Abstract
Background and Aims
Insufficient pollination is a function of quantity and quality of pollen receipt, and the relative contribution of each to pollen limitation may vary with intrinsic plant traits and extrinsic ecological properties. Community-level studies are essential to evaluate variation across species in quality limitation under common ecological conditions. This study examined whether endemic species are more limited by pollen quantity or quality than non-endemic co-flowering species in three endemic-rich plant communities located in biodiversity hotspots of different continents (Andalusia, California and Yucatan).
Methods
Natural variations in pollen receipt and pollen tube formation were analysed for 20 insect-pollinated plants. Endemic and non-endemic species that co-flowered were paired in order to estimate and compare the quantity and quality components of pre-zygotic pollination success, obtained through piecewise regression analysis of the relationship between pollen grains and pollen tubes of naturally pollinated wilted flowers.
Key Results
Pollen tubes did not frequently exceed the number of ovules per flower. Only the combination of abundant and good quality pollen and a low number of ovules per flower conferred relief from pre-zygotic pollen limitation in the three stochastic pollination environments studied. Quality of pollen receipt was found to be as variable as quantity among study species. The relative pollination success of endemic and non-endemic species, and its quantity and quality components, was community dependent.
Conclusions
Assessing both quality and quantity of pollen receipt is key to determining the ovule fertilization potential of both endemic and widespread plants in biodiverse hotspot regions. Large natural variation among flowers of the same species in the two components and pollen tube formation deserves further analysis in order to estimate the environmental, phenotypic and intraindividual sources of variation that may affect how plants evolve to overcome this limitation in different communities worldwide.
Keywords: Co-flowering community, Mediterranean, piecewise regression, pollen limitation, pollen tubes, pollination, stigmatic pollen load, Yucatan
INTRODUCTION
Pollen limitation of seed production may be the main or most proximate cause of reduced reproductive success in seed-limited plant populations (Wilcock and Neiland, 2002; Aguilar et al., 2006), and it is predicted to increase in association with habitat fragmentation, climate warming and pollinator decline (Aguilar et al., 2006; Memmott et al., 2007; Hegland et al., 2009; Potts et al., 2010; Winfree et al., 2011; Gilman et al., 2012). The magnitude of pollen limitation is highly variable among habitats and species (Larson and Barrett, 2000; Ashman et al., 2004), and contrasting results about its prevalence have been found among different plant communities (e.g. Motten, 1986; McMullen, 1987; Johnson and Bond, 1997; Hegland and Totland, 2008; García-Camacho and Totland, 2009). Thus, more community-level studies are required to uncover the links between characteristics of the co-flowering community and pollen limitation (Mitchell et al., 2009; Alonso et al., 2010).
Insufficient pollination can result from two main causes, insufficient quantity of pollen deposited on the stigma by scarce and/or inefficient floral visitors or limited quality of pollen receipt associated with selfing and mating between related plants (Ashman et al., 2004; Aizen and Harder, 2007). The specific effect of pollen quality has recently been stressed but remains largely unstudied in the wild, even though reduced pollen quality has been reported as the main cause of sexual reproductive failure in some natural populations (e.g. Chacoff et al., 2008; Amat et al., 2011). Further, variability among flowers within species in pollen receipt and pollen tube formation are important but rarely explored elements in understanding how often and how severely pollen tubes limit ovule fertilization, the most proximate estimate of pollination insufficiency (Herrera, 2002; Burd et al., 2009). Pollen tubes represent the intermediate phase between pollen arrival and seed production, and may better characterize the pollination phase of this two-stage process, without confounding the seed filling stage, wherein pollination success interacts with resource availability (Ashman et al., 2004; Wesselingh, 2007; Alonso et al., 2012). The relevance of quantity and quality of pollen receipt for pollen tube formation has been experimentally demonstrated, but their relative importance to overall pollen limitation in the wild remains largely unknown (Alonso et al., 2012, and references therein). Indeed, the two components may vary not only with intrinsic plant traits, but also with ecological conditions, and the extent to which co-existing plants compete for or facilitate pollination service (Ashman et al., 2004; Feldman et al., 2004; Mitchell et al., 2009; Alonso et al., 2010). The latter has been nicely demonstrated in Mimulus ringens, a species with a mixed mating system, in which, in contrast to monospecific stands, not only seed production but also the outcrossing rate was greatly reduced by the addition of a co-flowering species as a result of interspecific pollinator movements and improper conspecific pollen transfer (Bell et al., 2005). Thus, only community-level studies can directly reveal the causes of interspecific variation in the magnitude of quantity and quality components of pollination under common ecological conditions, and the intrinsic and extrinsic factors determining their relative role in limiting plant reproduction (Alonso et al., 2010).
A recent meta-analysis revealed stronger pollen limitation of plants in biodiversity hotspots (Vamosi et al., 2006). Further, endemic species, which contribute significantly to the species richness of biodiversity hotspots (Myers et al., 2000), seem to suffer increased pollen limitation in highly diverse regions (Alonso et al., 2010), and evidence exists to suggest that the roles of quantity and quality may differ for these compared with widespread species (reviewed in Alonso et al., 2010). For instance, the quality component could be particularly important for endemics because they frequently have reduced genetic diversity (Byers, 1995; Cole, 2003; Amat et al., 2011) or smaller population sizes (Kunin, 1993; Fischer et al., 2003; Amat et al., 2011). Quantity limitation could also be critical if they have low density populations (Kunin, 1993; Fischer et al., 2003; Feldman, 2008). Despite these compelling reasons, we currently have a limited comparative understanding of the relative drivers of pollen limitation between endemic and widespread species co-flowering in plant-rich communities (Alonso et al., 2010).
To address these gaps, we analysed natural variation in pollen tube formation of a large number of species in three highly diverse and endemic-rich, insect-pollinated co-flowering communities: two in Mediterranean-climate regions in Andalusia and California and a third one in the seasonally dry tropical area of the Yucatan. We applied a new method that allows estimation of the quantitative and qualitative components of pollination success based on the analysis of pollen grain–pollen tube dose–response curves of naturally pollinated wilted flowers (Alonso et al., 2012) and compared the relative strength of the two components in multiple pairs of co-flowering endemic and non-endemic species per community. Our specific questions were the following. (1) How variable is pollen receipt among flowers, species and communities? (2) Do paired endemic and non-endemic species differ in pollen quantity or quality, or both? If so, (3) are the sign and magnitude of differences consistent among pairs, which would suggest a common pattern for endemics vs. non-endemics? (4) How does quality of pollen receipt affect the strength of pollen limitation at the pre-zygotic phase?
