Abstract
We examined the metabolic characteristics that attend the development of type 2 diabetes (T2DM) in 441 impaired glucose tolerance (IGT) subjects who participated in the ACT NOW Study and had complete end-of-study metabolic measurements. Subjects were randomized to receive pioglitazone (PGZ; 45 mg/day) or placebo and were observed for a median of 2.4 years. Indices of insulin sensitivity (Matsuda index [MI]), insulin secretion (IS)/insulin resistance (IR; ΔI0–120/ΔG0–120, ΔIS rate [ISR]0–120/ΔG0–120), and β-cell function (ΔI/ΔG × MI and ΔISR/ΔG × MI) were calculated from plasma glucose, insulin, and C-peptide concentrations during oral glucose tolerance tests at baseline and study end. Diabetes developed in 45 placebo-treated vs. 15 PGZ-treated subjects (odds ratio [OR] 0.28 [95% CI 0.15–0.49]; P < 0.0001); 48% of PGZ-treated subjects reverted to normal glucose tolerance (NGT) versus 28% of placebo-treated subjects (P < 0.005). Higher final glucose tolerance status (NGT > IGT > T2DM) was associated with improvements in insulin sensitivity (OR 0.61 [95% CI 0.54–0.80]), IS (OR 0.61 [95% CI 0.50–0.75]), and β-cell function (ln IS/IR index and ln ISR/IR index) (OR 0.26 [95% CI 0.19–0.37]; all P < 0.0001). Of the factors measured, improved β-cell function was most closely associated with final glucose tolerance status.
The prevalence of type 2 diabetes mellitus (T2DM) has risen to epidemic proportions in the United States and worldwide (1), and is being driven by the epidemic of obesity (2). In high-risk individuals, it is reasonable to consider interventions that reduce the incidence of T2DM. Impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) are “high-risk” states with annual diabetes conversion rates ranging from 3 to 11% per year (3). Individuals with IGT have moderate-to-severe insulin resistance (IR) in muscle and impaired second-phase insulin secretion (IS), while those with IFG are characterized by hepatic IR and impaired first-phase IS with intact second-phase IS and normal/near-normal muscle insulin sensitivity (4–7). IGT conversion to T2DM is associated with a further and progressive decline in β-cell function with little worsening of IR, which is near maximally established in IGT (4,5,8–10). Treatment with thiazolidinediones improves IS (11) and IR (12–15), ameliorates lipotoxicity (12,14), and redistributes fat from muscle/liver/β-cells to subcutaneous fat depots (12,16). Therefore, they represent a logical choice for the treatment of IGT and IFG.
In the ACT NOW Study (17,18), over a 2.4-year period, the annual conversion rate of IGT to T2DM was 7.6% in placebo-treated vs. 2.1% in pioglitazone (PGZ)-treated subjects (hazard ratio 0.28, P < 0.0001) (18). PGZ is a potent insulin-sensitizing agent in muscle, liver, and adipocytes (reviewed in 12–14); augments IS; and preserves β-cell function (11,19). These effects are mediated, in part, via peroxisome proliferator–activated receptor-γ (PPAR-γ) receptor (13,20) and via reversal of lipotoxicity (12,14) and changes in adipocytokines (20). PGZ reduces plasma free fatty acid (FFA) levels, mobilizes fat out of muscle (21) and liver (22), and redistributes fat from visceral to subcutaneous depots (12,16).
In the current study, we examined which physiologic/metabolic/anthropometric changes (end-of-study versus baseline) in the ACT NOW Study (18) were associated with IGT progression to diabetes and reversion to normal glucose tolerance (NGT) in PGZ- and placebo-treated subjects.
RESEARCH DESIGN AND METHODS
Subjects.
A total of 602 high-risk IGT subjects comprised the study population (17,18). Demographic, anthropometric, and metabolic characteristics at baseline were similar in PGZ and placebo groups (Supplementary Table 1) and have been published (18). Here, we report on 441 subjects who completed the study (Supplementary Table 2). Subjects lost to follow-up or who dropped out and did not undergo end-of-study oral glucose tolerance tests (OGTTs) were not included. During screening, 120 subjects had undergone OGTTs but were not included in the ACT NOW Study because OGTT results were normal. These NGT subjects are included as the “control” group.
