Abstract
Moscatilin, a bibenzyl derivative from the orchid Dendrobium loddigesii, has been shown to possess anticancer activity. We examined the effect of moscatilin on human esophageal cancer cells, including squamous cell carcinoma (SCC) and adenocarcinoma (ADC) cells and its possible mechanisms. Moscatilin suppressed the growth of both the histological cell lines in a dose- and time-dependent manner. Morphological changes indicative of apoptosis and mitotic catastrophe were observed following moscatilin treatment. The population of cells in the sub-G1 phase and polyploidy phase significantly increased after treatment. Immunofluorescence revealed multipolar mitosis and subsequent multinucleation in moscatilin-treated cells, indicating the development of mitotic catastrophe. Western blot showed a marked increase in expressions of polo-like kinase 1 and cyclin B1 after exposure to moscatilin. In conclusion, moscatilin inhibits growth and induces apoptosis and mitotic catastrophe in human esophageal SCC- and ADC-derived cell lines, indicating that moscatilin has broad potential against esophageal cancer.
Key Words: esophageal cancer, moscatilin, polyploidization, sub-G1
Introduction
Chinese herbal medicine is widely used by skilled traditional Chinese medicine (TCM) practitioners, not only for primary health care but also cancer treatment for centuries. Nowadays, several natural compounds in cancer treatment (e.g., camptothecin derivatives, vinca alkaloids) come from Chinese herbal medicine and a half of the anticancer drugs approved are either natural products or their derivatives.1 Dendrobium species are widely used in TCM for fever reduction, body fluid replenishment, and as a tonic to nourish the gastrointestinal system. In addition, modern evidence suggests that several derivatives from dendrobium possess various pharmacological and biological activities, for instance, denbinobin inhibits human gastric cancer cells.2
Moscatilin (4,4′-dihydroxy-3,3′,5-trimethoxybibenzyl, C17H20O5) is a bibenzyl derivative from the Indian orchid Dendrobium moscatum and the stem of Dendrobium loddigesii.3,4 Previous reports have demonstrated that this compound inhibits platelet aggregation,5 exerts potent cytotoxic effects against stomach and lung cancer, and has antiproliferative effects in many cell lines derived from solid tumors. In addition, it has recently been demonstrated that moscatilin induces cell cycle arrest at the G2/M phase in human colorectal cancer cells.3 However, the effectiveness of and the underlying molecular response to moscatilin in esophageal cancer remain largely unknown.
Esophageal cancer is associated with a high mortality rate, with average 5-year survival rates not exceeding 25%.6 Locally advanced esophageal carcinoma is often refractory to current therapeutic approaches and its prognosis is poor.7,8 Patients with unresectable or inoperable disease are usually treated with chemotherapy and radiotherapy. Although various chemotherapy regimens are available, esophageal cancer carries a very poor prognosis, with a mean survival time of <8.1 months when treated with single agent or with combination therapy.9 Therefore, novel and potent compounds that can control or ameliorate both local and distant tumor progression in patients with esophageal cancer are urgently needed.
The incidence of esophageal adenocarcinoma (ADC) has risen rapidly over the past 25 years in the United States as well as in several Western European countries. In contrast, the major cell type of esophageal cancer in Asian countries is squamous cell carcinoma (SCC). Therefore, in the present study, we examined inhibitory effects of moscatilin against esophageal cancer using an SCC-derived cell line (CE81T/VGH) and an ADC-derived cell line (BE3) to cover interracial discrepancy.
Mitotic catastrophe, a type of mammalian cell death resulting from aberrant mitosis,10 is characterized by the presence of multiple micronuclei. Mitotic catastrophe as a prestage to necrosis is characterized by the presence of mitochondria and cell membrane swelling or rupture and organelle degradation.11 When damage to the mitotic apparatus is excessive, checkpoints will eventually arrest the cell cycle in the G2-M phase, thereby preventing a cell from entering into mitosis with broken or under-repaired DNA. Failure to arrest these cells at or before mitosis results in the formation of multinucleated, giant cells that contain abnormal nuclei, one of the most prominent morphological characteristics of mitotic catastrophe,12 which in turn leads to mitotic death. Studies have shown that chemotherapy and radiation therapy can induce mitotic catastrophe.13,14
In this study, we examined the cytotoxic effects of moscatilin in vitro using two esophageal cancer cell lines, one derived from SCC (cell line CE81T/VGH) and one from ADC (cell line BE3), to cover interracial discrepancy. Specifically, we examined the underlying mechanism governing moscatilin-induced growth inhibition and cell cycle arrest.
Materials and Methods
Chemicals
Moscatilin was isolated and characterized according to our previous report4 and dissolved in dimethyl sulfoxide (DMSO). Various concentrations were prepared by serially diluting the stock solution.
Cell culture
The cell lines used in this study included CE81T/VGH, an esophageal SCC cell line, and BE3, an ADC cell line. CE81T/VGH was kindly provided by Professor Cheng-Po Hu (Department of Life Science, Tunghai University, Taichung, Taiwan). Cell lines were maintained in the Dulbecco's modified Eagle's medium (Biosource) containing 10% heat-inactivated fetal bovine serum (Biological Industries) at 37°C in a humidified incubator containing 5% CO2 and 95% air. The cells were passaged every 2–3 days upon reaching 80% confluence and were maintained in exponential growth.
Cell viability
Cells were harvested 24–72 h after treatment with various concentrations of moscatilin. The trypan blue dye exclusion test was used to determine the cell viability. Viable cells were identified by dye exclusion and dead cells were characterized by the uptake of dye. All observations were made using an inverted light microscope (Nikon Eclipse TS100).
Morphology (Liu's and immunofluorescence stains)
After treatment with moscatilin or vehicle alone, cells were collected, cytospum onto a slide, and stained with Liu's dye to observe morphological changes under an Olympus light microscope at a magnification of 400× or 1000×.
