Abstract
Background
Women are twice as likely as men to develop Posttraumatic Stress Disorder (PTSD). Abnormal acquisition of conditioned fear has been suggested as a mechanism for the development of PTSD. While some studies of healthy humans suggest that women are either no different or express less conditioned fear responses during conditioning relative to men, differences in the acquisition of conditioned fear between men and women diagnosed with PTSD has not been examined.
Methods
Thirty-one participants (18 men; 13 women) with full or subsyndromal PTSD completed a fear conditioning task. Participants were shown computer-generated colored circles that were paired (CS+) or unpaired (CS−) with an aversive electrical stimulus and skin conductance levels were assessed throughout the task.
Results
Repeated measures ANOVA indicated a significant sex by stimulus interaction during acquisition. Women had greater differential conditioned skin conductance responses (CS + trials compared to CS− trials) than did men, suggesting greater acquisition of conditioned fear in women with PTSD.
Conclusions
In contrast to studies of healthy individuals, we found enhanced acquisition of conditioned fear in women with PTSD. Greater fear conditioning in women may either be a pre-existing vulnerability trait or an acquired phenomenon that emerges in a sex-dependent manner after the development of PTSD. Characterizing the underlying mechanisms of these differences is needed to clarify sex-related differences in the pathophysiology of PTSD.
Keywords: Sex differences, Learning, Conditioning, Fear, Posttraumatic stress disorder, Galvanic skin response
1. Introduction
Women develop posttraumatic stress disorder (PTSD) at twice the rate of men, despite greater trauma exposure in men (Adamson et al., 2008; Breslau et al., 1998). Fear conditioning has been proposed as a core process in the development of PTSD (Orr et al., 2000; Pitman et al., 2000). During traumatic stress exposure, severe and prolonged threat result in a cascade of biological stress responding (Pitman et al., 2001) leading to the release of stress hormones and neuromodulators that are believed to enhance fear conditioning and memory consolidation, subsequently resulting in chronic symptoms that are characteristic of PTSD (Pitman, 1989; Pitman et al., 2000). However, little is known about potential sex differences in conditioning mechanisms associated with PTSD.
In fear conditioning, a stressor (i.e., unconditioned stimulus; UCS) elicits fear and arousal (i.e., unconditioned response, UCR) that becomes associated with innocuous cues (i.e., conditioned stimulus; CS) (Orr et al., 2000; Pitman et al., 2000). Subsequently, presentations of the now conditioned stimuli are sufficient to elicit a conditioned response (CR) because of the prior CS–UCS associations. In conditioning models of PTSD, it is believed that neutral environmental cues present at the time of trauma exposure become associated with fear and arousal such that these previously innocuous stimuli can trigger conditioned fear responses even when danger is no longer present (Orr et al., 1997; Vermetten et al., 2007). Individuals who develop PTSD may be more “conditionable” in that they are more likely to acquire a conditioned fear response to a neutral stimulus and have more difficulty extinguishing this response once acquired (Orr et al., 2000). While PTSD has been associated with greater conditioned responses to cues signaling threat in several studies (Orr et al., 2000; Peri et al., 2000), it has not been found in others (Milad et al., 2008; Orr et al., 2006). Some studies have also found that PTSD was associated with greater reactivity to safety signals during fear conditioning, suggesting impaired stimulus discrimination in PTSD (Blechert et al., 2007; Grillon and Morgan, 1999; Peri et al., 2000). However, much of this research has been conducted in males or in studies that failed to examine the impact of sex, which may have led to considerable variability.
Growing evidence suggests that men and women may diverge in their responses to stress and fear acquisition. Brain regions implicated in both fear conditioning and PTSD (e.g., the amygdala, vmPFC, and hippocampus) are sexually dimorphic and contain receptors for sex-specific steroids such as estrogen (Goldstein et al., 2001). Additionally, estradiol, the primary form of estrogen in women during the reproductive years, has been found to modulate fear and extinction learning in animal and human studies (Chang et al., 2009; Milad et al., 2006, 2009; Zeidan et al., 2011). Although not always (Gupta et al., 2001), estradiol has been associated with enhanced trace eyeblink conditioning (Leuner et al., 2004), cued fear conditioning (Jasnow et al., 2006), contextual conditioning (Jasnow et al., 2006), and extinction consolidation (Milad et al., 2009, 2010; Zeidan et al., 2011).
Most studies of sex differences in fear conditioning in humans have examined nonclinical populations. Enhanced acquisition of conditioned fear was found in healthy women compared to men in one study (Guimaraes et al., 1991), while another study found no sex differences (Zorawski et al., 2005). Importantly, these studies did not control for menstrual phase, which is problematic because reproductive hormones can influence conditioning and extinction. A study that controlled for menstrual phase by comparing women in the follicular phase and men found less differential conditioned fear responding during acquisition in women, suggesting that the process(es) underlying or influencing fear conditioning likely differ between men and women (Milad et al., 2006).
