Abstract
Background and Aims
Although ammonium (NH4+) is the preferred form of nitrogen over nitrate (NO3−) for rice (Oryza sativa), lateral root (LR) growth in roots is enhanced by partial NO3− nutrition (PNN). The roles of auxin distribution and polar transport in LR formation in response to localized NO3− availability are not known.
Methods
Time-course studies in a split-root experimental system were used to investigate LR development patterns, auxin distribution, polar auxin transport and expression of auxin transporter genes in LR zones in response to localized PNN in ‘Nanguang’ and ‘Elio’ rice cultivars, which show high and low responsiveness to NO3−, respectively. Patterns of auxin distribution and the effects of polar auxin transport inhibitors were also examined in DR5::GUS transgenic plants.
Key Results
Initiation of LRs was enhanced by PNN after 7 d cultivation in ‘Nanguang’ but not in ‘Elio’. Auxin concentration in the roots of ‘Nanguang’ increased by approx. 24 % after 5 d cultivation with PNN compared with NH4+ as the sole nitrogen source, but no difference was observed in ‘Elio’. More auxin flux into the LR zone in ‘Nanguang’ roots was observed in response to NO3− compared with NH4+ treatment. A greater number of auxin influx and efflux transporter genes showed increased expression in the LR zone in response to PNN in ‘Nanguang’ than in ‘Elio’.
Conclusions
The results indicate that higher NO3− responsiveness is associated with greater auxin accumulation in the LR zone and is strongly related to a higher rate of LR initiation in the cultivar ‘Nanguang’.
Keywords: Auxin distribution, lateral root, nitrate, rice, Oryza sativa
INTRODUCTION
Plants exhibit various mechanisms to adapt to different nutrient supply conditions. Among these mechanisms, the plasticity of root development is vital. Plant root systems continuously branch and form lateral roots (LRs). These LRs develop from founder cells in the pericycle, the outermost layer of the vascular cylinder (stele) of the root (De Smet et al., 2006). Auxin plays a dominant role in the specification of founder cells that give rise to LR initiation and the later stages of LR development (Forde, 2002; De Smet et al., 2006), and differential distribution of auxin is required for LR organogenesis. An increase in auxin in single pericycle cells is sufficient to induce the initiation and formation of LRs from these cells in Arabidopsis thaliana (Dubrovsky et al., 2008). Various environmental and endogenous signals can be integrated to mediate changes in auxin distribution through effects on polar transport of auxin (Vanneste and Firml, 2009). Polar auxin transport is mediated by auxin influx (AUX/LAX) and efflux carriers (PIN genes and MDR/PGP genes) (Friml et al., 2003; Geisler et al., 2005). The initiation and emergence of LRs is regulated by AUX1, which facilitates the export of indole acetic acid (IAA) from newly developing leaf primordia, the unloading of IAA in the primary root apex and the import of IAA into developing LR primordia (Marchant et al., 2002). Detailed studies of PIN expression and localization as well as auxin distribution in pin mutants revealed that PIN proteins provide the basis for the directional distribution networks that mediate auxin gradients during LR growth (Vieten et al., 2007). Feraru and Friml (2008) developed a model for polar localization of PIN proteins and the direction of auxin flow in the root tip in arabidopsis. PIN1 is localized to the basal (root apex-facing) side of the root vasculature; PIN2 is expressed at the basal side of the cortical cells and at the apical (shoot apex-facing) side of the epidermal and root cap cells; PIN3 is distributed in an apolar manner in the columella cells of the root; PIN4 is localized to the basal side of cells in the central root meristem with less pronounced polarity in the cells of the quiescent centre; and PIN7 is localized to the basal side of the stele cells and is distributed in an apolar manner in columella cells.
Lateral root formation is regulated by both intrinsic developmental programmes and environmental conditions (Zhang and Forde, 2000). Changes in heterogeneous nitrogen (N) distribution and N forms in the soil induce plasticity in LR development (Zhang and Forde, 1998; Song et al., 2011). In several upland species, root proliferation in NO3−-rich regions leads to an increased ratio of root surface to explored soil volume, which facilitates the uptake of nutrients (Drew et al., 1973; Granato and Raper, 1989). Localized NO3− supply affects root architecture predominantly through its stimulation of the initiation and/or elongation of LRs (Drew, 1975; Zhang and Forde, 1998; Guo et al., 2005). Study of an arabidopsis NO3− reductase double mutant suggested that the local stimulation of LR growth is a consequence of the NO3− ion itself acting as a signal rather than as a nutrient (Zhang and Forde, 1998). The ANR1 and NRT1·1 genes, which encode a transcription factor and an NO3− transporter, respectively, have been proposed consecutively to control the stimulatory effect of NO3− on LR growth in arabidopsis (Zhang and Forde, 1998; Zhang et al., 1999; Remans et al., 2006). Lateral root elongation is not stimulated by localized NO3− supply in the auxin-insensitive mutant axr4, suggesting an overlap between the auxin and NO3− signalling pathways (Zhang et al., 1999). The NO3− and auxin signalling pathways are further linked by an effect on auxin transport through AtNRT1·1 (Krouk et al., 2010). Sattelmarcher and Thoms (1995) observed a transient increase in IAA in NO3−-rich Zea mays (maize) root segments after treatment for 3 d and hypothesized that a localized NO3− supply stimulates LR growth by increasing auxin unloading and accumulation in the NO3−-fed root segments. However, analysis of [3H]IAA movement showed that auxin levels in maize LRs decreased more in roots in an NO3−-fed compartment than in an NO3−-free compartment, and localized NO3− treatment appeared to inhibit shoot to root auxin transport (Liu et al., 2010). The role of auxin distribution and transport in localized NO3−-induced LR growth remains unclear.
Ammonium (NH4+) is the preferred form of N over NO3− in Oryza sativa L. (rice), due to its waterlogged growth environment. Although the predominant form of mineral N in bulk soil for paddy rice fields is likely to be NH4+, rice roots are exposed to partial NO3− nutrition (PNN) by nitrification in the rice rhizosphere (Li et al., 2008). The practice of intermittent flooding during rice cultivation, which causes an uneven distribution of NH4+ and NO3− within the soil horizon under field conditions, is being adopted by increasing numbers of Chinese farmers. Localized NO3− supply was shown to stimulate LR elongation in rice using a split-root experiment, consistent with responses in upland plants such as arabidopsis and maize (Wang et al., 2002a). Expression of the rice auxin efflux carrier protein gene OsREH1 showed a rapid increase with maximal expression at 4 h in roots supplied locally with NO3− (Wang et al., 2002b). However, in whole-plant rice culture, elongation of LRs was induced by NO3− deficiency rather than by NO3− supply (Wang et al., 2002a). This result was inconsistent with enhanced LR elongation in maize caused by NO3− supply rather than NO3− deficiency (Liu et al., 2010). In whole-plant culture, PNN stimulated LR initiation in a high NO3− response rice cultivar but not in a low NO3− response rice cultivar compared with plants grown with NH4+ as the sole N source (Song et al., 2011). Stimulation of LR initiation could be caused by an increased concentration of auxin in rice roots, but the distribution of auxin in the roots was not examined in the above study. To what extent, if any, auxin transport in rice roots is regulated by localized NO3− availability remains unclear.
