Abstract
Background and Aims
The Orchidaceae have a history of recurring convergent evolution in floral function as nectar production has evolved repeatedly from an ancestral nectarless state. However, orchids exhibit considerable diversity in nectary type, position and morphology, indicating that this convergence arose from alternative adaptive solutions. Using the genus Disa, this study asks whether repeated evolution of floral nectaries involved recapitulation of the same nectary type or diversifying innovation. Epidermis morphology of closely related nectar-producing and nectarless species is also compared in order to identify histological changes that accompanied the gain or loss of nectar production.
Methods
The micromorphology of nectaries and positionally equivalent tissues in nectarless species was examined with light and scanning electron microscopy. This information was subjected to phylogenetic analyses to reconstruct nectary evolution and compare characteristics of nectar-producing and nectarless species.
Key Results
Two nectary types evolved in Disa. Nectar exudation by modified stomata in floral spurs evolved twice, whereas exudation by a secretory epidermis evolved six times in different perianth segments. The spur epidermis of nectarless species exhibited considerable micromorphological variation, including strongly textured surfaces and non-secreting stomata in some species. Epidermis morphology of nectar-producing species did not differ consistently from that of rewardless species at the magnifications used in this study, suggesting that transitions from rewardlessness to nectar production are not necessarily accompanied by visible morphological changes but only require sub-cellular modification.
Conclusions
Independent nectary evolution in Disa involved both repeated recapitulation of secretory epidermis, which is present in the sister genus Brownleea, and innovation of stomatal nectaries. These contrasting nectary types and positional diversity within types imply weak genetic, developmental or physiological constraints in ancestral, nectarless Disa. Such functional convergence generated by morphologically diverse solutions probably also underlies the extensive diversity of nectary types and positions in the Orchidaceae.
Keywords: Disa, Disinae, Orchidaceae, orchid, deceit pollination, modified stoma, nectar, nectary, reward, rewardless, evolution, functional convergence
INTRODUCTION
Convergent adaptation in unrelated lineages can modify phenotypes and genotypes with differing specificity. Most strictly, convergence arises by recapitulation, either because of parallel changes in the same gene(s) (Conte et al., 2012) or from changes in genes that regulate the same biochemical pathway (e.g. Bosch et al., 2008). Less accurate convergence arises by innovation when the same function evolves via the establishment of alternative but similar physiological and/or morphological solutions in different lineages. All of these cases involve divergence from ancestral states within lineages, but lineages differ in the extent of phenotypic diversification. The production of floral nectar represents an example of functional convergence within angiosperms, reflecting physiological and morphological innovations within different lineages. Nectar is the most common floral reward employed by angiosperms to reinforce visitation by pollinators (e.g. Simpson and Neff, 1983; Proctor et al., 1996) and its characteristics affect various aspects of pollinator foraging (e.g. Zimmerman and Cook, 1985; Harder and Thomson, 1989; Fisogni et al., 2011). Despite these shared functions, nectaries vary extensively among species in morphology, anatomy and location, ranging from non-structural nectaries lacking histological differentiation (Daumann, 1970; Fahn, 1979) to complex structures with uni- or multicellular hairs, glands, or stomata (Fahn, 1979; Bernardello, 2007; Nepi, 2007). This nectary diversity, despite common function, probably reflects both repeated independent evolution of nectar production via different mechanisms and modification of nectaries within nectar-producing lineages (Fahn, 1979; Cronquist, 1988; Lee et al., 2005b). Both innovation and modification of nectaries are likely facilitated by their relatively simple structure and the associated simplicity of the genetic regulation of their development, location and functionality (Baum et al., 2001; Lee et al., 2005a, b). If so, nectary diversity likely reflects adaptive responses to contrasting morphological opportunities and pollination environments.
The Orchidaceae provide rich opportunities to explore nectary evolution, as it includes both nectarless and nectar-producing species, and among the latter the position and type of the sepal nectaries vary extensively (Pais, 1982; Figueiredo and Pais, 1992; Galetto et al., 1997; Stpiczyńska, 1997; Stpiczyńska and Matusiewicz, 2001; Stpiczyńska et al., 2003, 2005a; Davies et al., 2005; Davies and Stpiczyńska, 2007; Johnson et al., 2007; Bell et al., 2009, de Melo et al., 2010; Aguiar et al., 2012). Lack of floral nectar is apparently ancestral in this family (Benzing, 1987; Dressler, 1993), so that nectar production is derived. Furthermore, nectar and hence nectaries have been gained repeatedly and also subsequently lost in several orchid lineages (Johnson et al., 1998, 2013; Bateman et al., 2003; Singer and Koehler, 2004; Chase et al., 2005; Bell et al., 2009; Pansarin et al., 2012). This history raises the question of whether nectary evolution within orchid clades involves recapitulation of the same nectary type or diversifying innovation.
Despite the diversity of orchid flowers and the overall research effort dedicated to this family, few studies have examined orchid nectaries, and even less is known about the micromorphology of the floral spurs or labella of nectarless orchids (but see Bell et al., 2009; Bradshaw et al., 2010; Pansarin et al., 2013). All nectar-producing orchids examined to date have perigonal nectaries, in which the surface of the secretory epidermis is often enlarged by unicellular papillae or trichomes (Galetto et al., 1997; Stpiczyńska, 1997; Stpiczyńska and Matusiewicz, 2001; Stpiczyńska et al., 2005b; Davies and Stpiczyńska, 2007; Johnson et al., 2007; Pansarin, 2008; Bell et al., 2009, de Melo et al., 2010; Aguiar et al., 2012; Pansarin et al., 2012). Following secretion, nectar either seeps through fissures or pores in the cuticle (Figueiredo and Pais, 1992; Stpiczyńska, 1997; Stpiczyńska et al., 2005b; Davies and Stpiczyńska, 2007) or accumulates under the cuticle and eventually passes through without rupturing it (Galetto et al., 1997; Stpiczyńska and Matusiewicz, 2001; Stpiczyńska et al., 2003, 2005a; de Melo et al., 2010). Stomatal nectaries were considered absent in monocotyledons as a whole (Endress, 1995), despite being common in dicotyledons (Bernardello, 2007; Nepi, 2007), until Davies et al. (2005) described nectar exudation by modified stomata on the labellum of Maxillaria anceps (= Maxillariella anceps; Blanco et al., 2007). No other cases of stomatal nectaries have been reported in orchids.
Comparison of tissue morphology of closely related nectar-producing and nectarless species could provide insight into the histological changes that accompany the gain or loss of nectar production, reveal the number of times that nectar and specific nectary types have evolved, and identify histological processes underlying the evolution of nectar production in the Orchidaceae. To this end, we studied nectary structure in Disa, a large African genus (180 species; Bytebier et al., 2008) in which nectar production has evolved repeatedly from nectarless ancestors (Johnson et al., 1998, 2013). We extensively surveyed the micromorphology of nectaries in nectar-producing species and positionally equivalent tissues in nectarless species, representing all but one monotypic Disa section recognized by Bytebier et al. (2008). We identify and characterize morphological nectary types with stereomicroscopy and scanning electron microscopy (SEM), and include numerous nectarless species for intrageneric comparison. Following Bytebier et al. (2007) we also include three species of Brownleea, a small (seven species; Linder, 1981d, 1985), closely related (Douzery et al., 1999; Bellstedt et al., 2001), nectar-producing (Larsen et al., 2008) genus for extrageneric comparison. Because Disa is widely distributed in sub-Saharan Africa, with many rare or poorly accessible species (Linder, 1981a–c, e, f), fresh material could be examined with stereomicroscopy for only a subset of species. We therefore extended our survey with SEM of preserved material. We combined existing information on nectar production (Hobbhahn, 2012; Johnson et al., 2013) with stereomicroscopy and SEM results to infer the presence and types of nectaries in species for which fresh material was not available. Based on the existing molecular phylogeny of Disa (Bytebier et al., 2007), we specifically quantify the nature, frequency and order of the changes associated with the evolution of nectar production to assess whether the repeated evolution of nectar production (Johnson et al., 1998, 2013) involved recapitulation, or innovation that generated different nectary types.
MATERIALS AND METHODS
Plant material
We examined flowers of 68 Disa species and three Brownleea species (see Appendix and Supplementary Data Table S1 for geographical origin of collected material, collector information and herbarium accession numbers). The presence or absence of nectar production either had been established in previous studies (summarized in Johnson et al., 2013) or was established by repeatedly examining fresh, unpollinated flowers with hand lenses and probing potentially nectar-producing tissues with microcapillary tubes or filter-paper wicks. The presence of sugar in all floral exudates was confirmed with a hand-held refractometer adjusted for small volumes (Delta Refractometer, range 0–50 % sugar w/w, Bellingham & Stanley, Tunbridge Wells, UK) and, if only traces of nectar were found, high-pressure liquid chromatography. For eight of the examined Disa species fresh material was unavailable and reward status is uncertain; for completeness, their results are included in Supplementary Data Table S2, but were not included in the statistical analyses. Results for Brownleea are not included in the phylogenetic analyses of nectary characteristics and are not reported in detail, other than to illustrate micromorphological differences between Disa and a close relative. Depending on species rarity and material availability, one flower per inflorescence was examined for one to 11 inflorescences per species (Appendix).
Fresh material of 16 nectar-producing Disa species was examined with high-magnification stereomicroscopes to identify the floral tissues and cellular structures that exude nectar. Inflorescences were collected in the field and maintained with their stems in water-filled containers until stereomicroscopic examination, usually within 12 h of collection. Nectar production is confined to the floral spur in 14 of the 16 examined species (except D. longicornu and D. elegans). For these species, we dissected the spurs longitudinally to examine the inner epidermis. In most species, we chose buds 1–2 days prior to anthesis, when nectar production had started, but the limited volume allowed identification of the location and morphology of the nectar-exuding structures. In species for which only mature flowers were available (D. chrysostachya, D. crassicornis, D. zuluensis), we removed nectar from the spur sections with filter paper and incubated them at room temperature on moist filter paper in closed Petri dishes for 15–30 min before examination of resumed nectar production. Field observations indicated that D. longicornu produces nectar mostly on the petals, which extend far into the spur, and to a lesser extent on the inner spur epidermis. By comparison, in the spurless D. elegans nectar exudes from the petals and lip. Following sample incubation, we examined both the spur and petals for D. longicornu and the petals and lip for D. elegans.
We also used SEM to examine preserved flowers of 25 nectar-producing and 31 nectarless Disa species, eight Disa species with uncertain nectar status and three Brownleea species that we collected ourselves or obtained from the spirit collections of the Bolus Herbarium (BOL), University of Cape Town, South Africa, the Bews Herbarium (NU), University of KwaZulu-Natal Pietermaritzburg, South Africa, and several collectors.
