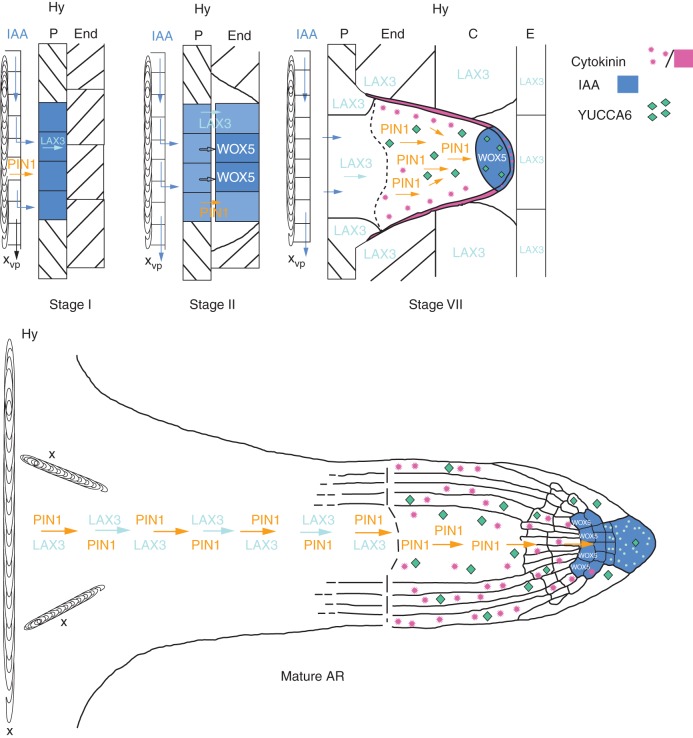
Abstract
Background and Aims
Adventitious roots (ARs) are part of the root system in numerous plants, and are required for successful micropropagation. In the Arabidopsis thaliana primary root (PR) and lateral roots (LRs), the quiescent centre (QC) in the stem cell niche of the meristem controls apical growth with the involvement of auxin and cytokinin. In arabidopsis, ARs emerge in planta from the hypocotyl pericycle, and from different tissues in in vitro cultured explants, e.g. from the stem endodermis in thin cell layer (TCL) explants. The aim of this study was to investigate the establishment and maintenance of the QC in arabidopsis ARs, in planta and in TCL explants, because information about this process is still lacking, and it has potential use for biotechnological applications.
Methods
Expression of PR/LR QC markers and auxin influx (LAX3)/efflux (PIN1) genes was investigated in the presence/absence of exogenous auxin and cytokinin. Auxin was monitored by the DR5::GUS system and cytokinin by immunolocalization. The expression of the auxin-biosynthetic YUCCA6 gene was also investigated by in situ hybridization in planta and in AR-forming TCLs from the indole acetic acid (IAA)-overproducing superroot2-1 mutant and its wild type.
Key Results
The accumulation of auxin and the expression of the QC marker WOX5 characterized the early derivatives of the AR founder cells, in planta and in in vitro cultured TCLs. By determination of PIN1 auxin efflux carrier and LAX3 auxin influx carrier activities, an auxin maximum was determined to occur at the AR tip, to which WOX5 expression was restricted, establishing the positioning of the QC. Cytokinin caused a restriction of LAX3 and PIN1 expression domains, and concomitantly the auxin biosynthesis YUCCA6 gene was expressed in the apex.
Conclusions
In ARs formed in planta and TCLs, the QC is established in a similar way, and auxin transport and biosynthesis are involved through cytokinin tuning.
Keywords: Adventitious root apex, Arabidopsis thaliana, auxin biosynthesis, auxin transport, cytokinin localization, quiescent centre, root meristemoids, stem endodermis, thin cell layers, WOX5
INTRODUCTION
Adventitious roots (ARs) emerge from organs other than the primary root (PR), such as hypocotyls, stems and leaves. They allow plant adaptation to environmental changes (Li et al., 2009), and are crucial for the survival of important crops, e.g. cereals. However, numerous plants fail to differentiate ARs in response to environmental stress, e.g. soil pollution, and this negatively affects their growth potentials. Also the roots formed by in vitro cultured explants are adventitious, and adventitious rooting recalcitrance causes failure of cuttings to grow, with economic losses. The model plant Arabidopsis thaliana forms ARs. They originate from the hypocotyl pericycle, at the hypocotyl–PR transition zone (Falasca and Altamura, 2003). Usually they are present in a low number, but mutants overproducing ARs, e.g. superroot2-1 (sur2-1), are known in this species (Delarue et al., 1998).
The indeterminate growth of the PR and lateral roots (LRs) is supported by the quiescent centre (QC), which co-ordinates the activity of the surrounding stem cells, with a central role in establishment, maintenance and elaboration of patterns in the apical meristem (Jiang and Feldman, 2005).
In arabidopsis PR and LRs, the QC is positioned in the centre of the stem cell niche, and is formed by four usually non-dividing cells surrounded by rapidly dividing initial cells, from which the derivative cells forming the tissues of the primary body originate. The QC maintains the undifferentiated state in the surrounding initials by local signalling (van de Berg et al., 1997; Sabatini et al., 2003). The PR is embryonic in origin and thus its QC is established in the embryo. In the arabidopsis embryo, the QC is produced by divisions in the upper hypophysis derivative cell (Jiang and Feldman, 2005). The LRs are post-embryonic, being formed by the PR pericycle cells. The QC is established in the LR tip at stage VII of primordium development (Malamy and Benfey, 1997). Moreover, in both PR and LRs, the QC identity is evidenced by the same QC promoter traps, e.g. QC25 and pAGAMOUS LIKE 42 (pAGL42) (Sabatini et al., 1999; Nawy et al., 2005; Della Rovere et al., 2010).
The ARs are post-embryonic as are LRs, but the establishment of their QC is still an open question. Clowes hypothesized (1956) that the QC was a ubiquitous feature of all Angiosperm root meristems, for at least part of their ontogeny. In arabidopsis, it is still unknown when/how the ARs specify the QC in planta, and how long they maintain it. Moreover, a different origin of the ARs, i.e. different founder cells in in vitro cultured explants with respect to in planta, might affect the establishment and/or the maintenance of the QC in the AR meristem.
Auxin transport via PIN-FORMED (PIN) auxin efflux carriers, e.g. PIN1, is necessary for QC positioning in the PR (Friml et al., 2003), and auxin biosynthesis is needed in PR and LR tips (Ljung et al., 2005), with YUCCA6, a gene important in tryptophan-dependent indole-3-acetic acid (IAA) biosynthesis (Mashiguchi et al., 2011), possibly involved (Kim et al., 2007). The AUXIN1 (AUX1)-LIKE AUX1 (AUX/LAX) proteins have auxin influx activity (Péret et al., 2012). Also these proteins are important for LR development (Marchant et al., 2002). Moreover, in PR and LRs, transport and biosynthesis of auxin contribute to establishing and maintaining auxin maxima centred in the QC and columella cells (Sabatini et al., 1999; Benková et al., 2003). In contrast, information about auxin transport/biosynthesis in the arabidopsis AR tip is lacking. It is however known that the synthetic auxin α-naphthalene acetic acid (NAA) increases ARs in planta (Falasca and Altamura, 2003), and high levels of endogenous IAA are present in AR-overproducing mutants, e.g. sur2-1 (Delarue et al., 1998).
In the PR, AtWUSCHEL RELATED HOMEOBOX5 (WOX5) is expressed in the QC, with the function of inhibiting differentiation in the initial distal cells (Sarkar et al., 2007). WOX5 is auxin inducible, acts downstream of auxin distribution (Ding and Friml, 2010) and seems to be involved in maintenance of the auxin maximum at the PR tip (Gonzali et al., 2005). Moreover, auxin has been suggested to specify the LR QC through WOX5 (Ditengou et al., 2008).
Cytokinin also seems to be involved in QC formation, even if its role needs further investigation. For example, in the PR apex of corn, QC removal causes its regeneration, with a concomitant reduction in cytokinin levels in the proximal meristem adjacent to the QC (Feldman, 1979).
Natural and synthetic auxins, applied alone or in combination with low levels of cytokinin, induce ARs in in vitro cultured explants of numerous plants, including arabidopsis. In arabidopsis TCLs, i.e. stem inflorescence tissues external to the vascular system, ARs are induced by indole-3-butyric acid (IBA) (10 µm) plus kinetin (Kin) (0·1 µm) (Falasca et al., 2004). The explants are cultured horizontally, epidermal side up, and ARs appear all along their surface and originate from a unique tissue, i.e. the stem endodermis (Falasca et al., 2004). Whether/how/when auxin polar transport is generated in the AR-forming IBA + Kin-cultured TCLs is unknown.
The aim of this study was to determine whether the QC is established in arabidopsis ARs, whether its establishment and maintenance are under auxin and cytokinin control, and whether the QC specification programme is shared by ARs of different origin, i.e. formed either by the hypocotyl pericycle cells in planta or by the stem endodermis cells in the TCLs.