The present study examined whether there are sex differences in the acquisition of conditioned fear in men and women with full or subsyndromal PTSD. Women were tested during the early follicular phase to control for the possible confounding effects of changing reproductive hormones in cycling women since estradiol and progesterone are both relatively low in the early follicular phase. Based on women’s higher rates of PTSD, we predicted that women with PTSD would have greater acquisition of conditioned fear (larger skin conductance responses to CS+ trials) compared to men with PTSD. Because the model of fear conditioning in PTSD also suggests that greater threat and stress reactivity may affect fear conditioning (Bremner, 2002; Pitman et al., 2001) we also examined whether anticipatory fear of the unconditioned stimulus differs between men and women, and might thereby explain differences in fear conditioning. Because this study was part of a larger clinical trial examining pharmacological agents administered during extinction, we were unable to examine sex differences associated with extinction.
2. Methods and materials
2.1. Participants
Participants were recruited from Veterans Affairs (VA) outpatient and community clinics, and local newspaper and internet advertisements. Potential participants underwent a diagnostic interview, medical history, and laboratory testing for determining study eligibility. Exclusion criteria included organic mental disorder, schizophrenia, bipolar disorder, alcohol dependence, drug abuse or dependence, seizure disorders, neurological disorders, previous moderate or severe head injuries, current infectious illness, and systemic illness affecting CNS function, or any other medical condition known to affect psychophysiological responses. Exclusionary medications included alpha and beta-adrenergic agents, antipsychotics, benzodiazepines, mood stabilizers, anticonvulsants, antihypertensives, sympathomimetics, and steroids. Additional attrition occurred for the following reasons: withdrawal from study (1 male), falling asleep during experiment (3 males), technical difficulties/experimenter error (1 male, 1 female). Data from individuals with very small skin conductance responses to the UCS (i.e., mean response less than .1 μs) (2 females) or to the CS+ (i.e., mean response less than .05 μs) (2 males, 9 females) were also excluded.
We present data from 31 participants (men: n = 18; women: n = 13), ages 18–65, who met DSM-IV criteria for full or subsyndromal current PTSD as their primary psychiatric complaint and completed the fear conditioning task. Participants reported the following traumatic events that triggered their PTSD symptoms: combat experiences (12), sexual assault (9), physical assault (8), and accidents (2). Comorbid Axis I disorders included: major depressive disorder (6), dysthymia (2), alcohol abuse (1), panic disorder without agoraphobia (1), agoraphobia (1), specific phobia (2), obsessive-compulsive disorder (1), and generalized anxiety disorder (1). Participants were alcohol- and drug-free during testing, as determined from self-report, urine drug screen, and breathalyzer.
2.2. Measures
2.2.1. Psychosocial measures
PTSD symptom levels were determined by interview using the Clinician Administered PTSD Scale (CAPS) (Blake et al., 1995). The CAPS provides information on current and lifetime PTSD symptoms and status, providing a diagnosis, frequency, and intensity of symptoms. Full PTSD diagnostic criteria for DSM-IV or subsyndromal PTSD (i.e., CAPS score > 30 and meeting the A1, A2, B, E, and F clusters, and either the C or D clusters) was determined by the CAPS and present for at least 3 months. Psychiatric and substance use disorders were assessed with the Structured Clinical Interview for DSM-IV, version 2.0 (SCID I-NP) (First et al., 2002). We used the Life Stressor Checklist-Revised (LSC-R) (Wolfe et al., 1996) interview to determine prior exposure to 21 stressful life events (e.g., experiencing or witnessing serious accidents, illnesses, sudden death, physical and sexual assault); whether exposure to events triggered emotions consistent with DSM-IV criterion A2 for PTSD; and the frequency and ages of exposure. All interviews were conducted by Master’s-level clinical psychologists, who calibrated their assessments weekly. Self-reported state anxiety prior to conditioning was assessed using the State-Trait Anxiety Inventory (STAI-state) questionnaire (Spielberger et al., 1983). The STAI-state includes 20 items assessing feelings of apprehension, tension, nervousness, and worry rated on a 4-point Likert-type scale from 1 (not at all) to 4 (very much).
2.2.2. Psychophysiological measures
A Coulbourn Modular Instrument System was used. Skin conductance was measured directly by a Coulbourn Isolated Skin Conductance coupler (S71-23) using a constant .5 V through 9 mm (sensor diameter) Sensor Medics Ag/AgCl electrodes placed on the hypothenar surface of the participant’s non-dominant hand in accordance with published guidelines (Fowles et al., 1981). The SC electrodes were separated by 14 mm. The SC level analog signal was digitized by a Coulbourn Lablinc Analog to Digital Converter (L25-12). A Microsoft Windows-based computer system was used for sampling and storing the digitized SC signal and controlling stimulus presentations.