Time-course experiments using a split-root system were used to examine the relationship between localized PNN and auxin transport and distribution in rice. Lateral root development and changes in auxin concentration as determined by HPLC were monitored for 9 d starting 7 d after germination in four regions of roots in two rice cultivars with high (‘Nanguang’) and low (‘Elio’) NO3− responses. Transgenic plants expressing a transgene construct containing the DR5 synthetic auxin-responsive promoter driving expression of a β-glucuronidase (GUS) transgene (DR5::GUS) were used to identify the pattern of auxin distribution and to determine how this pattern was affected by localized PNN in ‘Nanguang’. [3H]IAA was used to quantify polar auxin transport. The effects of the polar auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) were also evaluated. Semi-quantitative reverse transcription–PCR (RT–PCR) was used to identify the auxin influx/efflux genes involved in regulating auxin redistribution in LR regions in the two cultivars in response to localized NO3−. The results suggest that a higher responsiveness to NO3− in ‘Nanguang’ was associated with faster NO3−-responsive auxin transport from the shoot to the LR zone, greater auxin accumulation in the LR zone and a higher rate of LR initiation.
MATERIALS AND METHODS
Plant material
Two japonica rice (O. sativa) cultivars, ‘Nanguang’ and ‘Elio’, were selected from 177 japonica rice cultivars based on their similar growth duration and differential responses to N application in field trials conducted in 2003 and 2004. The agronomic traits of the two cultivars were significantly different under both field and hydroponic conditions (Duan et al., 2007a, b; Zhang et al., 2009). ‘Nanguang’ was identified as a high NO3− response cultivar because it exhibited high N accumulation and grain yield under PNN compared with NH4+ as the sole N source (Duan et al., 2007a, b) and was determined to have high N-use efficiency due to high biomass production and grain yield under low N supply and a positive response to increasing N supply (Zhang et al., 2009). Conversely, ‘Elio’ was identified as a low NO3− response cultivar due to low N accumulation and grain yield under PNN relative to NH4+ as a sole N source (Duan et al., 2007a, b). ‘Elio’ produced a lower biomass and grain yield under both low and high N supply and was thus identified as a low N-use efficiency cultivar (Zhang et al., 2009).
Nitrogen and NPA treatments
Plants were grown in a greenhouse under natural light at 30/18 °C day/night temperatures. The roots of two 7-day-old seedlings of uniform size and vigour were divided into two identical parts and transplanted into split-root boxes. Each part of the split-root box was filled with 1 L of nutrient solution (see below). Seedlings were subjected to two NH4+-N/NO3−-N ratios, i.e. 100/0 (NH4+) and 75/25 (PNN), by adding 1·43 mm N in the form of (NH4)2SO4 or a mixture of (NH4)2SO4 and NH4NO3. Each treatment included 12 replicates arranged in a randomized design to avoid edge effects in the greenhouse. The composition of the nutrient solution was: 0·3 mm NaH2PO4, 2·0 mm K2SO4, 1·0 mm CaCl2, 1·5 mm MgSO4·7H2O, 1·7 mm Na2SiO3, 20·0 µm Fe-EDTA, 9·1 µm MnCl2, 0·4 µm (NH4)6Mo7O24, 37·0 µm H3BO3, 0·8 µm ZnSO4 and 0·3 µm CuSO4. A nitrification inhibitor (dicyandiamide, 7·0 µm) was added to each pot to prevent NH4+ oxidation. The nutrient solution was renewed every 2 d. Nitrate was not detected in the nutrient solution with NH4+ as the sole N source. The pH of all nutrient solutions was adjusted to 5·5 daily with 0·1 m NaOH or 0·1 m HCl. Rice plants were harvested at different times during a 9 d period after treatments. For the determination of IAA and the relative expression of genes, roots samples were snap-frozen in liquid N2 and stored in a –40 °C freezer.
Seven-day-old seedlings were treated for 7 d to analyse the effects of NPA on plant growth. Localized application of NPA was performed by dispensing diluted agar containing 20 µm NPA directly across the root–shoot junction using a pipette.
Measurement of LR density and length and microscopic observations of LR initiation
Lateral root length and density in the adventitious and seminal roots were measured using a scanner-based image analysis system WinRhizo (Regent Instruments, Montreal, QC, Canada). Lateral root primordia were counted using a colour CCD camera (Olympus, http://www.olympus-global.com) at ×160 magnification. Stages of LR development were defined according to Malamy and Benfey (1997), with stages I–XII grouped here as unemerged primordia.
Determination of IAA concentration
The concentration of IAA in roots was determined essentially as described by Song et al. (2011). Four root zones were sampled: the root tip (RT, 0·5 cm), the zone where LR initiation and emergence occurred (LI, 0·5–4·0 cm), the LR elongation zone in which LRs were visible but their length was not as long as that of mature roots (LE, 4·0–8·0 cm) and the mature LR zone (ML, 8·0–12·0 cm). Fresh weights of samples were determined, followed immediately by freezing in liquid N. Sample preparation and measurement of free IAA by HPLC were carried out according to Song et al. (2011). A standard IAA sample was obtained from Sigma-Aldrich (St Louis, MO, USA).
To detect IAA distribution patterns in ‘Nanguang’ plants, the pDR5::GUS construct was transformed into ‘Nanguang’ plants using Agrobacterium tumefaciens (strain EHA105). The pDR5::GUS construct was kindly provided by Professor Ping Wu's group at Zhejiang University. The samples used for IAA quantification were also used for histochemical GUS staining. Tissues were treated with ethanol prior to observation to remove chlorophyll pigmentation. The stained tissues were photographed using an Olympus model Szx2-illk stereomicroscope with a colour CCD camera (Olympus).
[3H]IAA transport assay
[3H]IAA polar transport was assayed after localized PNN treatment as described by Liu et al. (2010). Ten replicate roots were sampled. The [3H]IAA solution contained 0·5 µm [3H]IAA (20 Ci mmol−1) in 2 % dimethylsulfoxide (DMSO), 25 mm MES (pH 5·2) and 0·25 % agar.