Stereomicroscopy
Flowers were examined with a Leica MZ16 stereomicroscope (magnification ×7·1 to ×112·5; Heerbrugg, Switzerland) with a KY-F1030 digital camera (JVC, Japan), a Nikon SMZ 1500 Stereoscopic Zoom Microscope (magnification ×7·5 to ×112·5; Tokyo, Japan) with a Nikon DS-5M digital camera (Tokyo, Japan), or a Wild M400 dissecting microscope (magnification ×12·6 to ×64; Heerbrugg, Switzerland) with a Zeiss Axiocam digital camera model 412–312 (Oberkochen, Germany). For each flower, we recorded age (bud, estimated days to anthesis, recently opened, mature flower), nectary location (spur, petal, lip), presence or absence of nectar on freshly dissected and incubated material, presence of stomata, papillae or trichomes, and whether nectar exudation correlated spatially with stomata or occurred in irregular patches on the epidermis. Incubated material was photographed to document our findings and for comparison with the SEM results.
Scanning electron microscopy
In preparation for SEM examination, all floral material was dissected in 70 % ethanol to isolate the focal tissue (e.g. spur), which was then transferred to 100 % ethanol for a 1-min wash before critical-point drying with liquid CO2 in a Hitachi HCP-2 critical-point drier (Tokyo, Japan). After sputter coating with gold–palladium alloy, specimens were examined with a Hitachi S-570 Scanning Electron Microscope (Tokyo, Japan), a Philips XI30 Environmental Scanning Electron Microscope (Eindhoven, Holland) or a Zeiss Evo LS15 Scanning Electron Microscope (Oberkochen, Germany) with accelerating voltages of 6–9, 10–12 and 8–9 kV, respectively.
We examined the floral spur for all spurred species and the dorsal sepal for spurless species, except that we examined both spur and petals for D. longicornu and the petals and lip for D. elegans. If stomata were present, we counted them while scanning the entire specimen along grid lines with the SEM. We used ImageJ software (version 1·43u; Rasband, 1997–2009) to measure stomatal dimensions on SEM photographs using the SEM scale bar as reference. On SEM scans that showed clearly distinguishable stomata in plan view and had a scale bar ≤500 μm, we measured both stoma length (l, distance between the outer guard-cell tips) and width (w, greatest distance between the outer guard-cell walls perpendicular to the longest axis). We calculated stomatal area (mm2) as πlw/4. When possible, we examined several flowers per species and measured five to ten stomata per flower. To determine whether nectar-producing tissues were thicker than non-secretory tissues, we assessed the thickness of spur walls by counting the number of cell layers in spur sections. Dissection compressed and distorted tissue, rendering linear measurements inaccurate. We did not count cell layers near veins to avoid overestimates of tissue thickness. Features like papillae or trichomes in spurs were noted.
Phylogenetic and statistical analyses
Phylogenetic data and character coding
Phylogenetic relationships were inferred from the molecular phylogeny of Disa by Bytebier et al. (2007, dated in Bytebier et al., 2011), which included all Disa species for which data were collected. We used the maximum clade credibility chronogram (rescaled to reflect median node heights for the contained clades and hereafter referred to as the MCC chronogram) from a sample of 1000 chronograms (extracted by sampling every 10 000th generation from a Markov chain Monte Carlo run in BEAST after excluding the initial 2·5 million generations to guarantee a conservative burn-in) for analyses. When appropriate, we repeated our analyses for all 1000 trees in the sample to account for phylogenetic uncertainty. Two misidentifications in Bytebier et al. (2007) were corrected: the specimens identified as D. atrorubens and D. zimbabweensis in Bytebier et al. (2007) were re-identified as D. comosa and D. rungweensis, respectively. Because D. comosa was already included in the phylogeny, the erroneous D. atrorubens terminal was deleted from all trees used here. The D. zimbabweensis terminal was re-named as D. rungweensis in all trees used here. Our analyses considered five traits. Binary coding (presence/absence) was used for the occurrence of stomata. Nectary types were coded as absent (0), stomatal nectary (1) and secretory epidermis (2) (Appendix). In species for which fresh material was not available, nectary presence and type were inferred by combining existing information on nectar production (Hobbhahn, 2012; Johnson et al., 2013) with SEM results. Correspondingly, all nectarless species were coded as nectary absent. Number of stomata (if present) per examined flower part, stoma area (mm2) and spur wall thickness (number of cell layers) were treated as continuous characters.
Taxa for which information was not available were pruned from the phylogeny before the respective analyses. Given variation in data availability for the different traits, the analyses considered between 22 (spur wall thicknesses of different nectary types) and 103 species (nectary type). Despite pruning, a minimum of nine of the 19 sections of Disa recognized by Bytebier et al. (2008) are represented in the data, and data on nectary type are available for representatives of all but the monotypic section Ovalifoliae.
Test for phylogenetic signal
We investigated whether the current distribution of nectary types and traits among species depends significantly on phylogenetic relatedness. For discrete traits we calculated the number of steps required for parsimony reconstruction over the MCC chronogram, and compared it with that of the same character re-shuffled 1000 times in Mesquite (version 2.75; Maddison and Maddison, 2011), while keeping the proportion of states constant. The null hypothesis of a phylogenetically random distribution is rejected if the observed state distribution lies outside the 95 % confidence interval of the randomized state distribution (Bytebier et al., 2011). For continuous traits we calculated the K statistic (Blomberg et al., 2003) and the probability associated with comparison of the variance of phylogenetically independent contrasts between the observed and 1000 randomized trait distributions over the phylogeny using the function ‘phylosignal’ in the R (version 2.15; R Development Core Team, 2010) package ‘picante’ (Kembel et al., 2010). To account for phylogenetic uncertainty, we estimated K for 1000 chronograms.
Ancestral state reconstruction and test for correlated evolution
To investigate the evolutionary histories of stomata and nectary type we estimated ancestral character states by parsimony and maximum likelihood (ML) using Mesquite (Maddison and Maddison, 2011). The ML results strongly supported the findings of the parsimony analyses, and revealed low rates of trait evolution for stomata (Mk1 rate [mean ± SE of 1000 chronograms]: 3·8 ± 1·3 × 10−5; n = 60 species) and nectary type (1·04 ± 0·3 × 10−5, n = 103 species). Under low rates of trait evolution, parsimony accurately reconstructs ancestral states, whereas ML methods suffer from insufficient information for correct parameter estimation (Harvey and Pagel, 1991; Mooers and Schluter, 1999; Pagel, 1999; Huelsenbeck et al., 2003; Pierie et al., 2012). We therefore report only the results of the parsimony analyses. Parsimony reconstruction identified optimal states for each internal node of the MCC chronogram and returned the number of trees in which each state was optimal. This enabled identification of the oldest internal nodes at which transitions between states occurred. We interpreted these nodes as transitional if the state identified as optimal in ≥75 % of trees differed from the state identified as optimal at older nodes. The number of state transitions under parsimony was summarized over 1000 chronograms.
The occurrence of stomata in both nectar-producing and nectarless species suggested that nectar production may have evolved in association with the evolution of stomata and that the presence of floral stomata may be a precondition for nectar production. We tested these hypotheses with Pagel's (1994) correlation test, as implemented in Mesquite (version 2.75; Maddison and Maddison, 2011), using the MCC chronogram. The probability that a model of dependent evolution fits the data significantly better than one of independent evolution was estimated with likelihood ratio tests involving 1000 Monte Carlo simulations of model parameters. For each simulation, ML estimates of model parameters were optimized with 500 iterations.
Phylogenetic generalized estimating equations
We analysed differences in quantitative characteristics with generalized linear models coupled with generalized estimating equations (GEEs) that accounted for interdependence among species owing to phylogenetic relatedness (Paradis and Claude, 2002) as implemented in ‘compar.gee’ in the R package ‘ape’ (version 3.0–2; Paradis et al., 2004). This approach was used to compare stomata number and size (mm2) and spur wall thickness between nectarless and nectar-producing species, to examine whether the independent evolution of stomatal nectaries in sections Monadenia and Micranthae (sensu Bytebier et al., 2008) is reflected in differences in stomata number and/or size, or spur wall thickness among sections, and whether spurs with a secretory epidermis differ in wall thickness from those with stomatal nectaries. Analysis of continuous dependent variables considered either a normal distribution (in some cases following log transformation) and identity link function, or a gamma distribution and inverse link function, when appropriate. Analyses were performed using species averages as within-species variation is beyond the scope of this study. To account for phylogenetic uncertainty, each analysis was repeated for 1000 chronograms.
RESULTS
Types and locations of floral nectaries
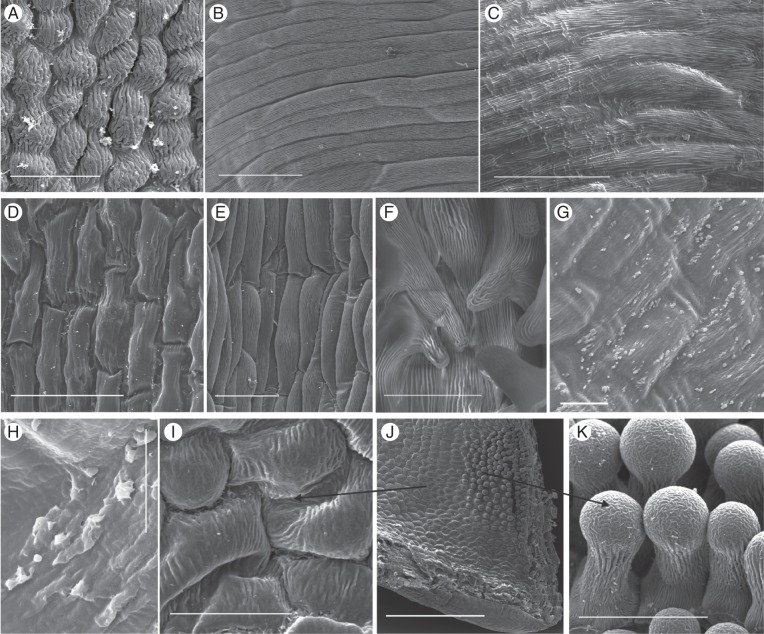
The Disa species for which we examined fresh material differed with respect to whether nectar is secreted by modified stomata or by a morphologically uniform epidermis that lacks trichomes or stomata (Figs 1 and 2, Appendix).
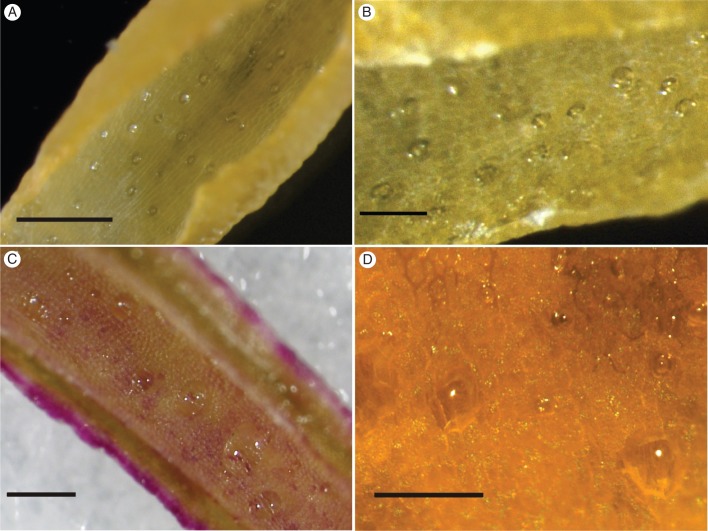
Fig. 1.