2.3. Procedures
During the initial study visit, study procedures were fully explained and written informed consent was obtained based on procedures approved by the University of California, San Francisco Institutional Review Board. Participants then underwent a structured diagnostic interview for psychiatric and medical history and provided blood and urine samples to assess medical conditions and use of medications or drugs that were exclusionary.
Next, eligible participants determined the level of electrical stimulation to be used as the UCS during the subsequent conditioning session (Orr et al., 2000). The experimental apparatus was located in the adjoining room and connected by wires. Participants were monitored via an unobtrusive video camera. The UCS was a 500 ms electrical pulse ranging from .5 to 5.0 mA generated by a Coulbourn Transcutaneous Aversive Finger Stimulator (E13-22). Electrodes for the electrical stimulus were attached to the second and third fingers of the participants’ dominant hand and participants were asked to choose the level of stimulation that is “highly annoying but not painful”, starting at a low level and gradually increasing until each participant said “stop”. Eligible participants were also given self-report questionnaires on demographics, health and psychological symptoms.
2.3.1. Fear conditioning
The aversive conditioning session occurred a minimum of one week later and during the early follicular phase for regularly cycling women.1 Sessions were scheduled between 1:00 pm to 4:00 pm to minimize potential diurnal effects. Participants were requested to abstain from caffeine, smoking, and eating for 1 h prior to the session and not to consume heavy amounts of alcohol or use illicit drugs for 72 h prior to participation.
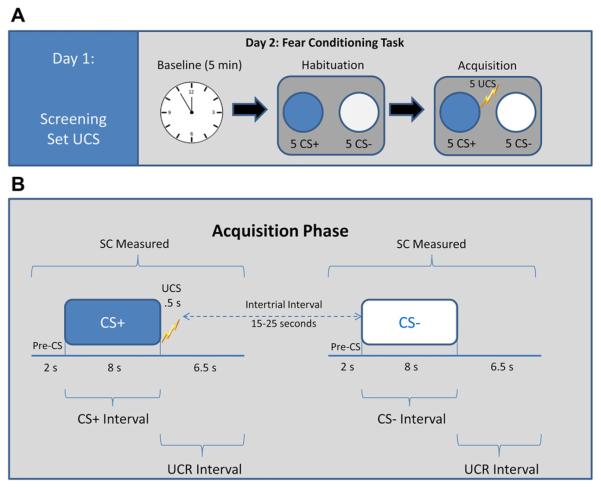
Electrodes for recording SC and those for UCS administration were attached. Administration of the task, adapted from Orr et al. (2000), began with a 5-min baseline recording period during which SC was sampled at 1000 Hz while the participant sat quietly. In this paradigm, the conditioned stimuli (paired = CS+; unpaired = CS−) were two different colored computer-generated 15.2 cm diameter circles; colors were randomly selected for each participant and presented for 8 s on a 28.5 × 21.5 cm monitor positioned 1 m in front of the participant. The UCS was a 500 ms electric pulse at the level previously identified by the participant as “highly annoying but not painful.” During habituation (Phase 1), participants were shown five presentations of each of the colored circles (to be CS+ and CS−) in the absence of the shock (UCS) with no more than 2 consecutive presentations of the same stimulus type. The CS duration was 8 s, and the intertrial interval (ITI) was 20 ± 5 s. This was followed by the acquisition phase (Phase 2), in which the CS+ (colored circle presented for 8 s followed by a 500 ms shock) and CS− (colored circle with no subsequent shock) stimuli was each presented five times in random order (Fig. 1A). After the task, participants were asked whether they could predict when the shock would occur, to identify the color of the CS+, and to rate their level of “annoyance” with the shock on a 5-point Likert-type scale.
Fig. 1.
A) Timeline of study procedures. Day 1: screening for psychiatric and medical history and setting US. Level ranged from .5 to 5.0 mA and self-determined to be “highly annoying but not painful”. Day 2: fear conditioning task: habituation (10 colored circles alone) was followed by acquisition (10 colored circles that were paired (CS+) or unpaired (CS−)) with US (US duration: 500 ms shock; CS duration: 8 s, intertrial interval (ITI) = 20 − 5 s). (B) Diagram to represent timing and measurement within the conditioned stimuli (CS+ and CS−) trial types during Acquisition. The SC response score for each CS interval was obtained by subtracting the mean level for the 2 s immediately preceding CS onset from the highest value among those recorded during the 8 s CS interval. The SC response score for the interval containing the UCR was obtained by subtracting the average SC level within 6–8 s following CS onset, from the maximum increase in SC level during the .5–6.5 s interval following CS offset (corresponding to the onset of the .5 s US).