To this aim, the activity of PR/LR QC markers, the expression patterns of the IAA-sensitive DR5::GUS reporter and of PIN1 and LAX3 auxin carriers, and the AR response of the AR-overproducing sur2-1 mutant were investigated under various auxin/cytokinin treatments, and YUCCA6 transcription and cytokinin presence were monitored.
The results show that the QC is established in the ARs. Independently of the founder cells, auxin accumulation and WOX5 expression characterize the early derivative cells involved in AR formation. By the activity of PIN1 and LAX3, an auxin maximum is determined to occur at the primordium tip, to which WOX5 expression is restricted, positioning the QC. Tip-localized auxin biosynthesis by YUCCA6 and the activity of the trans-zeatin riboside are necessary for QC establishment and maintenance.
MATERIALS AND METHODS
Plant material and growth conditions
Seeds of Arabidopsis thaliana Columbia (Col) and Wassilewskija (Ws) ecotypes, of QC25::GUS, DR5::GUS, PIN1::GUS, LAX3::GUS, pAGL42::GFP and pWOX5::GFP lines (all in the Col background) and of the sur2-1 mutant (Ws background) were stratified, sterilized and sown on Petri plates according to Airoldi et al. (2010), with minor modifications, i.e. full-strength salts of MS (Murashige and Skoog, 1962) and 1 % sucrose (i.e. hormone-free, HF, growth medium). Alternatively, either 10 µm IBA plus 0·1 µm Kin (Sigma-Aldrich; Falasca et al., 2004), 2 µm NAA (Sigma-Aldrich; Falasca and Altamura, 2003) or 0·1 µm Kin were added to the medium.
Twenty plates (12 × 12 cm, 15–20 seeds/plate) per genotype/line and treatment were placed in the vertical position, at 22 ± 2 °C, under continuous darkness for 14 d, after exposure to white light for 6 h (Takahashi et al., 2003). The plants used as the source of TCLs were grown on a commercial soil starting from seeds prepared as above. To allow stem elongation, 30 plants per genotype/line were grown until reproduction (i.e. day 40 after germination) in a growth chamber, at 22 ± 2 °C, 70 % humidity and long days (white light of 22 Wm−2 light intensity).
Histological analysis of ARs in planta, detection of GUS, and GFP epifluorescence
Thirty 14-day-old seedlings of Col and Ws grown under HF conditions were fixed in 70 % (v/v) ethanol, dehydrated by an ethanol series, embedded in Technovit 7100 (Heraeus Kulzer, Germany), longitudinally sectioned at 5 µm with a Microm HM 350 SV microtome (Microm, Germany), stained with 0·05 % toluidine blue and observed under a light microscope.
Stocks of 30 randomly selected QC25::GUS, DR5::GUS, PIN1::GUS and LAX3::GUS seedlings per growth medium were harvested at day 14 after sowing, and processed for β-glucuronidase (GUS) staining according to Willemsen et al. (1998). Samples of these lines and of their wild type were cleared with chloral hydrate solution (Weigel and Glazebrook, 2002), mounted on microscope slides and observed with Nomarski optics applied to a Leica DMRB microscope. Hypocotyl length was measured under a LEICA MZ8 stereomicroscope before seedling fixation, and AR number was expressed as mean density cm−1 (± s.e.). Stocks of 30 randomly selected pAGL42::GFP and pWOX5::GFP seedlings were harvested on the same day, and green fluorescent protein (GFP) fluorescence was observed under the same microscope equipped with a double wavelength filter set (EX BP 490/20 and BP 575/30; EM BPs 525/20 and 635/40). The images were acquired with a DC500 camera (Leica).
Thin cell layer culture and microscopic observations
One hundred TCLs (0·5 × 8 mm, six/seven cell layers thick, Supplementary Data Fig. S2A, B) per genotype/line were cultured for 22 d under continuous darkness, at 24 ± 2 °C, on the seedling growth medium to which 10 µm IBA and 0·1 µm Kin were added (Falasca et al., 2004). Forty TCLs of the sur2-1 mutant and of its wild type (Ws) were cultured either under the latter hormonal condition or under HF conditions.
Ten TCLs per genotype/line were harvested periodically for histological analysis in bright field (the wild type and GUS marker lines) and in epifluorescence (GFP lines). After the GUS assay, QC25::GUS, DR5::GUS, PIN1::GUS and LAX3::GUS explants were fixed and embedded as for wild-type seedlings, longitudinally sectioned at 13 µm and observed under light microscopy. pAGL42::GFP and pWOX5::GFP TCLs were observed using the Leica DMRB epifluorescence microscope. pAGL42::GFP TCLs were also observed under a Leica TCS-SP5 confocal microscope after counterstaining with propidium iodide (PI) at 10 mg L−1 for 5 min, evaluations being performed by argon laser (EX 488 nm), and detected by LAS software (Leica) with an LP 560 nm filter for PI and with a BP 525/20 nm filter for GFP. Sections (4 µm) of the wild type and pAGL42::GFP and pWOX5::GFP were also stained with 0·05 % (w/v) toluidine blue for light microscopy observations.
YUCCA6 in situ hybridization
RNA in situ hybridization on whole-mount 14-day-old Col and Ws seedlings, grown under HF conditions and continuous darkness, was performed according to García-Aguilar et al. (2005). Ten randomly selected seedlings per ecotype were fixed and stored according to Hejátko et al. (2006). The samples were treated with digoxigenin-labelled YUCCA6 antisense and sense RNA probes overnight at 55 °C. YUCCA6 mRNA detection was performed with 4-nitro blue tetrazolium chloride (NBT)/5-bromo-4-chloro-3-indolyl-phosphate (BCIP) overnight at room temperature. The probe was generated by in vitro transcription according to the DIG RNA Labeling Kit instructions (SP6/T7; Roche). cDNA used for probe transcription was synthesized using 5′-CAAACACAACGCTTATCTCTC-3' and 5′-GTAAACTAGCACATGACCACC-3′ as primers.
Hypocotyls from ten HF-grown seedlings of Col and Ws, and ten TCLs of sur2-1, Ws and Col cultured on HF and IBA + Kin media, were either processed as for whole-mount hybridizations or fixed in 0·5 % (w/v) glutaraldehyde and 3 % (w/v) paraformaldehyde in phosphate-buffered saline (PBS), dehydrated in an ethanol series, embedded in resin and sectioned (6 µm) for hybridization on sections. Sections were incubated overnight with YUCCA6 antisense and sense probes at the same concentration and temperature as for the whole mounts, and processed according to Takechi et al. (1999) with minor modifications. Sections were observed under light microscopy, and the absence of hybridization signal in the sense probe-treated materials was verified.
Cytokinin immunolocalization
Thirty 14-day-old seedlings of Col grown under HF conditions were processed and sectioned as for YUCCA6 hybridization in resin-embedded sections, and the sections were incubated overnight with 1 % trans-zeatin riboside primary antibody (OlChemlm Ltd, Czech Republic) in PBS at 4 °C, then with 1 % secondary antibody (Anti-Rabbit IgG, Sigma) with alkaline phosphatase activity for 3 h at room temperature, and finally treated according to Caboni et al. (2002). Sections were observed under light microscopy. Control sections were incubated without the primary antibody, and the absence of any immunostaining was verified.
Statistical analysis
Data were expressed as mean values (± s.e.). Fasciated ARs were counted as single ARs, and their number was expressed as a mean value (±s.e.) on the samples showing fasciation. One-way or two-way analysis of variance (ANOVA, P < 0·05) was used to compare effects of treatments and genotypes, and, if ANOVA showed significant effects, Tukey's post-test was applied. Alternatively, Student's t-test was used where appropriate (GraphPad Prism 6·0). All the experiments were repeated three times during 2 years, and similar results were obtained (data of the replicates of the second year are shown).
RESULTS
AR development in planta occurs like LR development, and IBA + Kin treatment enhances the process
Treatments with exogenous NAA and Kin alone caused a significant reduction in hypocotyl length in comparison with the HF treatment, with the highest reduction under NAA (Fig. 1A, inset). In contrast, the treatment with IBA + Kin did not cause significant changes in comparison with HF treatment (Fig. 1A, inset). The AR density was very low with Kin alone and under HF treatment, but became many-fold higher with IBA + Kin and with NAA (Fig. 1A).
Fig. 1.
