Abstract
Background and Aims
Extreme water stress episodes induce tree mortality, but the physiological mechanisms causing tree death are still poorly understood. This study tests the hypothesis that a potted tree's ability to survive extreme monotonic water stress is determined by the cavitation resistance of its xylem tissue.
Methods
Two species were selected with contrasting cavitation resistance (beech and poplar), and potted juvenile trees were exposed to a range of water stresses, causing up to 100 % plant death.
Key Results
The lethal dose of water stress, defined as the xylem pressure inducing 50 % mortality, differed sharply across species (1·75 and 4·5 MPa in poplar and beech, respectively). However, the relationships between tree mortality and the degree of cavitation in the stems were similar, with mortality occurring suddenly when >90 % cavitation had occurred.
Conclusions
Overall, the results suggest that cavitation resistance is a causal factor of tree mortality under extreme drought conditions.
Keywords: Water stress, embolism, hydraulic failure, resilience, vulnerability curves, mortality, beech, hybrid poplar, Fagus sylvatica, Populus deltoides × P. nigra
INTRODUCTION
Drought is one of the most common abiotic stresses that adversely affect plant growth and productivity through a series of morphological and physiological changes (Bréda et al., 2006). Soil water availability is also one of the most important factors determining plant survival and distribution along altitudinal and latitudinal gradients worldwide (Allen and Breshears, 1998; Kleidon and Mooney, 2000; Engelbrecht et al., 2007). Reduced water availability alters both soil–root and leaf–atmosphere interfaces, and threatens the liquid phase continuum integrity from soil to leaves, thereby reducing the capacity of plants to take up water from the soil (Sperry et al., 2002). A long-distance water transport through plant xylem under tension and in a metastable state makes it inherently vulnerable to cavitation that is a phenomenon by which xylem conduits become air filled and lose their functionality in sap conduction (Tyree and Sperry, 1989). This has direct consequences for physiological traits related to water movement and carbon fixation, such as stomatal performance (Sperry et al., 1998; Bréda et al., 2006; Sevanto et al., 2013), carbon assimilation rates (Brodribb and Feild, 2000; Urli et al., 2013), leaf water potential (Tognetti et al., 1995; Thomas, 2000; Mitchell et al., 2013), turgor loss point of leaf cells (Brodribb et al., 2003; Sevanto et al., 2013) and xylem water transport capability (Cochard et al., 1992, 2005; Maherali et al., 2004). Xylem hydraulic function, the connection for water columns between the soil and transpiring leaves, in particular should dictate the limits of a species' tolerance to water stress (Brendel and Cochard, 2011; Choat et al., 2012; López et al., 2013; Mitchell et al., 2013). Accordingly, the functional and ecological significance of variations in xylem hydraulics has received considerable attention over the past two decades.
Extensive comparisons over a wide range of woody plants have indicated that increased xylem resistance to drought-induced cavitation might be an advantageous characteristic for drought-resistant species (Tyree and Ewers, 1991; Pockman and Sperry, 2000; Jacobsen et al., 2007; Choat et al., 2012). However, drought-induced hydraulic failure has seldom been associated with the death of plants exposed to critical water stress (Rood et al., 2000; Davis et al., 2002; Tyree et al., 2002; Nardini et al., 2013). Therefore, whether resistance to cavitation is a causal factor for tree survival in cases of extreme dry-down (physiological resilience) clearly needs to be investigated at the individual scale, and not merely inferred from interspecific comparisons. Recently, some authors have suggested that the dynamics of xylem physiology could explain survival and recovery from extreme drought, even though the number of species investigated remains rather limited (Blackman et al., 2009; Brodribb and Cochard, 2009; Brodribb et al., 2010; Ogasa et al., 2013). Across conifer species, Brodribb and Cochard (2009) and Brodribb et al. (2010) demonstrated that tree mortality occurs when drought has caused >50 % loss of stem conductance, and Hartmann et al. (2013) provide a breakthrough in disentangling the mechanism by which plant mortality is induced by carbon starvation or drought in Norway spruce (Picea abies). However, the experimental evidence for such a close correlation between hydraulic failure and mortality is still largely lacking for broadleaved tree species (Mitchell et al., 2013; Nardini et al., 2013; Ogasa et al., 2013; Urli et al., 2013).