For immunofluorescence staining, treated cells were fixed in 4% paraformaldehyde for 10 min, and then permeabilized in 1% Triton-X-100 in phosphate-buffered saline (PBS) for 5 min. After washing with PBS, cells were exposed to 10% bovine serum albumin (BSA) in PBS before incubation with an anti-α-tubulin monoclonal antibody (1:100 dilution) (mAb; Zymed Laboratories) and a nondiluted mouse anti-γ-tubulin antibody in 2% BSA/PBS. After overnight primary antibody incubation, slides were rinsed with 1% BSA/PBS and then exposed to the secondary antibody with fluorescent isothiocyanate (FITC)-conjugated donkey anti-mouse IgG (1:100 dilution; Jackson ImmunoResearch Laboratories, Inc.). Cells were then incubated in Hoechst 33342 (Sigma-Aldrich) to localize cell nuclei. Slides were observed using an Axiophot fluorescent microscope (Carl Zeiss) equipped with a digital camera system (Carl Zeiss AxioCam HRm, v.2.0, black-white version). Experiments were repeated at least three times.
Cell cycle analysis and measurement of phosphorylated histone H3
After 3, 4, 16, 24, 48, and 72 h of treatment with 1.25, 2.5, 5, 10, and 20 μM moscatilin, cells were trypsinized, washed, centrifuged, and adjusted to 1×105 cells/mL. The cells were harvested, fixed at 4°C for 1 h with 70% ethanol, and then stained with a propidium iodide (PI) solution (PI, 0.5 mg/mL; RNase, 0.1 mg/mL) contained in a CycleTEST plus DNA reagent kit (Becton Dickinson) for 30 min. At least 10,000 cells were collected and analyzed by a FACSCalibur flow cytometer (Becton Dickinson). Cell cycle distribution was calculated using ModFIT cell cycle analysis software (Version 2.01.2; Becton Dickinson).
Cells were harvested after moscatilin treatment at various indicated times and costained according to the manufacturer's instructions with the phospho-histone H3 (Serine10) antibody, secondary antibody labeled with FITC (Cell Signaling Technology), and PI. The percentage of mitotic cells was determined by fluorescence-activated cell sorting analysis.
Apoptosis assay
To assess the development of apoptosis, cells were stained with Annexin V- FITC conjugate and PI. Viable cells do not take up either dye (FITC−/PI−), whereas cells undergoing early apoptosis reveal green fluorescence (FITC+/PI−), and cells in the late apoptotic phase take up both FITC and PI exhibiting both green and red fluorescence (FITC+/PI+). For this assay, at different designated times, cells were washed, and resuspended in 100 μL of Annexin-V binding buffer, and then incubated with 5 μL of FITC-conjugated Annexin-V (TACS Annexin-V-FITC Apoptosis Detection Kit; R&D Systems) for 15 min at room temperature. Then, 400 μL of ice-cold 1× binding buffer containing PI was added followed by fluorocytometric analysis.
Caspase substrate activity assay
After being treated by moscatilin, both cell types were harvested, washed, and counted. Then, the cells were lysed in the Triton X-100 buffer and incubated on ice for 10 min. Cell lysates were mixed with 50 μL of 2× reaction buffer, 1 μL 1,4-dithiothreitol, 5 μL of 1 mM AFC-conjugated substrates, and caspase substrates for 1 h at 37°C in the dark. Subsequently, the caspase activity was assayed by caspase fluorometric substrate set II plus (Medical & Biological Laboratories). A spectrofluorometer with a 400 nm excitation wavelength and a 505 nm emission filter were used for analysis. The degree of increased caspase activity was defined by a comparison with a vehicle control.
Western blot
Western blot analysis was performed using standard procedures for whole-cell extracts from cell lines. Lysates were prepared using the radioimmunoprecipitation assay buffer (Sigma-Aldrich). Equal amounts of protein lysates (50–100 μg) were separated by SDS-PAGE on 10% gels, electrotransferred to Immobilon-P membranes (Millipore), and probed with the indicated primary antibody. Antibodies used included Cdc25C (Abcam), phospho-Cdc2 (Tyr15; Cell Signaling), cyclin B1 (Santa Cruz Biotechnology), phospho-Cdc25C (Ser216; Cell Signaling), Chk1 (Santa Cruz Biotechnology), Cdc2 p34 (Santa Cruz Biotechnology), polo-like kinase 1 (Plk1; Abcam), survivin (R&D Systems), and β-actin (Sigma-Aldrich).
Statistical analysis
The results are expressed as the mean±standard deviation collected from at least three separate experiments. The Student's t-test was used for comparison and a P value of less than .05 was considered statistically significant.