Study design.
Descriptions of the study design (17) and results have been published (18). Eight centers participated in the study, which was approved by the institutional review board for each site. A total of 602 IGT subjects were randomized to receive PGZ or placebo and were observed for a median time of 2.4 years.
At baseline, subjects underwent 2-h 75-g OGTTs with plasma glucose (PG), insulin, C-peptide, and FFA concentrations measured at −30, −15, 0, and every 15 min in Central Laboratory (Texas Diabetes Institute) (17,18). Additional baseline assessments included the following: medical history, physical examination, body mass index (BMI), waist circumference, HbA1c level, lipid profile, screening blood tests, urinalysis, and electrocardiogram. Body fat was measured by dual-energy X-ray absorptiometry (model 4500; Hologic, Bedford, MA).
Participants were randomized to receive PGZ, 30 mg, or placebo. After 1 month, the PGZ dose was increased to 45 mg. Participants returned at 2, 4, 6, 8, 10, and 12 months during year 1, and every 3 months thereafter. Subjects were observed until they reached the primary end point of diabetes, dropped out, were lost to follow-up, or reached study end (2 years after the last subject was enrolled). The fasting PG (FPG) level was determined on each visit. HbA1c was measured every 6 months. An OGTT was performed annually. Baseline measurements were repeated at study end or the time of conversion to diabetes.
IGT conversion to diabetes.
The primary outcome was the development of diabetes (FPG ≥126 mg/dL or 2-h glucose ≥ 200 mg/dL). Diagnosis was confirmed by OGTT (2-h glucose ≥200 mg/dL or FPG ≥126 mg/dL).
Data analysis.
The Matsuda index (MI) of insulin sensitivity was calculated from PG and insulin levels obtained during the OGTT (23). The incremental areas under the curves (AUCs) of PG, insulin, C-peptide, and FFA during OGTTs were determined using the trapezoidal rule. The IS rate (ISR) was calculated from deconvolution of plasma C-peptide using standard C-peptide clearances (24). β-Cell function was calculated as the IS/IR (disposition) index (ΔI0–120/ΔG0–120 × MI; ΔISR0–120/ΔG0–120 × MI)(4–7). Adipocyte IR index was calculated as fasting plasma insulin × fasting FFA concentration (25).
Statistical analyses addressed the following question: what metabolic/physiologic/anthropometric changes were associated with protection from diabetes in IGT subjects treated with PGZ. Intention-to-treat analyses were conducted using all follow-up data. Continuous variables were compared between treatment groups or subgroups by t tests if normally distributed. Otherwise, nonparametric tests were used. Baseline insulin concentration, insulin AUC, MI of insulin sensitivity, adipose tissue IR index, and IS/IR index were ln transformed before comparisons. Changes from baseline to follow-up were compared between groups by t test when variables were normally distributed and by Wilcoxon test for variables non-normally distributed. Categorical variables were compared by Fisher exact test. Odds ratios (ORs) were estimated using logistic regression analyses adjusted for age, sex, and center.
Multivariate logistic regression analysis was performed in the entire cohort to examine factors associated with end-of-study glucose tolerance status (NGT/IGT vs. T2DM). We evaluated the separate impact of changes in plasma insulin response (ΔI0–120/G0–120), MI, ln IS/IR, ln ISR/IR, and all other metabolic/anthropometric data after accounting for age, sex, clinical center, BMI, and treatment used as independent variables for the calculation of OR.
Statistical significance was accepted for two-sided α < 0.05. Data are presented as the mean ± SE or median. Statistical analysis was performed using Statview and JMP from SAS.
RESULTS
Study cohort.