Shoot to root auxin transport in intact plants was monitored as follows. [3H]IAA solution (20 µL) was applied to the cut surface after rice shoots were removed at 2 cm above the root–shoot junction. After an 18 h (overnight) incubation in darkness, four root segments as defined above were sampled and weighed. Root segments were incubated in scintillation solution (4 mL) for 18 h. [3H]IAA radioactivity was detected using a Beckman Coulter LS6500 multipurpose scintillation counter.
The assay for basipetal auxin transport (transport away from the root tip) was performed using 3 cm long excised root tip segments. [3H]IAA solution (3 µL) was applied to the root tip placed horizontally on a plastic film. After incubation in a humid, dark environment for 18 h (overnight), root segments were cut into two parts: (1) the distal 1 cm from the root tip and (2) the remaining 2 cm. [3H]IAA radioactivity was measured in the 2 cm long segments.
Semi-quantitative RT–PCR analysis
Total RNA was isolated from plant tissues using the guanidine thiocyanate extraction method with TRIzol reagent (Invitrogen, Shanghai, China). First-strand cDNA synthesis was performed using M-MLV reverse transcriptase (Promega Madison, WI, USA) and approx. 1 µg of total RNA and oligo(dT) primer (Invitrogen, Shanghai, China) according to the manual. The mRNA levels were analysed by semi-quantitative RT–PCR using the gene-specific primers listed in Supplementary Data Table S1. The gene names and accession numbers or IDs for rice auxin carriers are OsAUX1 (AK068536), OsAXR4 (NM_001074582·1), OsPIN1a (AK103208), OsPIN1b (AK102343), OsPIN1c (Ak103181), OsPIN2 (AK101191), OsPIN5a (AK066552), OsPIN5b (Ak100297) and OsPIN9 (AK05922). OsACT (OsRac1, accession no. AB047313) mRNA was used as an internal standard. Two independent plant cultivations were conducted, and all samples were analysed three times by this technique.
Data analysis
Data from experiments were pooled for calculations of means and standard errors (s.e.) and analysed by one-way analysis if variance (ANOVA) followed by the least significant difference (LSD) test at P ≤ 0·05 to determine the statistical significance of the differences between individual treatments. All statistical evaluations were conducted using the SPSS (version 11·0) statistical software (SPSS Inc., Chicago, IL, USA).
RESULTS
Lateral root morphology
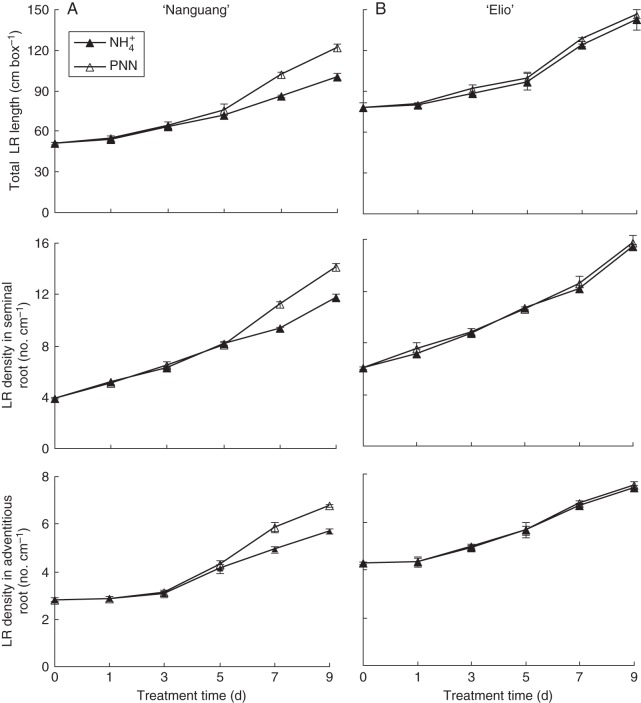
Lateral root length increased faster in response to PNN and was greater at 7 and 9 d for PNN than for NH4+ alone in ‘Nanguang’ (Fig. 1). No change in the length of seminal and adventitious roots was observed in ‘Nanguang’ and ‘Elio’ with PNN after 9 d compared with NH4+ (data not shown). The LR density in seminal and adventitious roots increased by approx. 19 % at 7 and 9 d in ‘Nanguang’ in response to PNN relative to NH4+ alone (Fig. 1). No significant changes in LR length or density were observed between the two N treatments for ‘Elio’. Lateral root length and density were greater in ‘Elio’ than in ‘Nanguang’ under both N treatments over the 9 d period.
Fig. 1.
Lateral root (LR) development in the cultivars ‘Nanguang’ (A) and ‘Elio’ (B). Seedlings were grown under sole NH4+ and partial NO3− nutrition (PNN) in a split-root system for 9 d. Data are means of 12 replications ± s.e.
A significant difference in the number of emerged root primordia between the two localized N treatments was detected by microscopic observation at 5 d in ‘Nanguang’ roots (Table 1). A marked increase in the numbers of emerged root primordia and LRs resulted in a 23 % increase in initiated primordia (number of primordia from the first divisions of pericycle cells to emergence plus the number of emerged laterals) in ‘Nanguang’ after incubation for 7 d with localized PNN compared with NH4+ alone. No difference was observed in the numbers of emerged root primordia for ‘Elio’ between the two N treatments (data not shown).
Table 1.
Microscopic observations of lateral root (LR) initiation events and the development of the seminal root that developed after transfer in ‘Nanguang’ plants
| Time (d) | Treatment | Unemerged LR primordia | Emerged LR primordia (≤0·5 mm) | No. of LRs (≥0·5 mm) | Total no. of initiated primordia |
|---|---|---|---|---|---|
| 5 | NH4+ | 64 ± 9a | 67 ± 2b | 114 ± 15a | 246 ± 19a |
| PNN | 61 ± 7a | 98 ± 1a | 131 ± 11a | 288 ± 20a | |
| 7 | NH4+ | 74 ± 7a | 68 ± 2b | 179 ± 10b | 321 ± 26b |
| PNN | 68 ± 6a | 106 ± 5a | 220 ± 16a | 394 ± 29a |
Seedlings were grown under sole NH4+ and partial NO3− nutrition (PNN) for 7 d.
Data are means of 12 replications ± s.e.
Stages of lateral root development were defined according to Malamy and Benfey (1997), with stages I–XII grouped here as unemerged primordia.
Different letters indicate significant differences in the same column at the same treatment time between means (P < 0·05).
