Abstract
Purpose
We hypothesize organic cation transporters (OCT) may have a potential role in determining the pharmacokinetics and toxicity of organic cation drugs applied topically. Hence, in the present in vivo study, we attempted to evaluate the role of OCT in modulating the transport of its substrates after topical application.
Methods
New Zealand albino rabbits of either sex were used. Transcorneal penetration of OCT substrates tetraethylammonium and metformin after single instillation was evaluated in the absence and presence of OCT blockers (quinidine and atropine). Aqueous humor (AH) samples were collected through paracentesis amounting to 70–100 μl under topical anesthesia at various time intervals. The samples were subjected for estimation of both substrate as well as blocker concentrations using liquid chromatography mass spectrometry.
Results
Topical pre-treatment (30 min before substrate) of OCT blockers significantly decreased the transcorneal penetration of OCT substrates after single topical administration. The levels of blockers reaching AH in the presence of substrates were also modulated at 60 min after its administration as compared with its control.
Conclusion
OCT are functionally active in the uptake of their substrates from tear to AH. Therefore, OCT in the corneal epithelium may be positioned from apical to basolateral. When administering their substrates/blockers topically, both may be competing for OCT for their uptake across the cornea, thereby decreasing the corneal penetration. Hence OCT can have a potential pharmacokinetic role in modulating the ocular bioavailability of their substrates administered topically, which are used as ocular therapeutics.
Keywords: organic cation transporters (OCT), transcorneal, pharmacokinetics, rabbits
Introduction
Topical administration of eye drops is a conventional and patient-compliant mode of drug administration to the eye.1 The majority of the topically applied drugs for treating anterior segment diseases utilize cornea as the main pathway to enter the eye from tear to aqueous humor (AH).2 Studies indicate that presence of a physiological protective mechanism like transporter proteins may be responsible for the uptake or altered penetration of drugs across the cornea.3, 4, 5 Most of the endogenous amines having a physiologically important role are organic cations (OC) and carry a positive charge, so they are unable to permeate across the cornea. Therefore, a specialized transport process has been reported to exist in the conjunctival and corneal epithelial cells involved in the regulation of the transport of various endogenous amines such as epinephrine, dopamine, histamine, and serotonin from tear fluid.6, 7, 8
Polyspecific organic cation transporters (OCT) mediate the facilitated transport of a wide variety of structurally diverse OC, including many drugs, toxins, and endogenous compounds. Few of the ophthalmic drugs that are transported by OCT are atropine, timolol, ofloxacin, and levofloxacin.9, 10, 11, 12 In eye, these transporters are expressed in various ocular tissues like the cornea, iris, ciliary body, conjunctiva, and retina.13 Targeting these transporters will be a smart way of drug delivery aimed at optimal ocular bioavailability.1 The expression of OCT in the cornea and conjunctiva has been well documented.13 Many of the pharmacologically important drugs in ophthalmic practice are substrates of OCT, thereby their bioavailability is getting altered.4, 14
Various studies in humans and animals showed the modulation in the ocular penetration of various OC. Additional administration of diclofenac sodium significantly increased the mean intraocular pressure (IOP) in patients undergoing latanoprost therapy.15 The effect of brimonidine in reducing the IOP was significantly reduced after oral administration of indomethacin.16 Even the possible drug-induced systemic toxicity was reduced when two OC, timolol, and phenylephrine were coadministered.17 Similarly, Lee et al18 showed that the coadministration of timolol with either pilocarpine or epinephrine significantly reduced the ocular absorption and thereby the concentration of timolol in the anterior segment tissues, possibly by its change during the precorneal clearance. All these findings have clinical importance; however, the reason for these significant findings of modulation in the ocular pharmacokinetics of OC is not clearly known.
We hypothesize OCT may have a potential role in determining the pharmacokinetics and toxicity of OC drugs applied topically. In previous studies, transport processes due to OCT were showed using ex vivo models in excised cornea,19 in vitro corneal cells,19 and excised conjunctiva20 for understanding the functional importance of OCT. However, from the literature it was also evident that cell line studies did not reveal (due to minimal expression of transporters in the cell lines) the active functional role of OCT in transporting the administered eye drop.19 Studying the functional importance of OCT in vivo can reveal their role in the transport of organic cations across the cornea, when administered topically. It is well known that tetraethylammonium chloride (TEA) and metformin are cation substrates of OCT, and atropine and quinidine can inhibit the uptake of cation substrates.9, 21, 22, 23, 24 Hence, in the present study we used these substrates and inhibitors to understand the role of OCT in modulating the transport of their substrates across the cornea after topical application.
Materials and methods
Drugs and chemicals
TEA, quinidine sulfate and atropine sulfate were purchased from Sigma-Aldrich (St Louis, MO, USA). Metformin potassium chloride was a generous gift from Microlabs, Bangalore, India. Homatropine hydrobromide was purchased from Boehringer Ingelheim, Ingelheim, Germany. All other chemicals and solvents purchased from their respective commercial sources.