The study population (n = 441; 57% female) had a mean age of 53.2 ± 0.6 years and a mean BMI of 33.9 ± 0.3 kg/m2. Three hundred and four subjects had IGT/IFG, and 137 subjects had isolated IGT. Baseline HbA1c, FPG, and 2-h PG levels were 5.53 ± 0.03% (37 ± 0.08 mmol/mol), 105 ± 0.5 mg/dL, and 169 ± 1 mg/dL, respectively. There were no significant differences in any clinical, anthropometric, or laboratory parameters between the placebo and PGZ groups (Supplementary Table 2). The 441 subjects who underwent OGTTs at baseline and at study end/time of conversion to diabetes had (P > 0.40) clinical, anthropometric, and laboratory values similar to those of the entire cohort (Supplementary Table 1).
Follow-up results.
Diabetes developed in 45 of 228 individuals (19.7%) in the placebo group and 15 of 213 individuals (7.0%) in the PGZ group during a median follow-up period of 2.4 years. The annual diabetes incidence rates, adjusting for age, sex, and center and calculated using person-years, were 8.2 and 3.1%, respectively (P < 0.001). These conversion rates for the 441 subjects in the current analysis were similar to those for the entire cohort of 602 subjects.
Values at baseline and time of diabetes development or study end are shown in Supplementary Table 3.
Effect of PGZ on HbA1c, PG, insulin, lipids, and BMI.
HbA1c levels differed between groups (P < 0.003) throughout the study, increasing by 0.28% (1.9 mmol/mol) in the placebo group and 0.07% (0.5 mmol/mol) in the PGZ group (P < 0.0001). At study end, decrements in FPG (−10.7 ± 0.9 vs. −4.0 ± 0.09 mg/dL) and 2-h PG (−28.7 ± 2.6 vs. −5.9 ± 2.6 mg/dL) levels were greater in the PGZ group versus placebo group (P < 0.0001). Fasting plasma insulin decreased by −3.3 ± 0.6 μU/mL in PGZ (P < 0.0001 vs. baseline) and did not change in placebo (Δ = 0.5 ± 0.5) (P < 0.0001, PGZ vs. placebo). Fasting plasma FFA levels did not change in the PGZ group and increased in the placebo group (P = 0.007). The adipocyte IR index decreased in the PGZ group (Δ = −1.8 ± 0.4 mmol/L × mU/L, P < 0.0001 vs. baseline) and increased in the placebo group (Δ = 0.7 ± 0.3, P = 0.03 vs. baseline, and P < 0.0001 vs. PGZ).
BMI increased in the placebo group (0.5 ±0.2 kg/m2, P < 0.006) and PGZ group (1.6±0.2 kg/m2, P < 0.0001), but the increment was greater with PGZ treatment (P < 0.0001). Compared with the placebo group, HDL cholesterol levels increased more (7.2±0.8 vs. 4.3 ± 0.7 mmol/L, P < 0.007), whereas plasma triglyceride levels decreased more (−14.1 ± 3.9 vs. −1.7 ± 4.1 mmol/L, P < 0.01) in the PGZ group.
Effect of PGZ on insulin sensitivity, IS, and β-cell function.
The MI of insulin sensitivity increased by 92% in the PGZ group (3.9 ± 0.2 to 7.5 ± 0.3, P < 0.0001), but only by 17% in the placebo group (4.0 ± 0.2 to 4.7 ± 0.3, P = 0.002) (P < 0.0001, PGZ vs. placebo). The fasting ISR (from plasma C-peptide deconvolution) increased in the placebo group (420 ± 14 to 558 ± 21 pmol/min, P < 0.0001 vs. baseline) and did not change in the PGZ group (411 ± 14 to 446 ± 19) (P < 0.0001, PGZ vs. placebo). The total insulin secreted during the OGTT (0–120 min) increased in both groups (PGZ, 165 ± 3 to 189 ± 5 nmol; placebo, 167 ± 3 to 202 ± 6) (P < 0.0001 vs. baseline in both groups; P = NS, PGZ vs. placebo). The IS/IR (disposition) index (ΔI0–120 /ΔG0–120 × MI) increased by 65% with PGZ treatment (3.2 ± 0.1 to 5.3 ± 0.3, P < 0.0001) and did not change significantly with placebo treatment (3.2 ± 0.1 to 3.7 ± 0.2, P = 0.13) (P < 0.0001, PGZ vs. placebo). When the IS/IR index was calculated as ΔISR0–120/ΔG0–120 × MI, the increase in β-cell function with PGZ (64 ± 3 to 198 ± 17, P < 0.0001) also was significantly greater than with placebo (66 ± 4 to 107 ± 8, P < 0.0001) (P < 0.0001, PGZ vs. placebo).