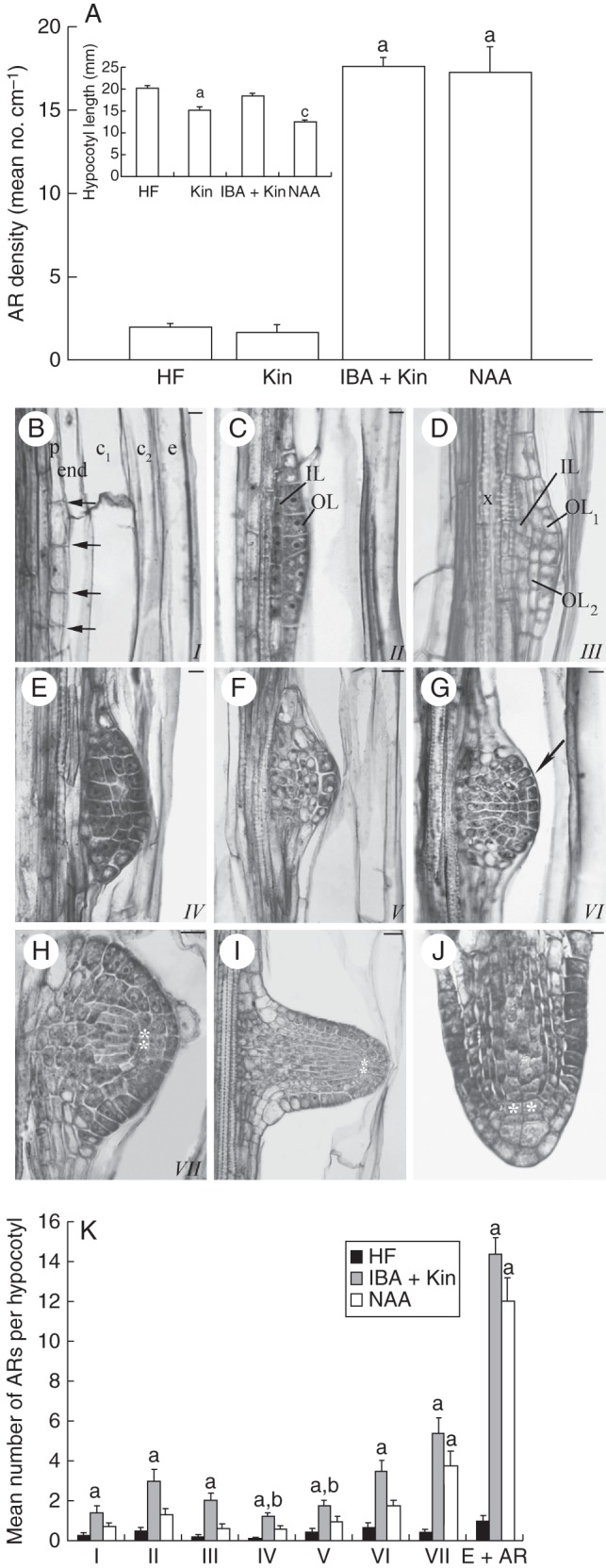
(A) AR mean density (± s.e.) in the hypocotyl of 14-day-old wild-type seedlings grown on HF, Kin (0·1 µm), IBA (10 µm) + Kin (0·1 µm) and NAA (2 µm) media, and hypocotyl mean length (inset). (B–J) Developmental stages of ARs in wild-type seedlings grown on HF medium. (B) First anticlinal divisions in the hypocotyl pericycle (arrows). (C) Outer (OL) and inner (IL) layers formed by periclinal divisions in the cells originated by the first anticlinal divisions. (D) OL periclinal doubling. (E) IL doubling, leading to a four-layered ARP. (F) ARP dome establishment. (G) ARP dome with protoderm specification (arrow). (H) Stage VII ARP showing cells with LR QC morphology (asterisks). (I) Developed ARP emerging from the hypocotyl (QC shown by the asterisks). (J) Apex of a mature AR (QC shown by the asterisks). (B–J) Histological longitudinal radial sections stained with toluidine blue (Ws ecotype). (K) Mean number (± s.e.) of ARs at different stages in wild-type seedlings grown on HF, IBA (10 µm) + Kin (0·1 µm) and NAA (2 µm) media. (A and K) a,cP < 0·01 differences from other treatments; bP < 0·05 difference between IBA + Kin and NAA. Columns with the same letter are not significantly different. n = 30 (Col ecotype). Scale bars: (B–G, J) = 10 µm; (H) = 20 µm; (I) = 30 µm. I–VII, developmental ARP stages, p, hypocotyl pericycle; x, protoxylem; c1–c2, cortex; end endodermis; e, epidermis.
The AR development was histologically investigated in the hypocotyl of 14-day-old HF-grown seedlings of Col and Ws ecotypes. In both ecotypes, the process followed seven stages before AR emergence, as exemplified by the Ws ecotype in Fig. 1B–H. The stages are indicated by Roman numbers, following the numbering proposed by Malamy and Benfey (1997) for LR developmental stages. The pericycle founder cells divided anticlinally (stage I, Fig. 1B), and then periclinally (stage II), forming an outer (OL) and an inner (IL) layer of derivative cells (Fig. 1C). When OL and IL cells expanded, the shape of the AR primordium (ARP) began to appear. At stage III, the OL again divided periclinally, giving rise to an ARP composed of three superimposed layers (Fig. 1D). At stage IV, the IL also divided (Fig. 1E). Anticlinal divisions in the central cells of the de novo formed layers occurred at stage V, and the ARP acquired a prominent dome shape (Fig. 1F). The ARP specified its protoderm soon after (stage VI, Fig. 1G). At stage VII, cells with a morphology similar to the QC cells in LR primordia (Malamy and Benfey, 1997) appeared in the ARP dome (Fig. 1H, asterisks). After the QC was established, the ARP rapidly protruded throughout the hypocotyl (Fig. 1I), and elongated, giving rise to a mature AR with a complete apex maintaining the QC position and features (Fig. 1J).
All stages of ARs were in present in similarly very low numbers in HF- and Kin-grown seedlings, as shown for HF in Fig. 1K, whereas their number increased greatly with both auxin treatments, and significantly more with IBA + Kin than with NAA (Fig. 1K). In contrast to the alternating pattern characterizing the appearance of the HF-formed ARs, opposite ARs appeared with both auxin treatments, but mainly with NAA (Supplementary Data Fig. S1A, B). ARs with a double tip (i.e. fasciated ARs, Supplementary Data Fig. S1C) also appeared, but their presence was sporadic with IBA + Kin, whereas they occurred in about 50 % of the seedlings, and with a mean number of 1·8 (±0·4), under the NAA treatment.
PR/LR QC markers are expressed in the AR QC in planta, but WOX5 is also expressed in the early derivative cells
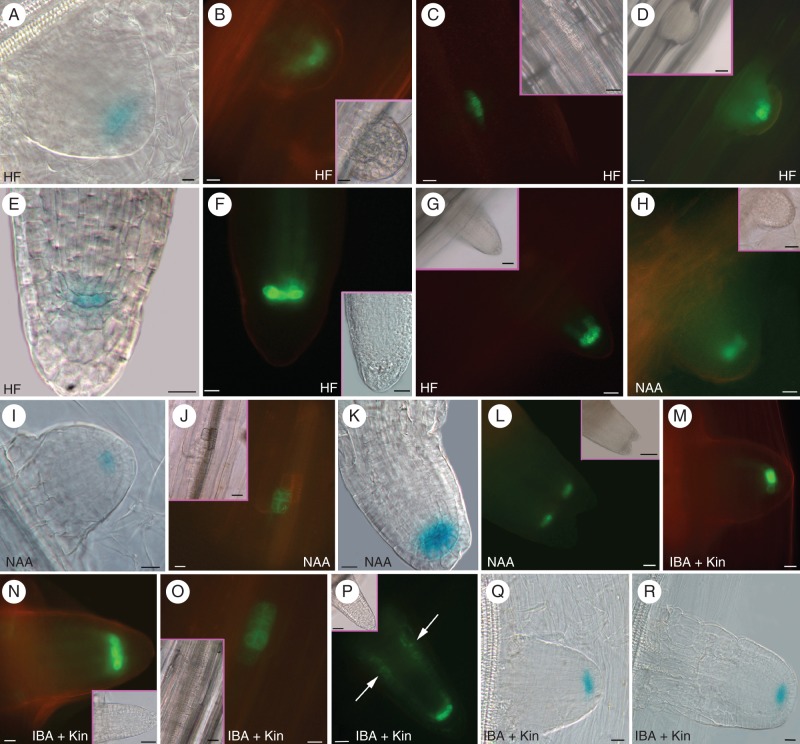
The analysis of QC25::GUS and pAGL42::GFP HF-grown seedlings revealed that the cells of stage VII ARPs resembling the LR QC cells (Fig. 1H, asterisks) expressed both these QC markers (Fig. 2A, B), and QC identity was confirmed by the expression of the pWOX5::GFP construct (Fig. 2D), another PR/LR QC marker. However, WOX5 was active from stages I–II (Fig. 2C). QC25, pAGL42 and WOX5 continued to mark the QC in the protruding ARs (Fig. 2E–G). Also in the seedlings grown with NAA and IBA + Kin pAGL42::GFP and QC25::GUS signals appeared at stage VII (Fig. 2H, I, M, Q), and WOX5::GFP before, i.e. around stage II (Fig. 2J, O), and all markers maintained expression in the QC of protruding/mature ARs (Fig. 2K, N, P, R). The markers were even expressed at the tip(s) of the fasciated ARs formed in the NAA treatment (Fig. 2L).
Fig. 2.