Nectar exudation by modified stomata in selected Disa species. Clearly defined nectar droplets accumulated above visible stomata in floral spurs of (A, B) D. polygonoides, (C) D. cooperi and (D) D. chrysostachya. Scale bars: (A) = 0·5 mm; (B) = 200 μm; (C) = 1 mm; (D) = 100 μm.
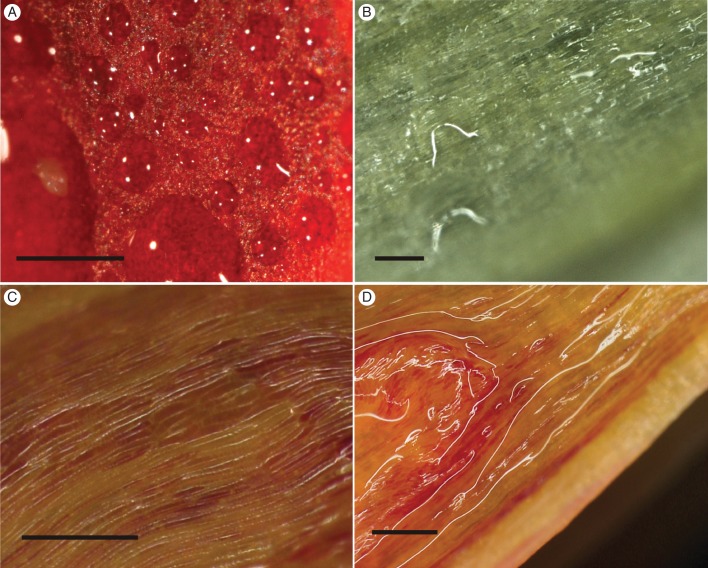
Fig. 2.
Nectar exudation by secretory epidermis in selected Disa species. Irregular nectar patches on a morphologically uniform epidermis of a petal of D. elegans (A) and the spur of D. longicornu (B) and D. uniflora (C, D). (C) Swollen spur epidermal cells after drying with filter paper and incubation in hydration chamber; (D) spur epidermis coated in nectar film. Scale bars: (A–C) = 0·5 mm; (D) = 1 mm.
Stomatal nectaries occurred in sections Monadenia (D. cylindrica) and Micranthae (D. cooperi, D. chrysostachya, D. polygonoides, D. rhodantha, D. scullyi and D. thodei). In these species, a fraction of the stomata did not secrete nectar during the observation period, and epidermal nectar secretion was never observed. Clearly defined nectar droplets accumulated over stomata, whereas the surrounding epidermis remained dry in buds of D. polygonoides (Fig. 1A, B), D. cylindrica, D. cooperi (Fig. 1C), D. rhodantha, D. scullyi and D. thodei and mature flowers of D. chrysostachya (Fig. 1D). These droplets increased until they collapsed and wetted the surrounding epidermis. Disa brevicornis (section Monadenia), D. crassicornis, D. versicolor and D. zuluensis (all Micranthae) also had stomata; however, stereomicroscopic observations were inconclusive concerning their involvement in nectar exudation. Disa brevicornis and D. versicolor produce minute nectar volumes (Johnson, 1995; N. Hobbhahn, unpubl. res.), which probably evaporated before detection and so were not observed. Only mature flowers picked several days before examination were available for D. crassicornis and D. zuluensis, and these did not resume nectar production in the hydration chamber after nectar removal.
Species with epidermal nectaries belonged to several sections, namely Disella (D. elegans), Phlebidia (D. longicornu), Disa (D. uniflora) and Atromaculiferae (D. vaginata and D. glandulosa). In these species, nectar accumulated in irregular patches on the epidermis (Fig. 2A, B). Disa elegans, which lacks a floral spur, exuded copious nectar from a morphologically uniform epidermis, devoid of papillae, trichomes or stomata, on the upper (adaxial) and lower (abaxial) surfaces of petals (Fig. 2A) and lip. Disa longicornu secreted nectar mainly from the oblong epidermal cells on the inner (abaxial) surface of the petals (Fig. 2B). The spur epidermis produced only traces of nectar, primarily above the veins and, to a lesser extent, the surrounding areas. Nectar was exuded primarily on the distal third of both petals and spur, and was not associated with veins in the petals. The outer (adaxial) epidermis of the petals appeared not to exude nectar. Disa uniflora, D. glandulosa and D. vaginata exuded nectar only from the spur epidermis. The epidermal cells of D. uniflora resumed nectar production after nectar removal, swelling noticeably (Fig. 2C) before small, irregular patches of nectar appeared that eventually coated the entire epidermis (Fig. 2D).
Tissue characteristics
Nectarless species
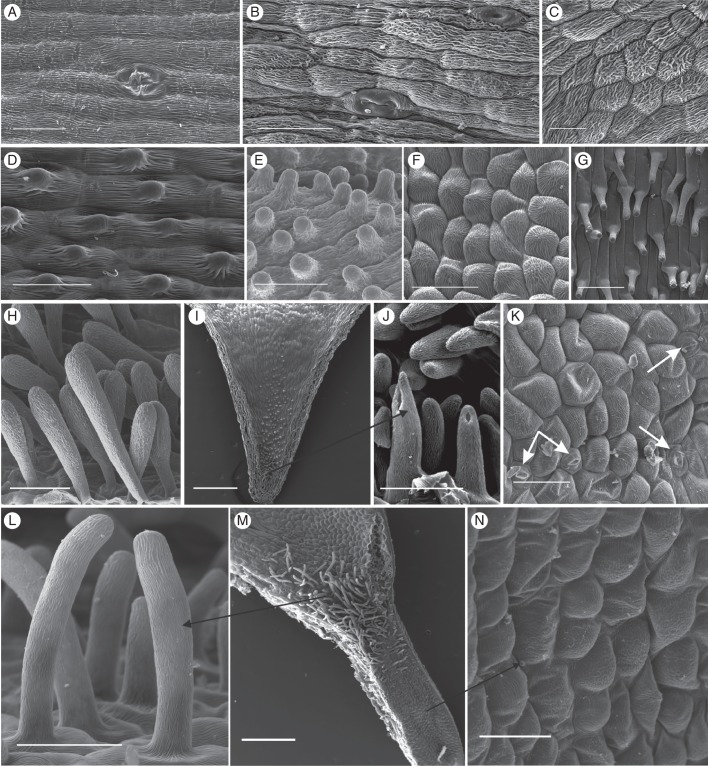
Nectarless Disa species displayed a variety of epidermal cell shapes and cuticular patterns. The slightly convex epidermis cells were oblong to isodiametric and tetra- to polygonal, with longitudinal, irregular or radiating cuticular striations (Fig. 3A–C, F, K, M). Epidermal cells in the spurs of D. graminifolia, D. hians, D. nervosa (Fig. 3D), D. obtusa subsp. hottentotica (Fig. 3E), D. patula var. transvaalensis and D. tripetaloides, and in the hood-shaped dorsal sepal of D. rosea had short central papillae with thick cuticular striations that radiated from the papilla tip but largely aligned in folds parallel to the longitudinal cell axis. The hood-shaped dorsal sepal of D. aconitoides subsp. aconitoides was lined with shortly papillate cells in its distal third; the remainder of the hood was lined with only slightly convex, polygonal cells with weak and irregular cuticular striations. In D. obliqua subsp. obliqua and D. uncinata the spur epidermis consisted of convex, isodiametric, polygonal cells (Fig. 3F, M), which in the spur entrance extended into papillae and unicellular trichomes, respectively, with longitudinal cuticular striations (Fig. 3G, L, M). Disa cephalotes subsp. cephalotes had unicellular, club-shaped hairs with thick cuticular striations in most of its spur (Fig. 3H); however, the spur tip was often devoid of hairs and lined with smooth cells. Disa caulescens had convex, oblong, tetragonal epidermal cells, and those in the distal third of the spur had short papillae with thick cuticular striations, which increased in length towards the tip (Fig. 3I, J). The spur epidermis of D. sagittalis exhibited numerous cuticular blisters that were evenly distributed over the thickly cuticularized cells and similar blisters occurred on the epidermis of the petals and exterior of the spur.
Fig. 3.
Scanning electron micrographs of the spur epidermis of selected nectarless Disa species. (A) Oblong epidermis cells with longitudinal striations and a malformed stoma near the spur tip of D. harveiana subsp. longicalcarata. (B) Mature and immature stoma near the spur tip of D. tysonii. (C) Thick, irregular cuticular striations characterize the entire spur epidermis of D. stachyoides. (D) Thickly cuticularized, papillate cells line the entire spur of D. nervosa. (E) Papillate epidermis cells line the entire spur of D. obtusa subsp. hottentotica. (F, G) Cells lining the spur (F) and papillae (G) in spur entrance of D. obliqua subsp. obliqua. (H) Club-shaped unicellular trichomes of D. cephalotes subsp. cephalotes. (I) Spur overview of D. caulescens. (J) Close-up of papillae in spur tip. The tips of the frontal two papillae were damaged during spur sectioning. (K) Scattered stomata in D. racemosa (indicated by arrows). (L–N) D. uncinata. (L) Spur epidermis; (M) overview of spur section showing field of trichomes in spur entrance; (N): close-up of unicellular trichomes. Scale bars: (A–F, H, J, L, N) = 50 μm; (G, K) = 100 μm; (I, M) = 0·5 mm.
Stomata were present in the flowers of 25 % of the nectarless species (Appendix). They were oriented parallel to the longitudinal spur axis in sections Reticulibractea (Fig. 3A) and Repandra (Fig. 3B). In D. aconitoides, stomata were oriented parallel to the longitudinal hood axis and occurred mainly near the hood tip. The dorsal sepal of both Disa filicornis and D. racemosa does not extend into spur, but instead has a shallow, central fold that is probed by visiting insects and is the location of all (D. filicornis), or almost all (D. racemosa; Fig. 3K), floral stomata in these species. Stomata were scattered throughout the bowl-shaped dorsal sepal of Disa bodkinii, but were sparse at the sepal base.