Relationship between final glucose tolerance status and measures of insulin sensitivity and IS.
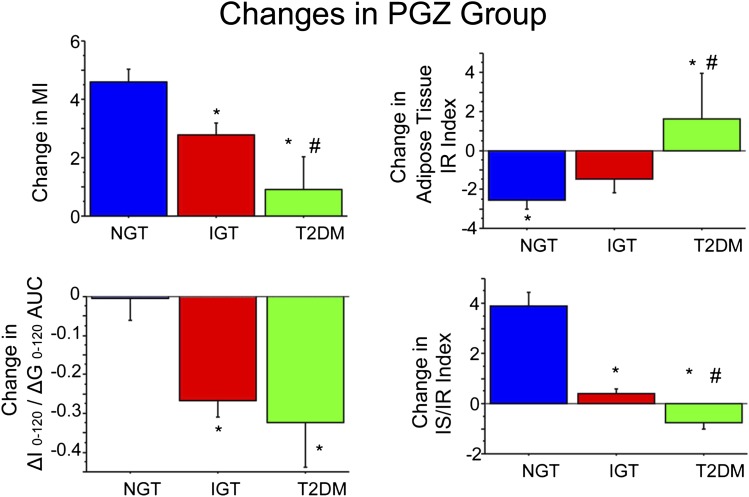
In PGZ-treated IGT subjects, the change in MI of insulin sensitivity was strongly related to final glucose tolerance status (Fig. 1). Subjects who reverted to NGT had the largest improvement in MI (4.6 ± 0.4, P < 0.0001), whereas subjects who converted to T2DM had no significant improvement in MI. The adipocyte IR index improved in IGT subjects who reverted to NGT and deteriorated in IGT subjects who progressed to T2DM (Fig. 1). Plasma insulin response to hyperglycemia (ΔI0–120/ΔG0–120) remained constant in subjects who reverted to NGT; consequently, the IS/IR index increased markedly (Fig. 1). In contrast, the plasma insulin response to hyperglycemia (ΔI0–120/ΔG0–120) declined significantly in IGT subjects who converted to T2DM, resulting in a modest but significant decrease in IS/IR index.
FIG. 1.
Change from baseline to study end in MI (top left), IS (ΔI0–120/ΔG0–120 AUC) (bottom left), IS/IR index (bottom right), and adipose tissue IR index (top right) in PGZ-treated subjects. Data are given as means ± SE. Change represents the difference between the absolute value at the study end minus baseline value. *P < 0.05 for NGT vs. IGT or T2DM; #P < 0.05 for IGT vs. T2DM using nonparametric tests.