MATERIALS AND METHODS
Study plant communities
We selected insect-pollinated species in three endemic-rich plant communities, two associated with soil-specific sites with Mediterranean climate, the sandy dolomite outcrops of the Baetic Ranges in Andalusia (Mota et al., 2008) and the serpentine seeps in California (Freestone and Inouye, 2006; Alexander et al., 2007), and one associated with clearings in the seasonally dry scrublands on the north coast of the Yucatan Peninsula in Mexico (Espadas Manrique et al., 2003). By making this selection, we focused on environments where flowering is seasonally restricted. In the Mediterranean climate, flowering is frequently year-round, but in our specific sites most species bloom from late spring to early summer, depending on species-specific tolerance to seasonal drying (Alexander et al., 2007). Thus, the study was conducted during May and June 2010 at the Natural Park of Cazorla, Segura y Las Villas (Andalusia, Spain) and during June and July 2010 at the Donald and Sylvia McLaughlin Natural Reserve (Northern California, USA). In the tropical dry forests flowering responds quickly to seasonal rainfall, promoting synchrony within populations and among species (Bullock, 1995). In Yucatan, flowering in 2010 started in July and the study was conducted in July–August, outside of any protected area on the northern coast of the Yucatan state. In each location we monitored 3–5 accessible sites (see Supplementary Data for a more detailed description).
Focal species
Across these communities we selected pairs of locally endemic and non-endemic species (Table 1). In all cases, endemic species were narrow endemics whose global distribution range was geographically restricted to the region and associated with the specific habitat studied, in contrast to the wider distribution ranges of their non-endemic partners (i.e. the dolomites in south-east Spain vs. southern Europe; Californian serpentines vs. non-serpentines; Yucatan coast–Caribbean vs. Mesoamerica). To provide some level of phylogenetic control, we paired species within families. This paired design was intended to control for the influence of species relatedness on floral characters and mating system when comparing pollination success (Knight et al., 2005). However, this was not always possible so we also selected pairs based on coexistence and co-flowering at a site to control for the ecological conditions associated with spatial and temporal changes in local pollinator fauna availability that could not be predicted a priori (Knight et al., 2005). In three cases (see Table 1), this resulted in pairs not within the same family but that had flowers with similar colour, symmetry and type of reward offered to pollinators. The final data set included ten study cases involving 20 species (Table 1). Mating system types (verified through controlled self-pollinations under greenhouse or field conditions by the authors) were most frequently shared by the paired species (Table 1).
Table 1.
Study pairs and floral features
| Region | Family | Pair | Taxa | Code | Range | Sites | Symmetry | Colour | Nectar | Mating | Ovules |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Andalusia | Cistaceae | 1 | Fumana baetica | Fub | E | 2 | Actino | Yellow | No | SC | 9 |
| Andalusia | Cistaceae | 1 | Helianthemum appeninum appeninum | Hcr | NE | 2 | Actino | Yellow | No | SI | 16·7 ± 1·3 |
| Andalusia | Cistaceae | 2 | F. paradoxa | Fup | E | 1 | Actino | Yellow | No | SC | 6 |
| Andalusia | Cistaceae | 2 | H. cinereum | Hci | NE | 1 | Actino | Yellow | No | SC | 6 |
| Andalusia | Caryophyllaceae | 3 | Silene psammitis lasiostyla | Spl | E | 2 | Actino | Pink | Yes | SC | 33·3 ± 0·9 |
| Andalusia | Caryophyllaceae | 3 | S. colorata | Sco | NE | 2 | Actino | Pink | Yes | SC | 53·8 ± 2·2 |
| Andalusia | Lamiaceae | 4 | Thymus orospedanus | Tho | E | 2 | Zygo | Pink | Yes | SC | 4 |
| Andalusia | Lamiaceae | 4 | Sideritis incana | Sii | E | 1 | Zygo | Yellow | Yes | SC | 4 |
| Andalusia | Lamiaceae | 4* | Teucrium polium | Tpo | NE | 2 | Zygo | Beige | Yes | SC | 4 |
| California | Liliaceae | 5 | Triteleia peduncularis | Trp | E | 5 | Actino | White | Yes | SI | 27·7 ± 0·8 |
| California | Liliaceae | 5 | Zigadenus venenosus | Ziv | NE | 5 | Actino | Beige | Yes | SI | 20·4 ± 0·4 |
| California | Ranunculaceae | 6 | Delphinium uliginosum | Deu | E | 5 | Zygo | Purple | Yes | SC | 31·4 ± 1·5 |
| California | Scrophulariaceae | 6 | Antirrhinum cornutum | Anc | NE | 5 | Zygo | Purple | Yes | SI | 62·3 ± 3·6 |
| California | Phyrmaceae | 7 | Mimulus nudatus | Min | E | 5 | Zygo | Yellow | No | SC | 179·2 ± 14·9 |
| California | Phyrmaceae | 7 | M. guttatus | Mig | NE | 5 | Zygo | Yellow | No | SC | 240·9 ± 28·3 |
| Yucatan | Malvaceae | 8 | Cienfuegosia yucatanensis | Cie | E | 2 | Actino | Yellow | Yes | SC | 16·4 ± 0·4 |
| Yucatan | Malvaceae | 8 | Sida acuta | Sid | NE | 2 | Actino | Yellow | Yes | SC | 5·9 ± 0·1 |
| Yucatan | Lythraceae | 9† | Cuphea gaumeri | Cup | E | 1 | Zygo | Purple | Yes | SC | 25·6 ± 1·6 |
| Yucatan | Scrophulariaceae | 9 | Angelonia angustifolia | Ang | NE | 1 | Zygo | Purple | Yes | SC | 53·5 ± 0·7 |
| Yucatan | Lythraceae | 10† | C. gaumeri | Cup | E | 1 | Zygo | Purple | Yes | SC | 25·8 ± 1·0 |
| Yucatan | Verbenaceae | 10 | Tamonea curassavica | Tam | NE | 1 | Zygo | Purple | Yes | SC | 4 |
Taxa were categorized according to distribution range, distinguishing between endemics (E) and non-endemics (NE); floral symmetry, distinguishing between actinomorphic (Actino) and zygomorphic (Zygo); presence of nectar as a reward; and mating system, distinguishing between self-incompatible (SI) and self-compatible (SC) plants. The number of ovules was constant in some taxa; for those that were variable, we show an average (± s.e.) obtained from a small independent sample (8 ≤ n ≤ 20). The number of sites where samples were collected is also indicated.