It is well known that plants permanently have to face the dilemma of dying by drying or being starved of carbon. It is therefore commonplace to think that an important imbalance between uptake and loss of either carbon or water would be responsible for tree death. Actually, drought-induced tree mortality would result from a non-mutually exclusive interaction of several mechanisms (Hartmann et al., 2013; Mitchell et al., 2013; Nardini et al., 2013; Sevanto et al., 2013). Among them, hydraulic failure, carbon starvation and biotic agent demographics seem to be the three most prominent mechanisms involved (McDowell et al., 2008; Sala et al., 2010; Anderegg and Callaway, 2012; Hartmann et al., 2013; Nardini et al., 2013; Sevanto et al., 2013; Urli et al., 2013,). According to Tyree and Sperry (1988, 1989), xylem tensions that cause >5–30 % loss of water transport capacity could generate a runaway embolism and lead to catastrophic xylem dysfunction that results in a total loss of conductivity, i.e. a hydraulic failure. Carbon starvation is an imbalance between carbon uptake and loss that results in a negative carbon balance. Carbon starvation is thus a consequence of avoidance of hydraulic failure through stomatal closure (McDowell et al., 2008; Sevanto et al., 2013). The mechanism of biotic agent demographics suggests that drought drives changes in demographics of mortality agents that subsequently drive tree mortality (McDowell et al., 2008; Plaut et al., 2012) because of climate-driven outbreaks of insects and pathogens. Finally, recent syntheses highlight that carbon-related and hydraulic-related mortality pathways are fundamentally inter-related in many ways, and their interconnections are largely unexplored (McDowell, 2011; Anderegg and Callaway, 2012). For instance, limitation of carbohydrate mobilization, translocation or transport could influence carbon starvation (Sala et al., 2010; McDowell, 2011; Sevanto et al., 2013), and declines in carbon availability could affect hydraulic capacity or defence against insects or pathogens (Davis et al., 2002; McDowell et al., 2011; Anderegg and Callaway, 2012).
Our aim in this study was to investigate the effects of an artificially imposed progressive reduction in soil moisture on stem xylem hydraulic failure and tree physiological resilience in beech and poplar, two contrasting temperate species with respect to their ability to survive water stress. We hypothesized that stem xylem hydraulic failure is a causal factor of tree mortality induced by extreme monotonic water stress in these species. We therefore analysed the response of beech saplings and poplar sprouts to prolonged soil water stress events, and examined the relationship between xylem hydraulic failure and xylem water potential, and tree mortality.
MATERIALS AND METHODS
Plant material, growth conditions and experimental design
We carried out experiments in a greenhouse at the INRA research station of Clermont-Ferrand in France (45°77′N, 3°14′E; altitude of 300 m) during 2008 for European beech Fagus sylvatica L. and 2010 for Populus deltoides Bartr. ex. Marsh × Populus nigra L. These two species are known to differ in their ecological distributions, and to vary substantially in controlling their transpiration and leaf water potential during water stress (Braatne et al., 1992; Leuschner et al., 2001; Bréda et al., 2006; Kim et al., 2008).
We excavated 159 saplings of beech from a nursery stand and transplanted them in November 2007 into 20 L pots filled with a mixture of homogenized clay loamy soil imported from a local forest and peat in the proportion of 2:1 (v/v). At that time, the beech saplings were 2 years old. They were left to acclimatize for 8 months in a greenhouse. Similarly, 135 cuttings of three unrelated poplar genotypes were planted into 20 L pots filled with the same mixture of soil as for beech in March 2010. The sprouts of the poplar hybrids were allowed to establish for 2 months prior to the onset of the water treatments. During their acclimation phase or initial establishment, we watered all the plants daily to field capacity.
The air temperature and relative humidity in the greenhouse were recorded every 30 min using a two-channel logger (Hobo H08 Temp/RH Logger, Prosensor, Amanvillers, France). We maintained the maximum temperature below 30 °C and the night-time air temperature above 4 °C. In summer, night-time air relative humidity was high (up to 94 %), but it could decrease gradually to 40 % by noon and fluctuated around this value until the mid-afternoon under an automatic cooling system. All the beech saplings and poplar cuttings were fed under partial shade representing approx. 32 % of full sunlight. The maximum diurnal photosynthetically active radiation (PAR) in the greenhouse was about 650 µmol m−2 s−1 over the waveband 400–700 nm at noon on cloudless days in summer. The PAR measurements were taken every 5 s (Li-190SA quantum sensor, Li-Cor Inc., Lincoln, NE, USA) and 10 min average values were stored in a 21X data logger (Campbell Scientific, Shepshed, Leics, UK).
For beech and poplar, we set up a drip irrigation controlled by a timer to supply the plants with tap water. We induced a progressive water stress by discontinuing watering. The beech saplings were divided into 12 blocks of 12 water-stressed individuals plus one block of 15 control individuals. We applied the water stress treatment on 30 June 2008 for two and a half months. From 15 September 2008 to 31 May 2009, all the plants involved in the experiment were well watered to ensure survival into the next growing season to check for their budburst status (a proxy of survivorship) (Barigah et al., 2013). Similarly, we divided the poplar sprouts into five blocks of eight water-stressed individuals per genotype plus one block of 15 control individuals. We applied the water stress treatment from 1 June to 27 July 2010.
To study the effects of progressive water stress on tree water status, weekly measurements were carried out on sub-sets of droughted plants and control plants of a given block. For clarity, we refer to the droughted plants on each sampling day as specimens. Because of the destructive nature of our sampling, we harvested one half of the specimens per block weekly between 0630 h and 0730 h solar time for hydraulic measurements. To monitor the extent of recovery from the water stress treatment, we first overwatered all the specimens at the end of each sampling date and on subsequent days we watered them daily to field capacity until their budburst status. The budburst occurred for poplar re-sprouts and beech saplings about 2 and 9 months, respectively, after re-watering.