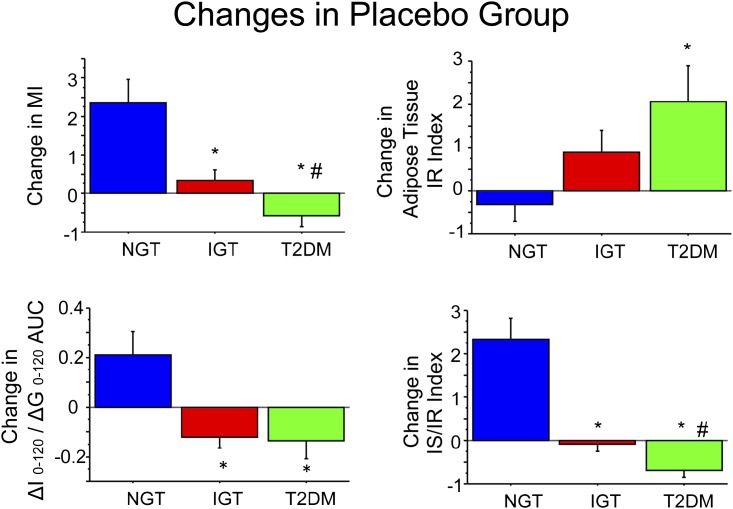
In placebo-treated IGT subjects, relationships between the change in MI of insulin sensitivity and the change in IS/IR index versus final glucose tolerance status were analogous to PGZ-treated subjects, although with different distributions of subjects in each final glucose tolerance category (Fig. 2). In IGT subjects who reverted to NGT and had received placebo, insulin sensitivity (MI) and IS/IR index improved, while the opposite was observed in subjects who progressed to T2DM.
FIG. 2.
Change in MI (top left), IS (ΔI0–120/ΔG0–120) (bottom left), IS/IR index (bottom right), and adipose tissue IR index (top right) in placebo-treated subjects. Data are given as means ± SE. Change represents the difference between the absolute value at the study end minus baseline value. *P < 0.05 for NGT vs. IGT or T2DM; #P < 0.05 for IGT vs. T2DM using nonparametric tests.
Relationship between IS/IR index and diabetes risk.
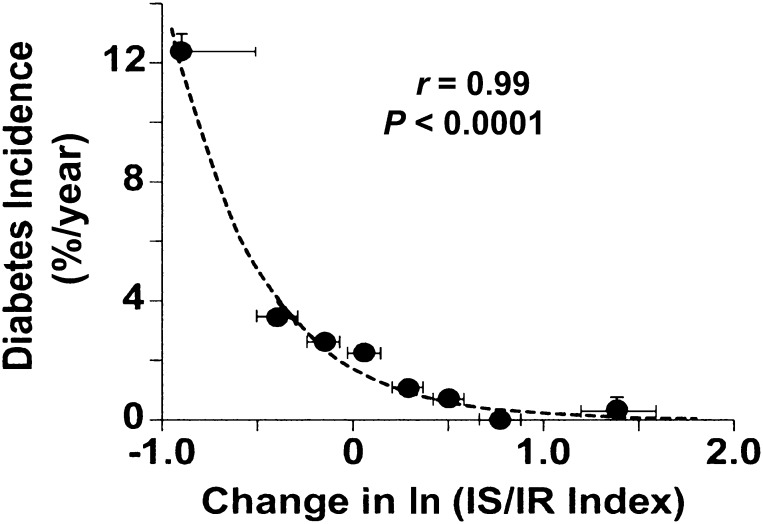
The risk of developing diabetes was strongly related to the ln of the IS/IR index. When all subjects (PGZ and placebo) were divided into eight equal groups (octiles), a strong curvilinear relationship between ln change in IS/IR index and diabetes incidence (r = 0.990, P < 0.0001) was observed (Fig. 3). In the placebo group, the relationship was shifted to the right (data not shown). IGT subjects with ≥80% improvement in IS/IR index had an ∼2% incidence of diabetes compared with a 14% incidence in subjects in whom the IS/IR index declined by 60–80%.
FIG. 3.
Relationship between the annual diabetes incidence rate and change in the IS/IR index in the combined PGZ-treated and placebo-treated groups. Cohort members were ordered from smallest to largest change and then divided into octiles. Means ± SEM of each octile are represented by solid circles. SEM values for diabetes incidence are not shown if they fall within the height of the solid circle.
When IGT subjects treated with PGZ (r = 0.988, P < 0.0001) or placebo (r = 0.987, P < 0.001) were analyzed separately, the change in ln IS/IR index also was strongly related to the diabetes incidence.
Relationship between plasma insulin response to hyperglycemia and insulin sensitivity.