*Teucrium polium was compared against two endemic species, although one of them was only collected in a study site in which the three coexist.
†Cuphea gaumeri only coexists with each paired species within a single study population; thus, population data were not merged.
Flower collection and style processing
Pollination success of each focal species was estimated by counting pollen grains and tubes on styles of wilted open-pollinated flowers collected from each species during peak flowering, monitored over the study period. Only 1–2 flowers per individual and ≤2 individuals every 5 m were collected to sample spatial variation effectively within each population. A pooled sample per species with approx. 150 styles was analysed by combining styles collected from all study sites where the species and its co-flowering partner were present (see Table 1 for details). All species sampled in >1 site showed continuous variation in the pollen grains–pollen tubes scatterplot. Samples were stored in 70 % ethanol until dissection. We used epifluorescence to visualize pollen tubes (see Kearns and Inouye, 1993 for details). As a rule, styles were softened in 1 n KOH at 65 °C for 20 min, rinsed with distilled water and stained for 20 min at 65 °C in decolorized aniline blue with small adjustments for species. Under the microscope, pollen grains were counted on the stigma and tubes at the base of the style and afterwards divided by the average number of ovules per flower to standardize among species (‘tubes’, hereafter). We characterized the mean number of ovules per flower for each species from a random sample of floral buds (8 ≤ n ≤ 20; Table 1).
Data set structure and statistical characterization of components of pollination success
Data set structure
All statistical analyses were conducted with SAS (SAS Institute, 2008). Definition of fixed and random effects is not always straightforward in GLMM (generalized linear mixed model) modelling (Bennington and Thayne, 1994). In our sampling scheme, endemism was a main fixed factor with two levels, namely endemic and non-endemic (see above). Also, community was entered as a main fixed effect with three levels (Andalusia, California and Yucatan) because the three plant communities studied were neither a random selection nor a high number of levels that could be eventually considered as representative of the ‘rich-plant communities worldwide’ variable. The endemism × community interaction was also included to test for the consistency in the sign and significance of the endemism effect at study locations. Further, species were not sampled randomly but rather paired for the benefit of controlling for the potential joint effect of relatedness and co-flowering, and ensuring a balanced sampling of endemics and non-endemics from different families within communities. The effect of pair was treated as random with ten levels because we studied as many suitable pairs as possible in our study sites and a species was only selected if it could be paired with another species, but they were not all the possible pairs existing in each plant community. Finally, when replicated at this level, species was modelled as a random subject effect to account for the non-independence of all data points within pairs.
Pollen quantity
Pollen load was characterized for each species by the mean number of conspecific pollen grains on the stigma and its coefficient of variation (CV). We also quantified the percentage of flowers collected that did not have any conspecific pollen grains on their stigmas (‘pollen-less stigmas’, hereafter).
Variation between endemics and non-endemics and among communities in CV and percentage of pollen-less stigmas was analysed by GLMMs with REML (procedure MIXED). Further, a GLMM (procedure GLIMMIX) with lognormal error distribution was applied to the complete sample of styles (n = 3036 excluding those with 0 pollen grains).
Pollen quality
For each study species, we used the ‘piecewise’ analysis approach of Alonso et al. (2012) to analyse the functional relationship between pollen tubes and grains of visited flowers. Linearity of the relationship between pollen tubes and grains was tested using generalized additive models (procedure GAM); a significant spline component indicated improvement of fit when the model includes a non-linear local component and, if so, piecewise regression was conducted. Piecewise regression allows the inclusion of a breakpoint in a linear regression when an abrupt change in the slope of the relationship is predicted, i.e. in the specific relationship between pollen tubes and grains the breakpoint has been interpreted as a shift in the relative importance of quantitative (Qt) and qualitative (Ql) components of pollination success (Alonso et al., 2012). Following the description in Alonso et al. (2012), at low pollen loads (region I in fig. 2 of Alonso et al., 2012), pollination success is predicted to be more limited by Qt. There, a high initial slope (b1) reflects receipt of high-quality pollen, and a low b1 (shallow rise) reflects the receipt of low-quality pollen. The value of c reflects the point at which the initial slope (b1) ceases to fit the data and the second regression (b2) becomes more appropriate (i.e. the ‘breakpoint’). The width of the confidence interval around c defines region II (in fig. 2 of Alonso et al., 2012) that reflects both the certainty of the breakpoint location and the range of pollen load sizes for which Ql and Qt are contributing with similar importance to pollination success. Finally, at high pollen loads (region III in fig. 2 of Alonso et al., 2012), Ql becomes a strong determinant of pollination success, associated with greater pollen tube competition. We fitted piecewise models to data from each species, as appropriate, using the NLIN procedure. Confidence intervals (BCa, hereafter) for all three parameter estimates (b1, b2 and c) were calculated using non-parametric bootstrapping (n = 1000).
Paired comparisons of slopes from piecewise regression (b1, b2) between endemic and non-endemic species were tested by splitting each data set at breakpoint (c) and for each subset applying analysis of covariance (ANCOVA) on pollen tubes where pollen load was a covariate and endemism a fixed factor (Alonso et al., 2012). Finally, the global effect of endemism and community on b1 was tested by ANCOVA on pollen tubes only for the portion of flowers receiving pollen loads less than the breakpoint (c). In this model, pollen load was a covariate and pair a random factor (procedure MIXED). In species that did not reach saturation, we used data from all flowers.
Pre-zygotic pollen limitation
For each species, we calculated the percentage of visited flowers with tube number less than the average number of ovules, which represents the proportion of flowers that despite receiving some pollen were unable to fertilize all ovules fully, i.e. pollen-limited flowers. Also, the number of pollen tubes per ovule at breakpoint (k) estimates the magnitude of pre-zygotic pollen limitation since k<1 indicates that pollen tube saturation is reached below the average number of ovules per flower (Alonso et al., 2012). For species that did not reach saturation, we estimated k as the expected value of pollen tubes per ovule at the highest pollen load recorded in the sample, assuming that saturation will be reached at higher values than observed. Variation between endemics and non-endemics and among communities in the percentage of pollen-limited flowers per species and k values was analysed by general linear models with REML (procedure MIXED).
RESULTS
Pollen quantity
High variance in pollen receipt was the rule in our study species, as reflected by their large CVs (from 54 to 248; Table 2), but the magnitude of variation differed marginally among the study communities (F2,8 = 4·05, P = 0·06). Variation in the percentage of pollen-less stigmas also differs among communities (F2,8 = 4·76, P = 0·04; Table 2). The effect of endemism and its interaction with community were far from significant in both analyses (P>0·30).