Soil moisture content monitoring and measurements
We carried out a survey of the volumetric soil moisture content (VSMC) to monitor the soil water status within the pots. To this end, buriable waveguides were installed in the pots of 30 of the plants to record and store daily at 1200 h solar time the instantaneous volumetric water content of the soil using time domain reflectometry sensors (TDR Trase system technology; Model 6050X1, Soil Moisture Equipment Corp., Goleta, CA, USA). Moreover, on each sampling date (every Wednesday), the VSMC was recorded manually in the vicinity of all specimen and control plants. We used the standard moisture table (BUN) to convert the measured apparent dielectric constant to VSMC (Topp et al., 1980; McCutchan and Shackel, 1992).
Xylem water potential measurements
During the time course of the experiment, we used a Scholander-type pressure chamber (PMS, Corvallis, Oregon, USA) to assess plant water status (Scholander et al., 1965; Turner, 1988) through the xylem water pressure potential (Ψx) for at least two fully exposed healthy leaves borne on current-year shoots (beech) or on sprouts (poplar). The measurements were completed between 1100 h and 1300 h solar time on covered leaves. To this end, aluminium foil was wrapped around the leaves and they were sealed in a plastic bag the evening before the sampling days to prevent transpiration and promote equilibrium with the plant axis overnight. We assumed the xylem water potential Ψx to be the same as that of the stem bearing them (McCutchan and Shackel, 1992), and we used it as a proxy of stem water potential.
Accelerated leaf desiccation and death are means by which plants reduce water requirement under drought stress. We therefore measured weekly, after water stress inception, the Ψx until the plants had shed almost all their leaves, within 6 weeks in the case of poplar. We discontinued measurement of the Ψx for the marcescent leaves of beech within 10 weeks when the balance pressures were no longer attainable. Beyond these thresholds, we estimated Ψx by fitting equations between the soil water content and the xylem pressure, assuming a steady-state decrease in volumetric soil moisture content over time.
Native steady-state embolism measurements and dehydration-based vulnerability curves
Native steady-state embolism is measured by the percentage loss of hydraulic conductance (PLC) that occurs as a consequence of the water stress experienced by an intact plant. The native steady-state percentage embolism was measured with a xylem embolism meter (XYL'EM, Bronkhorst, Montigny-Les-Cormeilles, France) on droughted and control plants. In the morning we severed current-year shoot samples under water and enclosed them in black plastic bags that had been previously misted inside to ensure high humidity and to prevent further cavitation. We brought them to the laboratory rapidly for immediate hydraulic conductance measurements. On each sampling day, we cut, under water, five 0·05 m and 0·12 m stem segments for beech and poplar from lateral twigs, the main shoot or sprout, and fitted them to water-filled tubing to evaluate native steady-state embolism. We connected one end of the stem segment to a tank of de-gassed, filtered (0·2 µm) 10 mm KCl and 1 mm CaCl2 solution. We recorded the flux of the solution through the stem section under low pressure (6 kPa) as the initial hydraulic conductance (ki). We then perfused the stem segment at least twice for 1–2 min with the same filtered solution at 0·2 MPa until the hydraulic conductance no longer increased in order to remove air from embolized vessels and determined the maximum hydraulic conductance (km) (Barigah and Cochard, 2012). The PLC was then determined for each sampled stem segment as PLC = 100 × (1 − ki/km). These data were computed and plotted as dehydration-based vulnerability curves, i.e. PLC vs. time and/or xylem water potential.
Plant survival
Survival of the plants was assessed after budburst (i.e. at the onset of the 2009 growing season for beech saplings and 8 weeks after the ultimate sampling date for poplar sprouts). We carried out a census of the surviving plants based on the ability of shoots to initiate a bud break and growth. The plant mortality rates were calculated as a percentage of dead beech seedlings at the onset of the 2009 growing season compared with the number of individuals in each set of specimens. The same protocol was applied to the poplar sprouts at least 2 months after they were re-watered.
Data analysis
To evaluate how soil moisture content varied with time, analyses of variance (ANOVA) were conducted on the sets of droughted and control plants with six replicates per sampling date. We included the values for each sampling date (week) as the repeated measure over time, and we considered weeks as random effects. The variables of interest were: xylem water potential, PLC and Ψ50. The vulnerability curve from each shoot was treated as a single replicate that yielded a single value of Ψ50. The means were compared with Tukey's multiple range tests at 0·05 levels when effects were significant. We subjected measured and derived data to statistical analysis using a software package (XLSTAT v7.5.3, Addinsoft, Paris, France) for ANOVA and the SigmaPlot 9.0.1 software package (SigmaPlot, Systat Software Inc., Point Richmond, CA, USA) for non-linear curve fitting.