Expression of PR/LR QC markers during AR formation in Col seedlings grown for 14 d under various hormonal treatments. (A–G; HF) QC25::GUS (A), pAGL42::GFP (B) and pWOX5::GFP (D) in the QC at stage VII, and pWOX5::GFP at stage II (C). QC25::GUS (E), pAGL42::GFP (F) and pWOX5::GFP (G) in the QC, and lateral initials (G) of emerged ARs. (H–L; NAA) pAGL42::GFP (H) and QC25::GUS (I) in the QC of stage VII ARPs. pWOX5::GFP at stage II (J), QC25::GUS at the tip of a regular AR (K) and pAGL42::GFP in the twin tip of a fasciated AR (L). (M–R; IBA + Kin) pAGL42::GFP in the QC of not yet emerged (M) and emerged (N) ARPs. pWOX5::GFP at stage II (O), and in the QC, lateral initials and pericycle cells forming LRs (arrows) in emerged ARPs (P). QC25::GUS in the QC at stage VII (Q), and in emerged ARPs (R). Insets in fluorescence pictures show corresponding bright-field images. Scale bars: = (A, B and inset, E, F, H–K, M–O, R) 10 µm; (C, D, G, L, P–Q, and insets in C, F, J, N–O) = 20 µm; (insets in D, G, H) = 30 µm; (inset in P) = 40 µm; (inset in L) = 100 µm.
Auxin accumulation precedes AR formation, and an auxin maximum is established at the ARP tip and maintained in the AR apex
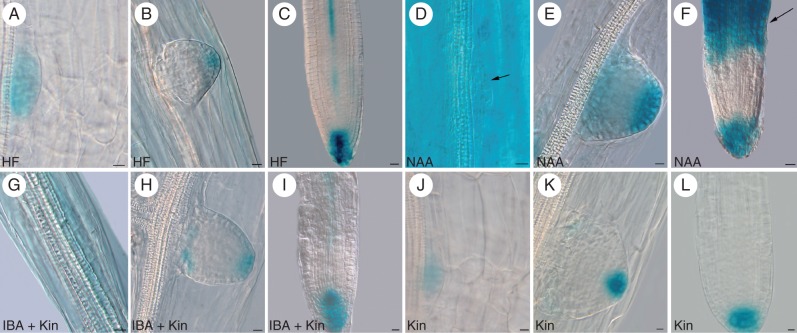
The histochemical analysis of the DR5::GUS seedlings grown without exogenous hormones showed that GUS staining, monitoring the presence of IAA, occurred in the vascular parenchyma of the transition zone before ARP formation (Supplementary Data Fig. S1D). Staining also occurred in the founder cells, and their derivatives, independently of the treatment (Fig. 3A, D, G, J); however, the staining was very faint in the presence of exogenous cytokinin alone (Fig. 3J). In the HF-growing seedlings, an IAA maximum was established to occur in the tip of stage VII ARPs (Fig. 3B), and was maintained in the QC, flanking initials and cap cells of the mature AR apex (Fig. 3C). Auxin was also present in the AR vasculature (Fig. 3C). The exogenous NAA caused an enhancement of staining during the entire process (Fig. 3D–F). Moreover, in the mature ARs, tissue differentiation occurred very near the tip, and the differentiated tissues were stained (Fig. 3F, arrow). Under IBA + Kin, the DR5 signal was more similar to HF than NAA treatment, in terms of both localization and intensity (Fig. 3G–I). Up to stage III (Fig. 3J), the pattern of GUS staining under Kin alone was weaker than under IBA + Kin, and even HF, and no signal was present in the hypocotyl vasculature. However, an auxin maximum was generated at stage VII (Fig. 3K), and maintained in the tip of the mature AR (Fig. 3L).
Fig. 3.
DR5::GUS, monitoring IAA presence in developing ARs from 14-day-old Col seedlings grown under different hormonal treatments. (A–C; HF) DR5 in a stage IV ARP located at the hypocotyl transition zone (A), in the tip of a stage VII ARP (B) and in the QC, flanking initials, columella and developing vasculature of a mature AR (C). (D–F; NAA) Strong DR5 at stage III (arrow) and in surrounding hypocotyl cells (D), at stage VII (E) and in the QC, surrounding initials, cap and differentiated tissues (arrow) of a mature AR (F). (G–I; IBA + Kin) DR5 at stage II (G), at the base and tip of a stage VII ARP (H) and in the QC, surrounding initials, columella and developing vasculature of a mature AR (I). (J–L; Kin) Faint DR5 signal in a stage III ARP located at the transition zone (J), stronger expression at the tip of a stage VII ARP (K) and in the QC, surrounding initials and columella of a mature AR (L). Scale bars: (A, B, D, E, G–L) = 10 µm; (C, F) = 20 µm.
AR development in planta is related to the expression of PIN1 auxin efflux and LAX3 auxin influx genes
The expression of the auxin efflux regulator PIN1, involved in generating auxin maxima in PR and LRs (Petrášek and Friml, 2009), was examined during AR development in planta. PIN1 was expressed in the hypocotyl vasculature and in the surrounding cells derived from the pericycle AR founder cells, independently of the treatment (Fig. 4A, D, G, J). However, in comparison with HF treatment (Fig. 4A), the signal was reinforced by the treatments with the exogenous auxins (Fig. 4D, G), and reduced by that with cytokinin alone (Fig. 4J). At stage VII, the signal was shown by the entire ARP, under both auxin treatments (Fig. 4E, H), and by its basal and middle parts under HF, and under Kin alone, in particular (Fig. 4B, K). Expression was also detected in the AR tip, i.e. niche and surrounding derivative cells, procambium and cap cells, and, again, at higher levels in the presence of the exogenous auxins (Fig. 4F, I) in comparison with HF, and with Kin alone, in particular (Fig. 4C, L). Moreover, expression was turned off in the differentiating cortical and epidermal cells of the ARs (Fig. 4C, I, L), except that in the NAA treatment (Fig. 4F).
Fig. 4.
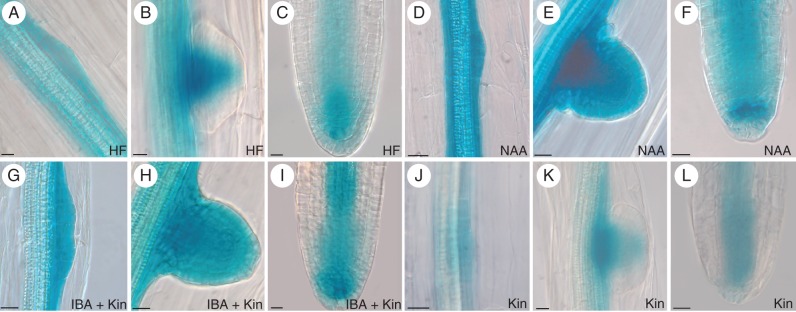
PIN1::GUS expression in developing ARs from 14-day-old Col seedlings grown under different hormonal treatments. (A–C; HF) PIN1 expression at stage III (A), at the base and in the middle of a stage VII ARP (B) and in the niche, cap and procambium of a mature AR (C). (D–F; NAA) High PIN1 expression at stage III (D), at stage VII (E) and in the whole apex of a mature AR (F). (G–I; IBA + Kin) High PIN1 expression at stage III (G) and VII (H), and in a mature AR tip, except for the differentiating cortex and epidermis (I). (J–L; Kin) Very weak PIN1 expression at stage I (J), higher expression at the base and middle part of a stage VII ARP (K) and faint expression in the niche and procambium of a mature AR apex (L). Scale bars: (A,B) = 10 µm; (C–L) = 20 µm.
In the HF treatment, LAX3::GUS expression was weak in the hypocotyl vasculature, but increased corresponding to the derivatives of the pericycle AR founder cells (Fig. 5A). Expression was reinforced at early ARP stages (Fig. 5B), becoming progressively stronger in the basal–middle portion of the developing ARP, and appearing in the surrounding hypocotyl peripheral tissues (Fig. 5C, D). From ARP emergence onwards, LAX3 signal also marked the ARP vasculature, extending up to the elongation zone (Fig. 5E). The apical meristem was without signal (Fig. 5E); however, in the mature AR tip, the signal appeared in the cap (Fig. 5F). Under the auxin treatments, expression was strongly detected in the hypocotyl, early AR stages and ARPs (Fig. 5G, H, L, M). From ARP emergence onwards, the expression pattern did not change in comparison with the HF treatment (Fig. 5I, J, N, O), with LAX3 continuing not to be expressed in the apical meristem (Fig. 5J, O). In the AR tip, the expression was again shown in the cap, and more extensively under NAA than under IBA + Kin (Fig. 5K, P). In the former treatment, the precociously differentiated tissues also showed expression (Fig. 5K). Under treatment with Kin alone, the hypocotyl vasculature showed expression at the transition region only. ARPs elongated in this region only, and the expression pattern during their development recapitulated that observed under HF, but with a weaker signal (Fig. 5Q–U).
Fig. 5.