Table 2.
Summary of results obtained as estimates of quantity and quality of pollination
| Region | Pair | Code | Pollen load |
Stigmas without pollen (%) | Regression parameters |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | CV | b1 | b2 | c (BCa) | k | ||||
| Andalusia | 1E | Fub | 49·4 ± 3·0** | 63·8 | 1·8 | 0·0406* | –0·0014 | 21·6 (13–35·5)* | 0·88 |
| Andalusia | 1NE | Hcr | 277·8 ± 15·4 | 53·7 | 2·1 | 0·0028 | 0·0010 | 170·4 (110·3–505·5) | 0·48 |
| Andalusia | 2E | Fup | 82·0 ± 11·7** | 114·1 | 1·5 | 0·0292* | 0·0010 | 85·8 (64·2–203·5) | 2·50 |
| Andalusia | 2NE | Hci | 203·4 ± 10·4 | 58·9 | 3·0 | 0·0078 | 0·0005 | 79 (28–145·2) | 0·62 |
| Andalusia | 3E | Spl | 103·3 ± 7·7NS | 104·3 | 9·7 | 0·0057NS | 0·0014 | 224·4 (120·8–289·7) | 1·28 |
| Andalusia | 3NE | Sco | 96·0 ± 9·9 | 124·4 | 9·7 | 0·0057 | 0·0003 | 202·2 (123·7–252·9) | 1·15 |
| Andalusia | 4E | Tho | 2·1 ± 0·3** | 204·7 | 44·3 | 0·1636NS | 0·0772 | 17·9 (4·7–18·8) | 2·93 |
| Andalusia | 4E | Sii | 55·9 ± 2·8** | 56·2 | 2·4 | 0·0758NS | 0·0067* | 28·1 (19–37·6)* | 1·88 |
| Andalusia | 4NE | Tpo | 13·9 ± 1·0 | 115·9 | 10·0 | 0·1753 | 0·0278 | 5·7 (3·6–7·9) | 1·00 |
| California | 5E | Trp | 164·0 ± 9·1** | 67·7 | 0 | 0·0038* | – | – | 1·97 |
| California | 5NE | Ziv | 88 ± 5·08 | 70·5 | 0 | 0·03 | 0·016 | 40 (23–63) | 1·20 |
| California | 6E | Deu | 254 ± 15·2** | 73·0 | 0 | 0·004* | -0·0008 | 344 (141–444) | 1·38 |
| California | 6NE | Anc | 158·0 ± 8·1 | 63·3 | 0 | 0·01 | 0·0002 | 143 (114–176) | 1·43 |
| California | 7E | Min | 203·0 ± 13·5** | 81·6 | 0 | 0·0010* | – | – | 0·75 |
| California | 7NE | Mig | 307 ± 20·7 | 82·8 | 0 | 0·008 | 0·002 | 168 (128–299) | 1·34 |
| Yucatan | 8E | Cie | 66·3 ± 3·2** | 76·7 | 0·8 | 0·0013* | – | – | 0·36 |
| Yucatan | 8NE | Sid | 8·1 ± 0·68 | 116·6 | 15·1 | 0·0136 | – | – | 0·83 |
| Yucatan | 9E | Cup | 43·2 ± 7·3NS | 247·8 | 29·2 | 0·0014NS | – | – | 1·35 |
| Yucatan | 9NE | Ang | 43·6 ± 7·3 | 162·6 | 28·7 | 0·0027 | – | – | 0·95 |
| Yucatan | 10E | Cup | 29·9 ± 4·5** | 219·7 | 21·0 | 0·0014** | 0·0003 | 39·6 (18·1–113·4) | 0·06 |
| Yucatan | 10NE | Tam | 10·3 ± 1·3 | 134·7 | 33·1 | 0·0519 | 0·0101 | 16·4 (6·4–27·7) | 0·85 |
Average (± s.e.) stigmatic pollen load and its coefficient of variation (CV = s.d./mean) were obtained from the complete sample of wilted flowers collected. The percentage of stigmas without pollen was also estimated. Regression between numbers of pollen tubes per ovule and pollen grains of the study species was tested and piecewise regression applied when a non-linear component was found to be significant.
The slopes of the relationship before and after the breakpoint are represented by b1 and b2, respectively. The breakpoint at which the slope of the relationship changes is indicated by c and k, denoting the number of pollen grains and the corresponding number of tubes, respectively. Bootstrapping confidence intervals (BCa) were estimated with n = 1000 bootstraps. In species with a linear relationship, b1 represents the linear regression parameter and k represents the value of y at the highest x recorded.
Significance of differences between locally paired endemic (E) and non-endemic (NE) species are indicated (*P < 0·05; **P < 0·0001); NS, non-significant.
Importantly, most paired endemic and non-endemic species exhibited significant differences in pollen load sizes, but the sign of the difference varied among pairs (Table 2), and therefore the effect of endemism and its interaction with community were far from significant in these two analyses (P>0·20). Further, we found a gradient of pollen receipt among communities from highest doses in serpentine seeps of California to lowest loads and increased variability in the clearings of coastal dry scrublands of the Yucatan (F2,8 = 6·64, P = 0·02; Table 2).This result was also confirmed for pollen loads from the complete sample of analysed styles when pollen-less stigmas were excluded (F2,3015 = 5·88, P = 0·003).
Pollen quality
The relationship between pollen tubes and grains was confirmed to involve a non-linear component in 14 species but to be linear in six (Table 2). In these six cases, pollen tubes increased linearly with pollen receipt (P<0·001; 0·26<R2<0·73), suggesting that saturation of the relationship did not arise and the initial slope was representative of the whole data range (Table 2). The relative importance of quantity and quality components cannot be estimated by the method used for these species, yet in all six cases styles did not frequently develop more tubes than ovules in most of the range of pollen receipt (Fig. 1C, D) despite the fact that their average pollen load surpassed the number of ovules in five cases, and therefore a low quality of pollen receipt is presumed (see below).
Fig. 1.