Results
Moscatilin inhibits cellular growth of esophageal cancer cell lines
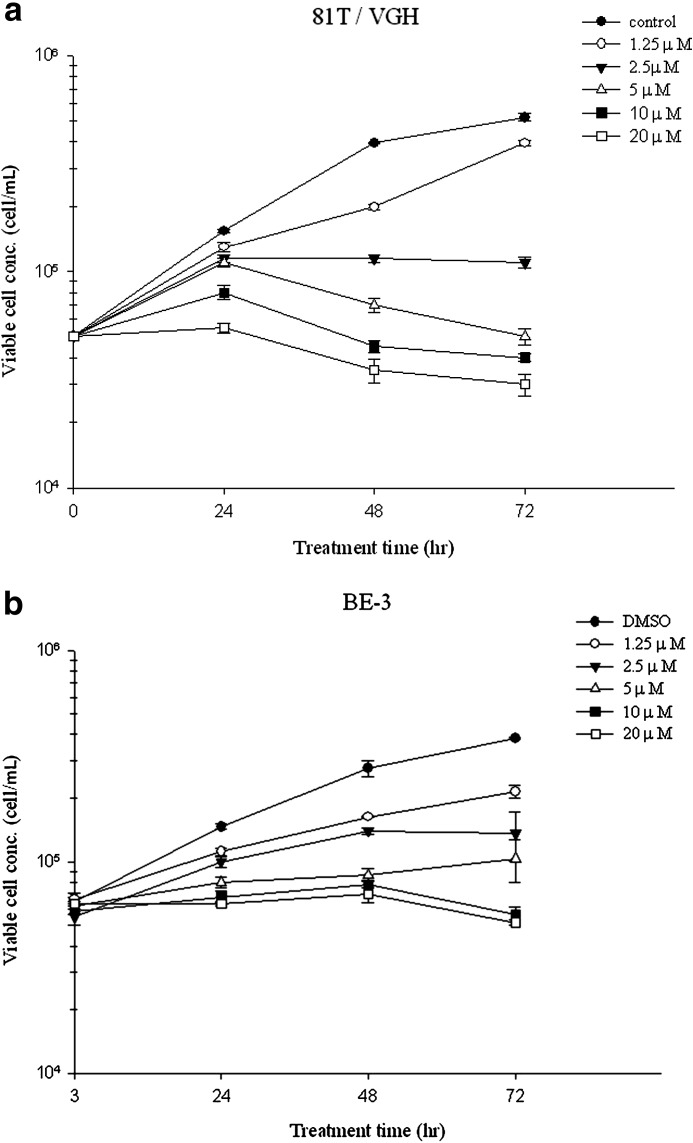
All cell lines were treated with 1.25, 2.5, 5, 10, and 20 μM of moscatilin for 24, 48, and 72 h. Trypan blue exclusion staining revealed that moscatilin induced a dose- and time-dependent reduction in viability (Fig. 1a, b). The IC50 at 24 h was ∼7.0 μM for CE81T/VGH and 6.7 μM for BE3.
FIG. 1.
Cell viability of (a) 81T/VGH and (b) BE3 cells treated with dimethyl sulfoxide (DMSO; control) and 1.25–20 μM moscatilin after 24–72 h. Data from three separate experiments are expressed as mean±standard deviation.
Moscatilin induces morphological changes in BE3 cells
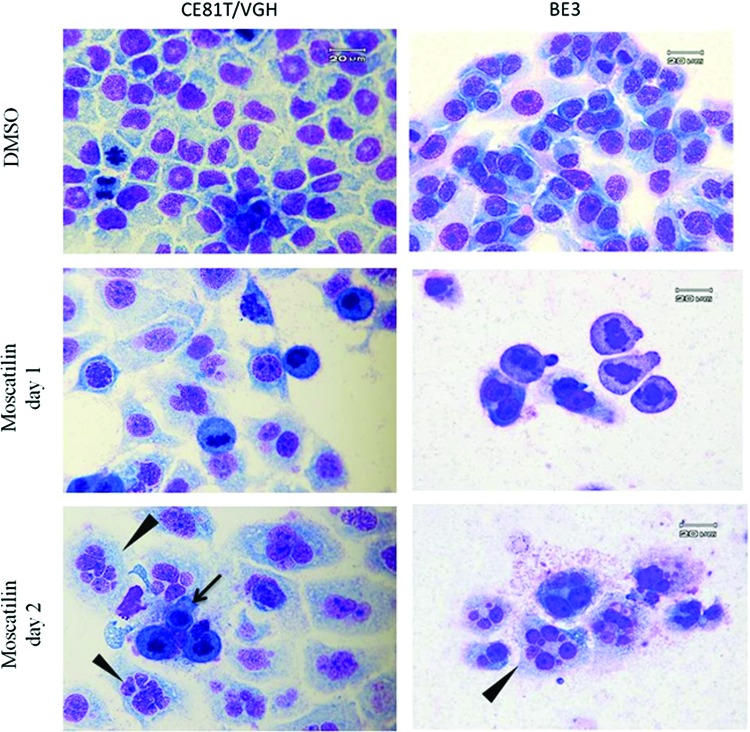
We first examined the changes in morphology after 1.25–5 μM moscatilin treatment using Liu's dye. After 24 h of treatment, there was an increase in the population of cells with chromatin condensation in moscatilin-treated CE81T/VGH and BE3 cells. At day 2 after the treatment, a different population of giant cells containing two or more uncondensed nuclei and micronuclei was observed, indicating mitotic catastrophe (Fig. 2). A few apoptotic cells were also noted.
FIG. 2.