2.4. Psychophysiological response scores
The SCR scores for CS and UCS intervals were calculated as described by Orr et al. (2000, 2006) and depicted in Fig. 1B. The SCR was square-root transformed to reduce heteroscedasticity prior to analysis. For a negative SCR, the square-root of the absolute value was calculated and given a negative sign. The SC resting level was determined by the mean SC during a 5 min baseline rest period. An SC orienting response was determined by averaging each individual’s response to the first presentation of the CS+ and CS− during the habituation phase.
3. Results
3.1. Sample characteristics
Means, standard deviations, and results of t-test comparisons between men and women for demographic and psychometric measures are presented in Table 1. There were no significant differences between men and women for age, education, ethnicity/race, PTSD symptom level, whether they met full criteria or subsyndromal PTSD or use of psychiatric medications (as indicated in Table 1).
Table 1.
Sample characteristics.
| Men (n = 18) |
Women (n = 13) |
Contrasts | ||
|---|---|---|---|---|
| N (%) or M (SD) | t(29) or χ2 | P | ||
| Age | 39.3 (11.5) | 33.0 (11.9) | 1.49 | .15 |
| Education | 6.69 | .08 | ||
| Some HS/HS grad/GED | 3 (16.7%) | 0 (0%) | ||
| Some college | 9 (50.0%) | 5 (38.5%) | ||
| AA/BA/BS | 5 (27.8%) | 3 (23.1%) | ||
| Post-graduate educ. | 1 (5.6%) | 5 (38.5%) | ||
| Ethnicity/race | 7.27 | .12 | ||
| Caucasian | 11 (61.1%) | 7 (53.8%) | ||
| Black/African American | 1 (5.6%) | 1 (7.7%) | ||
| Asian | 0 (0%) | 3 (23.1%) | ||
| Hispanic | 4 (22.2%) | 0 (0%) | ||
| Multi racial | 2 (11.1%) | 2 (15.4%) | ||
| CAPS total score (0–136) | 61.2 (17.0) | 66.0 (20.8) | −.71 | .48 |
| Subsyndromal PTSD | 5 (28.8%) | 3 (23.3%) | .09 | .77 |
| Comorbid major depression | 2 (11.1%) | 4 (30.8%) | 1.87 | .17 |
| Childhood traumaa | 12 (66.7%) | 10 (76.9%) | .34 | .53 |
| SSRIs | 3 (16.7%) | 1 (7.7%) | .54 | .46 |
| Other antidepressants | 1 (5.6%) | 1 (7.7%) | .06 | .81 |
| Psychophysiology measures | ||||
| UCS level (.5–5.0 mA) | 3.20 (1.73) | 2.04 (1.91) | 1.75 | .09 |
| SC resting level (sqrt uS) | 1.94 (.47) | 1.37 (.42) | 3.41 | .002 |
| OR (sqrt uS) | .49 (.34) | .40 (.25) | .85 | .40 |
| UCR (sqrt uS) | .93 (.38) | 1.18 (.48) | −1.60 | .12 |
Note: CAPS, Clinical Administered PTSD Scale; SSRIs, selective serotonin reuptake inhibitors; UCS level, unconditioned stimulus level: the highest level of stimulation that participants self-selected to be “highly annoying but not painful”; SC resting level, resting baseline skin conductance: mean SC level during 5 min rest period; OR, orienting response: SC response average to first presentation of the CS+ and CS− during the habituation phase; UCR, unconditioned response: average UCR for CS+ trials during the acquisition phase. SC variables were square-root transformed prior to analysis (for a negative SCR, the square-root of the absolute value was calculated and given a negative sign).
Childhood trauma-exposure was determined from responses to the LSC-R. Based on the extant literature, we considered those participants as childhood trauma-exposed who experienced traumatic life events with serious personal life threat or physical harm to the self (Elzinga et al., 2003; Otte et al., 2005; Pole et al., 2007) prior to the age of 14 years (Otte et al., 2005).
3.2. Baseline skin conductance and shock levels
Mean shock level, SC resting level during baseline, and the SC orienting response during the habituation phase are presented in Table 1. Results indicated a nonsignificant trend for sex differences in the level of shock selected with men selecting marginally higher levels of shock than women. A significant sex difference was found in resting SC level during the initial 5 min resting baseline indicating that men had higher mean baseline SCL than women. During the 5 min resting baseline, there was a nonsignificant trend for women to report greater fear of receiving the shock (M = 4.31, SD = 3.17 for women vs.M= 2.50, SD = 2.09 for men; t(29)=−1.91, p = .07) and state anxiety prior to conditioning (M = 48.00, SD = 10.58 for women vs. M = 42.89, SD = 8.14 for men; t(29) = −1.81, p = .08). The level of shock selected by participants, fear of shock, state anxiety and resting SC were not associated with differential fear acquisition. Thus, differences between men and women during the experimental phases of fear conditioning are not likely to be attributable to prior differences in shock level, anticipatory anxiety, or baseline SCL.