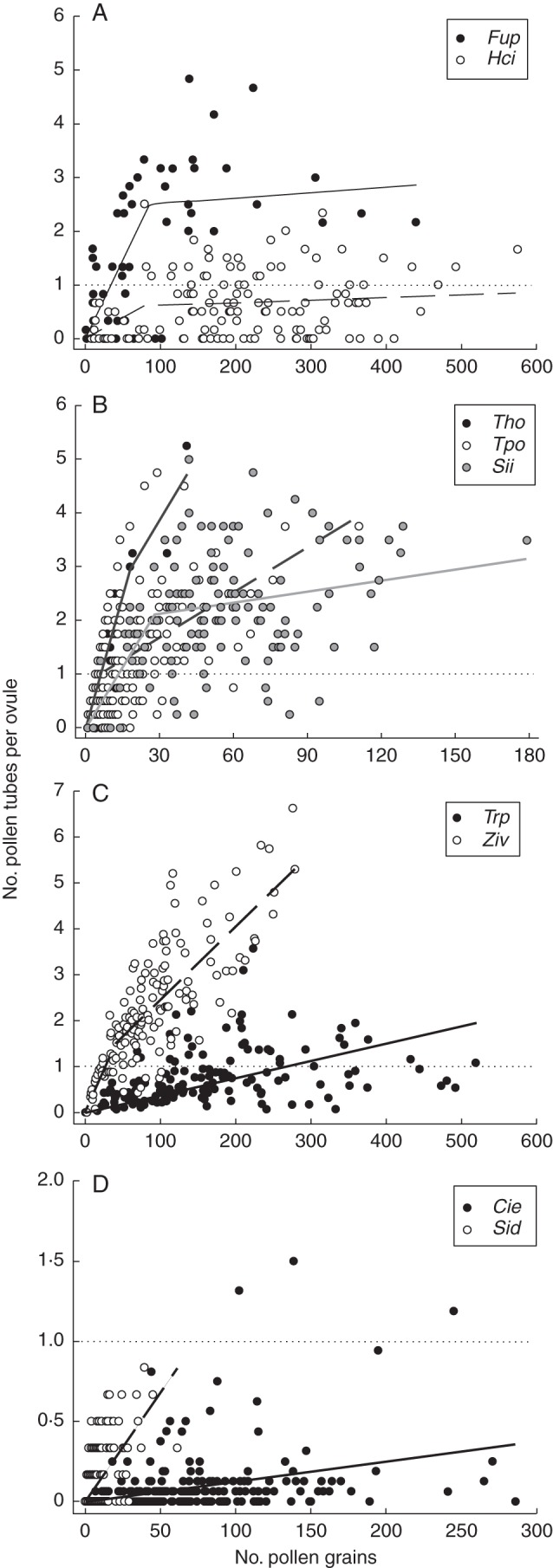
Piecewise regression analyses of paired endemic (filled circles, solid lines) and non-endemic (open circles, dashed lines) species representative of the whole range of variation recorded. (A) The endemic Fumana paradoxa and the non-endemic Helianthemum cinereum (Cistaceae) in Andalusia (pair 2). (B) The endemics Thymus orospedanus and Sideritis incana, and the non-endemic Teucrium polium (Lamiaceae) in Andalusia (pair 4). (C) The endemic Triteleia peduncularis and the non-endemic Zigadenus venenosus (Liliaceae) in California (pair 5). (D) The endemic Cienfuegosia yucatanensis and the non-endemic Sida acuta (Malvaceae) in Yucatan (pair 8). Dotted lines indicate when the number of pollen tubes equals the average number of ovules per flower. Taxa codes are as in Table 1. Note that scales were adjusted to data ranges.
For the 14 species that departed from a continuous linear regression, piecewise analysis was conducted. Investigation of these revealed that pollen loads at breakpoint (c) and b1 slopes were highly variable among species, in contrast to b2 slopes that in most cases did not differ from 0 (Table 2). Paired comparisons between endemics and non-endemics indicated significant differences in the slope of the initial linear relationship in seven out of 11 comparisons (Table 2; Fig. 1A, C, D).
A global ANCOVA indicated a significant community × endemism effect on the initial linear relationship between pollen grains and tubes in the complete sample (F2,1990 = 42,13, P<0·0001) and when only the seven divergent pairs were included (F2,1278 = 75·62, P<0·0001). When divided by community, we found that b1 was significantly higher in non-endemic than endemic species in serpentine seeps of California and dry scrublands of Yucatan, but the opposite pattern was found for pairs in the dolomite outcrops of Andalusia (Fig. 2).
Fig. 2.
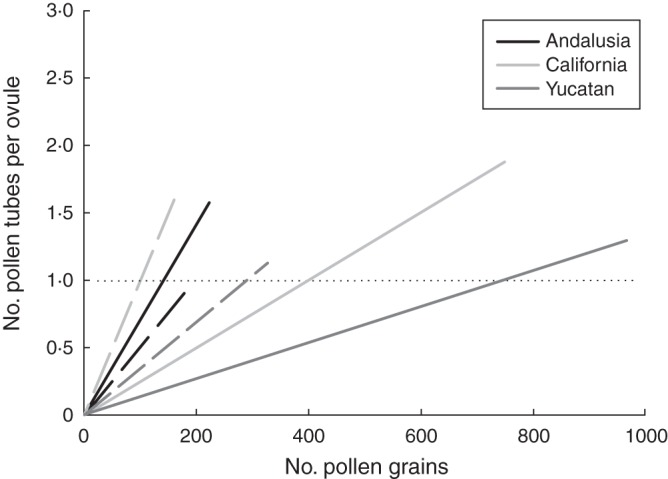
Initial linear relationship between number of pollen tubes per ovule and pollen grains in endemic (solid lines) and non-endemic (dashed lines) species studied in our three highly diverse communities, as indicated in the key.
Pre-zygotic pollen limitation
Study species varied widely in their k values (from 0·06 to 2·93; Table 2), even in spite of the fact that these were standardized by ovule number. This variation, however, was not explained by community, endemism or their interaction (P>0·14 in all cases).
In most species studied, pollen tubes did not exceed the number of ovules. In fact, <25 % of sampled flowers received more tubes than ovules (Fig. 3). Only in five out of 20 study species did >50 % flowers have more tubes than ovules, and only Zigadenus venenosus in California and Sideritis incana in Andalusia exhibited >75 % of flowers with more tubes than ovules. Large differences were found among communities in the percentage of pollen-unlimited flowers (F2,8 = 7·64, P = 0·014). In the dry scrublands of the Yucatan, almost no flowers had tubes in excess of ovules (Fig. 3). A significant community × endemism effect (F2,8 = 4·65, P = 0·046) was also found, with endemics having a higher proportion of pollen-limited flowers than non-endemics only for plants in the serpentine seeps of California. Here, the absence of saturation in some of the species (Fig. 1C) suggests that enduring quantity limitation of the pre-zygotic process is probably associated more with the high number of ovules per flower (e.g. particularly in Mimulus nudattus) than with the inability of most pollen grains to develop tubes.