Morphology of esophageal cancer cells with various treatments. Following DMSO and moscatilin treatments (1.25 μM for CE81T/VGH and 5 μM for BE3 cells), cells were stained with Liu's dye for morphological examination. The arrow indicates apoptotic cells, and the arrowhead indicates cells with mitotic catastrophe. Data shown are representative of three independent experiments. Color images available online at www.liebertpub.com/jmf
Moscatilin induces sub-G1 accumulation and polyploidization
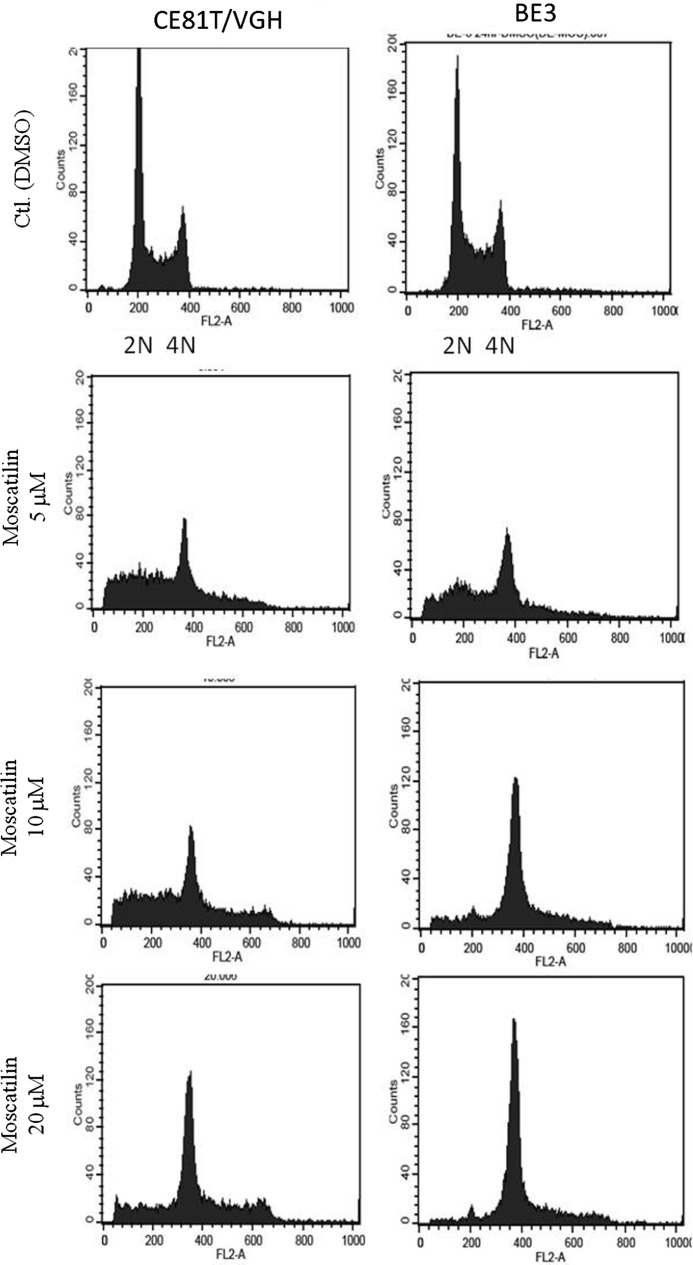
A DNA histogram was generated to assay the changes in the nuclear content and cell cycle distribution. Moscatilin treatment resulted in the accumulation of hypoploidal (sub-G1 population) and polyploidal cells (≥4 N) in both cell lines (Fig. 3). In addition, after exposure to doses greater than 5 μM for 24–72 h ∼20–50% of CE81T/VGH cells were hypoploidal indicating that cells were undergoing apoptosis (Table 1). A similar finding was noted in BE3 cells, although the percentage of cells in a hypoploidal state was lower. Another polyploidal population was noted following treatment with moscatilin in both cell lines but particularly in BE3 cells, where the percentage was almost three times higher than in the control group.
FIG. 3.
Cell cycle analysis of CE81T/VGH and BE3 cells treated with DMSO, 5, 10, and 20 μM moscatilin for 24 h.
Table 1.
Effects of Dimethyl Sulfoxide and Moscatilin on the Cell Cycle of CE81T/VGH and BE3 Cells
| |
Percentage of cells (mean±SD) |
|
|---|---|---|
| Sub-G1 | Polyploid | |
| CE81T/VGH | ||
| 24 h | ||
| DMSO | 0.69±0.24 | 23.62±1.24 |
| Moscatilin 5 μM | 27.77±5.29*** | 34.42±4.37 |
| Moscatilin 10 μM | 25.56±4.87*** | 41.92±7.67* |
| Moscatilin 20 μM | 13.18±2.41* | 68.94±4.60*** |
| 48 h | ||
| DMSO | 1.43±0.13 | 17.00±0.39 |
| Moscatilin 5 μM | 24.98±2.84*** | 45.92±2.92*** |
| Moscatilin 10 μM | 26.90±1.14*** | 44.12±3.64*** |
| Moscatilin 20 μM | 23.14±2.78*** | 58.86±1.78*** |
| 72 h | ||
| DMSO | 5.32±0.86 | 8.84±0.61 |
| Moscatilin 5 μM | 50.86±7.55*** | 25.40±5.30* |
| Moscatilin 10 μM | 41.54±6.14*** | 36.25±5.01*** |
| Moscatilin 20 μM | 55.59±3.50*** | 29.78±3.47*** |
| BE3 | ||
| 24 h | ||
| DMSO | 0.60±0.18 | 24.02±0.09 |
| Moscatilin 5 μM | 12.67±0.43*** | 41.46±1.64*** |
| Moscatilin 10 μM | 10.99±1.08*** | 57.73±1.38*** |
| Moscatilin 20 μM | 2.76±0.38 | 80.29±2.05*** |
| 48 h | ||
| DMSO | 0.46±0.18 | 24.56±0.39 |
| Moscatilin 5 μM | 10.37±0.96*** | 44.07±4.54*** |
| Moscatilin 10 μM | 12.08±0.50*** | 68.75±4.07*** |
| Moscatilin 20 μM | 3.14±0.22* | 88.68±0.47*** |
| 72 h | ||
| DMSO | 0.45±0.02 | 26.18±1.61 |
| Moscatilin 5 μM | 14.29±1.12*** | 43.22±2.75*** |
| Moscatilin 10 μM | 11.86±1.85*** | 64.79±3.53*** |
| Moscatilin 20 μM | 5.42±0.94*** | 84.87±1.75*** |
Compared with DMSO control, *P<.05; ***P<.001.
DMSO, dimethyl sulfoxide; SD, standard deviation.