Animals
New Zealand albino rabbits of either sex weighing 1.5–2.0 kg were used in this study. Animals were handled in accordance with the guidelines of Association for Research in Vision and Ophthalmology statement for the use of Animals in Ophthalmic and Vision Research. All the experimental procedures were reviewed and approved by the standing Institutional Animal Ethics Committee of All India Institute of Medical Sciences (File No. 479/IAEC/09).
Preparation and administration of drug formulations to rabbits
Formulations were prepared as described previously in Nirmal et al.21 In brief, required concentrations of OCT substrates TEA (7.85 mM) and metformin (7.85 mM), and blockers quinidine (6.13 mM) and atropine (3.47 mM) were dissolved in phosphate-buffered saline and pH maintained at 7.4. The resulting solutions were filtered through a 0.22-μm sterile Millipore filter (Millex GV filter, Millipore, Billerica, MA, USA) to achieve terminal sterilization. Fresh sterile solutions were prepared every time before the experiments. All formulations (substrate/blocker) amounting to 20 μl were instilled topically to the rabbit's eyes using a calibrated micropipette and the eyes were closed for a minute after instillation. In blocker pre-treated animals quinidine or atropine was instilled 30 min prior to the commencement of substrate instillation.
Transcorneal penetration of topical OCT blockers
The animals were randomized into two groups (four eyes for each time point). The groups were quinidine (group 1) and atropine (group 2). In both the groups, the AH was collected at different time intervals (15, 30, 60, and 120 min) and the AH kinetics of blockers were evaluated. For collecting the AH, the corneas were anesthetized with 4% lidocaine. AH was withdrawn with a 30-G sterile hypodermic needle via paracentesis amounting to 70–100 μl and stored at −80 °C until further analysis by liquid chromatography mass spectrometry (LC-MS/MS).
Transcorneal penetration of topical OCT substrates
The AH kinetics of OCT substrates (TEA and metformin) in the absence and presence of blockers (quinidine and atropine) was studied using rabbits. Animals were randomized into eight groups (4 eyes for each time point): TEA (group 3), metformin (group 4), TEA with quinidine (group 5), TEA with atropine (group 6), metformin with quinidine (group 7), and metformin with atropine (group 8). In all groups, the AH was collected at different time intervals (15, 30, 60, and 120 min) according to the procedure given above.
LC-MS/MS analysis
LC-MS/MS instrumentation for TEA, other substrates and blockers used in this study was used as described in our previous study.25 For all analytes electron spray ionization was used and the chromatographic eluents were subjected to ionization in the positive ion mode at 5500 V. TEA was quantified as described in Nirmal et al.25 The method used for the analysis of metformin was adopted from Mistri et al26 with slight modifications as described in our previous study.25, 27, 28 The LC conditions for metformin, quinidine, and atropine were followed as given. An isocratic mobile phase containing 80% acetonitrile with 0.1% formic acid and 20% aqueous 5 mM ammonium acetate containing 0.1% formic acid was used. The mobile phase was 0.22 μm, filtered, degassed online, and pumped at the rate of 0.5 ml/min. The samples were loaded into a 96-well tray and injected using an auto sampler and kept at 20 °C. Twenty microliters of the sample was subjected to quantification for 5 min run time.
Quantification of metformin was performed using the multiple reaction monitoring (MRM) mode, based on parent/product ion transitions 130/71. Compound-dependent parameters such as declustering potential, entrance potential, collision energy and cell exit potential were set at 41, 8, 35, and 5 V, respectively. Nitrogen gas flow for collision-induced dissociation and dwell time were kept at 4 (arbitrary value) and 100 ms, respectively.
The method used for the quantification of quinidine was adopted from Liu et al29 with minor modifications.27, 30 The MRM transitions were 325.2/172.1 (transition 1) and 325.2/79.1 (transition 2). Compound-dependent parameters such as declustering potential, entrance potential, collision energy, and cell exit potential were set at 58, 10, 47, and 10 V for transition 1, and at 57, 10, 61.64, and 5.5 V for transition 2, respectively. Nitrogen gas flow for collision-induced dissociation and dwell time were kept at 3 (arbitrary value) and 100 ms, respectively.
The method adopted from Chen et al31 with minor modifications27, 30 was used for the quantification of atropine. The MRM transitions were 290.11/124. Compound-dependent parameters such as declustering potential, entrance potential, collision energy, and cell exit potential were set at 79, 4, 35, and 10 V, respectively. Nitrogen gas flow for collision-induced dissociation and dwell time were kept at 3 (arbitrary value) and 100 ms, respectively.