LAX3::GUS expression during AR development in 14-day-old Col seedlings grown under different hormonal conditions. (A–F; HF) Expression at stage III (A) and IV (B), at the base of a stage VII ARP (C), in the forming vasculature of emerging (D) and elongating ARPs (E) and in a few cap cells, and the differentiating vasculature of a mature AR (F). (G–K; NAA) High and uniform expression at stage IV (G) and VI (H), in the ARP basal half, before (I) and after (J) protrusion, and in cap cells and precociously differentiated tissues in a mature AR (K). (L–P; IBA + Kin) High expression at stage III (L) and V (M), in the basal half of an emerging ARP (N), in the vasculature of an elongating ARP (O) and a mature AR (P), and in some cap cells in the latter (P). (Q–U; Kin, hypocotyl transition zone) LAX3 expression at stage II (Q) and IV (R), at the base of a stage VII ARP (S), in the developing vasculature of elongating ARPs (T) and mature ARs (U), and faintly in the AR cap (U). Scale bars: (A, B, H, I, K, M, Q–S) = 10 µm; (D, G, L, N–P, U) = 20 µm; (C, E, F, J, T) = 30 µm.
The ARs formed in vitro express the same QC markers of the ARs in planta and, similarly, WOX5 is expressed from the first divisions
Wild-type TCLs formed ARs only in the presence of IBA + Kin, confirming previous results (Falasca et al., 2004). Meristematic cell clusters originated from the stem endodermis (Fig. 6A, inset; Supplementary Data S2B, C), and formed root meristemoids (Fig. 6B, inset). Root meristemoids domed and grew into ARPs, which opened their way through the explant cortex (Fig. 6C, inset) and protruded from the explant epidermis, finally becoming mature ARs (Fig. 6D, inset). Fasciated ARs (Supplementary Data Fig. S2E) appeared on about 20 % of the explants, with a mean number of 2·08 (±0·4) per explant.
Fig. 6.
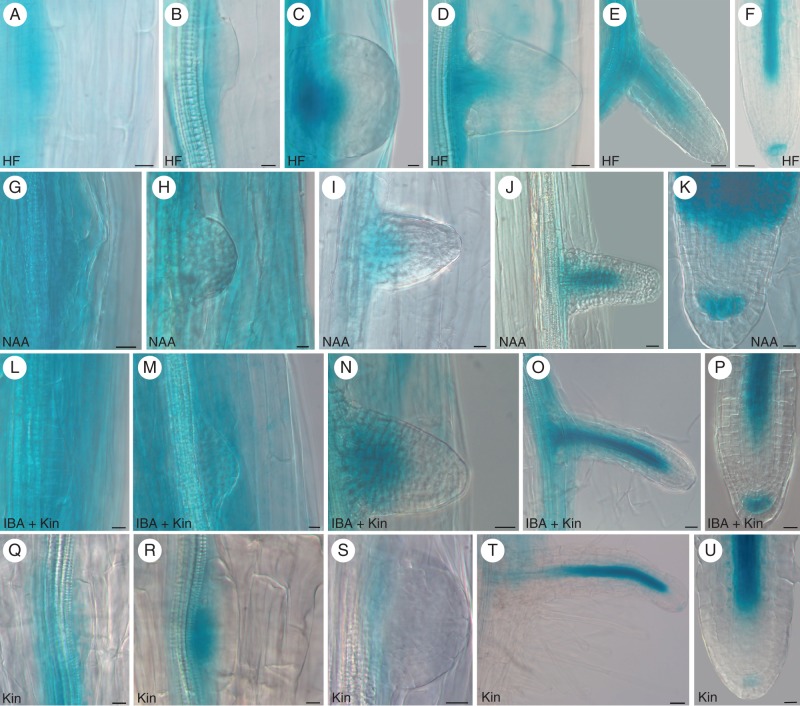
Expression of QC markers (A–J), auxin monitoring (K–O), and auxin efflux (P–R) and influx (S–W) gene expression during AR formation in IBA + Kin-cultured TCLs. (A, B) WOX5 expressed in meristematic cell clusters (A) and root meristemoids (B). Corresponding light microscopy images are shown in the insets. (C, D) Emerging ARPs (C) and apices of elongated ARs (D) showing WOX5 in the QC and lateral initials. Corresponding light microscopy images are shown in the insets. (E) Early-domed ARPs showing the appearance of pAGL42. (F) Emerging ARPs with pAGL42 expression in the QC. (G) Confocal microscopy image of mature AR apices with pAGL42::GFP signal in the QC. (H) No expression in early-forming ARPs of QC25::GUS TCLs, but expression in the QC of emerged ARPs (I) and elongated ARs (J). (K) Meristematic cell clusters with DR5::GUS expression. (L) Detail of a meristemoid showing signal. (M, N) Not yet protruded ARPs showing DR5 expression in the tip (arrows in N). (O) DR5::GUS expression in the niche and cap of mature ARs. The inset shows expression in the forming vasculature. (P) PIN1 expression in early-domed ARPs, corresponding to stage VII in planta, and endodermis-derived cells at the base. (Q) Not yet emerged ARPs showing PIN1 signal, mainly in the tip and forming vasculature. (R) Protruded ARP with PIN1 expression in the vasculature, procambium and apex. (S) LAX3 expression in forming meristemoids (square). (T) Not yet protruded ARPs with LAX3 expression at the base. (U, V) Elongating ARPs after protrusion, with LAX3 expression in the vasculature, but not in the apex. (W) Mature AR with LAX3 signal in the developing vasculature and some cap cells (arrow). Insets in A–D, toluidine blue section staining. Scale bars: (insets in A and C, G) = 10 µm; (A, C–F, H, J, O, inset in O, V, W) = 20 µm; (inset in B, I, T) = 30 µm; (inset in D, K–M, S, U) = 40 µm; (N, P–R) = 50 µm; (B) = 100 µm.
WOX5 was expressed early (days 5–7), marking meristematic cell clusters and meristemoids (Fig. 6A, B). Expression continued in the protruding ARPs, marking the QC, and the lateral initials (Fig. 6C). After 14 d of culture, the first elongated ARs were observed, and showed WOX5 signal in the QC and lateral initials in the apex (Fig. 6D). Expression was also shown by the apices of the fasciated ARs (Supplementary Data Fig. S2F).
pAGL42 expression began to be observed later than that of WOX5, i.e. in the early-domed ARPs, exhibiting a developmental stage comparable with stage VII ARPs in planta (Fig. 6E). In protruding ARPs, and normal/fasciated ARs, pAGL42 signal marked the QC, as it did in planta in the same hormonal treatment and stage (Fig. 6F, G; Supplementary Data S2G). As for pAGL42, QC25::GUS signal was not present at early AR stages (Fig. 6H), appearing in the QC of the domed ARPs, and continuing to be present in the QC of protruding ARPs and elongating ARs (Fig. 6I, J), as it did in planta under the same treatment. QC25::GUS was also shown in the tips of the fasciated ARs (Supplementary Data Fig. S2H).
Auxin accumulation occurs during AR formation in TCLs as well as during AR development in planta, and similarly involves PIN1 and LAX3 expression
For an in-depth insight into the relationship between ARs formed in vitro and in planta, IAA accumulation and transport were investigated in IBA + Kin-cultured TCLs excised from DR5::GUS, PIN1::GUS and LAX3::GUS plants.
During the first week of culture, IAA accumulated locally in the stem endodermis-derived meristematic cell clusters (Fig. 6K, L). In the early-domed ARPs, DR5::GUS activity became evident in a strict population of the apical cells (Fig. 6M), and this apical localization persisted in the protruding ARPs (Fig. 6N). In the ARs, IAA accumulated in the vasculature (Fig. 6O, inset) and in the apex, i.e. the QC, flanking initials and cap cells (Fig. 6O), defining an auxin maximum, as in planta under the same treatment and the HF condition.
PIN1 was diffusely expressed in the root meristemoids and early staged ARPs (Fig. 6P). The signal intensified in the developing ARPs, starting to mark the procambium (Fig. 6Q). A strong expression along the procambium occurred in the protruded ARPs (Fig. 6R) and ARs, reiterating the pattern observed in planta under the same treatment and the HF condition.
LAX3::GUS expression was diffuse in the meristemoids (Fig. 6S), like PIN1; however, in the forming ARPs, LAX3 signal became restricted to the ARP base (Fig. 6T), differently from PIN1. During further ARP development, expression extended acropetally along the procambium (Fig. 6U). In the elongating ARPs, and in the ARs, the expression was present in the vasculature and procambium up to the elongation zone, but was absent in the apical meristem (Fig. 6V, W), except some cap cells in mature ARs (Fig. 6W), collectively reiterating the LAX3 expression pattern observed in planta under the same treatment.
In contrast to the wild-type TCLs, about 27 % of TCLs from the auxin-hyperaccumulating sur2-1 mutant produced ARs in the HF medium, and with a mean number of 3·3 (±0·6). The ARs elongated and formed LRs (Supplementary Data Fig. S2I). Under IBA + Kin, ARPs and ARs, arranged in clumps, covered sur2-1 explants (Supplementary Data Fig. S2J, K). The comparison among treatments and genotypes showed that both the treatment and the genotype significantly (P < 0·0001) affected the response, with the sur2-1 TCLs cultured with IBA + Kin producing an AR number significantly (P < 0·0001) higher than wild-type TCLs under the same treatment (i.e. 242·2 ± 22·4 and 93·3 ± 8·8, respectively).