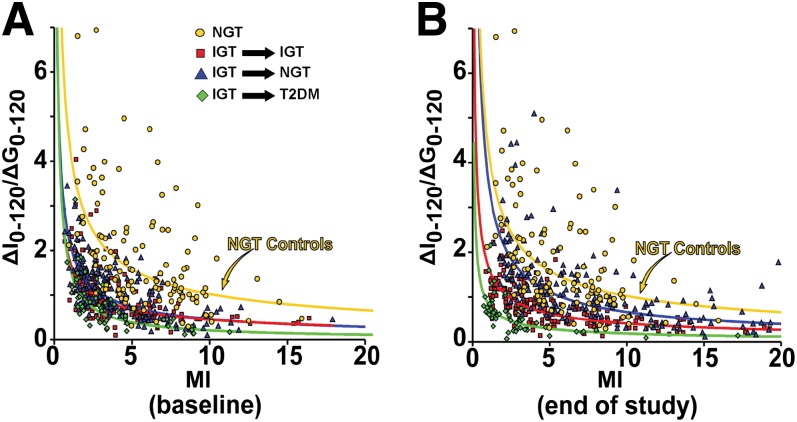
At baseline, the plot of insulin sensitivity (MI) versus plasma insulin response to hyperglycemia (ΔI0–120/ΔG0–120) during OGTT was curvilinear in all subjects, with little separation between the PGZ and placebo groups (data for separate groups not shown) (Fig. 4A). At study end or the time of diabetes diagnosis, the curve was shifted leftward in IGT subjects who converted to diabetes, was unchanged in subjects who remained with IGT, and was shifted rightward in subjects who reverted to NGT (Fig. 4B). For any level of IR, the plasma insulin response in T2DM subjects was less than that in subjects who remained with IGT, which, in turn, was less than in subjects who reverted to NGT. However, PGZ-treated IGT subjects who reverted to NGT still fell below the “control NGT” group, i.e., their plasma insulin response was not completely normalized (Fig. 4B).
FIG. 4.
Relationship between IS (ΔI0–120/ΔG0–120) and MI at baseline (A) and at study end (B). NGT subjects, yellow circles; IGT subjects who converted to NGT, blue triangles; IGT subjects who remained with IGT, red squares; IGT subjects who converted to T2DM, green diamonds.
When the ln of ΔI0–120/ΔG0–120 was plotted against the ln of the MI in subjects who reverted to NGT, a strong linear relationship with a similar slope was observed in control NGT subjects, placebo-treated IGT subjects, and PGZ-treated IGT subjects at study end (Supplementary Fig. 1).
Relationship between glucose tolerance status versus plasma insulin response and insulin secretory response in PGZ-treated subjects.
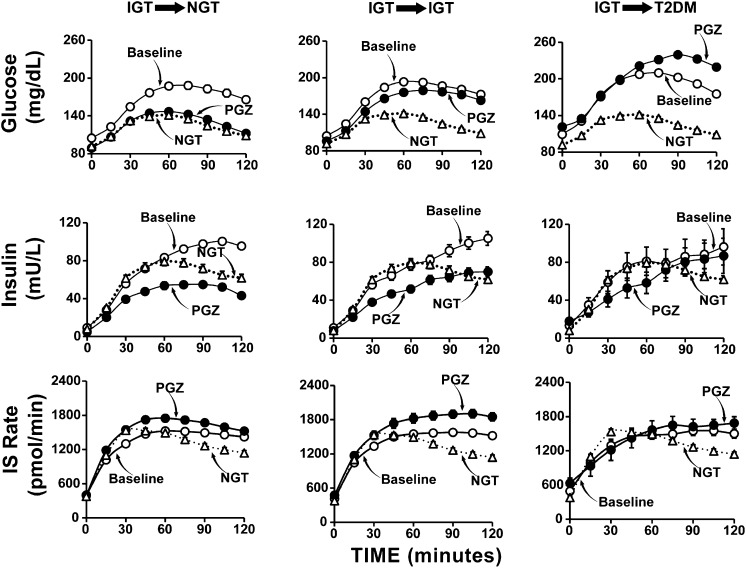
In PGZ-treated subjects who reverted to NGT, there was significant increase (P < 0.0001) in ISR (plasma C-peptide deconvolution) (Fig. 5). Nonetheless, plasma insulin response decreased markedly. Although not directly measured, this novel finding most likely is explained by a pronounced increase in the metabolic clearance rate (MCR) of insulin in response to PGZ. The change in insulin MCR cannot be explained by the change in glucose tolerance status, because PGZ-treated subjects who remained with IGT also manifested a significant increase in ISR, while the plasma insulin response still declined. These results clearly demonstrate that PGZ exerts independent effects on IS and insulin MCR, and that the balance between these two effects determines plasma insulin response.