RESULTS
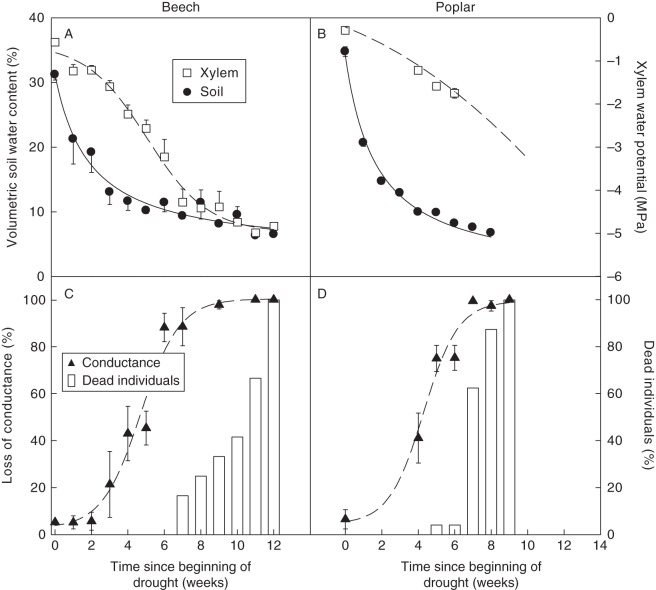
The volumetric soil water content declined sharply within the first 2–3 weeks following the onset of water stress for both species (Fig. 1A, B). With further soil drying, a roughly linear decrease to a slower rate of soil drying was observed until all available water had been used. Water stress also caused a sharp drop in plant xylem water potential Ψx; the lowest values were −4·74 ± 0·36 MPa (n = 6) for beech and −1·75 ± 0·11 MPa (n = 9) for poplar. In poplar, the determination of the lowest Ψx value was constrained by leaf shedding.
Fig. 1.
Temporal patterns of volumetric soil moisture content, xylem water potential, percentage loss of conductance, and percentage dead individuals in beech saplings and poplar sprouts exposed to a prolonged water stress. Water stress-induced mortality was observed for both species when xylem pressures reached a level that induced >80 % loss of conductance. Values are means ± s.e.
The native steady-state embolism was very low for both species before the onset of the water stress, but sharply increased after 4 weeks of treatment (Fig. 1C, D). The timing of embolism formation was thus relatively similar across species, but embolism occurred at much higher xylem pressures in poplar than in beech (Fig. 2). This is consistent with the well-established higher vulnerability to xylem embolism of poplar compared with beech.
Fig. 2.
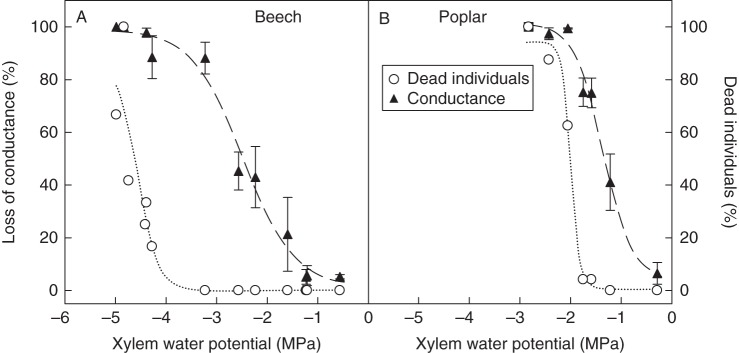
Percentage loss of conductance and percentage dead individuals in (A) beech and (B) poplar trees vs. xylem water potential. Poplar was more vulnerable to cavitation than beech, but mortality occurred when xylem tension fell below a critical threshold corresponding to >90 percentage loss of conductance in both species. Values are means ± s.e.
The juvenile trees of both species could withstand a relatively long period of time without water supply, as at least 7 weeks elapsed after water stress inception before the onset of mortality in beech, while this time was only 5 weeks for poplar. Unless the plants were re-watered, the desiccation phase led to death within 12 and 9 weeks, respectively, in beech and poplar (Fig. 1C, D). Meanwhile, none of the control plants died.
Incipient water stress-induced mortality was observed when the xylem pressure dropped below –4 MPa in beech and –1·75 MPa in poplar (Fig. 2). We defined a lethal xylem water potential as one inducing 50 % mortality. It occurred at –4·5 MPa for beech and at –2 MPa for poplar, corresponding for both species to a >90 % loss of xylem conductance.