Trans-zeatin riboside immunolocalization occurs concomitantly with YUCCA6 transcription
Trans-zeatin riboside, an endogenous cytokinin, was immunolocalized in wild-type seedlings grown under HF conditions. A faint staining appeared in the ARPs around stage IV, and increased in the following stages (Fig. 7A). In the not yet protruded ARPs the immunostaining became strong, marking the dome protoderm, in particular (Fig. 7B). After AR emergence, trans-zeatin riboside was immunolocalized in the apex only, marking the differentiating epidermis, in particular (Fig. 7C, inset). Since cytokinin is involved in PIN downregulation in the PR (Dello Ioio et al., 2008), the trans-zeatin riboside immunolocalization was compared with PIN1 expression under the same treatment. The results showed that the endogenous cytokinin accumulated preferentially where PIN1 was expressed at lower levels, both in the ARP (Fig. 4B) and in the AR tip (Fig. 4C).
Fig. 7.
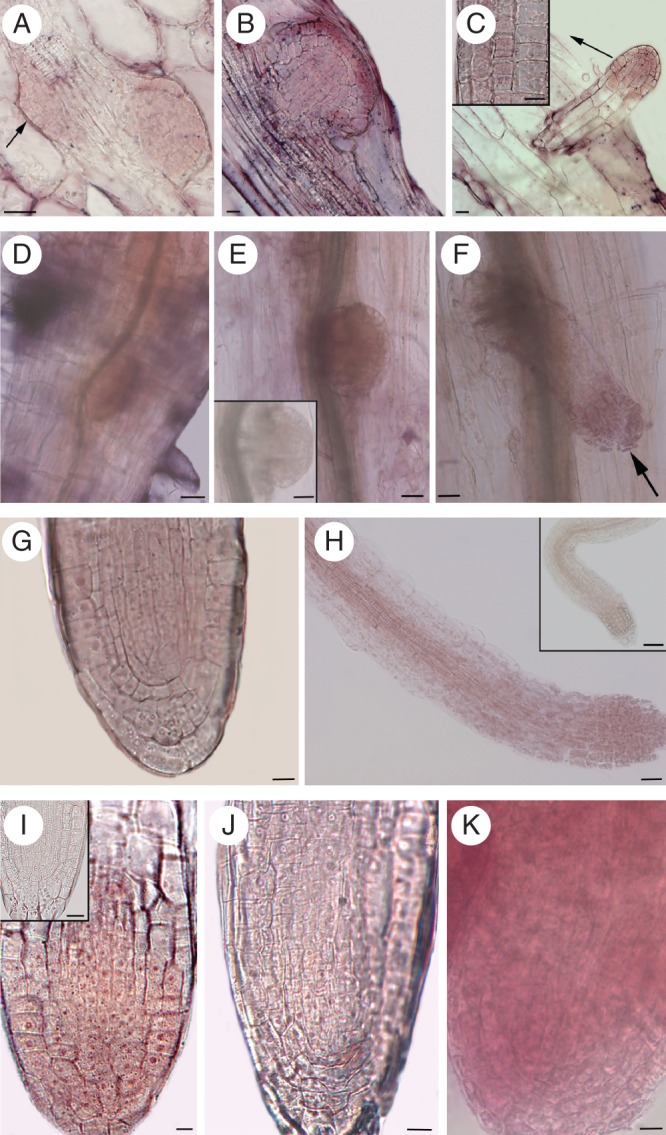
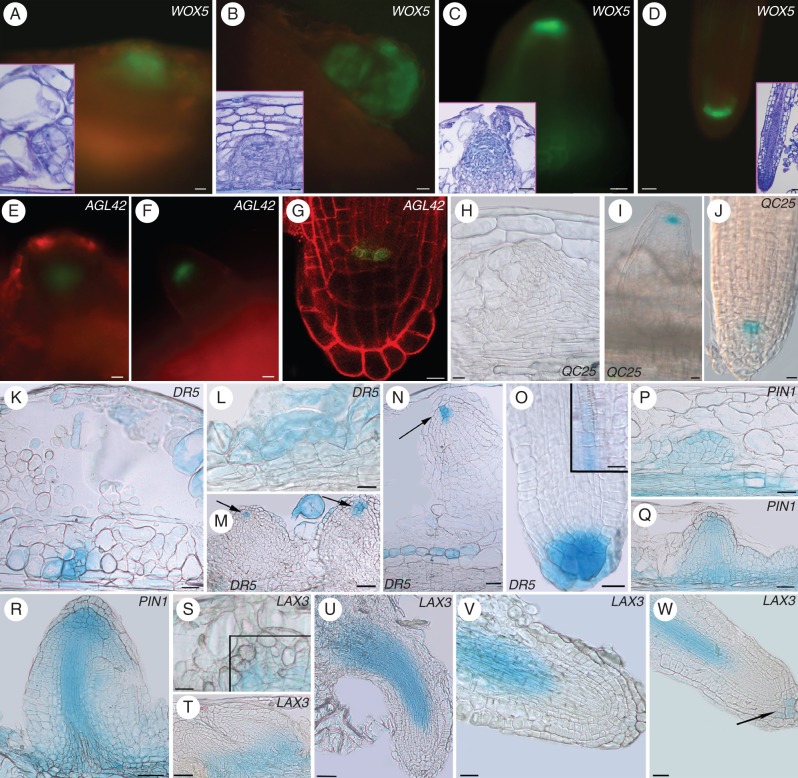
Localization of trans-zeatin riboside (A–C) and YUCCA6 transcription (D–H) during AR development in planta [14-day-old Col (A–F) and Ws (G, H) seedlings grown under HF treatment], and YUCCA6 transcription in ARs from TCLs cultured with/without IBA + Kin (I–K). (A) Stage IV (arrow) and stage VI ARPs showing a diffuse cytokinin immunostaining. (B) ARP just before protrusion showing high cytokinin immunostaining in the protoderm, in particular. (C) Elongating ARP with extensive cytokinin signal at the tip. Differentiating epidermis with staining (arrow) magnified in the inset (longitudinal tangential section). (D–E) In situ hybridizations showing YUCCA6 transcription at stage IV (D) and VI (E). Sense probe control shown in the inset of E. (F–H) YUCCA6 transcription at the tip (arrow) of protruded ARPs (F), all over the AR apex (G), and in the AR procambium and differentiating vasculature (H). Sense probe control shown in the inset of H. (I) YUCCA6 transcription in AR apices from IBA + Kin-cultured Ws TCLs. The sense probe control is shown in the inset. (J, K) Low (J) and high (K) YUCCA6 transcription in the apices of ARs formed by sur2-1 TCLs cultured without hormones and with IBA + Kin, respectively. (D–F, H, K) Whole-mount RNA in situ hybridizations; (G, I, J) RNA in situ hybridizations on longitudinal sections of resin-embedded ARs. Scale bars: (inset in C, G, I–K) = 10 µm; (A–C, inset in I) = 20 µm; (D–F, inset in E, H) = 30 µm; (inset in H) = 40 µm.
To verify whether auxin biosynthesis occurred during AR development, possibly to compensate for cytokinin action, YUCCA6 transcription was evaluated by in situ hybridizations in wild-type seedlings and TCLs, and in sur2-1 TCLs.
In HF-grown seedlings, YUCCA6 expression was high in the shoot apex and gradually decreased along the hypocotyl up to the transition zone. However, expression appeared in stage IV ARPs, and increased up to stage VII (Fig. 7D, E). The transcription of the gene was reinforced at the tip of protruded ARPs (Fig. 7F), and continued to be present in the apical meristem of the mature ARs, where it also occurred in the procambium and differentiating vasculature (Fig. 7G, H).
In the ARs formed by the wild-type TCLs cultured with IBA + Kin, YUCCA6 was more expressed than in the ARs of the HF-grown seedlings (compare Fig. 7I and G). The gene was weakly expressed in the apices of the ARs formed by the HF-cultured sur2-1 TCLs (Fig. 7J), but expression strongly increased in those formed under IBA + Kin (Fig. 7K).
DISCUSSION
Results show that arabidopsis ARs originating from cells from different tissues, i.e. from the hypocotyl pericycle in planta and the stem endodermis in in vitro cultured TCLs, similarly establish the QC in the apical meristem, and at the same developmental stage, which corresponds to the stage of QC establishment in the LR primordia. Auxin induces the AR process, and its gradient and biosynthesis sustain QC establishment and maintenance through cytokinin tuning and WOX5 activity.