3.3. Effect of sex on skin conductance responding to conditioned stimuli across experimental phases
The mean CRs to CS+ and CS− cues during habituation and acquisition phases of the experiment for men and women are shown in Fig. 2. A three-factor sex (males, females) × stimulus (CS+, CS−) × trials (the last 4 levels for each of the CS+ and CS−) repeated measures ANOVA with CR as the dependent variable was performed separately for the habituation and acquisition phases. We focused on the last 4 out of 5 presentations each of the CS+ and CS− trials to avoid orienting responses associated with initial presentation of stimuli. Because the UCS is administered after CS+ offset, any effects associated with the initial CS+ trial is likely to reflect an orienting response and not conditioning, since the CS and UCS have not yet been paired.
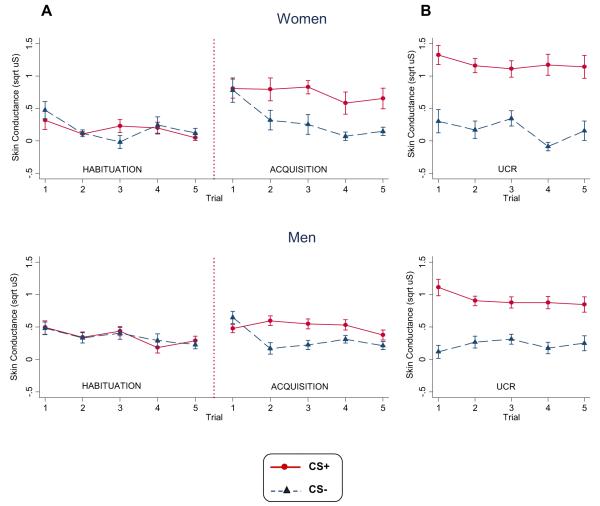
Fig. 2.
Men and women group mean skin conductance response scores for (A) the conditioned stimulus (CS) intervals of CS+ and CS− trials during habituation and acquisition and (B) the unconditioned stimulus intervals of CS+ and CS− trials during the acquisition phase. Data is square-root transformed. Error bars represent standard error.
3.3.1. Habituation
During habituation, the repeated measures ANOVA revealed a significant main effect for sex, F(1, 232) = 18.89, p < .001, ; men produced larger SC responses during presentation of the CS+ and CS− stimuli than women (M = .31, SD = .17 and M = .13, SD = .11 respectively). There were no significant differences in SC response magnitude to CS+ and CS− trials and no stimulus by sex interaction.
3.3.2. Acquisition
Acquisition of a differentially conditioned SC response is evident in a significant main effect for stimulus, F(1, 232) = 61.56, p < .001, . Fig. 2A shows that SC response magnitudes were larger for the reinforced CS+ trials compared to the non-reinforced CS− trials for both men and women. There was also a significant sex by stimulus interaction, F(1, 232) = 5.16, p < .05, with women having greater differential SC responses to CS+ than CS− cues than men (Fig. 3). Separate analyses of CS+ and CS− trials yielded a significant sex effect for CS+ trials, F(1, 116) = 6.33, p < .05, , with women (M= .71, SD= .47) having larger SC responses to the CS+ than men (M = .51, SD = .16). There were no significant main effects of sex or trials or sex × trial interactions for SC response to CS− trials. Thus, the enhanced differential conditioning in women is primarily due to enhanced responding the CS+, despite the significantly reduced baseline SCL and SC responses during habituation. Excluding participants with subsyndromal levels of PTSD similarly resulted in a significant sex × stimulus interaction, F(1, 168) = 5.05, p < .05, , and significant main effect for stimulus, F(1,168)= 45.07, p < .0001, . Similarly, exclusion of one female participant who reported to be menopausal did not alter the sex × stimulus interaction, F(1,224)= 5.28, p < .05, or main effect for stimulus, F(1, 224) = 59.17, p < .0001, .
Fig. 3.
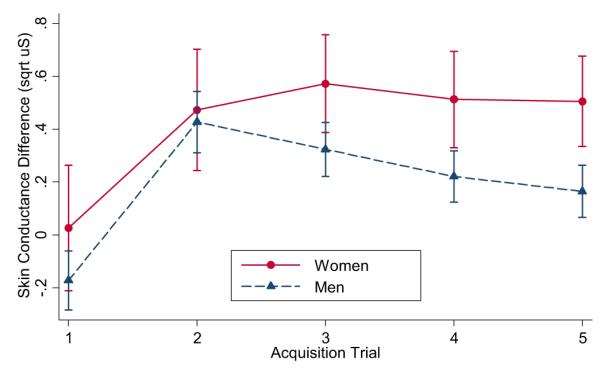
Differential scores (CS + minus CS−) for the conditioned stimulus (CS) intervals during the acquisition phase for men and women.