Fig. 3.
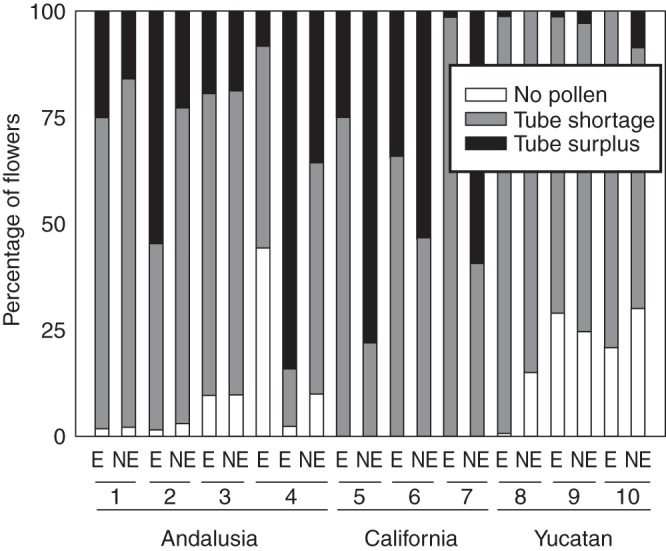
Differences among species in the three study communities in the percentage of flowers that did not have any pollen grains, those that received pollen but developed less than one pollen tube per ovule (tube shortage) and those able to develop the same or more pollen tubes than ovules (tube surplus). Species were ordered by pairs from left to right as in Table 1.
DISCUSSION
Our study of 20 species across three transcontinental diverse co-flowering plant communities corroborates others in regarding interfloral disparity of pollen receipt as a common rule in flowering plants (see Burd et al., 2009 for a recent meta-analysis; Herrera, 2009 for further examples). Moreover, it substantiates the claim that quality of pollen receipt also commonly limits ovule fertilization (Ashman et al., 2004; Aizen and Harder, 2007; Mitchell et al., 2009). However, while endemic and non-endemic co-flowering species frequently differed in pre-zygotic components of pollination success, there was no consistent pattern across the three communities.
Pollen quantity: variation between endemism and communities
When assessed under common ecological conditions, pollen loads differed between endemic and non-endemic species for the majority of pairs. The two Spanish Silene spp. and the distantly related Cuphea gaumeri and Angelonia angustifolia in the Yucatan were the only two exceptions in which both pollen load size and frequency of pollen-less stigmas were similar within the pair. More unexpectedly, the sign of the difference between endemic and non-endemic species was not consistent among communities: pollen loads were lower in the endemic species studied in the dolomite outcrops of Andalusia, as expected from the usually lower reproductive output of endemic species (Murray et al., 2002; Lavergne et al., 2004; Thompson et al., 2005), but higher for plants studied in the serpentine seeps of California and scrublands of the Yucatan. We cannot propose a simple ecological explanation for such an opposite pattern since the two extreme communities regarding pollination predictability and abundance exhibited a common outcome. We therefore conclude that endemism per se does not necessarily determine pollen load size of a species under common ecological conditions for pollination. Density of flowers and type and amount of rewards offered to pollinators could be specific traits that might affect the result of any specific comparison and maybe drive the inconsistent effect of endemism.
Further, pollination was stochastic in all of our three seasonal communities, but the degree of variability differed among communities and species. Specifically, significant differences among communities were found in the proportion of flowers without pollen grains, an important component of pollination not often emphasized (e.g. Richards et al., 2009; Alonso et al., 2012), but may better estimate frequency of pollination than visitation rate in unpredictable pollination environments. In our study season, the Yucatan species recorded the largest proportion of pollen-less flowers and the lowest amount of pollen per stigma, whereas the opposite extreme was represented by those studied in California.
Pollen quality: differences between paired species and global patterns
Our extensive comparative analysis of the relationship between pollen tubes and grains (Fig. 1) found that the shift from pollen quantity to pollen quality in driving pre-zygotic pollination success is a common feature (Alonso et al., 2012). Although the strength of pollen quality varied among co-flowering species, this co-ordinated across-species comparison demonstrates that pollen quality is key to determining ovule fertilization potential in the wild (Ashman et al., 2004; Aizen and Harder, 2007; Mitchell et al., 2009).
In contrast to our initial prediction, however, reduced quality of pollen was not characteristic of all endemic species, and in fact the direction of differences changed among communities (Fig. 2). Endemic species of the California and Yucatan communities, the two extreme communities in terms of quantity of pollen receipt (see above), had lower quality than non-endemics, but the opposite occurred in the Spanish community. These results suggest that endemism per se does not determine quality of pollen receipt but that intrinsic factors, ecological context or evolutionary history may be more important and should be more specifically addressed in future studies.
Mating system is recognized as a major intrinsic determinant of pollen limitation (Ashman et al., 2004). The unequal representation of mating system types among our study species prevented any formal test of related hypotheses regarding the influence of plant mating system on quality of pollen receipt (Aizen and Harder, 2007; Alonso et al., 2012). Here, we did not detect a distinctive natural pattern in quantity and quality of pollen receipt shared by the only four self-incompatible species studied. In addition, differences in quantity and quality of pollen were highly significant in the only pair involving two self-incompatible Californian lilies in which the reduced pollen quality of the endemic Triteleia peduncularis contrasted with the outstanding pollen quality of its co-flowering partner Z. venenosus, which reached the highest numbers of pollen tubes per ovule in California with the lowest recorded pollen load (Fig. 1C). Further, among self-compatible species, we found many flowers that recorded 0 tubes even after receiving large pollen loads (see, for example, Helianthemum cinereum in Andalusia in Fig. 1A and Cienfuegosia yucatanensis in Yucatan in Fig. 1D), a pattern that deserves further study because it could be indicative of autonomously self-deposited defective pollen. The non-manipulative method of Alonso et al. (2012) will be particularly fruitful in clarifying the effects of pollen quality in evolutionary transitions of mating system if applied to related species that co-flower and differ in self-compatibility (Eckert et al., 2010).