Stomatal nectaries
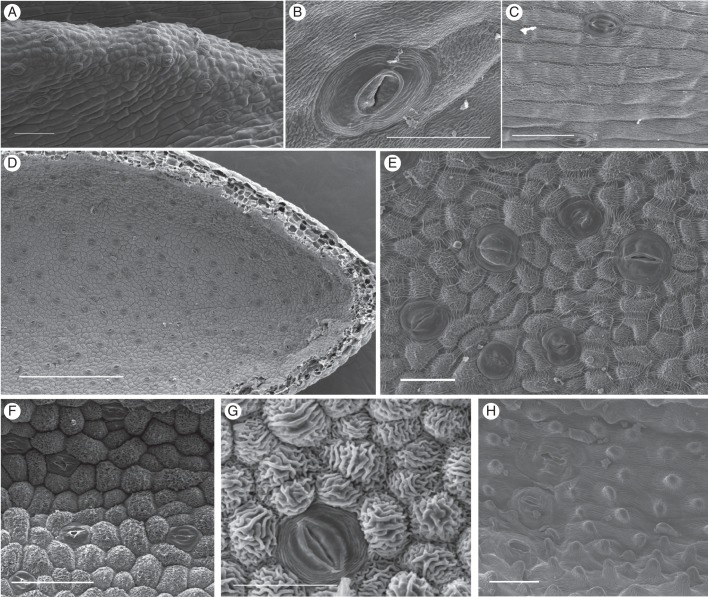
All members of sections Monadenia and Micranthae examined by SEM had numerous stomata in their floral spurs. Stomata were generally distributed throughout floral spurs, but their density often increased towards the tip. However, in D. brevicornis stomata were clustered along two ridges that protruded from the roof of the spur (Fig. 4A), whereas in D. cylindrica they were clustered on a callus on the base of the spur; in both species the remainder of spurs was mostly free of stomata. Most stomata were solitary, but paired stomata occurred occasionally. Stomata were elliptical to circular and generally oriented parallel to the longitudinal spur axis (Fig. 4A, C, H), except for D. chrysostachya, which had scattered stomata (Fig. 4D, E). The cuticular ledges of the guard cells formed an elliptical opening over the stomatal pore, which was partially occluded by protruding lateral guard-cell walls (Fig. 4B, C, G). The guard cells were covered with a smooth cuticle, but in most species were surrounded by a ring of concentric cuticular folds, which may have covered very small subsidiary cells (Fig. 4B, C, G, H), although subsidiary cells were not clearly distinguishable in the examined species.
Fig. 4.
Scanning electron micrographs of nectar-exuding tissues in Disa species with stomatal nectaries. (A) Stoma-studded ridge in spur of D. brevicornis. (B) D. sabulosa. (C) D. rufescens. (D, E) High density of modified stomata in the spur of D. chrysostachya. (F) D. scullyi. (G) D. versicolor. (H) D. crassicornis. Scale bars: (A, C, F) = 100 μm; (B) = 20 μm; (D) = 500 μm; (E, G, H) = 50 μm.
Epidermis cells of the predominantly nectar-producing sections Monadenia (Fig. 4A–C) and Micranthae (Fig. 4D–H) differed in shape and cuticular striation. In Monadenia, the epidermis surrounding stomata consisted of slightly convex, oblong, tetra- to polygonal cells. The pronounced cuticular striations were predominantly parallel to the longitudinal cell axis (Fig. 4A–C). In Micranthae, the predominantly polygonal, convex epidermis cells were more often isodiametric than elongate. Cuticular striations were generally pronounced and oriented irregularly across the cell surface (D. galpinii, D. sankeyi, D. cylindrica and D. scullyi; Fig. 4F), parallel to the longitudinal cell axis (D. cylindrica, D. woodii and D. versicolor; Fig. 4G), or radiated from a central papilla (D. crassicornis; Fig. 4H). More elaborate cuticular patterns consisted of an anticlinal frame of radiating striations surrounding a central field with striations that were predominantly parallel to the longitudinal cell axis (D. fragrans and D. cooperi) or irregular (D. cooperi and D. chrysostachya; Fig. 4E).
Secretory epidermis
As in several other orchid species with secretory epidermis (Figueiredo and Pais, 1992; Stpiczyńska and Matusiewicz, 2001; Stpiczyńska et al., 2005a; Davies and Stpiczyńska, 2007; Bell et al., 2009), epidermal nectaries in Disa were characterized by a morphologically uniform epidermis devoid of stomata (Appendix; Fig. 5), except for some specimens of D. uniflora and D. longicornu, which had a few stomata (Appendix). The secretory epidermis on petals and lip of D. elegans consisted uniformly of polygonal, isodiametric, slightly convex cells with pronounced radiating or parallel cuticular striations (Fig. 5A). The epidermis of the petals and spur of D. longicornu and the spurs of D. uniflora, D. vaginata, D. salteri, D. tenuis and D. rungweensis consisted of oblong, tetra- to polygonal, slightly convex cells with weak cuticular striations predominantly parallel to the longitudinal cell axis (Fig. 5B–E, G, I). The spur tips of D. salteri and D. tenuis contained several short papillae with pronounced cuticular striations (Fig. 5F, J, K), which resembled those observed in other orchids with a papillose secretory epidermis (Galetto et al., 1997; Stpiczyńska, 1997; Stpiczyńska and Matusiewicz, 2001; Stpiczyńska et al., 2005b; Davies and Stpiczyńska, 2007; Johnson et al., 2007; Bell et al., 2009; de Melo et al., 2010) and may be involved in nectar resorption (Stpiczyńska, 2003; Stpiczyńska et al., 2005b; Nepi and Stpiczyńska, 2007). In some specimens of D. rungweensis, examination at high magnification (maximum ×12 800) revealed a nectary cuticula distended into small, irregular protrusions (Fig. 5G), at the base of which the cuticula sometimes appeared to have holes (Fig. 5H). Nectar may flow into the spur lumen through these holes, making D. rungweensis the only study species with a secretory epidermis for which a mechanism by which nectar crosses the cuticula suggests itself. In all other Disa species with secretory epidermis, the mechanism by which nectar passes through the nectary cuticula remains to be resolved. The absence of collapsed nectary cells indicates that nectar exudation does not involve cell lysis. The magnification used in our SEM studies of other species with secretory epidermis (maximum ×1449) did not allow us to exclude the presence of microscopic pores or fissures in these species. However, the nectary cuticle can be permeable even in the absence of such microscopic outlets if nectar accumulating between the tangential epidermis cell wall and cuticle stretches the cuticle (e.g. Stpiczyńska et al., 2003; Stpiczyńska et al., 2005a; de Melo et al., 2010). The distinct swelling of epidermal cells of D. uniflora observed under the stereomicroscope may signal this process. The few stomata in D. uniflora spurs and on the petals of D. longicornu are likely not involved in nectar exudation, given their occurrence in only some specimens and the active excretion of nectar by epidermal cells in both species.
Fig. 5.
Scanning electron micrographs of nectar-exuding tissues in Disa species with secretory epidermis. (A) Petal of D. elegans. (B) Petal of D. longicornu. (C) Spur of D. uniflora. (D) Spur of D. vaginata. (E) Spur cells of D. salteri. (F) Papillae with pronounced cuticular striations in spur tip of D. salteri. (G, H) D. rungweensis. (G) Spur; (H) detail of cuticular blisters. (I–K) D. tenuis. (I) Spur epidermis; (J) overview of spur section showing field of short papillae; (K) Close-up of bulbous papillae. Scale bars: (A, D, F, G, I, K) = 50 μm; (B, C, E) = 100 μm; (H) = 10 μm; (J) = 0·5 mm.
All examined Brownleea species produce nectar (Larsen et al., 2008) but lacked floral stomata, so they likely have a secretory epidermis. The spur of B. galpinii was lined with convex, oblong, tetragonal cells with very weak cuticular striations (Supplementary Data Fig. S5A); those in the spur tip were isodiametric and strongly convex (Fig. S5B). The spur epidermis of B. macroceras consisted of slightly convex, oblong, tetragonal cells with short papillae and thick cuticular striations parallel to the longitudinal cell axis (Fig. S5C). Its spur tip was lined with isodiametric, tetragonal cells with very weak cuticular striations (Fig. S5D). The narrow spur entrance of B. parviflora was lined with long unicellular trichomes lacking cuticular striations (Fig. S5E, F), whereas the remainder of the spur epidermis cells were oblong-tetragonal and had short papillae and pronounced parallel or radiating cuticular striations (Fig. S5G).
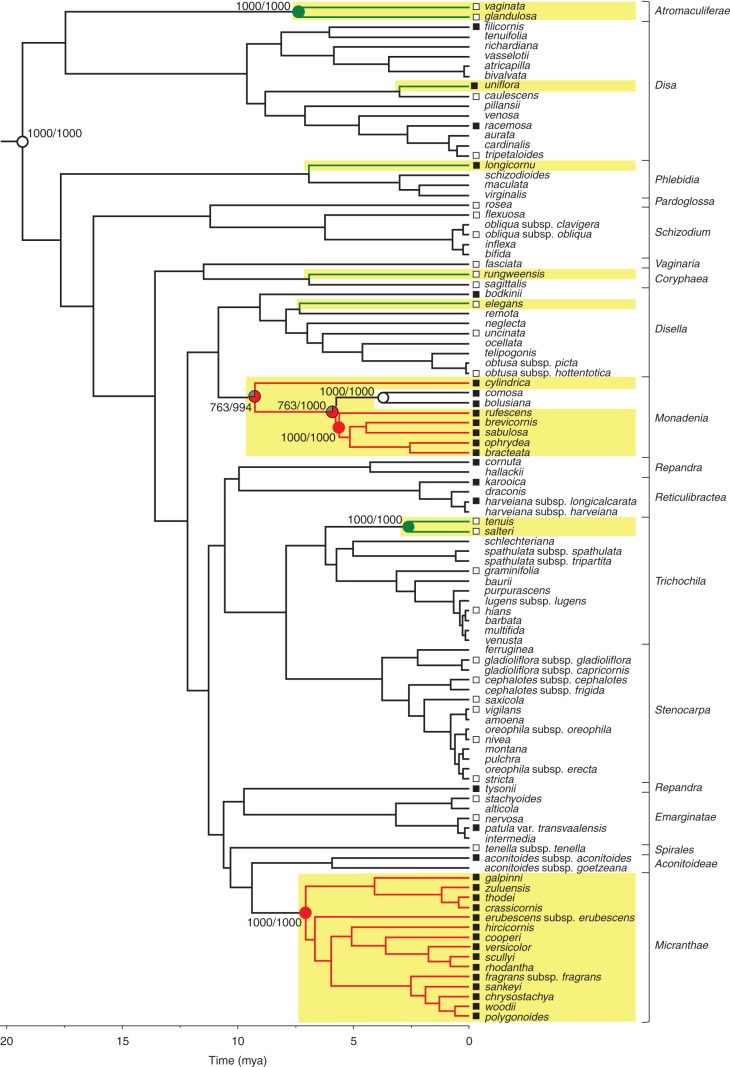
Evolution of nectaries and associated traits
The occurrence of stomata and nectary type, but not stomata number or size or spur wall thickness, exhibited significant phylogenetic conservatism (Table 1). Parsimony analysis correctly identified the nectarless root node (Johnson et al., 2013) as having no nectary (Fig. 6). Stomatal nectaries evolved between two and four times in the genus (mean estimate, 2·1, 103 species), whereas a secretory epidermis evolved six times. Stomatal nectaries were lost up to two times (mean estimate, 0·9) in a small nectarless clade consisting of D. comosa and D. bolusiana, which is embedded in the nectar-producing section Monadenia. By comparison, secretory epidermis was never lost after having evolved, and no transitions between nectary types were evident (Fig. 6). Ancestral state reconstruction for stomata was less conclusive, likely owing to the smaller sample (60 species). The root node was reconstructed as equivocal, even when the analysis included Brownleea. Stomata evolved between 0 and 10 times (mean estimate, 4·8, 1000 chronograms), and were lost times between 0 and 10 times (mean estimate, 4·7; see Supplementary Data Figs S1–S3, and Table S3 for node-state reconstructions). Given the lability of stoma occurrence, a model of dependent evolution of floral stomata and nectar production did not fit the data significantly better than a model of independent evolution (G4 = 7·65, P > 0·1, n = 60 species), indicating that the traits did not evolve in a correlated fashion and that the occurrence of stomata did not facilitate transitions to nectar production.