FIG. 5.
PG (top) and insulin (middle) concentrations and ISR (bottom) in PGZ-treated IGT subjects who reverted to NGT (left), remained with IGT (middle), or converted to T2DM (right). Data at baseline and at the end of PGZ treatment are shown. For comparison, data for control NGT individuals identified during the screening of participants for the ACT NOW Study are shown.
In subjects who converted to T2DM, PGZ had no effect on IS and the plasma insulin response to hyperglycemia did not change (declined slightly) significantly (Fig. 5).
Factors associated with improved β-cell function after PGZ treatment.
The IS/IR index (ΔISR0–120/ΔG0–120 × MI) is determined by the following: 1) plasma insulin response (ΔISR0–120), 2) increment in PG (ΔG0–120) (i.e., the stimulus for IS), and 3) IR (1/MI). In PGZ-treated subjects who reverted to NGT or remained with IGT, the ISR increased by ∼21% and the ISR related to increment in PG rose by 70%, whereas the MI of insulin sensitivity improved by 109% (IGT → NGT group) and 73% (IGT → IGT group), respectively. Thus, all three factors contributed to the improvement in β-cell function ([ΔISR0–120/ΔG0–120] × [MI]) (Fig. 5, bottom panels).
The plasma insulin concentration is the result of a balance between ISR and insulin MCR. In PGZ-treated subjects who reverted to NGT or remained with IGT, plasma insulin response declined (Fig. 5, middle panels), yet the ISR increased. This implies that the insulin MCR must have increased markedly. This is consistent with previous observations that IR is associated with a reduced insulin MCR (26), whereas improved insulin sensitivity is associated with an increased insulin MCR (27).
Chronic hyperglycemia, i.e., glucotoxicity, has been shown to impair β-cell function and cause IR in muscle and liver (8). Consistent with this, the reductions in FPG (r = −0.39, P < 0.0001; r = −0.45, P < 0.0001) and glucose AUC during OGTT (r = −0.63, P < 0.0001; r = −0.49, P < 0.0001) correlated with improvements in ΔI0–120/ΔG0–120 × MI and MI.
Metabolic and clinical parameters associated with final glucose tolerance status.
Using the entire cohort of 441 IGT subjects, we performed multivariate logistic regression analysis to examine which factors were associated with end-of-study glucose tolerance status (NGT/IGT vs. T2DM). In the first analysis (after accounting for age, sex, clinical center, and BMI), PGZ treatment was associated with an OR of 0.28 (95% CI 0.15–0.49, P < 0.0001) of developing T2DM. After accounting for the previous variables, including PGZ/placebo, we found that a reduction in plasma insulin response (ΔI0–120/G0–120) (OR 0.44 [95% CI 0.40–0.80]), improvement in the MI of insulin sensitivity (0.83 [0.75–0.93]), and increases in ln IS/IR (0.10 [0.05–0.20]) and ln ISR/IR (0.08 [0.03–0.19]) were protective against the development of T2DM (all P < 0.001).