DISCUSSION
Poplar sprouts and beech saplings displayed a response to water shortage typical of many temperate trees. Water depletion in the soil caused by leaf transpiration lowers soil and plant water potentials, inducing a progressive regulation of tree water loss by stomatal closure or by leaf shedding. Cavitation occurred in the stems only after a prolonged water stress, confirming that this process takes place only under severe drought conditions. After 2 months of intense water stress, the trees eventually died. In our study, poplar tended to suffer from irreversible water stress damage earlier and at a higher xylem pressure than beech, a result consistent with the known higher drought tolerance of beech (Bréda et al., 2006). However, this information is probably difficult to generalize, as for potted trees the timing of dehydration is strongly dependent on pot size, soil type and tree leaf area (Poorter et al., 2012a, b). Therefore, we postulated that the correlations we observe for our experimental purposes between mortality and physiological traits are the expression of an intrinsic property for each species. As a result, great care is required when some of the outputs are to be extrapolated to nature. However, we can hypothesize that the correlations we observed between mortality and physiological traits are the expression of an intrinsic property for each species.
The main objective of our study was to correlate water stress-induced hydraulic failure with tree mortality. The available information on the threshold water stress causing species mortality is very scant but is needed for an understanding of how forests are likely to respond to climate change-type droughts (Allen et al., 2010; Choat et al., 2012). The experimental protocol we used in our study was time consuming, but provided a simple yet very effective way to define such limits. Usually, trees experience a combination of static and dynamic water stress, but at times one component can dominate (Tyree and Ewers, 1991). Typically, the static water stress may predominate when Ψstem gets close to Ψsoil, i.e. when plants are about to die of thirst. Thus, the hydraulic threshold will not depend either on the monotonic or dynamic nature of droughts. Compared to dynamic droughts, monotonic ones are not so rare. They are widely spread over areas such as Mediterranean regions where long periods without rainfall are recorded annually and in drier places such as the Santa Monica Mountains of southern California, where, for example, Ceanothus crassifolius grows despite nearly 9 months without rain (Davis et al., 2002). The principle was to expose potted trees to progressive doses of water stress, measure relevant physiological traits, and note the plants' capacity to re-grow after re-watering. This protocol, based on stem measurements, enables the determination of the lethal xylem pressure and the corresponding degrees of xylem hydraulic failure for beech and poplar juvenile trees.
Nevertheless, our results must be carefully extrapolated with respect to drought-induced tree mortality in natural ecosystems since adverse conditions such as biotic demographic agents may act as facilitators. So far, evidence for carbon starvation-induced tree death is relatively scarce and some studies have reported high levels of non-structural carbohydrates (NSCs) in dying trees of Populus tremuloides (Galvez et al., 2011; Anderegg et al., 2012), Pinus sylvestris (Gruber et al., 2012) and Picea abies (Hartmann et al., 2013). However, in our study we did not measure samples assuming that water shortage is the leading disruptive factor (Gruber et al., 2012). Of course, we are aware of the major role that the stored carbohydrates may play during the recovery processes mainly for species such as poplars that re-sprout easily (Galvez et al., 2011; Barigah et al., 2013). Carbon starvation contributes to drought damage, but the use of stored carbon remains inhibited by drought-induced declines in hydration as Hartmann et al. (2013) have demonstrated.
The lethal dose of water stress was much higher in beech than in poplar. This can be quantified by the xylem pressure causing 50 % tree mortality (–4·5 MPa vs. –2 MPa, respectively). This finding is consistent with the ecological preferences and the physiology of these species. For instance, the capacity of a species to survive water stress was closely correlated with the vulnerability of their xylem tissue to cavitation (Tyree and Sperry, 1989; Choat et al., 2012; Vilagrosa et al., 2012; Nardini et al., 2013; Ogasa et al., 2013; Urli et al., 2013). However, the most striking finding of this work was that a unique relationship was observed between hydraulic failure and mortality (Fig. 3) although the two species differed substantially in their intrinsic cavitation resistance. Clearly, trees died only when >90 % embolism had occurred in the stem xylem tissue. These result in two angiosperm species, which are in line with those of Urli et al. (2013), contrast with the recent findings of Brodribb and Cochard (2009) and Brodribb et al. (2010) on conifers, which showed that mortality occurred near 50 % loss of xylem conductance.
Fig. 3.
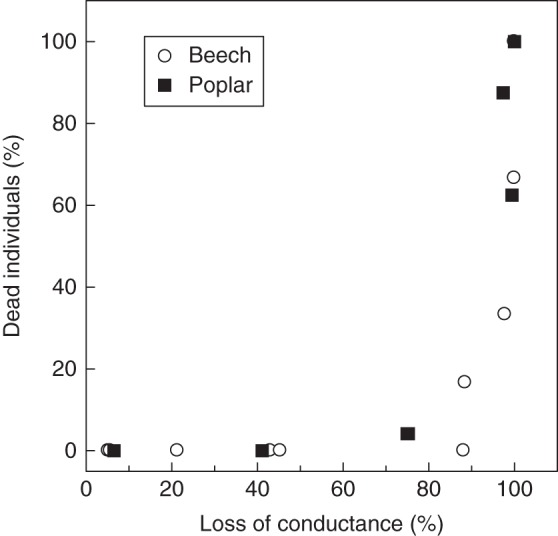
Correlation between stem hydraulic failure measured by the percentage loss of conductance and tree mortality for beech saplings and poplar sprouts exposed to a progressive dose of water stress. Incipient tree mortality was observed only when >90 percentage loss of conductance had occurred in the stems.