Table 1.
Phylogenetic signal of nectary-associated traits in Disa. For discrete traits (A), phylogenetic conservatism is indicated if the number of parsimony steps in the observed state distribution is outside the 95 % confidence interval (LCI, lower confidence interval; UCI, upper confidence interval) of the randomized state distribution [reported as mean (LCI − UCI)] in 1000 trait reshufflings. For continuous traits (B), P indicates the probability of a given K owing solely to sampling error (random trait distribution over phylogeny). K is reported as mean ± SE and P as the median (1st quartile, 3rd quartile) due to non-normality (see Supplementary Data Fig. S4). n represents the number of sampled species.
| (A) Discrete traits | |||
|---|---|---|---|
| Parsimony steps in observed state distribution | Parsimony steps in randomized state distribution | n | |
| Stomata | 10 | 19·7 (15·3, 23·5) | 60 |
| Nectary type | 9 | 24·1 (22·2, 27·4) | 103 |
| (B) Continuous traits | |||
|---|---|---|---|
| K | P | n | |
| Number of stomata (when present) | 0·55 ± 0·002 | 0·33 (0·23, 0·42) | 31 |
| Stoma size | 0·41 ± 0·001 | 0·24 (0·20, 0·28) | 31 |
| Spur wall thickness | 0·30 ± 0·001 | 0·22 (0·15, 0·30) | 48 |
Fig. 6.
Evolution of nectary types in 103 Disa species based on parsimony reconstruction. From an ancestor without nectaries, stomatal nectaries evolved at least twice (red branches) and nectar-secreting epidermis evolved six times (green branches). Nectar-producing clades are highlighted in yellow. Parsimony reconstruction of ancestral states over 1000 chronograms are summarized on the maximum-clade credibility chronogram. Empty circles indicate absence of (root) or loss of nectaries, filled circles mark nodes for which evolution of the respective nectary type is supported as optimal state by parsimony reconstruction in ≥75 % of trees containing the node (grey sectors represent the proportion of equivocal reconstructions). Support values indicate (number of trees in which state was identified as optimal/number of trees containing the node). Squares at branch tips indicate presence (filled symbols) or absence (open symbols) of stomata in 60 species examined by scanning electron microscopy or high-magnification light microscopy. For support values of all nodes see Supplementary Data Table S3. Rectangular brackets delimit Disa sections according to Bytebier et al. (2008).
After adjustment for phylogenetic relatedness, nectar-producing and nectarless species whose flowers had stomata did not differ significantly in either stomata number or area (Table 2). The examined Disa species had on average 7·0 stomata on the examined flower parts (lower standard error [LSE] = 4·45, upper standard error [USE] = 12·34) with an average (±SE) area of 1·46 ± 0·001 mm2. However, the spurs of nectar-producing species consisted of significantly more cell layers (mean = 7·7 cells, LSE = 0·74, USE = 0·92) than those of nectarless species (mean = 6·2 cells, LSE = 0·59, USE = 0·73; Table 2). This difference resulted from the atypically robust flowers of the nectar-producing D. uniflora, as exclusion of this species rendered the difference non-significant (Table 2; overall mean excluding D. uniflora = 6·5 cell layers, LSE = 0·69, USE = 0·57). The stomatal nectaries of the predominantly nectar-producing sections Monadenia and Micranthae did not differ significantly in stomata number, stoma area or spur wall thickness (Table 2). Furthermore, spur wall thickness did not differ significantly between species with secretory epidermis and those with stomatal nectaries (Table 2).
Table 2.
Comparison of nectary-associated traits between nectarless and nectar-producing Disa species, between sections Monadenia and Micranthae, and between nectary types. Summary of results of phylogenetic estimating equations (pGEEs) using 1000 chronograms to account for phylogenetic uncertainty. d.f., degrees of freedom of t-test in pGEE. t and P are reported as medians (1st quartile, 3rd quartile) due to non-normality (see Supplementary Data Fig. S4 for frequency distribution of P obtained from 1000 chronograms). n represents the number of sampled species: total species (species in first category of comparison, species in second category).
| Comparison | d.f. | t | P | n |
|---|---|---|---|---|
| Nectarless vs. nectar-producing species | ||||
| Stomata number (all examined flower parts) | 12·5 | 0·06 (0·03, 0·13) | 0·93 (0·89, 0·97) | 31 (12, 19) |
| Stomata number in spurs | 10·9 | 0·73 (0·66, 0·80) | 0·48 (0·45, 0·53) | 28 (7, 19) |
| Stoma area (all examined flower parts) | 12·5 | 1·89 (1·77, 2·01) | 0·09 (0·07, 0·10) | 31 (12, 19) |
| Stoma area (spurs only) | 10·9 | 0·55 (0·48, 0·62) | 0·36 (0·32, 0·39) | 28 (7, 19) |
| Spur wall thickness | 17·7 | 3·40 (2·88, 3·79) | 0·004 (0·0001- 0·009) | 48 (26, 22) |
| Spur wall thickness excluding D. uniflora | 17·5 | 0·29 (0·17, 0·42) | 0·77 (0·68, 0·87) | 47 (26, 21) |
| Sections Monadenia vs. Micranthae | ||||
| Stomata number in spurs | 8·3 | 0·21 (0·19, 0·24) | 0·84 (0·82, 0·85) | 19 (8, 11) |
| Stoma area (spurs only) | 8·3 | 0·07 (0·04, 0·10) | 0·95 (0·93, 0·97) | 19 (8, 11) |
| Spur wall thickness | 8·2 | 2·04 (1·98, 2·10) | 0·09 (0·08, 0·09) | 18 (8, 10) |
| Wall thickness of spurs with secretory epidermis vs. spurs with stomatal nectaries | 9·3 | 0·26 (0·18, 0·34) | 0·80 (0·74, 0·86) | 22 (6, 18) |
DISCUSSION
The repeated evolution of nectar production in Disa involved diversifying evolution of both nectary type and position, although both nectary types evolved repeatedly in the genus and therefore provide evidence for some recapitulation. Ancestral absence of nectaries indicates that both stomatal nectaries and secretory epidermis represent novelties in Disa. The other nectar-producing genera in the Diseae, Brownleea and Satyrium, secrete nectar from trichomes or a morphologically uniform spur epidermis (Brownleea, this study; Satyrium, Johnson et al., 2007; T van der Niet, Naturalis Biodiversity Institute, Netherlands, and N. Hobbhahn, unpublished observations of seven species). Consequently, the stomatal nectaries of Disa appear to be uniquely derived within the Diseae, whereas epidermal nectaries appear to be recapitulated within the tribe. Nectary diversification within Disa implies weak ancestral genetic, developmental or physiological constraints on nectar production, nectary type and position (cf. Baum et al., 2001). Even greater nectary diversity in the Orchidaceae (e.g. Galetto et al., 1997; Davies et al., 2005; Davies and Stpiczyńska, 2007; Johnson et al., 2007; Bell et al., 2009; de Melo et al., 2010) suggests widespread absence of such constraints in the family as a whole. Nevertheless, significant phylogenetic conservatism of nectary type, including the lack of direct transitions between stomatal and epidermal nectaries, indicates that once nectar production evolves, further evolution is restricted to limited modifications of an established nectary type, or occasionally loss of function.
Nectarless species
Although lack of nectar production is ancestral in Disa (Hobbhahn, 2012; Johnson et al., 2013), nectarless species differ extensively in features of their floral epidermis (Figs 2 and 4A–C). This variation is evident in the incidence and form of three-dimensional epidermal structures, which may help to retain floral visitors on rewardless flowers by providing tactile stimuli that require processing and stimulate exploration (cf. Davies and Stpiczyńska, 2010; Ellis and Johnson, 2010), and thereby promote pollination.
The role of stomata in the spurs of some nectarless species is largely puzzling. In D. comosa and D. bolusiana, which are rare cases of loss of nectar production within a nectar-producing clade (section Monadenia), floral stomata may be dysfunctional rudimentary nectaries. In other nectarless species, stomata on exposed flower parts, such as the spurless dorsal sepal of D. filicornis, D. racemosa and D. bodkinii, may be involved in scent emission and/or gas exchange (Effmert et al., 2005; de Melo et al., 2010). However, most nectarless Disa species do not produce discernible scent (e.g. Johnson and Steiner, 1997; Kurzweil et al., 1997; Johnson, 2000; S. D. Johnson, unpubl. res.). Floral stomata were not associated with green flowers or flower parts, so they are likely not involved in floral photosynthesis. Furthermore, stomata in spurs probably contribute little to gas exchange, as 63 % of the examined species lack them. Whatever their function, the presence of stomata does not strongly predispose to the evolution of stomatal nectaries, as is indicated most clearly by those few specimens of D. uniflora and D. longicornu that have a few stomata in their spur epidermis, but secrete nectar from a secretory epidermis.
Stomatal nectaries
Stomatal floral nectaries occur commonly in several angiosperm lineages (Bernardello, 2007; Nepi, 2007). However, they have been recorded only once previously in the Orchidaceae, namely in the Epidendroideae (Maxillaria; Davies et al., 2005), making Disa the first record for the Orchidoideae. Despite the apparent rarity of stomatal nectaries in orchids, the differences in the shape and cuticular striation of spur epidermal cells between Disa sections Monadenia and Micranthae indicate multiple independent origins of this nectary type within Disa. Interestingly, not all stomata secreted nectar in the species examined stereomicroscopically (also cf. Gaffal et al., 1998), suggesting that either some stomata retain their ancestral, if unknown, function or that they remain functionless throughout the flower's lifespan.
As in numerous other species with stomatal nectaries (Davies et al., 2005; Nepi, 2007; but see Daumann, 1974), the thickly cuticularized epidermis surrounding the stomata of Disa flowers appears not to be involved in nectar excretion. A thickly cuticularized epidermis is also correlated with nectarlessness in several Orchidinae species (Bell et al., 2009). Thick cuticle may impede nectar excretion by epidermis cells, necessitating excretion through modified stomata.