3.4. Effect of sex on skin conductance responding to unconditioned stimuli
Group mean SC responses for the UCS interval of CS+ and CS− acquisition phase trials are depicted in Fig. 2. We conducted a three-factor sex (males, females) × stimulus (shock vs. no shock) × trials (4 levels for each UCS condition) repeated measures ANOVA with SC response magnitude during the UCS interval as the dependent variable to evaluate potential sex differences in response to the unconditioned stimuli. As expected, there was a significant main effect for the electric stimulus such that larger SC responses were produced during the UCS interval of CS+ trials, compared to CS− trials, F(1, 232) = 209.66, p < .001, . We also found a significant stimulus × sex interaction, F(1, 232) = 11.18, p = .001, , which reflected a significantly larger SC response magnitude during the UCS interval of CS+ trials for women compared to men, M = 1.18, SD = .39 vs. M = .92, SD = .39, respectively, F(1,232) = 11.48, p < .001, . There were no other significant effects for sex, trial, or for the interactions of sex × trial, stimulus × trial, or sex × stimulus × trial, all Fs < 2.10, ps > .27.
Following acquisition, 24 of 31 individuals (13 men; 11 women) correctly identified the relationship between the CS+ and shock; indicating explicit awareness of the CS–UCS contingency in the majority of participants. The correct identification of the CS–UCS contingency did not differ by sex, χ2 (n = 31) = .66, p = .44, UCS level, PTSD severity, or acquisition (all ts < 1.19, ps > .18). Rerunning analyses, excluding non-aware participants, resulted in a significant gender × stimulus interaction, F(1, 176) = 4.68, p < .05 and significant main effect for stimulus, F(1, 176) = 48.33, p < .0001. Women reported greater annoyance with the shock, t(1, 28) = −3.64, p < .001 and annoyance with shock was associated with greater differential fear acquisition, r = .44, p < .05.
4. Discussion
Our findings suggest that women with PTSD had larger differential skin conductance responses to presentations of a fear conditioned stimulus during acquisition, compared to men with PTSD. In particular, women were more reactive to cues signaling threat compared to men, whereas there was no sex difference in the magnitude of their responses to cues signaling safety. In contrast, men diagnosed with PTSD exhibited elevated baseline SC levels and enhanced responding to novel cues during habituation. These findings suggest greater differential fear conditioning in women with PTSD, and perhaps enhanced general fear/arousal in men with PTSD. A greater propensity to acquire a conditioned fear response could explain the persistent emotional and physiological reactivity to cues that is characteristic of PTSD (Pitman, 1989; Pitman et al., 2000).
In addition, women had larger SC responses to the shock UCS. However, the sex difference in response to the shock does not fully explain the sex difference in conditioned responding because in this fear conditioning protocol, the interval in which the CS is presented occurs prior to delivery of the shock UCS. Since the CS interval temporally precedes the UCS interval, the response occurs during a time in which participants may be experiencing fear or anticipatory anxiety while expecting an aversive outcome, but are not receiving it.
An important question is whether men and women differ in their UCRs because they did not experience shock in the same way. Some studies have found that women have a lower pain threshold and tolerance and report higher ratings of pain intensity and unpleasantness in experimental manipulations of pain (Fillingim et al., 2009). To control for potential differences in pain sensitivity, participants were asked to self-determine a level of shock that they found “highly annoying but not painful”. Despite this, women still reported significantly higher annoyance with shock exposure following the task. In the future, examining conditioning to other types of non-painful stimuli (e.g., airpuff) would provide additional evidence to confirm that sex differences in the cued conditioned response was due to associative learning irrespective of potential differences in sensitivity to stimuli.
Another question is whether greater responsiveness to unconditioned and conditioned stimuli in women was due to greater perceived threat and anticipation in general, since prior studies have shown that threat of shock (Spence et al., 1954) and attentional biases toward threat (Fani et al., 2012) were associated with greater acquisition of conditioned fear. Consistent with this possibility, prior to the conditioning task, women tended to select slightly lower levels of shock and reported marginally more fear of receiving the shock and greater state anxiety although these were nonsignificant trends. However, this was not corroborated by physiological evidence, since there was no association with fear acquisition and men had higher SC levels during baseline and habituation. Thus, it does not appear that threat and global anticipatory anxiety explains differences in acquisition.
The heightened conditionability observed in this sample of women with PTSD stands in contrast to the sex differences in fear acquisition that has been found in some studies of healthy, non-trauma exposed humans in which females tend to either not differ from males or exhibit lower conditioned responding. One possible explanation for this difference is that greater fear conditioning in women could be a marker of vulnerability that is specific to those exposed to trauma who develop PTSD. A promising candidate that may explain a sex-specific vulnerability to greater fear conditioning and PTSD is pituitary adenylate cyclase-activating polypeptide (PACAP), a peptide that modulates stress circuits in the brain and throughout the periphery, and is modulated by estrogen. Ressler et al. (2011) reported a sex-specific association between PACAP and fear conditioning in traumatized women. Women, but not men, with higher levels of PACAP had greater PTSD symptom severity and startle to both CS+ and CS− cues in a fear-potentiated acoustic startle conditioning paradigm, suggesting impaired fear discrimination. Furthermore, variation in a gene SNP encoding for the PAC1 receptor that is in an estrogen response element (ADCYAP1R1 rs2267735) was also associated with greater reactivity to the CS− (indicating decreased fear discrimination) and with PTSD in women, but not in men.