IAA concentration and distribution in the root
Both the above results and published data suggest that auxin may be involved in the adaptive response of roots to localized PNN. A significant increase in IAA concentration was observed in the roots of both cultivars with both N treatments with increasing time in split-root culture (Fig. 2). PNN-induced increases in IAA concentration in the roots of ‘Nanguang’ at 5 and 7 d were 21 and 25 %, respectively, compared with NH4+ alone. IAA concentrations were higher in ‘Elio’ than in ‘Nanguang’ throughout the experiment.
Fig. 2.
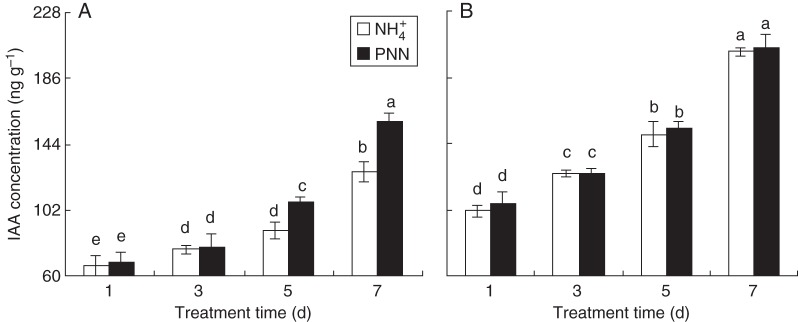
Root auxin concentration in the cultivars ‘Nanguang’ (A) and ‘Elio’ (B). Seedlings were grown under sole NH4+ and partial NO3− nutrition (PNN) in a split-root system for 7 d. Data are means of 12 replications ± s.e. Different letters indicate significant differences between means.
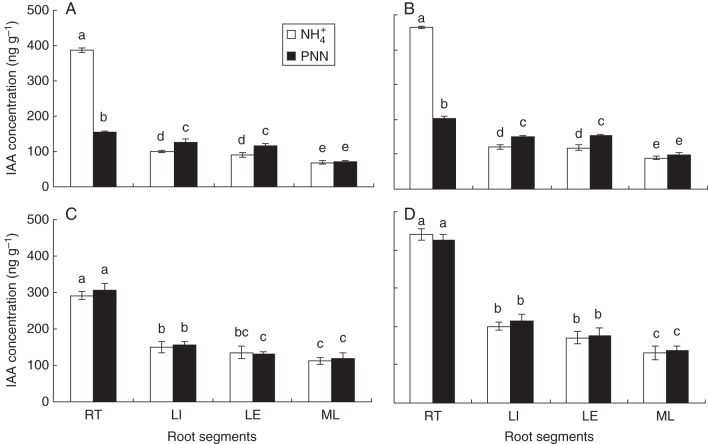
Differences in IAA concentrations in the four root sections were found at 5 and 7 d under both local N treatments only in ‘Nanguang’ (Fig. 3). Interestingly, the IAA concentration decreased by approx. 58 % at the root tip and increased by approx. 28 % in the LI and LE zones with localized PNN compared with NH4+ alone. Due to a small proportion of the actual size for the root tip relative to the size of the LR zone, NO3−-reduced IAA concentration at room temperature did not affect enhanced IAA concentration in the root of ‘Nanguang’ compared with NH4+ alone. IAA concentration changed in the ‘Elio’ root zones in the same manner in both N treatments due to this cultivar's insensitivity to the 25 % NO3− supplied in the PNN treatment.
Fig. 3.
Auxin concentration in four root zones of the cultivars ‘Nanguang’ and ‘Elio’. (A) ‘Nanguang’, 5 d; (B) ‘Nanguang’, 7 d; (C) ‘Elio’, 5 d; (D) ‘Elio’, 7 d. RT, root tip; LI, lateral root initiation and emergence zone; LE, lateral root elongation zone; ML, mature root zone. Seedlings were grown under sole NH4+ and partial NO3− nutrition (PNN) in a split-root system for 7 d. Data are means of 12 replications ± s.e. Different letters indicate significant differences between means.
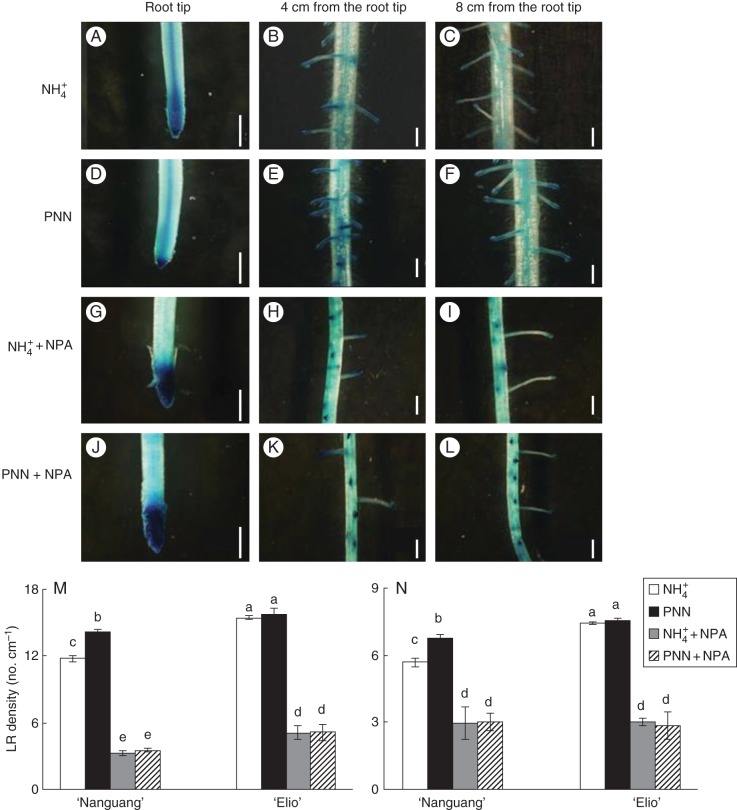
The effect of PNN on the distribution of auxin was further investigated using transgenic ‘Nanguang’ plants expressing the DR5::GUS construct (Fig. 4). Localized PNN treatment changed the histochemical localization of GUS activity relative to localized NH4+ treatment in the DR5::GUS transgenic plants. Although the maximum IAA concentration in the root tip was similar under both N treatments, the IAA distribution was more restricted in the root tip in response to PNN than to NH4+ alone. Between 4 and 8 cm from the root tip, IAA was more widely distributed in response to localized PNN compared with localized NH4+ alone mainly due to greater LR density. Between 8 and 12 cm from the root tip, similar IAA distributions were observed under both N treatments.
Fig. 4.