Furthermore, Nardini et al. (2013) have also measured a PLC as high as 90 % in Quercus pubescens and Acer monspessulanum in a karstic woodland during a extreme summer drought. Even though these values were high enough to block water supply to the foliage and cause extensive leaf desiccation, the trees survived (Tyree et al., 2002; Brodribb and Cochard, 2009; Barigah et al., 2013). In addition, Choat et al. (2012) found that the difference between the minimum xylem pressure and the Ψ50 value was much higher in conifers than in angiosperms, interpreted as a higher safety margin for conifers. However, if it is confirmed, when more information becomes available, that hydraulic failure occurs at Ψ90 for angiosperms and Ψ50 for conifers, the actual safety margin for mortality may not be so different across all these species.
The mechanism by which hydraulic failure causes plant mortality during water stress is still not fully understood. In a recent study, Munne-Bosch and Alegre (2004) obtained some insights into this mechanism by studying the impact of stem cavitation on bud physiology in poplar. They found that bud water content sharply decreased to lethal levels only after the xylem tissue became fully embolized. Bud water content was closely associated with xylem cavitation for two reasons. First, vessel cavitation can free enough water to compensate for bud water loss and hence buffer water status during drought (Choat et al., 2012). Secondly, when most vessels have cavitated, buds can become hydraulically disconnected from the plant and soil water reservoirs, and water supply to the buds may no longer compensate for their water loss. Under these hypotheses, bud vitality and a plant's ability to recover from a severe water stress may be directly associated with the maintenance of a minimal transport capacity.
Our study does not exclude the involvement of other important causes of drought-induced mortality in trees, such as carbon starvation or vulnerability to pests. However, under the extreme monotonic water stress condition of our experiment, hydraulic failure was probably the primary cause of mortality (Hartmann et al., 2013). The situation is probably different under other experimental conditions. For instance, carbon starvation is likely to cause death of plants exposed to prolonged and relatively mild water stress conditions. In this situation, carbon starvation is probably due to reduced carbon uptake following stomatal control of cavitation (Sperry et al., 1998; Mitchell et al., 2013; Ogasa et al., 2013). Therefore, here again hydraulic failure may be seen as a primary cause of mortality.
In conclusion, our work demonstrates that massive cavitation is probably a causal factor for tree mortality under extreme water stress conditions. This explains why cavitation resistance correlates so closely with species' ecological preferences (Maherali et al., 2004; Choat et al., 2012; López et al., 2013).
ACKNOWLEDGEMENTS
We thank C. Bodet, C. Serre, P. Conchon, P. Chaleil and A. Faure for taking care of the plant material and for their field assistance. We also thank the anonymous reviewers for evaluating this manuscript and for their comments, and N. Boirie, J. Garcia-Sanches and ATT for language editing. This work was supported by the Ministry of the Environment, France through the Office of the Executive of the Programme National/ACI, Ecosphère continentale: processus et modélisation; Écologie pour la Gestion des Écosystèmes et de leurs Ressources (ECOGER).
LITERATURE CITED
- Allen CD, Breshears DD. Drought-induced shift of a forest–woodland ecotone: rapid landscape response to climate variation. Proceedings of the National Academy of Sciences, USA. 1998;95:14839–14842. doi: 10.1073/pnas.95.25.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management. 2010;259:660–684. [Google Scholar]
- Anderegg WRL, Callaway ES. Infestation and hydraulic consequences of induced carbon starvation. Plant Physiology. 2012;159:1866–1874. doi: 10.1104/pp.112.198424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, Field CB. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proceedings of the National Academy of Sciences, USA. 2012;109:233–237. doi: 10.1073/pnas.1107891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barigah TS, Bonhomme M, Lopez D, et al. Modulation of bud survival in Populus nigra sprouts in response to water stress-induced embolism. Tree Physiology. 2013;33:261–274. doi: 10.1093/treephys/tpt002. [DOI] [PubMed] [Google Scholar]
- Barigah TS, Cochard H. Xylem Embolism Meter (Xyl'EM) 2012 http://prometheuswiki.publish.csiro.au/ [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ. Leaf hydraulics and drought stress: response, recovery and survivorship in four woody temperate plant species. Plant, Cell and Environment. 2009;32:1584–1595. doi: 10.1111/j.1365-3040.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- Braatne JH, Hinckley TM, Stettler RF. Influence of soil water on the physiological and morphological components of plant water balance in Populus trichocarpa, Populus deltoides and their F1 hybrids. Tree Physiology. 1992;11:325–339. doi: 10.1093/treephys/11.4.325. [DOI] [PubMed] [Google Scholar]