Pre-zygotic pollen limitation: relative relevance of quantity and quality components
In this study, we found that most flowers in the three communities received some (and highly variable) pollen but did not develop enough pollen tubes to fertilize their full set of ovules, with important differences among species and communities (Fig. 3). In understanding those figures (see below), we must take into account that, despite the fact that we have statistically controlled for it, the number of ovules per flower varied among study species, from a constant value of four in Spanish Lamiaceae, to the outstanding Californian Mimulus spp. with variable and >180 ovules per flower, with a majority of species showing moderate number and reduced variability (Table 1).
Our results suggest that the quantity and quality components do indeed limit ovule fertilization but their relative contribution is largely unpredictable among species, frequently differing among co-flowering species paired by phylogenetic relatedness or floral features. Indeed, we could identify the four scenarios proposed by Alonso et al. (2012) in their fig. 3 for comparison among data sets. First, high pollen quantity and quality (their option a), together with a low frequency of pollen-less stigmas, characterized S. incana and Fumana paradoxa in Andalusia, and the three non-endemic species in California (Z. venenosus, Antirrhinum cornutum and Mimulus guttatus). Reduced pollen quality was the main constraint (their option b) in four cases, two in Andalusia (Helianthemum appeninum appeninum and H. cinereum) and two in California (T. peduncularis and M. nudatus), whereas reduced pollen quantity was the main constraint (their option c) at least in three cases in Andalusia (Silene psammitis lasiostyla, Silene colorata and Thymus orospedanus). Finally, in Yucatan, reduced quantity and quality (their option d) jointly contributed to severe limitation in all study species in this particular year (Fig. 1D).
Only concurrent abundant and good quality pollination led to a boost of ovule fertilization. Certainly, only the five species with high quantity and quality pollen exhibited <50 % flowers with pollen tube deficiency (Table 2, Fig. 3). Furthermore, only when combined with a low to moderate number of ovules (four in S. incana and 20 in Z. venenosus, respectively) was pollination particularly successful and possibly unlimiting for seed production. In seasonal environments like those studied here, it seems that the high achievement of the two pollination components apparently required to overcome pollen limitation is exceptional and pollen limitation could thus shape evolutionary pathways in a range of floral features that deserve further study (see, for example, Burd et al., 2009; Herrera, 2009).
Conclusions and future directions
Our transcontinental multicommunity evaluation showed that quality of pollen received was as variable as its quantity and was a major contributor to differences in ovule fertilization potential across species. Furthermore, the large natural variation observed among flowers of the same species in quantity and quality of pollen receipt, and subsequently in pollen tube formation, deserves further analysis if we want to estimate the environmental, phenotypic and intraindividual sources of variation that may condition the evolutionary response of plants to stochastic pollination environments (e.g. Herrera, 2002; Bernasconi et al., 2007; Herrera, 2009, and references therein).
These results also contribute to understanding why the consequences of disruption of pollination service are not universal but probably geographically or even community dependent (Kremen and Ricketts, 2000, and references therein).
Documenting pollen tube formation and analysing its relationship with pollen grain receipt in the wild is a promising research route to clarify when quality of pollination service is critical in limiting plant reproduction and how plants may evolve to overcome this limitation in different regions.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Luis Abdala, Patricia Téllez, Nataly Celaya, Luis Salinas, Clare Kohler, Katie Schuller, Anneka Jankowiaka, María del Mar Alonso and Curro Molina for contributions to field work and pollen tube analysis, and Abelardo Aparicio and Alfredo Benavente for assistance with plant identification. The Consejería de Medio Ambiente, Junta de Andalucía and the University of California Davis are thanked for authorizing the work and providing facilities in Cazorla and McLaughlin Natural Reserve, respectively. This work was supported by the FBBVA through the research project ENDLIMIT [BIOCON08/125]. T.L.A. and G.A.M. were also supported by the NSF [OISE 0852846, DEB 1020523], and G.A.G. by CONACYT [211982] and SEP fellowships.
LITERATURE CITED
- Aguilar R, Ashworth L, Galetto L, Aizen M. Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecology Letters. 2006;9:968–980. doi: 10.1111/j.1461-0248.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- Aizen MA, Harder LD. Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology. 2007;88:271–281. doi: 10.1890/06-1017. [DOI] [PubMed] [Google Scholar]
- Alexander EB, Coleman RG, Keeler-Wolf T, Harrison SP. Serpentine geoecology of western North America. New York: Oxford University Press; 2007. [Google Scholar]
- Alonso C, Vamosi JC, Knight TM, Steets JA, Ashman T-L. Is reproduction of endemic plant species particularly pollen limited in biodiversity hotspots? Oikos. 2010;119:1192–1200. [Google Scholar]
- Alonso C, Herrera CM, Ashman T-L. A piece of the puzzle: a method for comparing pollination quality and quantity across multiple species and reproductive events. New Phytologist. 2012;193:532–542. doi: 10.1111/j.1469-8137.2011.03932.x. [DOI] [PubMed] [Google Scholar]
- Amat ME, Vargas P, Gómez JM. Pollen quality limitation in the Iberian critically endangered genus Pseudomisopates (Antirrhinaceae) Plant Ecology. 2011;212:1069–1078. [Google Scholar]
- Ashman T-L, Knight TM, Steets JA, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Bell JM, Karron JD, Mitchell RJ. Interspecific competition for pollination lowers seed production and outcrossing in Mimulus ringens. Ecology. 2005;86:762–771. [Google Scholar]
- Bennington CC, Thayne WV. Use and misuse of mixed model analysis of variance in ecological studies. Ecology. 1994;75:717–722. [Google Scholar]
- Bernasconi G, Lang DJ, Schmid B. Microgametophyte population sizes and plant reproductive output in the insect-pollinated Prunella grandiflora (Lamiaceae) New Phytologist. 2007;173:393–400. doi: 10.1111/j.1469-8137.2006.01920.x. [DOI] [PubMed] [Google Scholar]
- Bullock SH. Plant reproduction in neotropical dry forest. In: Bullock SH, Mooney HA, Medina E, editors. Seasonally dry tropical forest. Cambridge: Cambridge University Press; 1995. pp. 277–303. [Google Scholar]