Histochemical localization of DR5:GUS activity in the cultivar ‘Nanguang’ and lateral root (LR) density at 9 d in ‘Nanguang’ and ‘Elio’. (A–C) NH4+; (D–F) PNN; (G–I) NH4+ + NPA; (J–L) PNN + NPA. DR5::GUS is a specific reporter that contains seven repeats of a highly active synthetic auxin response element and can reflect the in vivo auxin level; NPA is the polar auxin transport inhibitor. In (A–L), seedlings were grown under sole NH4+ and partial NO3− nutrition (PNN) with or without NPA for 7 d. Plants were stained for GUS activity for 1 h at 37 °C. Scale bar = 0·1 mm. (M) Seminal root; (N) adventitious root. In (M) and (N), seedlings were grown under sole NH4+ and PNN with or without NPA for 9 d. Different letters indicate significant differences between means.
The effect of NPA on IAA transport in response to both N treatments was visualized by DR5::GUS activity in the roots (Fig. 4G–L). When NPA was applied at the root–shoot junction, GUS activity was restricted to the root tip and decreased GUS activity was observed in the LRs between 4 and 8 cm and between 8 and 12 cm from the root tip for both N treatments. NPA application at the root–shoot junction significantly inhibited LR initiation (Fig. 4H, I, K, L–N) due to inhibition of polar auxin transport from the shoot to the root.
[3H]IAA transport
To monitor changes in auxin levels in rice roots, we measured auxin transport using radiolabelled IAA. Plants were assayed using the split-root system after 1 d of treatment. The IAA transport from the shoot to the root (acropetal) was determined in response to PNN and NH4+ treatments and IAA transport in the two halves of the split-root system was compared (Fig. 5). Partial NO3− nutrition enhanced auxin transport in the LR initiation, emergence and elongation zones, and decreased auxin transport at the root tip compared with the NH4+ treatment (Fig. 5A). Basipetal (away from the root tip) transport of IAA was substantially reduced in PNN-treated roots (Fig. 6). Independent measurement of acropetal transport was possible because the roots were excised for the assay. This result confirmed the increased acropetal IAA transport by PNN shown in Fig. 2.
Fig. 5.
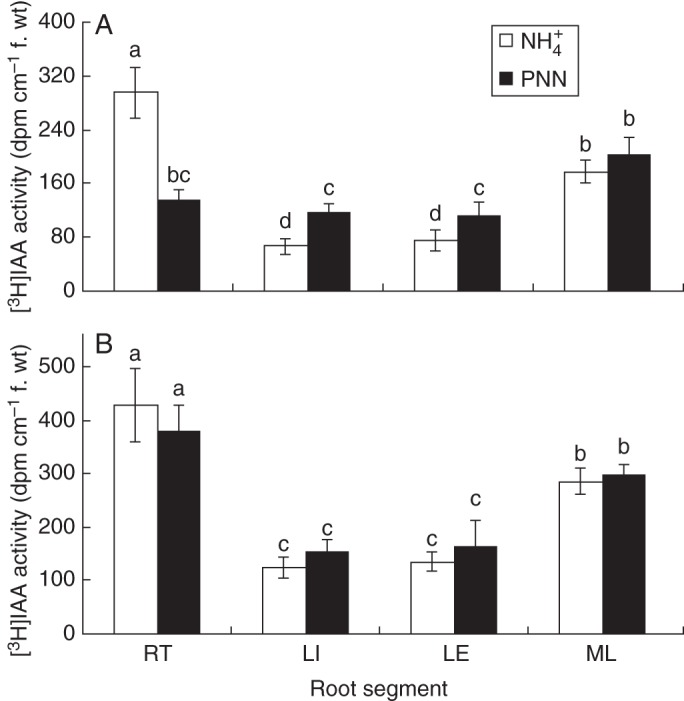
[3H]IAA transport in the cultivars ‘Nanguang’ (A) and ‘Elio’ (B). Radiolabelled IAA was given after 1 d of treatment, as described in the Materials and Methods. RT, root tip; LI. lateral root initiation and emergence zone; LE, lateral root elongation zone; ML, mature lateral root zone. Plants were kept in the dark for 18 h before being assayed for radioactivity in the indicated zones. Different letters indicate significant differences between means.
Fig. 6.
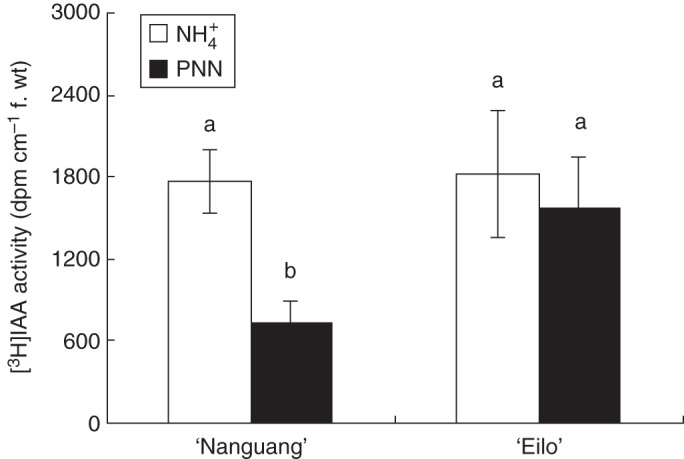
[3H]IAA basipetal transport: radiolabelled IAA was given after 1 d of treatment, as described in the Materials and Methods. Plants were kept in the dark for 18 h before being assayed for radioactivity in the indicated zones. Different letters indicate significant differences between means.