The source-dependent parameters of the mass spectrometer were gas 1 (30 psi); gas 2 (60 psi); curtain gas (10 psi); ion spray voltage (5500 V); and temperature (350 °C) for metformin, quinidine and atropine. The transitions for homatropine were 276.1/142. Homatropine was used as an internal standard (IS) for all the drugs, and the conditions were followed as described in Nirmal et al.25
Standard and sample preparation
The stock concentrations of TEA, metformin, quinidine, and atropine used were 7.84, 7.85, 6.13, and 3.468 μmol/ml. The stock solution was diluted with 50% methanol containing 0.1% formic acid to reach the required concentrations ranging from low to high. The stock solution (3.63 μmol/ml) of IS was further diluted to reach 1.8 nmol/ml in the extraction solvent containing 70% acetonitrile with 0.1% formic acid. Twenty microliters of AH sample was extracted using 200 μl of extraction solvent, vortexed for 1 min, and subjected to centrifugation. The clear supernatant of 100 μl was transferred to 96-well plates and subjected to quantification.
Pharmacokinetics and statistical analysis
The area under the curve (AUC) of AH concentration upto 2 h (AUC0–2) was calculated by using the linear trapezoidal rule using standard formulae.32
The results are represented as mean±SEM. Student's t-test (unpaired) was used to compare the significance between the control and blocker pre-treated groups using SigmaStat statistical software programme ver.2.0 (Jandel, San Jose, CA, USA).
Results
Transcorneal penetration of OCT blockers in the absence of substrates
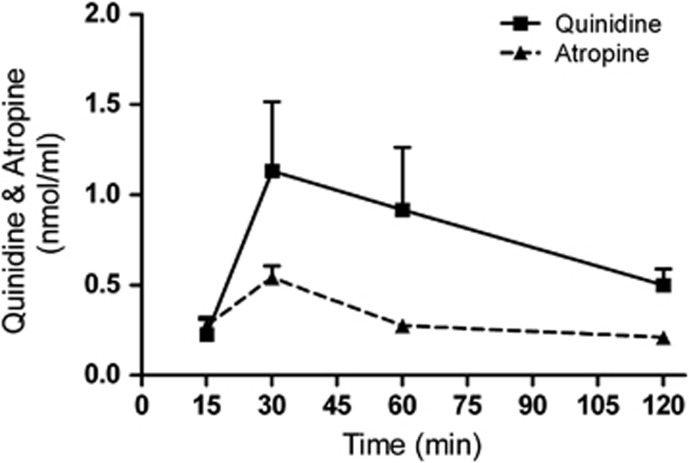
The transcorneal penetration shows the maximum concentration of quinidine and atropine reached at 30 min was 1.13±0.38 and 0.54±0.06 nmol/ml, respectively, after a single topical application (Figure 1). Based on this observation the blocker pre-treatment time has been fixed as 30 min before the substrate administration.
Figure 1.
Transcorneal penetration of quinidine and atropine in the absence of substrate. Mean concentration–time profile of quinidine and atropine in the AH was measured after single topical administration in rabbit's eye. The values are shown as mean±SEM.
Effect of OCT blockade in the transcorneal penetration of TEA
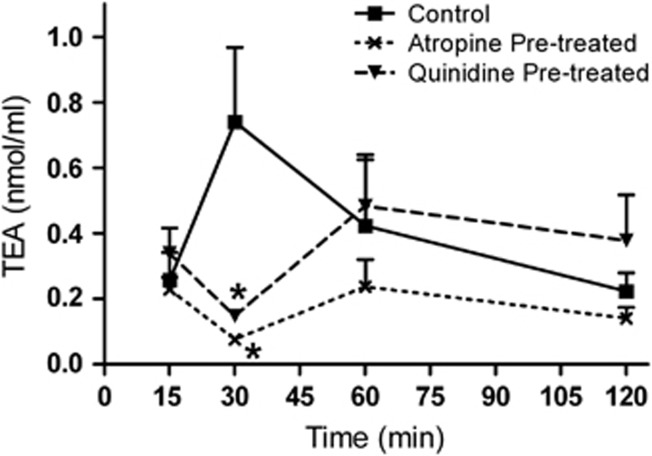
TEA (referred to as control) was administered either alone or in the presence of quinidine or atropine (referred to as quinidine pre-treated or atropine pre-treated). AH levels of TEA in the control group reached its Tmax at 30 min after the single topical instillation. The TEA levels in AH in the quinidine pre-treated group (0.14±0.01 nmol/ml) were significantly (P<0.05) reduced when compared with the control group (0.73±0.22 nmol/ml) at 30 min (Figure 2). The transcorneal penetration of TEA in the blocker pre-treated groups showed that the blocker was effective till 30 min after TEA instillation (Figure 2). Percentage penetration of the control and quinidine pre-treated groups was found to be 0.47% and 0.41%, respectively. The AUCs of the control and quinidine pre-treated groups were 0.73 and 0.64 nmol/ml·h, respectively. The Cmax of TEA in the control group was 0.73±0.22 nmol/ml. At the same time point, blocker pre-treatment decreased the TEA penetration to 0.14±0.016 nmol/ml, which was found to be 5.2-fold less compared to control (Table 1). Atropine had a significant effect on the transcorneal penetration of TEA. The Cmax of TEA in the control group (0.73±0.22 nmol/ml) was significantly (P<0.05) decreased to 0.076±0.002 nmol/ml at 30 min in the atropine pre-treated group (Figure 2). In case of percentage penetration, the atropine pre-treated group was 2.5-fold less than the control group. The AUC and Cmax of TEA were decreased to 2.4- and 10.4-fold in the presence of atropine (Table 1).