Moscatilin causes apoptosis and increases caspase activity
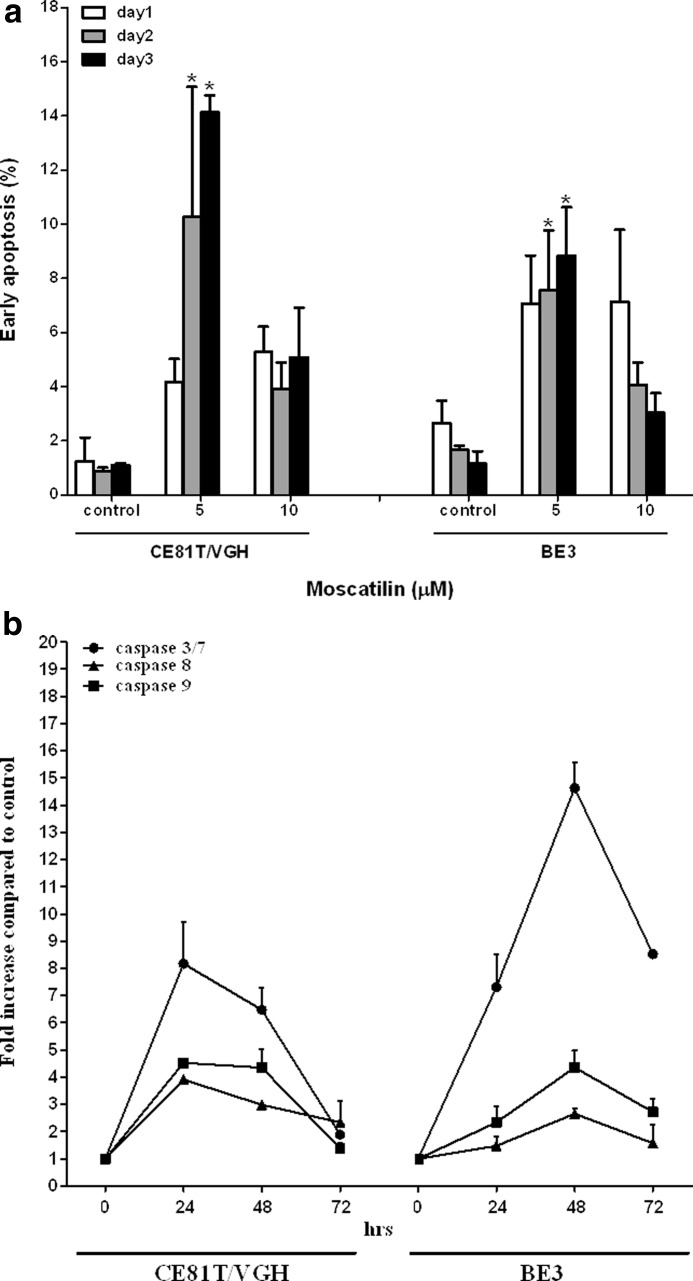
To examine whether cells undergo apoptosis, untreated or moscatilin-treated CE81T/VGH and BE3 esophageal cancer cells were stained with annexin V and PI. As shown in Figure 4a, exposure with 5, 10 μM moscatilin resulted in a higher population of early apoptotic cells (annexin-V+ and PI−). It was significantly increased in a time-dependent manner after treatment with 5 μM moscatilin in both cell lines. We also observed an increase of caspase 3/7 activity after 5 μM moscatilin treatment, which peaked at 24 h in CE81T/VGH cells and at 48 h in BE3 cells (Fig. 4b).
FIG. 4.
Effects of moscatilin on apoptosis induction and caspase activity. (a) Percentage of early apoptotic CE81T/VGH and BE3 cells after treatment with 5, 10 μM moscatilin. Annexin-V/PI staining was performed for detection of early apoptotic cells. (b) Fold increase of caspase 3/7, 8, 9 activities in the moscatilin (5 μM)-treated CE81T/VGH and BE3 cells against vehicle (DMSO)-treated cells at various time intervals. We expressed the result as means±SEM from three separate experiments. *P<.05.
Moscatilin gives rise to multipolar mitosis and mitotic catastrophe
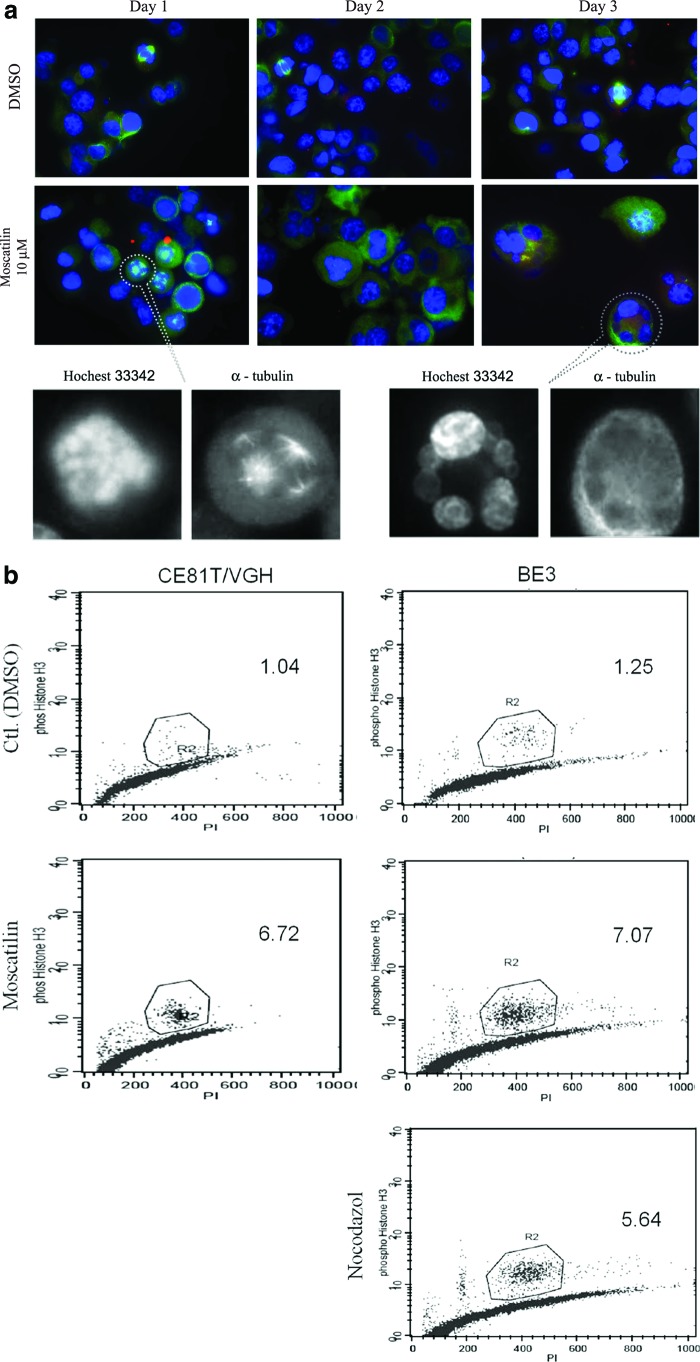
To further verify mitosis and multinucleation, moscatilin-treated BE3 cells were visualized by immunostaining with antibodies directed against α-tubulin and/or γ-tubulin counterstained with Hoechst 33342 (for DNA). Mitotic spindles of cells treated with DMSO were bipolar. In contrast, following moscatilin treatment, chromosome misalignments, aberrant spindle patterns (asymmetric dipolar spindles and/or tripolar and tetrapolar spindles), and multipolar mitoses were noted on day 1 (Fig. 5a). Another distinct feature in the moscatilin-treated BE3 cells on day 2 and 3 was a pattern of mitotic catastrophe, including micronuclei and multinucleated giant cells, which is compatible with the result obtained from Liu's staining.
