration QTL in MRL x CAST. Mammalian Genome. 2005;16:918–924. doi: 10.1007/s00335-005-0077-0. [DOI] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8(3):277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv E, Hu D. Genetic variation in insulin/IGF-1 signaling pathways and longevity. Ageing Res Rev. 2011;10(2):201–204. doi: 10.1016/j.arr.2010.09.002. [DOI] [PubMed] [Google Scholar]