Figure 2.
Transcorneal penetration of TEA in the absence and presence of blockers. Mean concentration–time profile of TEA in the AH was measured after single topical administration in rabbit's eye. Note: TEA alone (control); TEA in the quinidine or atropine pre-treatment group (quinidine or atropine pre-treated). The values are shown as mean±SEM. *P<0.05.
Table 1. The calculated pharmacokinetic parameters of TEA in AH after topical administration.
| Parameters | Control | Quinidine pre-treated | Fold (decrease) | Atropine pre-treated | Fold (decrease) |
|---|---|---|---|---|---|
| Percentage penetration (%) | 0.47 | 0.41 | 1.2 | 0.19 | 2.5 |
| AUC0–2 (nmol/ml h) | 0.73 | 0.64 | 1.5 | 0.30 | 2.4 |
| Concentration at 30 min (nmol/ml) | 0.73 | 0.14 | 5.2 | 0.07 | 10.4 |
Note: TEA (control); TEA in the quinidine or atropine topical pre-treatment group (quinidine or atropine pre-treated). The decrease in fold was calculated from control vs quinidine or atropine pre-treated.
Fate of quinidine and atropine in the presence of TEA
Quinidine/atropine (referred to as control) was administered either alone or in the presence of TEA (referred to as experimental). The estimation of quinidine levels in AH showed that the transcorneal penetration of quinidine was higher than that of TEA in the control and quinidine pre-treated groups. In the presence of substrate (TEA) penetration of blocker to AH decreased drastically (Figure 1a, Supplementary Data). Atropine levels reached AH in both the control and experimental groups (Figure 1b, Supplementary Data). However, atropine levels in both groups were less when compared with TEA (control) (Figures 2 and 1b, Supplementary Data).
Effect of OCT blockade in the transcorneal penetration of metformin
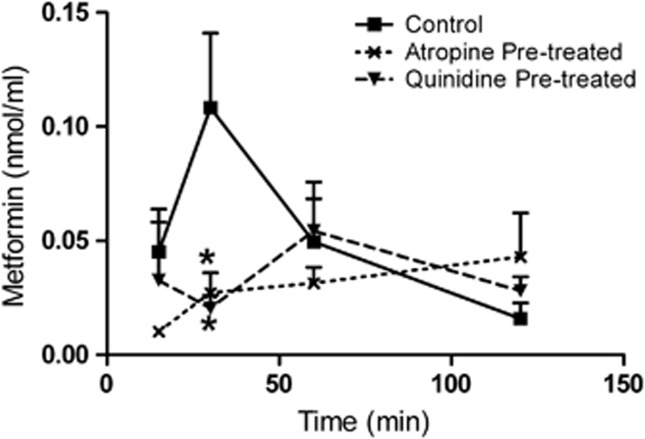
Metformin (referred to as control) was administered either alone or in the presence of quinidine or atropine (referred to as quinidine pre-treated or atropine pre-treated). The maximum aqueous level of metformin (Cmax) for the control group was attained at 30 min, and was found to be 0.108±0.03 nmol/ml. In the quinidine pre-treated group, the metformin levels were significantly decreased to 0.02±0.005 nmol/ml at 30 min (P<0.05). It shows that there was a 5.4-fold decrease in the penetration of metformin in the quinidine pre-treated group compared with the control group. The above results imply that the blocker was effective till 30 min after metformin instillation (Figure 3). The percentage penetration of metformin in the quinidine pre-treated group was 0.042%, whereas in the control group it was 0.058% . This shows that the blocker pre-treated group was 1.6-fold less than the control group. The AUC of the control and quinidine pre-treated groups was 0.091 and 0.066 nmol/ml·h, respectively (Table 2). The levels of metformin in the atropine pre-treated group were less at the initial time points and gradually increased at later time points. Metformin levels in the atropine pre-treated group, as shown in Figure 3, were significantly (P<0.05) decreased as compared with the control at 30 min. The aqueous concentration of metformin at 30 min was 0.027±0.008 nmol/ml in the atropine pre-treated group and was found to be four fold less than that in the control group. In case of percentage penetration, the blocker pre-treated group was 1.9-fold less than the control.
Figure 3.
Transcorneal penetration of metformin in the absence and presence of blockers. Mean concentration–time profile of metformin in the AH was measured after single topical administration in rabbit's eye. Note: Metformin alone (control); metformin in the quinidine or atropine pre-treatment group (quinidine or atropine pre-treated). The values are shown as mean±SEM. *P<0.05.