An IAA gradient is necessary for AR formation in planta and in TCLs
In the pericycle of arabidopsis PR, endogenous auxin accumulates in the founder cells of LRs, and in their early derivatives, and this accumulation is enhanced by exogenous auxin, e.g. by NAA (Benková et al., 2003). The present results verify that early auxin accumulation is a common event in post-embryonic rhizogenesis. In fact, in the hypocotyl of arabidopsis, endogenous auxin accumulated in the vascular parenchyma adjacent to the pericycle founder cells of the ARs, in the latter cells and in their early derivatives. Moreover, the intensity of the auxin signal increased in the presence of NAA and IBA + Kin, whereas it decreased when cytokinin was applied alone. It is known that auxin moves from shoot to root through the hypocotyl vascular parenchyma (Blakeslee et al., 2007). The exogenous auxins might enhance the hormone export from the perivascular cells towards the pericycle cells, increasing the possibility that an auxin content sufficient for AR induction is formed in the latter cells. The PIN1 auxin efflux carrier is auxin inducible (Vieten et al., 2005). The auxin accumulation in the early derivatives of the hypocotyl pericycle founder cells agrees well with the observed expression of PIN1 in the same cells, and with the increase in expression caused by the exogenous auxins. This supports that PIN1 might be involved in AR induction, promoting auxin lateral efflux from the hypocotyl vasculature towards the pericycle founder cells. However, other PIN genes might also be involved, because the PIN family members are functionally redundant (Blilou et al., 2005). In arabidopsis, the formation of ARs in stem segments cultured in vitro is induced by IBA and inhibited by 3,4,5-triiodobenzoic acid, an inhibitor of polar auxin transport (Ludwig-Müller et al., 2005). Moreover, anomalies increase in root-forming NAA-cultured hypocotyl segments when an inhibitor of auxin efflux, i.e. 1-naphthylphthalamic acid, is also applied (Pernisová et al., 2009). These results confirm the well known role of natural/synthetic exogenous auxins for AR induction in vitro, but also support that an auxin transport via efflux carriers might be active in the explants. Accordingly, the present results demonstrate that auxin accumulated in the endodermis-derived cell clusters and meristemoids of the AR-forming IBA + Kin-cultured TCLs, with a PIN1 expression pattern parallel to that observed in planta. IBA is a natural precursor of IAA (Strader and Bartel, 2011), and an efficient IBA to IAA conversion is important for arabidopsis LR formation (Strader et al., 2011). It is possible that exogenous IBA is rapidly converted to IAA in the TCL cells close to the medium, and PIN1 directs IAA efflux to the endodermis, inducing in some cells of this tissue an IAA accumulation sufficient for AR initiation. The results obtained with the sur2-1 mutant, characterized by an endogenous high content of IAA (Delarue et al., 1998), support the hypothesis, because ARs were formed in sur2-1 TCLs cultured without hormones, whereas AR formation in the wild-type TCLs needs IBA + Kin. In addition, the latter treatment enhanced AR formation in sur2-1 TCLs, showing an additive function of the exogenous IBA on the endogenous IAA.
LAX (LIKE AUX1) proteins are active in auxin cellular uptake. LAX3 is auxin inducible and a PR stelar marker (Swarup et al., 2008). Under both exogenous auxin treatments (i.e. NAA and IBA + Kin), we observed LAX3 expression in the hypocotyl vasculature, and a reinforcement of expression in early AR phases. Moreover, LAX3 and PIN1 expression patterns were similar during the first AR phases in planta and TCLs. Coupling the DR5-monitored auxin accumulation with PIN1 and LAX3 expression patterns, we believe that a co-ordinated auxin efflux influx, involving the two carriers, caused the IAA gradient essential to the early AR events and building up of the ARP, in planta and in TCLs. However, at later developmental stages, LAX3 and PIN1 expression patterns differed, i.e. LAX3 expression was confined at the ARP base, whereas PIN1 expression extended up to the ARP tip. Also during LR formation, after an initial uniform expression, LAX3 is excluded by the primordium tip (Swarup et al., 2008).
In addition, LAX3 seemed necessary for AR emergence because it was expressed in the hypocotyl endodermal, cortical and epidermal cells adjacent to the protruding ARP. A similar expression pattern is shown by LAX3 in the PR during LR emergence, and a relationship between LAX3 and gene expression leading to protrusion has been proposed (Swarup et al., 2008). Moreover, we observed LAX3 expression in the columella cells. This suggests that the carrier is also necessary for the gene expression required for cell to cell separation in the root cap.
WOX5 is an early marker of the AR process
We show that WOX5 is precociously activated during the AR process, both in planta and in in vitro culture. In agreement with this, WOX5 is expressed in the QC precursor cells in the early globular embryo (Haecker et al., 2004), and in the LR founder cells (Ditengou et al., 2008).
Because WOX5 is induced by auxin (Gonzali et al., 2005), and auxin is present in the LR founder cells (Dubrovsky et al., 2008), auxin has been proposed to specify the QC in the LRs through WOX5 expression (Ditengou et al., 2008). We can advance a similar hypothesis for the ARs formed both in planta and in in vitro culture. In fact, based on the expression patterns of WOX5 and PIN1 and the observed localization of auxin gradients and maxima, an auxin flow directed towards the tip of the forming ARP might progressively restrict the initial expression domain of WOX5, resulting into the apical positioning of the QC at stage VII of development.
Cytokinin negatively affects PIN1 and LAX3 expression
In the PR, the bulk of cytokinin is synthesized in the tip, in the cap in particular, and is exported through the xylem, whereas, in LRs, it is produced in the entire tip and appears not to be transported through the stele (Aloni et al., 2005). We observed that in planta exogenous Kin, when applied alone, reduced early auxin accumulation. In PR and LRs, cytokinin is known to regulate auxin negatively by inducing Aux/IAA proteins which downregulate the abundance of PIN proteins (e.g. of PIN1) (Moubayidin et al., 2009; Su et al., 2011). It is possible that a cytokinin-induced reduction of auxin flow occurred at the onset of the AR process to counteract a possible excess in auxin-induced founder cell formation and activity. The relevant immunolocalization of trans-zeatin riboside at stage VII suggests a direct role for the hormone also in ARP growth. The cytokinin mainly accumulated in the outermost layers of the ARP, where an almost total absence of PIN1 expression and auxin accumulation were observed. This suggests that endogenous cytokinin is locally synthesized to downregulate PIN1 and block the auxin flow in the outermost part of the ARP, forcing PIN1 activity, and auxin flow, to occur along the middle cells up to the ARP tip, here establishing the auxin maximum required for WOX5-related QC positioning. Moreover, the application of cytokinin alone to the seedlings shows that the hormone is also involved in the restriction of the LAX3 expression domain to the ARP base.
Local auxin biosynthesis is needed for ARP development
In planta the establishment of an auxin maximum in the ARP tip, and its maintenance in the AR, were also observed when kinetin was applied alone to the growth medium. Based on the negative effect of the hormone on the expression of PIN1 and LAX3, it is possible that a local auxin biosynthesis was needed to compensate the cytokinin-reduced auxin flow to the tip and to maintain apical auxin homeostasis and the QC-related gene expression (e.g. that of WOX5). In agreement with this, auxin biosynthesis contributes to the auxin homeostasis at the PR tip (Petersson et al., 2009). Moreover, cytokinin is known to promote auxin biosynthesis in PR and LRs (Jones et al., 2010). The present results support the hypothesis, because YUCCA6 expression and trans-zeatin riboside immunolocalization initiated at the same stage (i.e. stage IV), i.e. before QC establishment, increased at stage VII, and were similarly localized in the tip at further stages.
The response of sur2-1 TCLs is also informative. YUCCA genes are not involved in the IAA synthesis pathway which is upregulated by sur2 mutation (Barlier et al., 2000; Mashiguchi et al., 2011). In agreement with this, YUCCA6 was weakly expressed in the tip of the ARs formed by the mutant TCLs cultured without hormones. In contrast, expression increased greatly at the tip of the ARPs formed both by sur2-1 and by wild-type TCLs cultured with IBA + Kin, suggesting that this similar enhancement was directly/indirectly caused by cytokinin application, supporting the regulative role proposed for the hormone on AR formation in planta.
In conclusion, the ARs organize a QC; its establishment and maintenance are independent of the origin of the founder cells, a co-ordinated action of auxin and cytokinin is needed, and involves expression of specific genes, as proposed in the model of Fig. 8.
Fig. 8.
Model of auxin flow, gene expression and cytokinin localization during AR formation in planta. At stage I, auxin (IAA) is diverted from the basipetal flow along the vascular parenchyma cells (vp) adjacent to the protoxylem (x) of the hypocotyl (Hy) towards the pericycle (P) cells by PIN1, activating LAX3 and auxin accumulation (blue colour) in the founder cells. At stage II, auxin is maintained in the first-formed inner and outer AR layers by PIN1 (yellow arrow) and LAX3 (light-blue arrows), and WOX5 is expressed. At stage VII, PIN1 drives auxin flow towards the ARP tip throughout the middle cell files, because cytokinin (pink colour) downregulates PIN1 in the peripheral ARP layers. Cytokinin also downregulates LAX3, limiting the carrier activity at the ARP base (up to the dotted line). The auxin flow driven by PIN1 towards the tip results in an apical auxin maximum, limiting WOX5 expression at the distal tip, and here establishing the position of the QC. Auxin biosynthesis by YUCCA6 (green diamonds) contributes to auxin maximum positioning in the tip. LAX3 is also active in the Hy endodermis (End), cortex (C) and epidermis (E) around the ARP, possibly favouring protrusion. In the mature AR, the auxin maximum (blue colour) encompasses the QC, flanking initials and cap cells (columella, in particular), and WOX5 QC expression is maintained. Auxin biosynthesis by YUCCA6 is also maintained (green diamonds), contributing to the persistence of apical auxin accumulation. Also cytokinin is present at the AR tip (pink stars), contributing to the maintenance of auxin homeostasis by a downregulation of PIN1 in the forming epidermis/cortex, and of LAX3 in the entire tip, except the cap (light-blue dots). PIN1 and LAX3 are expressed in the AR vasculature, and LAX3 expression stops at the elongation zone border (dotted line).