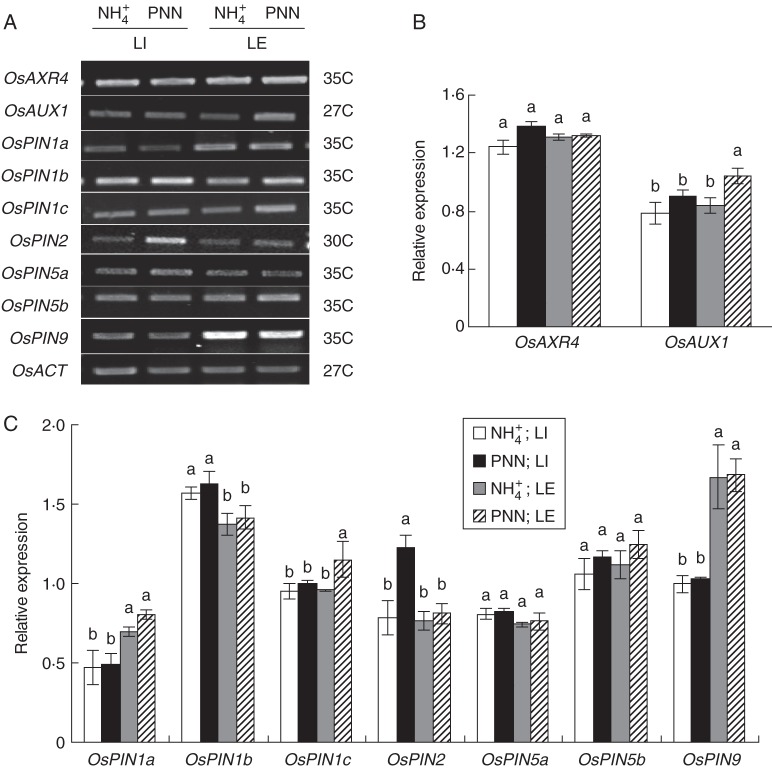
Expression of OsPIN genes, OsAUX1 and OsAXR4
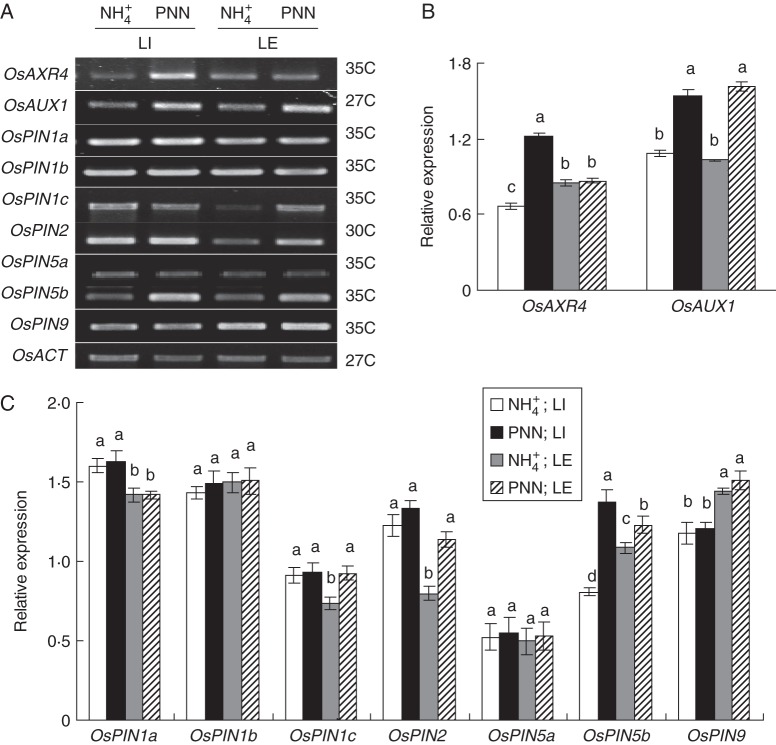
The expression patterns of seven rice PIN genes that have root-specific expression (Wang et al., 2009) were analysed in the LI and LE zones of roots. RT–PCR analysis showed that the expression levels of OsPIN1a, OsPIN1b, OsPIN5a and OsPIN9 genes were unaffected by PNN or NH4+ treatments in either of the cultivars (Figs 7 and 8). Partial NO3− nutrition enhanced OsPIN1c expression in the LE zone and OsPIN2 expression in both root zones in both cultivars relative to the NH4+ treatment. Partial NO3− nutrition enhanced expression of OsPIN5b in both root zones in ‘Nanguang’ but not in ‘Elio’ relative to NH4+ alone.
Fig. 7.
RT–PCR analysis of OsAXR4, OsAUX1 and OsPIN family genes in the cultivar ‘Nanguang’. OsACT was used as a control. (A) Expression patterns of OsAXR4, OsAUX1 and OsPIN family genes (semi-quantitative RT–PCR) in lateral root initiation and emergence (LI) and elongation (LE) zones of ‘Nanguang’ plants under sole NH4+ and partial NO3− nutrition (PNN) in a split-root system. The number of PCR cycles is given on the right. ‘Nanguang’ seedlings were cultured under sole NH4+ nutrition for 1 week from germination and supplied with nutrient solution containing sole NH4+ and PNN for 24 h. Total RNA was isolated from two root sections. (B, C) Relative mRNA levels for individual genes relative to OsACT. Values are shown with mean ± s.e. (n = 5). Different letters indicate a significant difference at P <0·05 of the same gene between four treatments.
Fig. 8.
RT–PCR analysis of OsAXR4, OsAUX1 and OsPIN family genes in the cultivar ‘Elio’. OsACT was used as a control. (A) Expression patterns of OsAXR4, OsAUX1 and OsPIN family genes (semi-quantitative RT–PCR) in lateral root initiation and emergence (LI) and elongation (LE) zones of ‘Elio’ plants under sole NH4+ and partial NO3− nutrition (PNN) in a split-root system. The number of PCR cycles is given on the right. ‘Elio’ seedlings were cultured under sole NH4+ nutrition for 1 week from germination and supplied with nutrient solution containing sole NH4+ and PNN for 24 h. Total RNA was isolated from two root sections. (B, C) Relative mRNA levels for individual genes relative to OsACT. Values are shown with mean ± s.e. (n = 5). Different letters indicate significant difference at P <0·05 of the same gene between four treatments.
Expression of the OsAUX1 and OsAXR4 genes was higher in the LI zone of ‘Nanguang’ roots in response to PNN than to NH4+ alone (Fig. 7). Expression of OsAUX1 was higher in the LE zone in the roots of both cultivars in response to PNN than to NH4+ alone (Fig. 8).
DISCUSSION
A significant feature of root architecture is its plasticity in response to nutrient supply. One of the best known examples of the plasticity of root development is the localized stimulation of LR growth in the roots of many plant species when exposed to a localized source of NO3−. In upland species such as maize and arabidopsis, the primary effect of a localized NO3− treatment was to stimulate LR elongation compared with NO3− starvation (Zhang and Forde, 1998; Guo et al., 2005) although Linkohr et al. (2002) showed that LR density in arabidopsis was also affected by localized NO3− supply. Wang et al. (2002a) showed that localized NO3− supply also stimulated LR elongation in rice compared with an NO3−-free nutrient supply. In whole-plant culture, however, elongation of LRs was induced by NO3− deficiency rather than NO3− supply (Wang et al., 2002a). Due to the different nitrification activities in the rhizosphere, which are dependent on rice genotype and the intermittent flooding cultivation practices of Chinese farmers, NH4+ and NO3− are unevenly distributed within the soil horizon under field conditions. In the whole-root system, PNN enhanced LR formation and adventitious root initiation after 5 weeks compared with NH4+ alone in ‘Nanguang’, which has a high NO3− response, but not in ‘Elio’, which has a low NO3− response (Song et al., 2011). With localized PNN, significant enhancement in LR root length was observed 7 d after treatment due to enhanced emergence of LR primordia at 5 d (Table 1) and LR density at 7 d (Fig. 1). The inconsistency between these results and those of Wang et al. (2002a) may be due to different growing environments. When part of the root system is supplied with sufficient NO3− while another part suffers N starvation, the response to the localized NO3− supply occurs in a background where half of the root system presumably continues to emit an NO3− starvation signal (Wang et al., 2002a). However, in our experiment, differences in LR development occurred in the two halves of the split-root system even with exposure to different N forms at the same concentration. Our results also showed that the emergence of LRs (Fig. 1) rather than adventitious roots (data not shown) was found when ‘Nanguang’ was cultured at 7 and 9 d. The change in the development of adventitious roots was not observed in our experiments, which might result from the shorter cultivation time than that used by Song (2011). Similar results have been observed in other plant species, such as arabidopsis. Linkohr et al. (2002) observed changes in primary root length in 18-day-old-plants over a range of NO3− concentrations, while Zhang and Forde (1998) did not observe such changes in 14-day-old-plants.