FIG. 5.
After moscatilin treatment for 24, 48, and 72 h, BE3 cells were stained with an anti-α-tubulin (green) for mitotic spindles, anti-γ-tubulin (red) for centrosomes, and Hochest33342 for nuclei (blue) (a). The original magnification is 1000× for all panels. (b) Representative of flow cytometric analysis of DNA content and phospho-histone H3 levels in cells treated with the indicated concentrations of moscatilin for 24 h. Nocodazole was used as a positive control. Color images available online at www.liebertpub.com/jmf
Moscatilin increases mitotic index in BE3 cells
The accumulation of cells with polyploidy content indicates arrest of the cell cycle in either the G2 or M phase. Treated cells were stained with anti-phosphohistone H3 as a surrogate of the M phase marker. The percentage of cells classified as positive responders increased after moscatilin treatment (Fig. 5b). Expression of phospho-histone H3 varied from 1.5% to 2.0% in the control group. After treatment of BE3 cells with 2.5 and 5 μM moscatilin for 8, 16, and 24 h, the percentage of phospho-histone H3-positive cells significantly increased to 6.4% and 20.4%, respectively, indicating that the proportion of cells in the M phase had increased (Table 2).
Table 2.
Effects of Dimethyl Sulfoxide and Moscatilin on Percentage of Phospho-Histone H3-Positive Cells
| DMSO | Moscatilin 2.5 μM | Moscatilin 5 μM | Nocodazole | |
|---|---|---|---|---|
| 8 h | 2.00±0.25 | 20.41±1.06* | 19.68±022* | 15.35±0.66* |
| 16 h | 1.59±0.29 | 15.80±1.25* | 15.93±2.23* | 11.52±2.17* |
| 24 h | 1.52±0.15 | 7.23±0.74* | 6.87±0.87* | 6.46±0.54* |
Values are presented as mean±SD.
Compared with DMSO control, *P<.05.
Moscatilin changes the expression of protein related to mitotic catastrophe
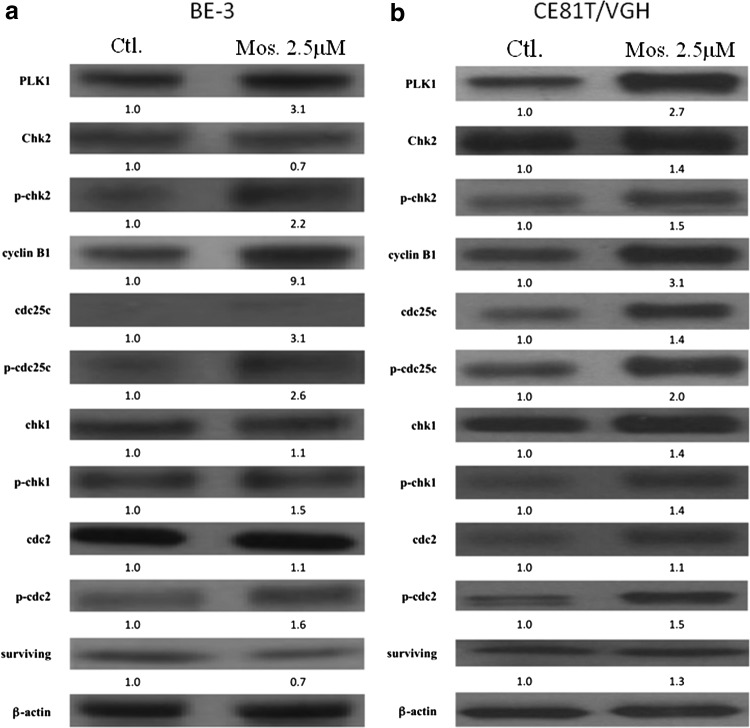
To examine the molecular mechanisms mediating the effects of moscatilin on growth suppression and mitotic catastrophe, we analyzed the expression of cell cycle regulatory proteins related to mitotic catastrophe. Moscatilin treatment resulted in the upregulation of Plk1 and cyclin B1 protein expression in both cell lines (Fig. 6a, b). In addition, a slight increase in Cdc25c phosphorylation was also noted in both cell lines.
FIG. 6.