Table 2. The calculated pharmacokinetic parameters of metformin in AH after topical administration.
| Parameters | Control | Quinidine pre-treated | Fold (decrease) | Atropine pre-treated | Fold (decrease) |
|---|---|---|---|---|---|
| Percentage penetration (%) | 0.058 | 0.042 | 1.6 | 0.036 | 1.9 |
| AUC0–2 (nmol/ml h) | 0.091 | 0.066 | 1.3 | 0.056 | 1.6 |
| Concentration at 30 min (nmol/ml) | 0.108 | 0.020 | 5.4 | 0.027 | 4.0 |
Note: Metformin (control); metformin in the quinidine or atropine topical pre-treatment group (quinidine or atropine pre-treated). The decrease in fold was calculated from control vs quinidine or atropine pre-treated.
Fate of quinidine and atropine in the presence of metformin
Quinidine/atropine (referred to as control) was administered either alone or in presence of metformin (referred to as experimental). Aqueous quinidine level in the control was slightly less than that in the experimental group (Figure 2a, Supplementary Data). The levels of blocker reaching AH were higher when compared with the substrate (metformin). The levels of atropine in the control and experimental groups were higher than metformin levels, indicating higher aqueous penetration of the blocker than the substrate (Figure 2b, Supplementary Data).
Discussion
Many of the clinically interesting ophthalmic drugs are OC, due to which their transport across the corneal epithelium heavily depends on transporter carriers.4 Despite the restriction offered by the corneal epithelium, transcorneal penetration is the major route of entry for drugs from tears to AH.5 Hence, cationic drugs may utilize some uptake transporters for their permeation across biological membranes.19, 20, 28 Presence of transporters like OCT in the conjunctival and corneal epithelial cells to reabsorb various endogenous amines in the tear fluid has been well recognized.6, 7, 8 Moreover, evidence from molecular biology studies from our laboratory and others showed the presence of various OCT in cornea.13, 27, 30 OCTN1 and OCTN2 were suggested to be present at the apical region of cornea.33 Drug absorption can be greatly enhanced by targeting endogenously expressed transport systems.34 Hence the objective of this study was to investigate the functional importance of OCT-mediated carrier system on in vivo corneal penetration using appropriate substrates and blockers as pharmacological tools. To achieve this, TEA20, 23, 35, 36, 37 and metformin23, 38, 39, 40, 41, 42 were used as OCT substrates. Quinidine23, 36, 43, 44, 45 and atropine9, 23 were used as OCT blockers. The substrates and blockers were quantified in AH using LC-MS/MS and their representative peaks were shown (Figure 3, Supplementary Data). For the first time in this study we showed the functional importance of OCT in the cornea for transport of topically applied xenobiotics using an in vivo rabbit model.
The factors contributing to precorneal disappearance of topically administered drugs include drainage through the lacrimal fluid pathway, absorption through the conjunctival pathway, corneal absorption, and non-specific absorption in other precorneal tissues. Although the functional role of OCT is known to be active from apical to basolateral in the conjunctiva,20 the role of OCT in the cornea is not clear as of now. Some transport process does exist in the corneal epithelia and is known to reabsorb the cationic endogenous amines from the tear. A study conducted in our laboratory to evaluate the effect of OCT blockade on the tear kinetics of OCT substrates administered topically concluded that OCT are involved functionally in precorneal disposition of topically applied drugs.27, 30 The precorneal disposition route of xenobiotics and corneal uptake of endogenous amines probably utilize OCT to transport from tear to AH across the cornea. A similar observation has been found using timolol coadministration with either pilocarpine or epinephrine and showed significant reduction in ocular absorption of timolol possibly due to its alteration during precorneal clearance. But the possible role of OCT in cornea was not known at that time.18
Hence, investigating the transcorneal penetration of topically applied OCT substrates can reveal the role of OCT in the cornea. To understand the role of OCT in corneal penetration of its topically applied substrates, AH of the rabbits was sampled by paracentesis at various time intervals for the quantification of both substrate as well as their topically applied blockers. To reduce the volume-induced tear drainage, the applied volume was kept at a minimum of 20 μl having either the substrate or the blocker. The dose was fixed based on the IC50 value of TEA, metformin, atropine, and quinidine for OCT interaction from the literature.23
To determine the blocker pre-treatment time, Tmax of the blockers quinidine and atropine reaching AH after single topical administration was studied individually in controlled conditions. Therefore, the blocker pre-treatment was chosen at 30 min before the administration of substrates to study the modulation of transcorneal penetration of substrates. Topically applied TEA showed a maximum aqueous concentration at 30 min after its instillation. In this experiment, quinidine (blocker) pre-treatment significantly reduced the concentration of TEA at 30 min to the extent of 5.2-fold. When atropine was used as the blocker, the concentration of TEA was reduced to an extent of 10.4-fold in the AH (Table 1).