Although >70 arabidopsis genes have been shown to affect LR development, auxin and its polar transport have emerged as central regulators of LR development (Benková et al., 2003; De Smet et al., 2006). The dynamic, differential distribution of auxin within the LR zone regulated by basipetal and acropetal auxin transport controls LR initiation and elongation, which tailor root morphology to environmental conditions such as NO3− supply (Grieneisen et al., 2007; Vanneste and Firml, 2009; Kourk et al., 2010). Auxin levels in roots are reduced with increasing NO3− supply using uniform NO3− treatment in soybean (Caba et al., 2000), arabidopsis (Walch-Liu and Forde, 2008) and maize (Tian et al., 2008). Although modulating auxin transport clearly affects LR growth under low NO3− treatment (Kourk et al., 2010), the role of auxin distribution and transport in localized NO3−-induced LR growth remains unclear. Liu et al. (2010) found that local application of NO3− reduced acropetal and basipetal transport compared with N-free treatment and decreased auxin distribution in the LR zone to a level more suitable for LR elongation in maize. In addition, localized treatment with NH4+ also reduced IAA levels, to about the same extent as in the localized NO3− treatment, but did not stimulate LR growth. Therefore, the results in maize showed that the change in auxin transport in response to two localized N forms was not coincident with the change of LR growth (Guo et al., 2009; Liu et al., 2010).
Song et al. (2011) found that a uniform PNN treatment increased auxin synthesis and auxin transport from the shoot to the root relative to an NH4+ treatment only in rice with a high NO3− response. The split-root system was used here to better account for the effect of a local PNN treatment on auxin transport form the shoot to root and auxin distribution in roots. After cultivation for 5 d, auxin transport from the shoot to root was enhanced in response to PNN relative to NH4+ alone only in ‘Nanguang’ (Fig. 3). This indicates that both uniform and localized PNN enhanced auxin transport from the shoot to root. Radiotracer experiments using [3H]IAA further showed that localized PNN enhanced auxin acropetal transport; however, it decreased auxin basipetal transport compared with NH4+ treatment (Figs 5 and 6). These results were inconsistent with the results in maize (Liu et al., 2010). Many studies showed that the change of auxin distribution in roots might reflect N status in the plants rather than a specific response to a localized NO3− supply (Walch-Liu et al., 2006; Bao et al., 2007; Tian et al., 2008; Liu et al., 2010). The main reason for this discrepancy was probably a result of N sufficiency in the other half of the rice root in the present study and N deficiency in the other half of the maize root (Liu et al., 2010). However, more experiments are needed to explain where the increased auxin in the LR initiation and elongation zones came from.
The polarity of auxin transport is determined by the asymmetric localization of the AUX1 and PIN auxin influx and efflux facilitators (Kramer, 2004). Genetic studies suggest that AXR4 functions in the same pathway as AUX1. Loss of AXR4 resulted in abnormal accumulation of AUX1 in the endoplasmic reticulum of epidermal cells, indicating that the axr4 agravitropic phenotype is caused by defective AUX1 trafficking in the root epidermis (Dharmasiri et al., 2006). Expression of AUX1 was high in arabidopsis roots treated with low NO3− (Bao et al., 2007). Wang et al. (2002b) showed that expression of the auxin efflux carrier REH1 increased rapidly and peaked after 4 h in response to localized NO3− supply. Therefore, we hypothesize that a localized NO3− supply regulates the expression of auxin influx and efflux transporters and causes an uneven auxin distribution in rice roots. Higher expression of OsAXR4 was observed in the LI and LE zones in ‘Nanguang’ but not in ‘Elio’ in response to PNN, which corresponded to the higher OsAUX1 expression within the same root zones. Higher OsAUX1 expression was also observed in the LI and LE zones in ‘Nanguang’. Partial NO3− nutrition enhanced OsPIN1c expression in the LE zone and OsPIN2 expression in the LI and LE zones in both cultivars relative to NH4+ alone. Partial NO3− nutrition enhanced expression of the OsPIN5b gene in the LI and LE zones in ‘Nanguang’ but not in ‘Elio’. One of seven root-specific PIN genes, OsPIN1c, was expressed predominantly in the stele of seminal roots and specifically expressed in LR primordia (Wang et al., 2009). Increased OsPIN1c expression in response to PNN was observed in the LR zone in both cultivars. Wang et al. (2009) reported that OsPIN5b was primarily expressed in the meristem. However, increased OsPIN5b expression was observed in the LI and LE zones in ‘Nanguang’ but not in ‘Elio’. These results indicated that localized PNN increased the expression of auxin influx (OsAUX1 and OsAXR4) and efflux (OsPIN5b) carriers, creating a robust auxin flux to the LR zones and enhanced LR initiation.
In conclusion, PNN enhanced auxin polar transport from the shoot to root and induced greater auxin flux into the LR zone and enhanced LR proliferation in the high NO3−-responsive ‘Nanguang’. These results indicate that stronger NO3− responses are associated with greater auxin accumulation in the LR zone and a higher rate of LR initiation in ‘Nanguang’.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was funded by the National Nature Science Foundation of China (nos 31071846 and 31172022), by the Ministry of Science and Technology of China (no. 2011CB100302), by the State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science (no. Y052010013), by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and by Innovative Research Team Development Plan of the Ministry of Education of China (IRT1256).