Secretory epidermis
Nectar excretion by secretory epidermis evolved independently at least six times; five of the six origins were reconstructed in mostly nectarless sections of Disa, generating diversity of nectary location and morphology of secretory epidermal cells. In most Disa species with epidermal nectaries, the secretory cells occur in the spur formed by the dorsal sepal (outer perianth whorls) and are morphologically similar among species. The similarity of the secretory epidermis cells of D. uniflora and closely related species in section Atromaculifera may represent a common origin, whereas the papillae in the spur tips of D. salteri and D. tenuis support an independent origin of nectar production in these species. Further independent origins of nectar production are evidenced by the occurrence of nectaries on the inner perianth whorl (petals and lip) in D. elegans and on parts of the inner (petals) and outer perianth whorl (spurred dorsal sepal) in D. longicornu. The dissimilarity of the secretory epidermis cells of D. elegans from those of other species with the same nectary type further supports an independent origin of nectar production in this species. Disa elegans is pollinated mainly by cetoniine beetles (Scarabaeidae; S. D. Johnson, et al., unpubl. res.), which have short mouthparts and could not access the nectar if it was concealed in a spur. Easily accessible nectar secreted on the exposed surfaces of petals and lip draws nectar-foraging beetles into the flower centre, where reproductive structures are located. Interestingly, nectar exudes from both surfaces of petals and lip. The nectar exuded on the adaxial surfaces is more difficult to reach, forcing beetles to move around on the flower, thereby increasing the likelihood of pollen removal and deposition. In the long-spurred D. longicornu, which is probably pollinated by long-tongued flies, nectar is produced mostly by the long, narrow petals, which are concealed in the spur. Other rewarding Disa species pollinated by long-tongued flies (e.g. D. scullyi, D. rhodantha and D. zuluensis) produce nectar in a spur lacking three-dimensional structures, such as trichomes, grooves or ridges, and their petals do not extend into the spur or exude nectar. This absence of three-dimensional structures suggests that long-tongued flies do not require a tactile stimulus to feed. Therefore, the unusual position of the nectar-producing structures in D. longicornu represents an alternative solution to providing nectar rewards in the spur, which may have been necessitated by structural or functional constraints on nectar production by the spur nectary (e.g. poorer vascular supply, reduced functionality or activity of nectary cells).
Convergence and diversification
The recurring evolution of nectar production in Disa clearly illustrates convergent functional evolution achieved by both recapitulation and innovation. Recapitulation is suggested by the repeated evolution of secretory epidermis, which apparently also occurs in the sister genera Brownleea and Satyrium. Nevertheless, the morphological and positional diversity of the secretory epidermis among Disa species suggests that it has not evolved simply by reactivation of the same developmental and physiological pathways, and so involves some innovation. Furthermore, the evolution of stomatal nectaries, especially in clades that otherwise lack floral stomata, clearly represents morphological innovation. The conditions that favoured one of these solutions over another are obscure. Importantly, whether a particular nectary type evolved in response to specific ecological conditions (e.g. water economy) or because it was subject to the weakest genetic and developmental constraints remains to be determined. In the absence of consistent differences in epidermis morphology between rewarding and rewardless species, examination of the genetic architecture of nectar production and comparative studies of the development and histology of nectar-producing and positionally equivalent non-secreting tissues are required to elucidate the sub-cellular modifications required for transitions between rewardlessness and nectar production. Regardless of the mechanism, the frequent evolution of nectar production implies that it evolves readily when it promotes mating with relatively limited resource costs (Harder and Barrett, 1992; Golubov et al., 1999; Hobbhahn, 2012). Furthermore, the Disa example clearly illustrates the contribution of functional convergence to phenotypic diversification.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: origin of plant material of Disa and Brownleea examined. Table S2: mean stomata and spur characteristics of Disa species with uncertain nectar status that were examined by scanning electron microscopy but excluded from analysis. Table S3: reconstructed states from parsimony analyses of stomata occurrence and nectary type in Disa. Figure S1: ancestral state reconstruction of the occurrence of stomata in Disa by parsimony. Figure S2: node numbers associated with ancestral state reconstruction of stomata occurrence on the maximum-clade credibility chronogram containing 60 species. Figure S3: node numbers associated with ancestral state reconstruction of nectary type by parsimony on the maximum-clade credibility chronogram containing 103 species. Figure S4: frequency distributions of P values obtained from 1000 chronograms used to account for phylogenetic uncertainty in tests for phylogenetic signal in continuous traits, and in comparisons using phylogenetic generalized estimating equations. Figure S5: scanning electron micrographs of spur tissues of the examined Brownleea species.
ACKNOWLEDGEMENTS
For use of pickled flower material we thank the Bolus Herbarium of the University of Cape Town, the Bews Herbarium of the University of KwaZulu-Natal and Timotheüs van der Niet (Naturalis Biodiversity Institute, Netherlands). We thank the Centre for Electron Microscopy at the University of KwaZulu-Natal, Pietermaritzburg, the Electron Microscope Unit at the University of Cape Town and Petra Muller from the Zoology Department, University of Cape Town, for microscope access and assistance with sample preparation and microscopy. Ruth Cozien is thanked for help with specimen collection in the field. Alexandre Antonelli and Michael D. Pirie are thanked for helpful discussions of phylogenetic analyses. This work was supported by the Alberta Ingenuity Fund (N. H.), the Natural Sciences and Engineering Research Council of Canada (L. D. H., E. C. Y.) and the South African National Science Foundation (S. D. J., B. B.).
APPENDIX
Appendix.
Microscopic analysis, incidence of nectar, nectary type and location, number of modified stomata per examined flower part, stoma size (mm2) and spur wall thickness (cell layers) in the examined species of Disa and Brownleea.
| Taxon | Analysis | Nectar | Nectary type and location | Number of stomata | Stoma size (mm2) | Spur wall thickness (cell layers) |
|---|---|---|---|---|---|---|
| Brownleea | ||||||
| B. galpinii subsp. major | SEM | 1 | S | 0 (2) | – | – |
| B. macroceras | SEM | 1 | S | 0 (2) | – | – |
| B. parviflora | SEM | 1 | S | 0 (2) | – | – |
| Disa | ||||||
| D. caulescens | SEM | 0 | 0 | 0 (6) | – | 6 ± 0·2 (17, 2) |
| D. filicornis | SEM | 0 | 0 | 10·5 ± 1·5 (2)* | 0·83 ± 0·045 (9, 1) | 6 ± 0·5 (2, 1) |
| D. racemosa | SEM | 0 | 0 | 90 ± 52 (2)* | 1·49 ± 0·055 (25, 1) | 10 ± 0·5 (2, 1) |
| D. tripetaloides | SEM | 0 | 0 | 0 (2) | – | 8 ± 0·4 (10, 2) |
| D. uniflora | SM, SEM | 1 | 2, S | 3·9 ± 2·4 (11) | 2·78 ± 0·404 (7, 3) | 13 ± 0·6 (17, 3) |
| Atromaculiferae | ||||||
| D. glandulosa | SM, SEM | 1 | 2, S | 0 (3) | – | – |
| D. vaginata | SM, SEM | 1 | 2, S | 0 (2) | – | 6 ± 0·3 (11, 2) |
| Phlebidia | ||||||
| D. longicornu | SM, SEM | 1 | 2, P, (S) | 0·3 ± 0·2 (7) | 1·69 ± 0·495 (2, 2) | 8 ± 0·4 (12, 2) |
| Pardoglossa | ||||||
| D. rosea | SEM | 0 | 0 | 0 (2) | – | 5 ± 0 (2, 1) |
| Schizodium | ||||||
| D. flexuosa | SEM | 0 | 0 | 0 (2) | – | 6 ± 0·2 (5, 1) |
| D. obliqua subsp. obliqua | SEM | 0 | 0 | 0 (4) | – | 5 ± 0·2 (20, 3) |
| Vaginaria | ||||||
| D. fasciata | SEM | 0 | 0 | 0 (1) | – | 6 ± 0·3 (3, 1) |
| Coryphaea | ||||||
| D. sagittalis | SEM | 0 | 0 | 0·7 ± 0 (4) | 0·64 (1, 4) | 5 ± 0·3 (11, 4) |
| D. rungweensis | SEM | 1 | 2, 0 | 0 (3) | – | 6 ± 0·2 (14, 3) |
| Disella | ||||||
| D. bodkinii | SEM | 0 | 0 | 233·5 ± 4·5 (2)* | 1·61 ± 0·107 (7, 2) | – |
| D. elegans | SM, SEM | 1 | 2, P, L | 0 (3) | – | – |
| D. obtusa subsp. hottentotica | SEM | 0 | 0 | 0 (4) | – | 5 ± 0·2 (6, 2) |
| D. uncinata | SEM | 0 | 0 | 0 (2) | – | 5 ± 0·6 (3, 1) |
| Monadenia | ||||||
| D. bolusiana | SEM | 0 | 0 | 44·5 ± 0·5 (2) | 1·24 ± 0·078 (6, 1) | 6 ± 0·3 (7, 1) |
| D. bracteata | SEM | 1 | 1, S | 31 ± 6·1 (6) | 1·17 ± 0·065 (7, 3) | 4 ± 0·2 (10, 2) |
| D. brevicornis | SM, SEM | 1 | 1, S | 77 (1) | 1·14 ± 0·069 (5, 1) | 6 ± 0·2 (7, 1) |
| D. comosa | SEM | 0 | 0 | 30·5 ± 0·5 (2) | 1·7 ± 0·154 (9, 1) | 7 ± 0·4 (6, 1) |
| D. cylindrica | SM, SEM | 1 | 1, S | 39·3 ± 11·9 (3) | 1·31 ± 0·164 (5, 2) | 5 ± 0·2 (13, 2) |
| D. ophrydea | SEM | 1 | 1, S | 11·5 ± 3·5 (2) | 0·94 ± 0·064 (4, 1) | 5 ± 0·4 (14, 2) |
| D. rufescens | SEM | 1 | 1, S | 26 ± 1 (2) | 1·92 ± 0·142 (7, 2) | 7 ± 0·7 (7, 1) |
| D. sabulosa | SEM | 1 | 1, S | 30 ± 2 (2) | 1·73 ± 0·148 (5, 2) | 6 ± 0·2 (9, 2) |
| Reticulibractea | ||||||