- Burd M, Ashman T-L, Campbell DR, et al. Ovule number per flower in a world of unpredictable pollination. American Journal of Botany. 2009;96:1159–1167. doi: 10.3732/ajb.0800183. [DOI] [PubMed] [Google Scholar]
- Byers DL. Pollen quantity and quality as explanations for low seed set in small populations exemplified by Eupatrium (Asteraceae) American Journal of Botany. 1995;82:1000–1006. [Google Scholar]
- Chacoff NP, Garcıía D, Obeso JR. Effects of pollen quality and quantity on pollen limitation in Crataegus monogyna (Rosaceae) in NW Spain. Flora. 2008;203:499–507. [Google Scholar]
- Cole CT. Genetic variation in rare and common plants. Annual Review of Ecology, Evolution and Systematics. 2003;34:213–237. [Google Scholar]
- Eckert CG, Kalisz S, Geber MA, et al. Plant mating systems in a changing world. Trends in Ecology and Evolution. 2010;25:35–43. doi: 10.1016/j.tree.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Espadas Manrique C, Durán R, Argáez J. Phytogeographic analysis of taxa endemic to the Yucatán Peninsula using geographic information systems, the domain heuristic method and parsimony analysis of endemicity. Diversity and Distributions. 2003;9:313–330. [Google Scholar]
- Feldman TS, Morris WF, Wilson WG. When can two plant species facilitate each other's pollination? Oikos. 2004;105:197–207. [Google Scholar]
- Feldman TS. The plot thickens: does low density affect visitation and reproductive success in a perennial herb, and are these effects altered in the presence of a co-flowering species? Oecologia. 2008;156:807–817. doi: 10.1007/s00442-008-1033-y. [DOI] [PubMed] [Google Scholar]
- Fischer M, Hock M, Paschke M. Low genetic variation reduces cross-compatibility and offspring fitness in populations of a narrow endemic plant with a self-incompatibility system. Conservation Genetics. 2003;4:325–336. [Google Scholar]
- Freestone AL, Inouye BD. Dispersal limitation and environmental heterogeneity shape scale-dependent diversity patterns in plant communities. Ecology. 2006;87:2425–2432. doi: 10.1890/0012-9658(2006)87[2425:dlaehs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- García-Camacho R, Totland Ø. Pollen limitation in the Alpine: a meta-analysis. Arctic, Antarctic, and Alpine Research. 2009;41:103–111. [Google Scholar]
- Gilman RT, Fabina NS, Abbott KC, Rafferty NE. Evolution of plant–pollinator mutualisms in response to climate change. Evolutionary Applications. 2012;5:2–16. doi: 10.1111/j.1752-4571.2011.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegland SJ, Totland Ø. Is the magnitude of pollen limitation in a plant community affected by pollinator visitation and plant species specialisation levels? Oikos. 2008;117:883–891. [Google Scholar]
- Hegland SJ, Nielsen A, Lázaro A, Bjerknes A-L, Totland Ø. How does climate warming affect plant–pollinator interactions? Ecology Letters. 2009;12:184–185. doi: 10.1111/j.1461-0248.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- Herrera CM. Censusing natural gametophyte populations: variable spatial mosaics and extreme fine-graininess in winter-flowering Helleborus foetidus. American Journal of Botany. 2002;89:1570–1578. doi: 10.3732/ajb.89.10.1570. [DOI] [PubMed] [Google Scholar]
- Herrera CM. Multiplicity in unity. Plant subindividual variation and interactions with animals. Chicago, IL: University of Chicago Press; 2009. [Google Scholar]
- Johnson SD, Bond WJ. Evidence for widespread pollen limitation of fruiting success in Cape wild flowers. Oecologia. 1997;109:530–534. doi: 10.1007/s004420050113. [DOI] [PubMed] [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Niwot, CO: University Press of Colorado; 1993. [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, et al. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology, Evolution and Systematics. 2005;36:467–497. [Google Scholar]
- Kremen C, Ricketts T. Global perspectives on pollination disruptions. Conservation Biology. 2000;14:1226–1228. [Google Scholar]
- Kunin WE. Sex and the single mustard: population density and pollinator behavior effects on seed-set. Ecology. 1993;74:2145–2160. [Google Scholar]
- Larson BMH, Barrett SCH. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society. 2000;69:503–520. [Google Scholar]
- Lavergne S, Thompson JD, Garnier E, Debussche M. The biology and ecology of narrow endemic and widespread plants: a comparative study of trait variation in 20 congeneric pairs. Oikos. 2004;107:505–518. [Google Scholar]
- McMullen CK. Breeding systems of selected Galápagos Islands Angiosperms. American Journal of Botany. 1987;74:1694–1705. [Google Scholar]
- Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant–pollinator interactions. Ecology Letters. 2007;10:710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Flanagan RJ, Brown BJ, Waser NM, Karron JD. New frontiers in competition for pollination. Annals of Botany. 2009;103:1403–1413. doi: 10.1093/aob/mcp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota JF, Medina-Cazorla JM, Navarro FB, et al. Dolomite flora of the Baetic Ranges glades (South Spain) Flora. 2008;203:359–375. [Google Scholar]
- Motten AF. Pollination ecology of the spring wildflower community of a temperate deciduous forest. Ecological Monographs. 1986;56:21–42. [Google Scholar]
- Murray BR, Thrall PH, Gill AM, Nicotra AB. How plant life-history and ecological traits relate to species rarity and commonness at varying spatial scales. Austral Ecology. 2002;27:291–310. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: trends, impacts and drivers. Trends in Ecology and Evolution. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Richards SA, Williams NM, Harder LD. Variation in pollination: causes and consequences for plant reproduction. American Naturalist. 2009;174:382–398. doi: 10.1086/603626. [DOI] [PubMed] [Google Scholar]
- SAS. SAS for Windows (version 9·2) Cary, NC: SAS Institute; 2008. [Google Scholar]
- Thompson JD, Lavergne S, Affre L, Gaudeul M, Debussche M. Ecological differentiation of Mediterranean endemic plants. Taxon. 2005;54:967–976. [Google Scholar]
- Vamosi JC, Knight TM, Steets JA, Mazer SJ, Burd M, Ashman T-L. Pollination decays in biodiversity hotspots. Proceedings of the National Academy of Sciences, USA. 2006;103:956–961. doi: 10.1073/pnas.0507165103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh RA. Pollen limitation meets resource allocation: towards a comprehensive methodology. New Phytologist. 2007;174:26–37. doi: 10.1111/j.1469-8137.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- Wilcock C, Neiland R. Pollination failure in plants: why it happens and when it matters. Trends in Plant Science. 2002;7:270–277. doi: 10.1016/s1360-1385(02)02258-6. [DOI] [PubMed] [Google Scholar]
- Winfree R, Bartomeus I, Cariveau DP. Native pollinators in anthropogenic habitats. Annual Review of Ecology, Evolution and Systematics. 2011;42:1–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