Estrogen has also been found to have regulatory actions on adrenergic, GABAnergic, and serotonergic systems (McEwen, 2002). Inhibitory interneurons within the amygdala, vmPFC, and hippocampus are rich with receptors for estrogen (Goldstein et al., 2001). Estrogen administration to ovariectomized mice has been found to enhance contextual conditioning (Jasnow et al., 2006), facilitate cued fear conditioning (Jasnow et al., 2006) and trace eyeblink conditioning (Leuner et al., 2004). NMDA and glucocorticoid-mediated mechanisms of learning and memory in fear conditioning and extinction may be affected by estrogen; estrogen has been shown to increase NMDA receptor transmission and long-term potentiation (Smith and McMahon, 2005) and regulate HPA axis function (Chrousos et al., 1998). While studies have found that higher estrogen levels were associated with greater extinction retention in healthy humans (Milad et al., 2010) and low estrogen levels have been associated with extinction deficits in women with PTSD (Glover et al., 2012), no associations were found for fear acquisition.
Alternatively, enhanced conditioning observed in women may be acquired with trauma exposure. Since our study was cross-sectional it is unknown whether enhanced acquisition of conditioned fear in women predates trauma exposure and confers risk, is a consequence of trauma exposure, or a correlate of symptomatology. More prospective studies of high-risk individuals will be important to disentangle possible temporal or causal relationships in conditionability and the possibility that it varies by sex prior to trauma exposure.
There are several limitations of this study to consider. Because we wanted to limit the possible effects of changes in reproductive hormones on fear conditioning, women were scheduled for the conditioning task during the early follicular phase, although a minority of women did not have regular menstrual cycles or were on hormonal birth control. Menstrual status information was obtained by self-report and not confirmed by blood levels of hormones, limiting conclusions regarding the role of menstrual phase or status on fear conditioning. In addition, other studies have found that deficits in extinction retention are associated with PTSD and differ by sex in healthy individuals (Milad et al., 2006, 2010). In the future, we hope to include extinction and extinction retention tests in our research. Despite these limitations, our findings along with mounting evidence of sex differences in fear conditioning and extinction in healthy humans (Guimaraes et al., 1991; Milad et al., 2006, 2010), structural and functional sex differences in fear networks in the brain (Felmingham et al., 2010; Goldstein et al., 2001), and a hormonal milieu that influences aspects of conditioning processes (Glover et al., 2012; Jasnow et al., 2006; Milad et al., 2009, 2010; Zeidan et al., 2011) suggest that there may be important sex differences in fear conditioning that are relevant to PTSD.
Acknowledgments
We would like to thank Roger Pitman, MD and Christian Otte, MD for their valuable scientific contributions to the design of this study; Lauren Zitner, Ph.D., Emily Hu, Jonathan Varbel, Evelyn Rucker, MA, and Randall Coeshott, Ph.D. for study coordination, recruitment and data collection; Kristin Samuelson, Ph.D., Laura Natta, MA, Shilpa Reddy, MA, and Leighann DeJesse, Ph.D. for conducting diagnostic assessments; Brigitte Apfel, MD and Pierre-Cedric Crouch, NP for conducting medical evaluations; and Gary Tarasovsky for his assistance with data management.
Role of the funding source This research was supported by a grant from the Department of Veteran Affairs CSR&D (CDA-2-037-07F) to Dr. Inslicht, and the Mental Illness Research and Education Clinical Center (MIRECC) of the US Veterans Health Administration. Resources and the use of facilities were provided by the Veterans Administration Medical Center, San Francisco California and the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health, through a UCSF-CTSI Grant (#KL2 RR024130). Dr. Pineles was supported by the Department of Veteran Affairs CSR&D (CDA-2-042-07F). The sponsors had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
One female participant reported menopausal status. All other participants reported regular menstrual cycles.
Conflict of interest Dr. Neylan has received experimental medication from Actelion for a study funded by the Department of Defense. The authors report no other financial, personal, or other relationships with any entities that might inappropriately influence, or be perceived to have influenced the research presented in this manuscript.