Similar to TEA, metformin has also shown a maximum aqueous concentration (Cmax) at a Tmax of 30 min. Blocker pre-treatment with quinidine and atropine showed 5.4- and 4-fold reduction in the aqueous concentration of metformin at the 30-min time point. The difference in the inhibitory effects of quinidine and atropine toward the uptake of TEA and metformin may be due to the difference in the contribution of individual OCT to the uptake of TEA and metformin. In the presence of TEA or metformin, blocker concentrations were generally found to be reduced as compared with their control. From the above observation it was understood that topical blocker pre-treatment significantly affected the uptake of OCT substrates from the precorneal area to the AH. Therefore, it was evident that OCT are present in the cornea from apical to basolateral, and may have a functional role in the uptake of their substrates across the cornea when applied topically as it has been reported in the conjunctiva.20
At equimolar concentration, TEA showed 7 times higher aqueous Cmax compared to metformin in the control experiments. Quinidine and atropine both showed almost similar percentage of aqueous penetration in the control studies at 30 min post instillation. This shows that both of them were unlikely to have any difference in the available OCT isoform susceptibility in cornea. Further studies are needed to clarify the substrate specificity to the various isoforms of OCT. OCT may be having a vital role in the uptake of topically applied drugs having OCT susceptibility. When administering OCT substrates/blockers topically, both may be competing for OCT for their uptake across the cornea, thereby decreasing the corneal penetration. The altered levels of OCT blockers in the presence of OCT substrates as compared with their control levels further support the above finding (Figures 1 and 2, and Supplementary Data). The observation from the present study would be having therapeutic relevance when two OCT substrates/blockers are administered concomitantly in ocular topical drug therapy. This interaction might render low aqueous bioavailability of topically applied drugs, especially when alkaloids like atropine and their derivatives are extensively used along with other cationic drugs like anti-infectives, anti-glaucoma, anti-inflammatory, and anti-histaminics as ocular medication.
To conclude, OCT in the cornea may be functionally active from tear fluid to AH. OCT can have a potential pharmacokinetic role in modulating the ocular bioavailability of their substrates administered topically, which are used as ocular therapeutics.
Acknowledgments
We thank CSIR for providing SRF to Dr J Nirmal and the All India Institute of Medical Sciences for providing the intramural grant (F.6-1/2010-Acad) for this research work.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
Presented at annual meeting of American Association of Pharmaceutical Scientists in 2010.
Supplementary Material
References
- Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57 (14:2010–2032. doi: 10.1016/j.addr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Dey S, Gunda S, Mitra AK. Pharmacokinetics of erythromycin in rabbit corneas after single-dose infusion: role of P-glycoprotein as a barrier to in vivo ocular drug absorption. J Pharmacol Exp Ther. 2004;311 (1:246–255. doi: 10.1124/jpet.104.069583. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Yamamura K, Mukai T, Nishida K, Nakamura J, Nakashima M, et al. Enhancement of ocular drug penetration. Crit Rev Ther Drug Carrier Syst. 1999;16 (1:85–146. [PubMed] [Google Scholar]
- Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 2006;58 (11:1136–1163. doi: 10.1016/j.addr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58 (11:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Van Haeringen NJ. Clinical biochemistry of tears. Surv Ophthalmol. 1981;26 (2:84–96. doi: 10.1016/0039-6257(81)90145-4. [DOI] [PubMed] [Google Scholar]
- Martin XD, Brennan MC. Dopamine and its metabolites in human tears. Eur J Ophthalmol. 1993;3 (2:83–88. doi: 10.1177/112067219300300206. [DOI] [PubMed] [Google Scholar]
- Martin XD, Brennan MC. Serotonin in human tears. Eur J Ophthalmol. 1994;4 (3:159–165. doi: 10.1177/112067219400400305. [DOI] [PubMed] [Google Scholar]
- Muller J, Lips KS, Metzner L, Neubert RH, Koepsell H, Brandsch M. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT) Biochem Pharmacol. 2005;70 (12:1851–1860. doi: 10.1016/j.bcp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Foote EFaH CE. Effects of probenecid and cimetidine on renaldisposition of ofl oxacin in rats. Antimicrob Agents Chemother. 1998;42:456–458. doi: 10.1128/aac.42.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka K, Kawazu K, Nishida K, Nakamura J, Nakashima M, Nakamura T, et al. Transport of timolol and tilisolol in rabbit corneal epithelium. Biol Pharm Bull. 2006;29 (10:2143–2147. doi: 10.1248/bpb.29.2143. [DOI] [PubMed] [Google Scholar]