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Dr S. Sabatini, L. Colombo, M. Bennett, P. Benfey, B. Scheres and C. Bellini for providing QC25::GUS and DR5::GUS, PIN1::GUS, LAX3::GUS, pAGL42::GFP, pWOX5::GFP and sur2-1 seeds, respectively. We also thank Dr P. Tavladoraki for assistance in confocal analysis, and D. Paolo for support in YUCCA6 probe preparation. The research was supported by Progetto d'Ateneo 2009, Sapienza University and PRIN 2008 (to M.M.A.).
LITERATURE CITED
- Airoldi CA, Della Rovere F, Falasca G, et al. The Arabidopsis BET bromodomain factor GTE4 is involved in maintenance of the mitotic cell cycle during plant development. Plant Physiology. 2010;152:1320–1334. doi: 10.1104/pp.109.150631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R, Langhans M, Aloni E, Dreieicher E, Ullrich CI. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. Journal of Experimental Botany. 2005;56:1535–1544. doi: 10.1093/jxb/eri148. [DOI] [PubMed] [Google Scholar]
- Barlier I, Kowalczyk M, Marchant A, et al. The SUR2 gene of Arabidopsis thaliana encodes the cytochrome P450 CYP83B1, a modulator of auxin homeostasis. Proceedings of the National Academy of Sciences, USA. 2000;97:14819–14824. doi: 10.1073/pnas.260502697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature. 1997;390:287–289. doi: 10.1038/36856. [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Lee OR, et al. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. The Plant Cell. 2007;19:131–147. doi: 10.1105/tpc.106.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Caboni E, D'Angeli S, Chiappetta A, Innocenti AM, Onckelen HV, Damiano C. Adventitious shoot regeneration from vegetative shoot apices in pear and putative role of cytokinin accumulation in the morphogenetic process. Plant Cell, Tissue and Organ Culture. 2002;70:199–206. [Google Scholar]
- Clowes FAL. Nucleic acids in root apical meristem of Zea. New Phytologist. 1956;55:29–35. [Google Scholar]
- Delarue M, Prinsen E, Onckelen VH, Caboche M, Bellini C. Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. The Plant Journal. 1998;14:603–611. doi: 10.1046/j.1365-313x.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- Della Rovere F, Airoldi CA, Falasca G, et al. The Arabidopsis BET bromodomain factor GTE4 regulates the mitotic cell cycle. Plant Signaling and Behavior. 2010;56:677–680. doi: 10.4161/psb.5.6.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, et al. A genetic framework for the control of cell division and differentiation in the root meristem. Science. 2008;322:1380–1384. doi: 10.1126/science.1164147. [DOI] [PubMed] [Google Scholar]
- Ding Z, Friml J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proceedings of the National Academy of Sciences, USA. 2010;107:12046–12051. doi: 10.1073/pnas.1000672107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditengou FA, Teale WD, Kocherspenger P, et al. Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 2008;105:18818–18823. doi: 10.1073/pnas.0807814105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proceedings of the National Academy of Sciences, USA. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasca G, Altamura MM. Histological analysis of adventitious rooting in Arabidopsis thaliana (L.) Heynh seedlings. Plant Biosystems. 2003;137:265–274. [Google Scholar]
- Falasca G, Zaghi D, Possenti M, Altamura MM. Adventitious root formation in Arabidopsis thaliana thin cell layers. Plant Cell Reports. 2004;23:17–25. doi: 10.1007/s00299-004-0801-3. [DOI] [PubMed] [Google Scholar]
- Feldman LJ. Cytokinin biosynthesis in roots of corn. Planta. 1979;145:315–321. doi: 10.1007/BF00388355. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, et al. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- García-Aguilar M, Dorantes-Acosta A, Pérez-España V, Vielle-Calzada JP. Whole-mount in situ mRNA localization in developing ovules and seeds of Arabidopsis. Plant Molecular Biology Reporter. 2005;23:279–289. [Google Scholar]
- Gonzali S, Novi G, Loreti E, et al. A turanose-insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana. The Plant Journal. 2005;44:633–645. doi: 10.1111/j.1365-313X.2005.02555.x. [DOI] [PubMed] [Google Scholar]
- Haecker A, Gross-Hardt R, Geiges B, et al. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131:657–668. doi: 10.1242/dev.00963. [DOI] [PubMed] [Google Scholar]
- Hejátko J, Blilou I, Brewer PB, Friml J, Scheres B, Benková E. In situ hybridization technique for mRNA detection in whole mount Arabidopsis samples. Nature Protocols. 2006;1:1939–1946. doi: 10.1038/nprot.2006.333. [DOI] [PubMed] [Google Scholar]
- Jiang K, Feldman LJ. Regulation of root apical meristem development. Annual Review of Cell and Developmental Biology. 2005;21:485–509. doi: 10.1146/annurev.cellbio.21.122303.114753. [DOI] [PubMed] [Google Scholar]
- Jones B, Gunneras SA, Petersson SV, et al. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. The Plant Cell. 2010;22:2956–2969. doi: 10.1105/tpc.110.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Sharkhuu A, Jin JB, et al. yucca6, a dominant mutation in Arabidopsis, affects auxin accumulation and auxin-related phenotypes. Plant Physiology. 2007;145:722–735. doi: 10.1104/pp.107.104935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Xue L, Xu S, Feng H, An L. Mediators, genes and signaling in adventitious rooting. Botanical Review. 2009;75:230–247. [Google Scholar]
- Ljung K, Hull AK, Celenza J, et al. Sites and regulation of auxin biosynthesis in Arabidopsis roots. The Plant Cell. 2005;17:1090–1104. doi: 10.1105/tpc.104.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J, Vertocnik A, Town CD. Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. Journal of Experimental Botany. 2005;56:2095–2105. doi: 10.1093/jxb/eri208. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, et al. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant Cell. 2002;14:589–597. doi: 10.1105/tpc.010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, et al. The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2011;108:18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubayidin L, Di Mambro R, Sabatini S. Cytokinin–auxin crosstalk. Trends in Plant Science. 2009;14:557–562. doi: 10.1016/j.tplants.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Nawy T, Lee JY, Colinas J, et al. Transcriptional profile of the Arabidopsis root quiescent center. The Plant Cell. 2005;17:1908–1925. doi: 10.1105/tpc.105.031724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernisová M, Klíma P, Horák J, et al. Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proceedings of the National Academy of Sciences, USA. 2009;106:3609–3614. doi: 10.1073/pnas.0811539106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson SV, Johansson AI, Kowalczyk M, et al. An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. The Plant Cell. 2009;21:1659–1668. doi: 10.1105/tpc.109.066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrášek J, Friml J. Auxin transport routes in plant development. Development. 2009;136:2675–2688. doi: 10.1242/dev.030353. [DOI] [PubMed] [Google Scholar]
- Péret B, Swarup K, Ferguson A, et al. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. The Plant Cell. 2012;24:2874–2885. doi: 10.1105/tpc.112.097766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes and Development. 2003;17:354–358. doi: 10.1101/gad.252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AK, Luijten M, Miyashima S, et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446:811–814. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- Strader LC, Bartel B. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Molecular Plant. 2011;4:477–486. doi: 10.1093/mp/ssr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Wheeler DL, Christensen SE, et al. Multiple facets of Arabidopsis seedling development require indole-3-butyric acid-derived auxin. The Plant Cell. 2011;23:984–999. doi: 10.1105/tpc.111.083071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YH, Liu YB, Zhang XS. Auxin–cytokinin interaction regulates meristem development. Molecular Plant. 2011;4:616–625. doi: 10.1093/mp/ssr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nature Cell Biology. 2008;10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- Takahashi F, Sato-Nara K, Kobayashi K, Suzuki M, Suzuki H. Sugar-induced adventitious roots in Arabidopsis seedlings. Journal of Plant Research. 2003;116:83–91. doi: 10.1007/s10265-002-0074-2. [DOI] [PubMed] [Google Scholar]
- Takechi K, Sakamoto W, Katsuhara M, Murata M, Motoyoshi F. In situ RNA hybridization using Technovit resin in Arabidopsis thaliana. Plant Molecular Biology Reporter. 1999;17:43–51. [Google Scholar]
- Vieten A, Vanneste S, Wiśniewska J, et al. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development. 2005;132:4521–4531. doi: 10.1242/dev.02027. [DOI] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J. Arabidopsis: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- Willemsen V, Wolkenfelt H, de Vrieze G, Weisbeek P, Scheres B. The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development. 1998;125:521–531. doi: 10.1242/dev.125.3.521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.