Contributors Dr. Inslicht designed the study, wrote the protocol, and wrote the first draft of this manuscript. Mr. Metzler undertook the statistical analysis and interpretation of findings and statistical analysis and results sections of this manuscript. Ms. Garcia managed literature searches, coordinated data collection, and screened and analyzed data, and reviewed the manuscript for accuracy of interpretation. Dr. Pineles established the database, assisted with interpretation of variables, contributed to the scientific interpretation of findings and edited the manuscript. Dr. Milad, Dr. Orr, Dr. Marmar, and Dr. Neylan assisted with the design of the study, interpretation of findings, and all drafts of this manuscript. All authors contributed to and have approved the final manuscript.
References
- Adamson DM, Burnam MA, Burns RM, Caldarone LB, Cox RA, D’Amico E, et al. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. In: Tanielian T, Jaycox L, editors. RAND Center for Military Health Policy Research. Rand Corporation; Santa Monica, CA: 2008. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behaviour Research and Therapy. 2007;45:2019–33. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Current Psychiatry Reports. 2002;4:254–63. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit area survey of trauma. Archives of General Psychiatry. 1998;55:626–32. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus. 2009;19:1142–50. doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic–pituitary–adrenal axis and the female reproductive system: clinical implications. Annals of Internal Medicine. 1998;129:229–40. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–65. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychological Medicine. 2012;42:533–43. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, et al. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. Journal of Abnormal Psychology. 2010;119:241–7. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. Journal of Pain. 2009;10:447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders, research version, non-patient edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, Venables PH. Committee report. Publication recommendations for electrodermal measurements. Psychophysiology. 1981;18:232–9. doi: 10.1111/j.1469-8986.1981.tb03024.x. [DOI] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerler K, Bradley B, Ressler KJ, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological Psychiatry. 2012;72:19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11:490–7. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf war veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–42. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, Hellewell J, Hensman R, Wang M, Deakin JF. Characterization of a psychophysiological model of classical fear conditioning in healthy volunteers: influence of gender, instruction, personality and placebo. Psychopharmacology (Berl) 1991;104:231–6. doi: 10.1007/BF02244184. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Research. 2001;888:356–65. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Hormones and Behavior. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–90. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Progress in Hormone Research. 2002;57:357–84. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, et al. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience. 2006;120:1196–203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–95. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. Journal of Psychiatric Research. 2008;42:515–20. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–8. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:290–8. [PubMed] [Google Scholar]
- Orr SP, Milad MR, Metzger LJ, Lasko NB, Gilbertson MW, Pitman RK. Effects of beta blockade, PTSD diagnosis, and explicit threat on the extinction and retention of an aversively conditioned response. Biological Psychology. 2006;73:262–71. doi: 10.1016/j.biopsycho.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Orr SP, Solomon Z, Peri T, Pitman RK, Shalev AY. Physiologic responses to loud tones in Israeli veterans of the 1973 Yom Kippur war. Biological Psychiatry. 1997;41:319–26. doi: 10.1016/s0006-3223(95)00671-0. [DOI] [PubMed] [Google Scholar]
- Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, et al. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biological Psychiatry. 2005;57:27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological Psychiatry. 2000;47:512–9. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Post-traumatic stress disorder, hormones, and memory. Biological Psychiatry. 1989;26:221–3. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Shalev A, Orr S. Post-traumatic stress disorder, emotion, conditioning and memory. In: Gazzaniga M, editor. The cognitive neurosciences. Plenum Press; New York: 2000. pp. 1133–48. [Google Scholar]
- Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. Journal of Clinical Psychiatry. 2001;62(Suppl. 17):47–54. [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Metzler TJ, Best SR, Henn-Haase C, et al. Associations between childhood trauma and emotion-modulated psychophysiological responses to startling sounds: a study of police cadets. Journal of Abnormal Psychology. 2007;116:352–61. doi: 10.1037/0021-843X.116.2.352. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–7. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. Journal of Neuroscience. 2005;25:7780–91. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence KW, Farber IE, Taylor E. The relation of electric shock and anxiety to level of performance in eyelid conditioning. Journal of Experimental Psychology. 1954;48:404–8. doi: 10.1037/h0055739. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for state-trait anxiety inventory (form Y) Consulting Psychologists Press; California: 1983. [Google Scholar]
- Vermetten E, Schmahl C, Southwick SM, Bremner JD. Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacology Bulletin. 2007;40:8–30. [PMC free article] [PubMed] [Google Scholar]
- Wolfe J, Kimerling R, Brown PJ, Chresman KR, Levin K. Psychometric review of the life stressor checklist-revised. In: Stamm BH, editor. Measurement of stress, trauma, and adaptation. Sidran Press; Lutherville, MD: 1996. pp. 676–9. [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry. 2011;70:920–7. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorawski M, Cook CA, Kuhn CM, LaBar KS. Sex, stress, and fear: individual differences in conditioned learning. Cognitive, Affective and Behavioral Neuroscience. 2005;5:191–201. doi: 10.3758/cabn.5.2.191. [DOI] [PubMed] [Google Scholar]