- Mulgaonkar A, Venitz J, Grundemann D, Sweet DH. Human organic cation transporters 1 (SLC22A1), 2 (SLC22A2), and 3 (SLC22A3) as disposition pathways for fluoroquinolone antimicrobials. Antimicrob Agents Chemother. 2013;57:2705–2711. doi: 10.1128/AAC.02289-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Xiang CD, Gale D, Carreiro S, Wu EY, Zhang EY. Drug transporter and cytochrome P450 mRNA expression in human ocular barriers: implications for ocular drug disposition. Drug Metab Dispos. 2008;36 (7:1300–1307. doi: 10.1124/dmd.108.021121. [DOI] [PubMed] [Google Scholar]
- Lee VH. Membrane transporters. Eur J Pharm Sci. 2000;11 (Suppl 2:S41–S50. doi: 10.1016/s0928-0987(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Sorkhabi R, Alipanahi R, Eftakhari-Milani A, Ghojazadeh L. The influence of topical diclofenac sodium on the ocular hypotensive effect of latanoprost in glaucoma patients. J Glaucoma. 2011;20 (4:240–243. doi: 10.1097/IJG.0b013e3181e07933. [DOI] [PubMed] [Google Scholar]
- Sponsel WE, Paris G, Trigo Y, Pena M, Weber A, Sanford K, et al. Latanoprost and brimonidine: therapeutic and physiologic assessment before and after oral nonsteroidal anti-inflammatory therapy. Am J Ophthalmol. 2002;133 (1:11–18. doi: 10.1016/s0002-9394(01)01286-7. [DOI] [PubMed] [Google Scholar]
- Jarvinen K, Urtti A. Cardiac effects of different eyedrop preparations of timolol in rabbits. Curr Eye Res. 1992;11 (5:469–473. doi: 10.3109/02713689209001801. [DOI] [PubMed] [Google Scholar]
- Lee VH, Luo AM, Li SY, Podder SK, Chang JS, Ohdo S, et al. Pharmacokinetic basis for nonadditivity of intraocular pressure lowering in timolol combinations. Invest Ophthalmol Vis Sci. 1991;32 (11:2948–2957. [PubMed] [Google Scholar]
- Xiang CD, Batugo M, Gale DC, Zhang T, Ye J, Li C, et al. Characterization of human corneal epithelial cell model as a surrogate for corneal permeability assessment: metabolism and transport. Drug Metab Dispos. 2009;37 (5:992–998. doi: 10.1124/dmd.108.026286. [DOI] [PubMed] [Google Scholar]
- Ueda H, Horibe Y, Kim KJ, Lee VH. Functional characterization of organic cation drug transport in the pigmented rabbit conjunctiva. Invest Ophthalmol Vis Sci. 2000;41 (3:870–876. [PubMed] [Google Scholar]
- Nirmal J, Velpandian T, Singh SB, Biswas NR, Azad R, Thavaraj V, et al. Evaluation of the functional importance of organic cation transporters on the ocular disposition of its intravitreally injected substrate in rabbits. Curr Eye Res. 2012;37 (12:1127–1135. doi: 10.3109/02713683.2012.715715. [DOI] [PubMed] [Google Scholar]
- Masago M, Takaai M, Sakata J, Horie A, Ito T, Ishida K, et al. Membrane transport mechanisms of quinidine and procainamide in renal LLC-PK1 and intestinal LS180 cells. Biol Pharma Bull. 2010;33 (8:1407–1412. doi: 10.1248/bpb.33.1407. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24 (7:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- Horie A, Sakata J, Nishimura M, Ishida K, Taguchi M, Hashimoto Y. Mechanisms for membrane transport of metformin in human intestinal epithelial Caco-2 cells. Biopharm Drug Dispos. 2011;32 (5:253–260. doi: 10.1002/bdd.755. [DOI] [PubMed] [Google Scholar]
- Nirmal J, Velpandian T, Singh SB, Biswas NR, Thavaraj V, Azad R, et al. Development and validation of a highly sensitive LC-MS/MS method for organic cation transporter (OCT) substrate tetraethylammonium (TEA) in rabbits. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879 (9-10:585–590. doi: 10.1016/j.jchromb.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Mistri HN, Jangid AG, Shrivastav PS. Liquid chromatography tandem mass spectrometry method for simultaneous determination of antidiabetic drugs metformin and glyburide in human plasma. J Pharm Biomed Anal. 2007;45 (1:97–106. doi: 10.1016/j.jpba.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Velpandian T, Nirmal J, Sirohiwal A, Singh SB, Vasantha T, Azad RV.Evaluation of the Modulation of Organic Cation Transporter (OCT) in the Tear Disposition of its Substrates in Rabbits Assoc Res Vis Ophthalmol USA20125333/A5358
- Nirmal J, Velpandian T, Singh SB, Ranjan Biswas N, Azad R, Thavaraj V, et al. Evaluation of the functional importance of organic cation transporters on the ocular disposition of its intravitreally injected substrate in rabbits. Curr Eye Res. 2012;37:1127–1135. doi: 10.3109/02713683.2012.715715. [DOI] [PubMed] [Google Scholar]
- Liu X, Van Natta K, Yeo H, Vilenski O, Weller PE, Worboys PD, et al. Unbound drug concentration in brain homogenate and cerebral spinal fluid at steady state as a surrogate for unbound concentration in brain interstitial fluid. Drug Metab Dispos. 2009;37 (4:787–793. doi: 10.1124/dmd.108.024125. [DOI] [PubMed] [Google Scholar]
- Nirmal J, Velpandian T, Biswas NR, Azad RV, Vasantha T, Bhatnagar A, Ghose S.Evaluation of the relevance of OCT blockade on the transcorneal kinetics of topically applied substrates using rabbits FIP 2010 World Congress in Association with AAPS, New OrleansUSA2010. SA8208/T3427.