| D. harveiana subsp. longicalcarata | SEM | 0 | 0 | 8 ± 2·5 (3) | 1·73 ± 0·126 (7, 1) | 9 ± 0·4 (15, 2) |
| D. karooica | SEM | 0 | 0 | 2·5 ± 0·5 (2) | 2 ± 0·327 (3, 2) | 8 ± 0·3 (9, 1) |
| Repandra | ||||||
| D. cornuta | SEM | 0 | 0 | 59 (1) | 1·1 ± 0·102 (7, 1) | – |
| D. tysonii | SEM | 0 | 0 | 69·7 ± 29 (3) | 0·97 ± 0·059 (36, 2) | 8 ± 0·3 (15, 3) |
| Trichochila | ||||||
| D. graminifolia | SEM | 0 | 0 | 0 (1) | – | 7 ± 0·7 (5, 1) |
| D. hians | SEM | 0 | 0 | 0 (2) | – | 6 ± 0·3 (7, 1) |
| D. salteri | SEM | 1 | 2, S | 0 (3) | – | 5 ± 0·3 (16, 3) |
| D. tenuis | SEM | 1 | 2, S | 0 (1) | – | 6 ± 0·4 (5, 1) |
| Stenocarpa | ||||||
| D. cephalotes subsp. cephalotes | SEM | 0 | 0 | 0 (1) | – | 5 ± 0·2 (5, 1) |
| D. gladioliflora subsp. gladioliflora | SEM | 0 | 0 | 0 (2) | – | 6 ± 0·3 (11, 2) |
| D. nivea | SEM | 0 | 0 | 0 (1) | – | 7 ± 0·6 (5, 1) |
| D. saxicola | SEM | 0 | 0 | 0 (2) | – | 6 ± 0·3 (8, 1) |
| D. stricta | SEM | 0 | 0 | 0 (2) | – | 4 ± 0·2 (7, 1) |
| D. vigilans | SEM | 0 | 0 | 0 (1) | – | 7 ± 0·5 (4, 1) |
| Emarginatae | ||||||
| D. nervosa | SEM | 0 | 0 | 0 (1) | – | 7 ± 0·3 (3, 1) |
| D. patula var. transvaalensis | SEM | 0 | 0 | 2 (1) | 0·9 ± 0·161 (5, 1) | 7 ± 0·4 (7, 1) |
| D. stachyoides | SEM | 0 | 0 | 0 (1) | – | 6 ± 0·3 (4, 1) |
| Spirales | ||||||
| D. tenella subsp. tenella | SEM | 0 | 0 | 0 (2) | – | 7 ± 0·3 (9, 2) |
| Aconitoideae | ||||||
| D. aconitoides subsp. aconitoides | SEM | 0 | 0 | 3 ± 3 (2) | 1·11 ± 0·183 (3, 2) | 4 (1, 1) |
| Micranthae | ||||||
| D. chrysostachya | SM, SEM | 1 | 1, S | 718·6 ± 62·3 (11) | 1·38 ± 0·042 (45, 5) | – |
| D. cooperi | SM, SEM | 1 | 1, S | 300·5 ± 19·5 (2) | 2·14 ± 0·185 (13, 2) | 9 ± 0·4 (13, 2) |
| D. crassicornis | SM, SEM | 1 | 1, S | 107 (1) | 2·21 ± 0·114 (15, 3) | 10 ± 0·4 (9, 1) |
| D. erubescens subsp. erubescens | SEM | 1 | 1, S | 7 (1) | 0·64 ± 0·134 (3, 1) | 9 ± 0·9 (8, 1) |
| D. fragrans subsp. fragrans | SEM | 1 | 1, S | 41 ± 1·2 (3) | 1·55 ± 0·072 (19, 2) | 6 ± 0·2 (7, 3) |
| D. galpinii | SEM | 1 | 1, S | 96 (1) | 1·21 ± 0·063 (7, 1) | 8 ± 0·5 (10, 1) |
| D. hircicornis | SEM | 1 | 1, S | 32 ± 7 (2) | 1·74 ± 0·134 (9, 1) | 8 ± 0·6 (4, 1) |
| D. polygonoides | SM | 1 | 1, S | + (5) | – | – |
| D. rhodantha | SM | 1 | 1, S | + (3) | – | – |
| D. sankeyi | SEM | 1 | 1, S | 31·5 ± 5·5 (2) | 0·94 ± 0·05 (15, 3) | 9 ± 0·6 (17, 3) |
| D. scullyi | SM, SEM | 1 | 1, S | 354 (1) | 1·1 ± 0·066 (20, 1) | 9 ± 0·5 (7, 1) |
| D. thodei | SM | 1 | 1, S | + (2) | – | – |
| D. versicolor | SM, SEM | 1 | 1, S | 11 ± 2·7 (4) | 1·01 ± 0·167 (4, 1) | 8 ± 0·5 (11, 2) |
| D. woodii | SEM | 1 | 1, S | 14·3 ± 1·2 (3) | 0·65 ± 0·047 (8, 2) | 5 ± 0·2 (9, 3) |
| D. zuluensis | SM | 1 | 1, S | + (2) | – | – |
Nectar column: 1, nectar-producing; 0, nectarless. Nectary type: 0, no nectary; 1, modified stomata; 2, secretory epidermis. Nectary location: S, spur; P, petals, L, lip. Values are means ± standard error. Sample sizes for number of stomata are given as (nspecimens), those for stomata size and spur-wall thickness as (nmeasurement, nspecimens).
* Spur absent; + stomata present, no count available; SM, stereomicroscopy.
LITERATURE CITED
- Aguiar JMRBV, Pansarin LM, Ackerman JD, Pansarin ER. Biotic versus abiotic pollination in Oeceoclades maculata Lindl. (Orchidaceae) Plant Species Biology. 2012;27:86–95. [Google Scholar]
- Bateman RM, Hollingsworth PM, Preston J, Yi-Bo L, Pridgeon AM, Chase MW. Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae) Botanical Journal of the Linnean Society. 2003;142:1–40. [Google Scholar]
- Baum SF, Eshed Y, Bowman JL. The Arabidopsis nectary is an ABC-independent floral structure. Development. 2001;128:4657–4667. doi: 10.1242/dev.128.22.4657. [DOI] [PubMed] [Google Scholar]
- Bell AK, Roberts DL, Hawkins JA, Rudall PJ, Box MS, Bateman RM. Comparative micromorphology of nectariferous and nectarless labellar spurs in selected clades of subtribe Orchidinae (Orchidaceae) Botanical Journal of the Linnean Society. 2009;160:369–387. [Google Scholar]
- Bellstedt DU, Linder HP, Harley EH. Phylogenetic relationships in Disa based on non-coding trnL-trnF chloroplast sequences: evidence of numerous repeat regions. American Journal of Botany. 2001;88:2088–2100. [PubMed] [Google Scholar]
- Benzing DH. Major patterns and processes in orchid evolution: a critical synthesis. In: Arditti J, editor. Orchid biology. Reviews and perspectives, IV. Ithaca, NY: Cornell University Press; 1987. pp. 33–78. [Google Scholar]
- Bernardello G. A systematic survey of floral nectaries. In: Nicolson SW, Nepi M, Pacini E, editors. Nectaries and nectar. Dordrecht: Springer; 2007. pp. 19–128. [Google Scholar]
- Blanco M, Carnevali G, Whitten M, et al. Generic realignments in Maxillariinae (Orchidaceae) Lankesteriana. 2007;7:515–538. [Google Scholar]
- Blomberg SP, Garland T, Jr, Ives AR. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Bosch JA, Heo K, Sliwinski MK, Baum DA. An exploration of LEAFY expression in independent evolutionary origins of rosette flowering in Brassicaceae. American Journal of Botany. 2008;95:286–293. doi: 10.3732/ajb.95.3.286. [DOI] [PubMed] [Google Scholar]
- Bradshaw E, Rudall PJ, Devey DS, Thomas MM, Glover BJ, Bateman RM. Comparative labellum micromorphology of the sexually deceptive temperate orchid genus Ophrys: diverse epidermal cell types and multiple origins of structural colour. Botanical Journal of the Linnean Society. 2010;162:504–540. [Google Scholar]
- Bytebier B, Bellstedt DU, Linder HP. A molecular phylogeny for the large African orchid genus Disa. Molecular Phylogenetics and Evolution. 2007;43:75–90. doi: 10.1016/j.ympev.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Bytebier B, Bellstedt DU, Linder HP. A new phylogeny-based sectional classification for the large African orchid genus Disa. Taxon. 2008;57:1233–1251. [Google Scholar]
- Bytebier B, Antonelli A, Bellstedt DU, Linder HP. Estimating the age of fire in the Cape flora of South Africa from an orchid phylogeny. Proceedings of the Royal Society B Biological Sciences. 2011;278:188–195. doi: 10.1098/rspb.2010.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MW, Hanson L, Albert VA, Whitten WM, Williams NH. Life history evolution and genome size in subtribe Oncidiinae (Orchidaceae) Annals of Botany. 2005;95:191–199. doi: 10.1093/aob/mci012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte GL, Arnegard ME, Peichel CL, Schluter D. The probability of genetic parallelism and convergence in natural populations. Proceedings of the Royal Society of London B Biological Sciences. 2012;279:5039–47. doi: 10.1098/rspb.2012.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronquist A. The evolution and classification of flowering plants. New York, NY: Botanical Garden; 1988. [Google Scholar]
- Daumann E. Das Blütennektarium der Monocotyledonen unter besonderer Berücksichtigung seiner systematischen und phylogenetischen Bedeutung. Feddes Repertorium. 1970;80:463–590. [Google Scholar]
- Daumann E. Zur Frage nach dem Vorkommen eines Septalnektariums bei Dicotyledonen. Preslia. 1974;46:97–109. [Google Scholar]
- Davies KL, Stpiczyńska M. Micromorphology of the labellum and floral spur of Cryptocentrum Benth. and Sepalosaccus Schltr. (Maxillariinae: Orchidaceae) Annals of Botany. 2007;100:797–805. doi: 10.1093/aob/mcm165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Stpiczyńska M. Structure and distribution of floral trichomes in Lycaste and Sudamerlycaste (Orchidaceae: Maxillariinae s.l.) Botanical Journal of the Linnean Society. 2010;164:409–421. [Google Scholar]
- Davies KL, Stpiczyńska M, Gregg A. Nectar-secreting floral stomata in Maxillaria anceps Ames & C. Schweinf. (Orchidaceae) Annals of Botany. 2005;96:217–227. doi: 10.1093/aob/mci182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douzery EJP, Pridgeon AM, Kores P, Linder HP, Kurzweil H, Chase MW. Molecular phylogenetics of Diseae (Orchidaceae): a contribution from nuclear ribosomal ITS sequences. American Journal of Botany. 1999;86:887–899. [PubMed] [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Portland, OR: Dioscorides Press; 1993. [Google Scholar]
- Effmert U, Grosse J, Rose USR, Ehrig F, Kagi R, Piechulla B. Volatile composition, emission pattern, and localization of floral scent emission in Mirabilis jalapa (Nyctaginaceae) American Journal of Botany. 2005;92:2–12. doi: 10.3732/ajb.92.1.2. [DOI] [PubMed] [Google Scholar]
- Ellis AG, Johnson SD. Floral mimicry enhances pollen export: the evolution of pollination by sexual deceit outside of the Orchidaceae. The American Naturalist. 2010;176:E143–E151. doi: 10.1086/656487. [DOI] [PubMed] [Google Scholar]
- Endress PK. Major evolutionary traits of monocot flowers. In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ, editors. Monocotyledons: systematics and evolution. London: Royal Botanic Gardens, Kew; 1995. [Google Scholar]
- Fahn A. Secretory tissues in plants. New York: Academic Press; 1979. [Google Scholar]
- Figueiredo ACS, Pais MS. Ultrastructural aspects of the nectary spur of Limodorum abortivum (L) Sw. (Orchidaceae) Annals of Botany. 1992;70:325–331. [Google Scholar]
- Fisogni A, Cristofolini G, Rossi M, Galloni M. Pollinator directionality as a response to nectar gradient: promoting outcrossing while avoiding geitonogamy. Plant Biology. 2011;13:848–856. doi: 10.1111/j.1438-8677.2011.00453.x. [DOI] [PubMed] [Google Scholar]