Moscatilin regulated mitotic catastrophe-associated proteins. BE3 (a) and 81T/VGH (b) cells were treated with moscatilin (Mos.) or DMSO (Ctl.), lysed at the indicated time point, and subjected to immunoblot with antibodies against Cdc25C, phospho-Cdc2 (Tyr15), cyclin B1, phospho-Cdc25C (Ser216), Chk1, Cdc2, Plk1, and survivin. β-actin was used for normalization. Data are representative of at least three different experiments.
Discussion
Moscatilin has been shown to be a potentially effective anticancer agent in stomach and colorectal cancer cells. To the best of our knowledge, this study is the first to explore the efficacy of and molecular mechanisms governing moscatilin treatment in esophageal carcinoma cell lines. We found that moscatilin suppressed the growth of SCC-derived CE81T/VGH cells and ADC-derived BE3 cells in a dose- and time-dependent manner. Moscatilin-treated cells displayed significant morphological changes characteristic of apoptosis and mitotic catastrophe. Treatment with moscatilin also triggered hypo- and polyploid accumulation in both cell lines. The moscatilin-induced mitotic catastrophe was accompanied by G2/M arrest. Furthermore, the putative mechanism of action might involve the proteins regulating mitosis.
The induction of mitotic cell arrest has proven useful in cancer treatment. Cells stuck in mitosis will ultimately either undergo apoptosis or exit mitosis.15,16 When cells exit mitosis, they escape apoptosis without division (mitotic slippage), resulting in the formation of multinucleated cells, microtubule misalignment, multipolar mitoses, and aneuploidy leading to eventual cell death. Ionizing radiation and several antimitotic chemotherapeutic agents elicit cell death or growth arrest by inducing mitotic arrest or mitotic slippage.17 Mitotic arrest is thought to be a major factor influencing the cytotoxicity of paclitaxel (Taxol) and the vinca alkaloids.18 In our study, moscatilin treatment resulted in mitotic arrest within 24 h. The number of cells in mitotic arrest decreased and the number of polyploidal cells increased after exposure for 48 and 72 h. These results suggest mitotic arrest is critical for moscatilin toxicity. Furthermore, cells in the G2/M phase are particularly susceptible to radiation. Whether moscatilin is an effective radiation sensitizer warrants further study.
Mitotic catastrophe is used to delineate cell death occurring during or after a faulty mitosis. Ionizing radiation and various antitumor drugs have been described to induce mitotic catastrophe as the principal form of cell death.13,19 Eriksson et al. showed that irradiation of HeLa Hep2 cells resulted in transient G2-M arrest and that premature re-entry into the cell cycle induced several mitotic disturbances, including an increase of anaphase bridges, lagging chromosomal material, and multipolar mitotic spindles. Chemotherapy has been shown to induce aberrant mitosis or accumulation of deficiencies in the mitotic spindle checkpoint.20,21 These perturbations cease in polyploid cells together with the formation of giant cells containing multiple nuclei. However, some studies show that mitotic catastrophe can be followed by apoptosis. Whether mitotic catastrophe is a specific death process or functions as a trigger for apoptosis is still debatable.22 In our study, immunofluorescence revealed aberrant mitosis in moscatilin-treated cells; however, no apoptotic features were visible in Hoechst 33342-stained cells. In contrast, giant cells with multinucleation were observed, indicating mitotic catastrophe. Based on our findings, we support the hypothesis that mitotic catastrophe is a type of cell death. Generally, cells can be either apoptosis prone or apoptosis reluctant23,24 and induction of mitotic catastrophe has been exploited as a means of eradicating apoptosis-resistant cancer cells.25 Several antitumor drugs such as doxorubicin and oxaliplatin showed a similar tendency.26,27 Our results suggest that moscatilin, perhaps, also has the potential to induce either apoptosis or cell death through mitotic catastrophe in different cell types.
Mammalian cells have evolved surveillance checkpoints to monitor the structure of chromosomes and coordinate repair and cell cycle progression. Cells can recover from DNA damage-induced arrest once the damage has been repaired. Plk1 regulates cell cycle progression after damage-induced G2/M arrest by activating Cdk1.28 Following moscatilin treatment, we found that the mitotic index increased in BE3 cells within 24 h and that the protein expression of Plk1 and cyclin B1 was upregulated during this period. At the same time, immunofluorescence staining of DNA, α-tubulin, and γ-tubulin showed incremental increases in multipolar mitosis, improper chromosome alignment, and improper chromosome segregation, all of which are characteristics of aberrant mitosis. Afterward, mitotic arrest disappeared followed by the presence of multinucleation at day 2 and 3. A previous study also noted that the overproduction of Plk1 protein in HeLa cells delayed the progression through mitosis and disturbed the completion of mitosis and cytokinesis, thereby giving rise to multinucleation.29 Disabling of mitotic checkpoint signaling due to overexpression of Plk1 has been shown to lead to immature cell division without proper chromosome alignment and segregation.30 In addition, mitotic entry in the presence of DNA damage has been demonstrated to lead to cell death through mitotic catastrophe.31 The roles Plk1 plays in cancer treatment warrant more exploration.
In conclusion, our results demonstrate that moscatilin can cause apoptosis and mitotic catastrophe in different types of esophageal cancer. The mechanism underlying this effect involves early promotion of M phase cell cycle blockade and premature release of damaged cells back into the cell cycle.