- Bréda N, Huc R, Granier A, Dreyer E. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Annals of Forest Science. 2006;63:625–644. [Google Scholar]
- Brendel O, Cochard H. How plant species cope with water stress. In: Birot Y, Gracia C, Palahi M, editors. Water for forest and people in the Mediterranean: a challenging balance. European Forest Institute; 2011. pp. 76–80. [Google Scholar]
- Brodribb T, Cochard H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiology. 2009;149:575–584. doi: 10.1104/pp.108.129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS. Stem hydraulic supply is linked to leaf photosynthetic capacity: evidence from New Caledonian and Tasmanian rainforests. Plant, Cell and Environment. 2000;23:1381–1388. [Google Scholar]
- Brodribb TJ, Holbrook NM, Edwards EJ, Gutierrez MV. Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant, Cell and Environment. 2003;26:443–450. [Google Scholar]
- Brodribb TJ, Bowman D, Nichols S, Delzon S, Burlett R. Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytologist. 2010;188:533–542. doi: 10.1111/j.1469-8137.2010.03393.x. [DOI] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491:752–756. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- Cochard H, Cruiziat P, Tyree MT. Use of positive pressures to establish vulnerability curves: further support for air-seeding hypothesis and implications for pressure–volume analysis. Plant Physiology. 1992;100:205–209. doi: 10.1104/pp.100.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Damour G, Bodet C, Tharwat I, Poirier M, Ameglio T. Evaluation of a new centrifuge technique for rapid generation of xylem vulnerability curves. Physiologia Plantarum. 2005;124:410–418. [Google Scholar]
- Davis SD, Ewers FW, Sperry JS, Portwood KA, Crocker MC, Adams GC. Shoot dieback during prolonged drought in Ceanothus (Rhamnaceae) chaparral of California: a possible case of hydraulic failure. American Journal of Botany. 2002;89:820–828. doi: 10.3732/ajb.89.5.820. [DOI] [PubMed] [Google Scholar]
- Engelbrecht BMJ, Comita LS, Condit R, et al. Drought sensitivity shapes species distribution patterns in tropical forests. Nature. 2007;447:80–83. doi: 10.1038/nature05747. [DOI] [PubMed] [Google Scholar]
- Galvez DA, Landhaeusser SM, Tyree MT. Root carbon reserve dynamics in aspen seedlings: does simulated drought induce reserve limitation? Tree Physiology. 2011;31:250–257. doi: 10.1093/treephys/tpr012. [DOI] [PubMed] [Google Scholar]
- Gruber A, Pirkebner D, Florian C, Oberhuber W. No evidence for depletion of carbohydrate pools in Scots pine (Pinus sylvestris L.) under drought stress. Plant Biology. 2012;14:142–148. doi: 10.1111/j.1438-8677.2011.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann H, Ziegler W, Kolle O, Trumbore S. Thirst beats hunger – declining hydration during drought prevents carbon starvation in Norway spruce saplings. New Phytologist. 2013 doi: 10.1111/nph.12331. in press. doi:10.1111/nph.12331. [DOI] [PubMed] [Google Scholar]
- Jacobsen A, Pratt B, Ewers F, Davis S. Cavitation resistance among 26 Chaparral species of Southern California. Ecological Monographs. 2007;77:99–115. [Google Scholar]
- Kim H-S, Oren R, Hinckley TM. Actual and potential transpiration and carbon assimilation in an irrigated poplar plantation. Tree Physiology. 2008;28:559–577. doi: 10.1093/treephys/28.4.559. [DOI] [PubMed] [Google Scholar]
- Kleidon A, Mooney HA. A global distribution of biodiversity inferred from climatic constraints: results from a process-based modelling study. Global Change Biology. 2000;6:507–523. [Google Scholar]
- Leuschner C, Backes K, Hertel D, Schipka F, Schmitt U, Terborg O, Runge M. Drought responses at leaf, stem and fine root levels of competitive Fagus sylvatica L. and Quercus petraea (Matt.) Liebl. trees in dry and wet years. Forest Ecology and Management. 2001;149:33–46. [Google Scholar]
- López R, López de Heredia U, Collada C, Cano FJ, Emerson BC, Cochard H, Gil L. Vulnerability to cavitation, hydraulic efficiency, growth and survival in an insular pine (Pinus canariensis) Annals of Botany. 2013;111:1167–1179. doi: 10.1093/aob/mct084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85:2184–2199. [Google Scholar]
- McCutchan H, Shackel KA. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv. French) Journal of the American Society for Horticultural Science. 1992;117:607–611. [Google Scholar]
- McDowell NG. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology. 2011;155:1051–1059. doi: 10.1104/pp.110.170704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell N, Pockman WT, Allen CD, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends in Ecology and Evolution. 2011;26:523–532. doi: 10.1016/j.tree.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, O'Grady AP, Tissue DT, White DA, Ottenschlaeger ML, Pinkard EA. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytologist. 2013;197:862–872. doi: 10.1111/nph.12064. [DOI] [PubMed] [Google Scholar]