- Chen H, Chen Y, Du P, Han F, Wang H, Zhang H. Sensitive and specific liquid chromatographic-tandem mass spectrometric assay for atropine and its eleven metabolites in rat urine. J Pharm Biomed Anal. 2006;40 (1:142–150. doi: 10.1016/j.jpba.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Katragadda S, Talluri RS, Mitra AK. Simultaneous modulation of transport and metabolism of acyclovir prodrugs across rabbit cornea: An approach involving enzyme inhibitors. Int J Pharm. 2006;320 (1-2:104–113. doi: 10.1016/j.ijpharm.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Garrett Q, Xu S, Simmons PA, Vehige J, Flanagan JL, Willcox MD. Expression and localization of carnitine/organic cation transporter OCTN1 and OCTN2 in ocular epithelium. Invest Ophthalmol Vis Sci. 2008;49 (11:4844–4849. doi: 10.1167/iovs.07-1528. [DOI] [PubMed] [Google Scholar]
- Steffansen B, Nielsen CU, Brodin B, Eriksson AH, Andersen R, Frokjaer S. Intestinal solute carriers: an overview of trends and strategies for improving oral drug absorption. Eur J Pharm Sci. 2004;21 (1:3–16. doi: 10.1016/j.ejps.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nakanishi T, Kobayashi D, China K, Kosugi Y, Nezu J, et al. Involvement of OCTN1 (SLC22A4) in pH-dependent transport of organic cations. Mol Pharm. 2004;1 (1:57–66. doi: 10.1021/mp0340082. [DOI] [PubMed] [Google Scholar]
- Zhang N, Kannan R, Okamoto CT, Ryan SJ, Lee VH, Hinton DR. Characterization of brimonidine transport in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2006;47 (1:287–294. doi: 10.1167/iovs.05-0189. [DOI] [PubMed] [Google Scholar]
- Franke RM, Kosloske AM, Lancaster CS, Filipski KK, Hu C, Zolk O, et al. Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in urinary N-acetyl-beta-D-glucosaminidase. Clin Cancer Res. 2010;16 (16:4198–4206. doi: 10.1158/1078-0432.CCR-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser MJ, Gray AT, Giacomini KM. Kinetic and selectivity differences between rodent, rabbit, and human organic cation transporters (OCT1) J Pharmacol Exp Ther. 2000;292 (3:1146–1152. [PubMed] [Google Scholar]
- Kimura N, Masuda S, Tanihara Y, Ueo H, Okuda M, Katsura T, et al. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet. 2005;20 (5:379–386. doi: 10.2133/dmpk.20.379. [DOI] [PubMed] [Google Scholar]
- Choi MK, Song IS. Organic cation transporters and their pharmacokinetic and pharmacodynamic consequences. Drug Metab Pharmacokinet. 2008;23 (4:243–253. doi: 10.2133/dmpk.23.243. [DOI] [PubMed] [Google Scholar]
- Takane H, Shikata E, Otsubo K, Higuchi S, Ieiri I. Polymorphism in human organic cation transporters and metformin action. Pharmacogenomics. 2008;9 (4:415–422. doi: 10.2217/14622416.9.4.415. [DOI] [PubMed] [Google Scholar]
- Chen L, Pawlikowski B, Schlessinger A, More SS, Stryke D, Johns SJ, et al. Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenet Genomics. 2010;20 (11:687–699. doi: 10.1097/FPC.0b013e32833fe789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi H, Tamai I, Nezu J, Sakamoto K, Oku A, Shimane M, et al. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J Pharmacol Exp Ther. 1999;289 (2:768–773. [PubMed] [Google Scholar]
- Kwok B, Yamauchi A, Rajesan R, Chan L, Dhillon U, Gao W, et al. Carnitine/xenobiotics transporters in the human mammary gland epithelia, MCF12A. Am J Physiol Regul Integr Comp Physiol. 2006;290 (3:R793–R802. doi: 10.1152/ajpregu.00087.2005. [DOI] [PubMed] [Google Scholar]
- Umehara KI, Iwatsubo T, Noguchi K, Usui T, Kamimura H. Effect of cationic drugs on the transporting activity of human and rat OCT/Oct 1-3 in vitro and implications for drug-drug interactions. Xenobiotica. 2008;38 (9:1203–1218. doi: 10.1080/00498250802334409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.