LITERATURE CITED
- Bao J, Chen F, Gu R, Wang G, Zhang F, Mi G. Lateral root development of two Arabidopsis auxin transport mutants, aux1-7 and eir1-1, in response to nitrate supplies. Plant Science. 2007;173:417–425. [Google Scholar]
- Benková E, Michniewicz M, Sauer M, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Caba JM, Centeno ML, Fernández B, Gresshoff PM, Ligero F. Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a supernodulation mutant and the wild type. Planta. 2000;211:98–104. doi: 10.1007/s004250000265. [DOI] [PubMed] [Google Scholar]
- De Smet I, Vanneste S, Inzé D, Beeckman T. Lateral root initiation or the birth of a new meristem. Plant Molecular Biology. 2006;60:871–887. doi: 10.1007/s11103-005-4547-2. [DOI] [PubMed] [Google Scholar]
- Dharmasiri S, Swarup R, Mockaitis K, et al. AXR4 is required for localization of the auxin influx facilitator AUX1. Science. 2006;312:1218–1220. doi: 10.1126/science.1122847. [DOI] [PubMed] [Google Scholar]
- Drew MC. Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytologist. 1975;75:479–490. [Google Scholar]
- Drew MC, Saker LR, Ashley TW. Nutrient supply and the growth of the seminal root system in barley I. The effect of nitrate concentration on the growth of axes and laterals. Journal of Experimental Botany. 1973;24:1189–1202. [Google Scholar]
- Duan YH, Zhang YL, Ye LT, Fan XR, Xu GH, Shen QR. Responses of rice cultivars with different nitrogen use efficiency to partial nitrate nutrition. Annals of Botany. 2007a;99:1153–1160. doi: 10.1093/aob/mcm051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Zhang Y, Wang S, Shen Q. Effect of NH4+ to NO3− ratio (NH4+/NO3−) on biological characteristics of rice with different nitrogen use efficiency. Journal of Nanjing Agricultural University. 2007b;30:73–77. Available at http://nauxb.njau.edu.cn/qw/show.php?id=3198 . [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proceedings of the National Academy of Sciences, USA. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraru E, Friml J. PIN polar targeting. Plant Physiology. 2008;147:1553–1559. doi: 10.1104/pp.108.121756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BG. Local and long-range signaling pathways regulating plant responses to nitrate. Annual Review of Plant Biology. 2002;53:203–224. doi: 10.1146/annurev.arplant.53.100301.135256. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, et al. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. The Plant Journal. 2005;44:179–194. doi: 10.1111/j.1365-313X.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- Grieneisen VA, Xu J, Marée AFM, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- Granato TC, Raper CD., Jr Proliferation of maize (Zea mays L.) roots in response to localized supply of nitrate. Journal of Experimental Botany. 1989;40:263–275. doi: 10.1093/jxb/40.2.263. [DOI] [PubMed] [Google Scholar]
- Guo Y, Chen F, Zhang F, Mi G. Auxin transport from shoot to root is involved in the response of lateral root growth to localized supply of nitrate in maize. Plant Science. 2005;169:894–900. [Google Scholar]
- Kramer EM. PIN and AUX/LAX proteins: their role in auxin accumulation. Trends in Plant Science. 2004;9:578–582. doi: 10.1016/j.tplants.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, et al. Nitrate-regulated auxin transport by NRT1·1 defines a mechanism for nutrient sensing in plants. Developmental Cell. 2010;18:927–937. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Li YL, Fan XR, Shen QR. The relationship between rhizosphere nitrification and nitrogen-use efficiency in rice plants. Plant, Cell and Environment. 2008;31:73–85. doi: 10.1111/j.1365-3040.2007.01737.x. [DOI] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser O. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. The Plant Journal. 2002;29:751–760. doi: 10.1046/j.1365-313x.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- Liu J, An X, Cheng L, et al. Auxin transport in maize roots in response to localized nitrate supply. Annals of Botany. 2010;106:1019–1026. doi: 10.1093/aob/mcq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, et al. AUX1 promotes lateral formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant Cell. 2002;14:589–597. doi: 10.1105/tpc.010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattelmarcher B, Thoms K. Morphology and physiology of the seminal root system of young maize (Zea mays L.) plants as influenced by a locally restricted nitrate supply. Zeitschrift für Pflanzenernährung und Bodenkunde. 1995;158:493–497. [Google Scholar]
- Remans T, Nacry P, Pervent M, et al. The Arabidopsis NRT1·1 transporter participates in the signalling pathway triggering root colonization of nitrate-rich patches. Proceedings of the National Academy of Sciences, USA. 2006;103:19206–19211. doi: 10.1073/pnas.0605275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Makeen K, Wang D, et al. Nitrate supply affects root growth differentially in two rice cultivars differing in nitrogen use efficiency. Plant and Soil. 2011;343:357–368. [Google Scholar]
- Tian Q, Chen F, Liu J, Zhang F, Mi G. Inhibition of maize root growth by high nitrate supply is correlated to reduced IAA levels in roots. Journal of Plant Physiology. 2008;165:942–951. doi: 10.1016/j.jplph.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends in Plant Science. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Wang JR, Hu H, Wang GH, Li J, Chen JY, Wu P. Expression of PIN genes in rice (Oryza sativa L.). Tissue specificity and regulation by hormones. Molecular Plant. 2009;2:823–831. doi: 10.1093/mp/ssp023. [DOI] [PubMed] [Google Scholar]
- Wang XB, Wu P, Hu B, Chen Q. Effect of nitrate on the growth of lateral root and nitrogen absorption in rice. Acta Botanica Sinica. 2002a;44:678–683. [Google Scholar]
- Wang X, Wu P, Xia M, Wu Z, Chen Q, Liu F. Identification of genes enriched in rice roots of the local nitrate treatment and their expression patterns in split-root treatment. Gene. 2002b;297:93–102. doi: 10.1016/s0378-1119(02)00870-3. [DOI] [PubMed] [Google Scholar]
- Walch-Liu P, Forde BG. Nitrate signalling mediated by the NRT1·1 nitrate transporter antagonises l-glutamate-induced changes in root architecture. The Plant Journal. 2008;54:820–828. doi: 10.1111/j.1365-313X.2008.03443.x. [DOI] [PubMed] [Google Scholar]
- Walch-Liu P, Ivanov II, Filleur S, Gan Y, Remans T, Forde BG. Nitrogen regulation of root branching. Annals of Botany. 2006;97:875–881. doi: 10.1093/aob/mcj601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde BG. Regulation of Arabidopsis root development by nitrate availability. Journal of Experimental Botany. 2000;51:51–59. [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde BG. Dual pathways for regulation of root branching by nitrate. Proceedings of the National Academy of Sciences, USA. 1999;96:6529–6534. doi: 10.1073/pnas.96.11.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YL, Fan JB, Wang DS, Shen QR. Genotypic differences in grain yield and physiological nitrogen use efficiency among rice cultivars. Pedosphere. 2009;19:681–691. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.