- Gaffal KP, Heimler W, El-Gammal S. The floral nectary of Digitalis purpurea L., structure and nectar secretion. Annals of Botany. 1998;81:251–262. [Google Scholar]
- Galetto L, Bernardello G, Rivera G. Nectar, nectaries, flower visitors, and breeding system in five terrestrial Orchidaceae from central Argentina. Journal of Plant Research. 1997;110:393–403. [Google Scholar]
- Golubov J, Eguiarte LE, Mandujano MC, Lopez-Portillo J, Montana C. Why be a honeyless honey mesquite? Reproduction and mating system of nectarful and nectarless individuals. American Journal of Botany. 1999;86:955–963. [PubMed] [Google Scholar]
- Harder LD, Barrett SCH. The energy cost of bee pollination for Pontederia cordata (Pontederiaceae) Functional Ecology. 1992;6:226–233. [Google Scholar]
- Harder LD, Thomson JD. Evolutionary options for maximizing pollen dispersal of animal-pollinated plants. American Naturalist. 1989;133:323–344. [Google Scholar]
- Harvey PH, Pagel M. The comparative method in evolutionary biology. Oxford: Oxford University Press; 1991. [Google Scholar]
- Hobbhahn N. Correlates and consequences of repeated nectar evolution in the ancestrally rewardless orchid genus. University of Calgary, Calgary: Canada; 2012. Disa. PhD Thesis. [Google Scholar]
- Huelsenbeck JP, Nielsen R, Bollback JP. Stochastic mapping of morphological characters. Systematic Biology. 2003;52:131–158. doi: 10.1080/10635150390192780. [DOI] [PubMed] [Google Scholar]
- Johnson SD. The pollination of Disa versicolor (Orchidaceae) by anthophorid bees in South Africa. Lindleyana. 1995;9:209–212. [Google Scholar]
- Johnson SD. Batesian mimicry in the non-rewarding orchid Disa pulchra, and its consequences for pollinator behaviour. Biological Journal of the Linnean Society. 2000;71:119–132. [Google Scholar]
- Johnson SD, Steiner KE. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Linder HP, Steiner KE. Phylogeny and radiation of pollination systems in Disa (Orchidaceae) American Journal of Botany. 1998;85:402–411. [PubMed] [Google Scholar]
- Johnson SD, Ellis A, Dotterl S. Specialization for pollination by beetles and wasps: the role of lollipop hairs and fragrance in Satyrium microrrhynchum (Orchidaceae) American Journal of Botany. 2007;94:47–55. doi: 10.3732/ajb.94.1.47. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Hobbhahn N, Bytebier B. Ancestral deceit and labile evolution of nectar production in the African orchid genus Disa. Biology Letters. 2013;9:20130500. doi: 10.1098/rsbl.2013.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel SW, Cowan PD, Helmus MR, et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- Kurzweil H, Liltved WR, Linder HP. Disa introrsa sp. nov. (Orchidaceae) from the Western Cape of South Africa, with notes on the phylogeny of Disa sect. Disella. Nordic Journal of Botany. 1997;17:353–360. [Google Scholar]
- Larsen MW, Peter C, Johnson SD, Olesen JM. Comparative biology of pollination systems in the African-Malagasy genus Brownleea (Brownleeinae: Orchidaceae) Botanical Journal of the Linnean Society. 2008;156:65–78. [Google Scholar]
- Lee JY, Baum SF, Alvarez J, Patel A, Chitwood DH, Bowman JL. Activation of CRABS CLAW in the nectaries and carpels of Arabidopsis. Plant Cell. 2005a;17:25–36. doi: 10.1105/tpc.104.026666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Baum SF, Oh SH, Jiang CZ, Chen JC, Bowman JL. Recruitment of CRABS CLAW to promote nectary development within the eudicot clade. Development. 2005b;132:5021–5032. [Google Scholar]
- Linder HP. Taxonomic studies in the Disinae (Orchidaceae). IV. A revision of Disa Berg. sect. Micranthae Lindl. Bulletin du Jardin Botanique National de Belgique. 1981a;51:255–346. [Google Scholar]
- Linder HP. Taxonomic studies in the Disinae. V. A revision of the genus Monadenia. Bothalia. 1981b;13:339–363. [Google Scholar]
- Linder HP. Taxonomic studies in the Disinae. VI. A revision of the genus Herschelia. Bothalia. 1981c;13:365–388. [Google Scholar]
- Linder HP. Taxonomic studies on the Disinae: 1. A revision of the genus Brownleea Lindl. Journal of South African Botany. 1981d;47:13–48. [Google Scholar]
- Linder HP. Taxonomic studies on the Disinae: 2. A revision of the genus Schizodium Lindl. Journal of South African Botany. 1981e;47:339–371. [Google Scholar]
- Linder HP. Taxonomic studies on the Disinae. III. A revision of Disa Berg. excluding sect. Micranthae Lindl. Cape Town: Bolus Herbarium; 1981f. [Google Scholar]
- Linder HP. Notes on the orchids of southern tropical Africa I: Brownleea and Herschelia. Kew Bulletin. 1985;40:125–129. [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. 2011 Version 2·75. www.mesquiteproject.org . [Google Scholar]
- de Melo MC, Borba EL, Paiva EAS. Morphological and histological characterization of the osmophores and nectaries of four species of Acianthera (Orchidaceae: Pleurothallidinae) Plant Systematics and Evolution. 2010;286:141–151. [Google Scholar]
- Mooers AO, Schluter D. Reconstructing ancestor states with maximum likelihood: support for one- and two-rate models. Systematic Biology. 1999;48:623–633. [Google Scholar]
- Nepi M. Nectary structure and ultrastructure. In: Nicolson SW, Nepi M, Pacini E, editors. Nectaries and Nectar. Dordrecht: Springer; 2007. pp. 129–166. [Google Scholar]
- Nepi M, Stpiczyńska M. Nectar resorption and translocation in Cucurbita pepo L. and Platanthera chlorantha Custer (Rchb.) Plant Biology. 2007;9:93–100. doi: 10.1055/s-2006-924287. [DOI] [PubMed] [Google Scholar]
- Pagel M. Detecting correlated evolution on phylogenies – a general method for the comparative analysis of discrete characters. Proceedings of the Royal Society of London B Biological Sciences. 1994;255:37–45. [Google Scholar]
- Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Systematic Biology. 1999;48:612–622. [Google Scholar]
- Pais MSS. Les nectaires florales d'Epipactis atropurpurea Rafin. Quelques aspects inframicroscopiques de l'assise nectarifere. Bulletin de la Société Botanique de France. 1982;129:103–107. [Google Scholar]
- Pansarin ER. Pollen and nectar as a reward in the basal epidendroid Psilochilus modestus (Orchidaceae: Triphoreae): a study of floral morphology, reproductive biology and pollination strategy. Flora. 2008;203:10–10. [Google Scholar]
- Pansarin ER, Salatino A, Pansarin LM, Sazima M. Pollination systems in Pogonieae (Orchidaceae: Vanilloideae): a hypothesis of evolution among reward and rewardless flowers. Flora. 2012;207:849–861. [Google Scholar]
- Pansarin ER, Aguiar JMRBV, Pansarin LM. Floral biology and histochemical analysis of Vanilla edwallii Hoehne (Orchidaceae: Vanilloideae): an orchid pollinated by Epicharis (Apidae: Centridini) Plant Species Biology. 2013 in press. doi:10.1111/1442-1984.12014. [Google Scholar]
- Paradis E, Claude J. Analysis of comparative data using generalized estimating equations. Journal of Theoretical Biology. 2002;218:175–185. doi: 10.1006/jtbi.2002.3066. [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Pierie MD, Humphreys AM, Antonelli A, Galley C, Linder HP. Model uncertainty in ancestral area reconstruction: a parsimonious solution? Taxon. 2012;61:652–664. [Google Scholar]
- Proctor M, Yeo P, Lack A. The natural history of pollination. Portland, OR: Timber Press; 1996. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. 2010 Version 2·12·1. Vienna, Austria, R Foundation for Statistical Computing. www.r-project.org . [Google Scholar]
- Rasband WS. ImageJ. Bethesda, MD: National Institutes of Health; 1997–2009. 1·43u. [Google Scholar]
- Simpson BB, Neff JL. Evolution and diversity of floral rewards. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York: Scientific and Academic Editions; 1983. [Google Scholar]
- Singer RB, Koehler S. Pollinarium morphology and floral rewards in Brazilian Maxillariinae (Orchidaceae) Annals of Botany. 2004;93:39–51. doi: 10.1093/aob/mch009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczyńska M. The structure of nectary of Platanthera bifolia L Orchidaceae. Acta Societatis Botanicorum Poloniae. 1997;66:5–11. [Google Scholar]
- Stpiczyńska M. Nectar resorption in the spur of Platanthera chlorantha Custer (Rchb.) Orchidaceae – structural and microautoradiographic study. Plant Systematics and Evolution. 2003;238:119–126. [Google Scholar]
- Stpiczyńska M, Matusiewicz J. Anatomy and ultrastructure of spur nectary of Gymnadenia conopsea (L.) (Orchidaceae) Acta Societatis Botanicorum Poloniae. 2001;70:267–272. [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Nectary structure and nectar secretion in Maxillaria coccinea (Jacq.) LO Williams ex Hodge (Orchidaceae) Annals of Botany. 2003;93:87–95. doi: 10.1093/aob/mch008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Comparative account of nectary structure in Hexisea imbricata (Lindl.) Rchb.f. (Orchidaceae) Annals of Botany. 2005a;95:749–756. doi: 10.1093/aob/mci081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczyńska M, Milanesi C, Faleri C, Cresti M. Ultrastructure of the nectary spur of Platanthera chlorantha (Custer) Rchb. (Orchidaceae) during successive stages of nectar secretion. Acta Biologica Cracoviensia Series Botanica. 2005b;47:111–119. [Google Scholar]
- Zimmerman M, Cook S. Pollinator foraging, experimental nectar-robbing and plant fitness in Impatiens capensis. American Midland Naturalist. 1985;113:84–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.