Acknowledgments
This work was supported by grant MMH-9637 from the Mackay Memorial Hospital and grant NSC-95-2314-B-195-014 from the National Science Council, Taiwan.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Newman DJ. Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 2.Song JI. Kang YJ. Yong HY. Kim YC. Moon A. Denbinobin, a phenanthrene from dendrobium nobile, inhibits invasion. Oncol Rep. 2012;27:813–818. doi: 10.3892/or.2011.1551. [DOI] [PubMed] [Google Scholar]
- 3.Chen TH. Pan SL. Guh JH, et al. Moscatilin induces apoptosis in human colorectal cancer cells: a crucial role of c-Jun NH2-terminal protein kinase activation caused by tubulin depolymerization and DNA damage. Clin Cancer Res. 2008;14:4250–4258. doi: 10.1158/1078-0432.CCR-07-4578. [DOI] [PubMed] [Google Scholar]
- 4.Ho CK. Chen CC. Moscatilin from the orchid Dendrobrium loddigesii is a potential anticancer agent. Cancer Invest. 2003;21:729–736. doi: 10.1081/cnv-120023771. [DOI] [PubMed] [Google Scholar]
- 5.Chen CC. Wu LG. Ko FN. Teng CM. Antiplatelet aggregation principles of Dendrobium loddigesii. J Nat Prod. 1994;57:1271–1274. doi: 10.1021/np50111a014. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A. Siegel R. Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 7.Bedenne L. Michel P. Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 8.Kranzfelder M. Buchler P. Lange K. Friess H. Treatment options for squamous cell cancer of the esophagus: a systematic review of the literature. J Am Coll Surg. 2010;210:351–359. doi: 10.1016/j.jamcollsurg.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Chiarion-Sileni V. Corti L. Ruol A, et al. Phase II trial of docetaxel, cisplatin and fluorouracil followed by carboplatin and radiotherapy in locally advanced oesophageal cancer. Br J Cancer. 2007;96:432–438. doi: 10.1038/sj.bjc.6603585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roninson IB. Broude EV. Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat. 2001;4:303–313. doi: 10.1054/drup.2001.0213. [DOI] [PubMed] [Google Scholar]
- 11.Okada H. Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 12.Waldman T. Lengauer C. Kinzler KW. Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson D. Lofroth PO. Johansson L. Riklund KA. Stigbrand T. Cell cycle disturbances and mitotic catastrophes in HeLa Hep2 cells following 2.5 to 10 Gy of ionizing radiation. Clin Cancer Res. 2007;13:5501s–5508s. doi: 10.1158/1078-0432.CCR-07-0980. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M. Bastians H. Mitotic drug targets and the development of novel anti-mitotic anticancer drugs. Drug Resist Updat. 2007;10:162–181. doi: 10.1016/j.drup.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Jordan MA. Wendell K. Gardiner S. Derry WB. Copp H. Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56:816–825. [PubMed] [Google Scholar]
- 16.Woods CM. Zhu J. McQueney PA. Bollag D. Lazarides E. Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol Med. 1995;1:506–526. [PMC free article] [PubMed] [Google Scholar]
- 17.Rieder CL. Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Jordan MA. Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 19.Strauss SJ. Higginbottom K. Juliger S, et al. The proteasome inhibitor bortezomib acts independently of p53 and induces cell death via apoptosis and mitotic catastrophe in B-cell lymphoma cell lines. Cancer Res. 2007;67:2783–2790. doi: 10.1158/0008-5472.CAN-06-3254. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Vargas H. Palacios J. Moreno-Bueno G. Telling cells how to die: docetaxel therapy in cancer cell lines. Cell Cycle. 2007;6:780–783. doi: 10.4161/cc.6.7.4050. [DOI] [PubMed] [Google Scholar]
- 21.Morse DL. Gray H. Payne CM. Gillies RJ. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol Cancer Ther. 2005;4:1495–1504. doi: 10.1158/1535-7163.MCT-05-0130. [DOI] [PubMed] [Google Scholar]
- 22.Castedo M. Perfettini JL. Roumier T. Andreau K. Medema R. Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 23.Blagosklonny MV. Robey R. Sheikh MS. Fojo T. Paclitaxel-induced FasL-independent apoptosis and slow (non-apoptotic) cell death. Cancer Biol Ther. 2002;1:113–117. doi: 10.4161/cbt.53. [DOI] [PubMed] [Google Scholar]
- 24.Demidenko ZN. Blagosklonny MV. Flavopiridol induces p53 via initial inhibition of Mdm2 and p21 and, independently of p53, sensitizes apoptosis-reluctant cells to tumor necrosis factor. Cancer Res. 2004;64:3653–3660. doi: 10.1158/0008-5472.CAN-04-0204. [DOI] [PubMed] [Google Scholar]
- 25.Vitale I. Galluzzi L. Castedo M. Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12:385–392. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- 26.Eom YW. Kim MA. Park SS, et al. Two distinct modes of cell death induced by doxorubicin: apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Oncogene. 2005;24:4765–4777. doi: 10.1038/sj.onc.1208627. [DOI] [PubMed] [Google Scholar]
- 27.Ngan CY. Yamamoto H. Takagi A, et al. Oxaliplatin induces mitotic catastrophe and apoptosis in esophageal cancer cells. Cancer Sci. 2008;99:129–139. doi: 10.1111/j.1349-7006.2007.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Vugt MA. van de Weerdt BC. Vader G, et al. Polo-like kinase-1 is required for bipolar spindle formation but is dispensable for anaphase promoting complex/Cdc20 activation and initiation of cytokinesis. J Biol Chem. 2004;279:36841–36854. doi: 10.1074/jbc.M313681200. [DOI] [PubMed] [Google Scholar]
- 29.Mundt KE. Golsteyn RM. Lane HA. Nigg EA. On the regulation and function of human polo-like kinase 1 (PLK1): effects of overexpression on cell cycle progression. Biochem Biophys Res Commun. 1997;239:377–385. doi: 10.1006/bbrc.1997.7378. [DOI] [PubMed] [Google Scholar]
- 30.Kops GJ. Weaver BA. Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 31.Nitta M. Kobayashi O. Honda S, et al. Spindle checkpoint function is required for mitotic catastrophe induced by DNA-damaging agents. Oncogene. 2004;23:6548–6558. doi: 10.1038/sj.onc.1207873. [DOI] [PubMed] [Google Scholar]