- Munne-Bosch S, Alegre L. Die and let live: leaf senescence contributes to plant survival under drought stress. Functional Plant Biology. 2004;31:203–216. doi: 10.1071/FP03236. [DOI] [PubMed] [Google Scholar]
- Nardini A, Battistuzzo M, Savi T. Shoot desiccation and hydraulic failure in temperate woody angiosperms during an extreme summer drought. New Phytologist. 2013 doi: 10.1111/nph.12288. in press. doi:10.1111/nph.12288. [DOI] [PubMed] [Google Scholar]
- Ogasa M, Miki NH, Murakami Y, Yoshikawa K. Recovery performance in xylem hydraulic conductivity is correlated with cavitation resistance for temperate deciduous tree species. Tree Physiology. 2013;33:335–44. doi: 10.1093/treephys/tpt010. [DOI] [PubMed] [Google Scholar]
- Plaut JA, Yepez EA, Hill J, et al. Hydraulic limits preceding mortality in a pinon–juniper woodland under experimental drought. Plant, Cell and Environment. 2012;35:1601–1617. doi: 10.1111/j.1365-3040.2012.02512.x. [DOI] [PubMed] [Google Scholar]
- Pockman WT, Sperry JS. Vulnerability to xylem cavitation and the distribution of Sonoran desert vegetation. American Journal of Botany. 2000;87:1287–1299. [PubMed] [Google Scholar]
- Poorter H, Buhler J, van Dusschoten D, Climent J, Postma JA. Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Functional Plant Biology. 2012a;39:839–850. doi: 10.1071/FP12049. [DOI] [PubMed] [Google Scholar]
- Poorter H, Fiorani F, Stitt M, et al. The art of growing plants for experimental purposes: a practical guide for the plant biologist. Functional Plant Biology. 2012b;39:821–838. doi: 10.1071/FP12028. [DOI] [PubMed] [Google Scholar]
- Rood S, Patino S, Coombs K, Tyree M. Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods. Trees-Structure and Function. 2000;14:248–257. [Google Scholar]
- Sala A, Piper F, Hoch G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytologist. 2010;186:274–281. doi: 10.1111/j.1469-8137.2009.03167.x. [DOI] [PubMed] [Google Scholar]
- Scholander PF, Hammel HT, Bradstreet ED, Hemmongsen EA. Sap pressure in vascular plants. Science. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Sevanto S, McDowell NG, Dickman LT, Pangle R, Pockman WT. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant, Cell and Environment. 2013 doi: 10.1111/pce.12141. in press. doi:10.1111/pce.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Adler FR, Campbell GS, Comstock JP. Limitation of plant water use by rhizosphere and xylem conductance: results from a model. Plant, Cell and Environment. 1998;21:347–359. [Google Scholar]
- Sperry JS, Hacke UG, Oren R, Comstock JP. Water deficits and hydraulic limits to leaf water supply. Plant, Cell and Environment. 2002;25:251–263. doi: 10.1046/j.0016-8025.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- Thomas F. Growth and water relations of four deciduous tree species (Fagus sylvatica L., Quercus petraea [Matt.] Liebl., Q. pubescens Willd., Sorbus aria [L.] Cr.) occurring at Central European tree line sites on shallow calcarous soils: physiological reactions of seedlings to severe drought. Flora. 2000;195:104–115. [Google Scholar]
- Tognetti R, Johnson JD, Michelozzi M. The response of European beech (Fagus sylvatica L.) seedlings from two Italian populations to drought and recovery. Trees. 1995;9:348–354. [Google Scholar]
- Topp GC, Davis JL, Annan AP. Electromagnetic determination of soil water content: measurements in coaxial transmission lines. Water Resources Research. 1980;16:574–582. [Google Scholar]
- Turner NC. Measurement of plant water status by the pressure chamber technique. Irrigation Research. 1988;9:289–308. [Google Scholar]
- Tyree MT, Ewers FW. The hydraulic architecture of trees and other woody plants. New Phytologist. 1991;119:345–360. [Google Scholar]
- Tyree MT, Sperry JS. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Plant Physiology. 1988;88:574–580. doi: 10.1104/pp.88.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. Vulnerability of xylem to cavitation and embolism. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:19–38. [Google Scholar]
- Tyree MT, Vargas G, Engelbrecht BMJ, Kursar TA. Drought until death do us part: a case study of the desiccation-tolerance of a tropical moist forest seedling-tree, Licania platypus (Hemsl.) Fritsch. Journal of Experimental Botany. 2002;53:2239–2247. doi: 10.1093/jxb/erf078. [DOI] [PubMed] [Google Scholar]
- Urli M, Porté AJ, Cochard H, Guengant Y, Burlett R, Delzon S. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiology. 2013;33:672–683. doi: 10.1093/treephys/tpt030. [DOI] [PubMed] [Google Scholar]
- Vilagrosa A, Chirino E, Peguero JJ, Barigah TS, Cochard H, Gil-Pelegrin E. Xylem cavitation and embolism in plants living in water-limited ecosystems. In: Aroca R, editor. Plant responses to drought stress. Berlin: Springer-Verlag; 2012. pp. 63–109. [Google Scholar]