DISCUSSION
Troglitazone (28,29) and rosiglitazone (30) have been shown to reduce IGT/IFG conversion to T2DM. However, troglitazone is no longer available, and the use of rosiglitazone has been greatly restricted because of cardiovascular safety concerns. In contrast, PGZ does not increase the number of cardiovascular events (31,32). In the ACT NOW Study (18), we reported that PGZ decreased IGT conversion to T2DM by 72%, and that 42% of PGZ-treated subjects reverted to NGT. Herein, we examine physiologic mechanisms associated with reduced IGT conversion to T2DM and reversion to NGT in ACT NOW Study participants after PGZ therapy. Our results demonstrate that an increase in ln IS/IR (disposition) index (OR 0.10, P < 0.0001) and ln ISR/IR index (OR 0.08, P < 0.0001) were strongly associated with protection against the development of diabetes. Improved MI of insulin sensitivity (OR 0.83, P < 0.001) also was associated with protection against diabetes. However, from the quantitative standpoint increased IS/IR and ISR/IR were most strongly related with protection against diabetes.
When all subjects were examined collectively at study end, changes from baseline in ISR (ΔISR0–120/ΔG0–120), plasma insulin response (ΔI0–120/ΔG0–120), whole-body insulin sensitivity, β-cell compensation for IR, and adipose tissue insulin sensitivity (Fig. 1) were individually associated with final glucose tolerance status. However, in a multivariate analysis, changes in ln IS/IR and ln ISR/IR were the only independent correlates of final glucose tolerance status. These results demonstrate that β-cell function is the primary determinant of the development of diabetes or regression to NGT, independent of treatment. The fact that β-cell compensation was higher in PGZ- versus placebo-treated patients points to the preservation or restoration of β-cell function as a fundamental mechanism for diabetes prevention with PGZ treatment, as previously reported for troglitazone (28). This is clearly evident if one plots the incidence of diabetes against the change in ln IS/IR index (Fig. 3).
The critical role of β-cell function in determining final glucose tolerance status is further substantiated by examining the relationship between IS (ΔI0–120/ΔG0–120) and insulin sensitivity (Fig. 4). People in whom diabetes developed failed to increase insulin levels in response to glucose despite large decreases in insulin sensitivity. In subjects who remained with IGT, a decline in insulin sensitivity was offset by an increase in IS compared with subjects who progressed to diabetes. Subjects who reverted to NGT manifested a similar improvement in insulin sensitivity, but mounted a twofold to fourfold greater increase in insulin response compared with subjects who progressed to T2DM. Of note, IGT subjects who reverted to NGT did not reach the level of β-cell compensation observed in the control NGT group (Fig. 4). Thus, although PGZ improved β-cell function in people who reverted from IGT to NGT, it did not return it completely to normal status.
In the placebo-treated group, more IGT subjects converted to T2DM and fewer reverted to NGT. However, like the PGZ group, reversion to NGT was associated with improved β-cell function (ΔI0–120/ΔG0–120 × MI) and enhanced insulin sensitivity, whereas progression to T2DM was associated with deterioration in both parameters (Supplementary Fig. 2).
Not all PGZ-treated subjects remained with IGT or reverted to NGT. To gain insight into responders versus nonresponders, we divided PGZ-treated subjects into the following three groups: those who converted to diabetes, those who remained at IGT, and those who reverted to NGT (Fig. 5). In IGT subjects who converted to diabetes, there was no change in MI (Fig. 1), no significant change in plasma insulin response to hyperglycemia (Fig. 5), and no improvement in β-cell function (Fig. 1). In contrast, subjects who reverted to NGT had improved insulin sensitivity (Fig. 1), a marked decline in plasma insulin response to hyperglycemia (Fig. 5), and an increase in IS/IR index (Fig. 1). These findings are consistent with prior observations in Hispanic women with gestational diabetes mellitus who were treated with troglitazone (9,28) and PGZ (19), and in multiethnic IGT subjects treated with troglitazone (33). They also are consistent with prospective (10) and longitudinal (2,3,10,34–37) studies demonstrating poor β-cell compensation for IR in IGT individuals who progress to diabetes.
A novel finding was dissociation between ISR and plasma insulin response in PGZ-treated subjects (Fig. 5). In IGT individuals who reverted to NGT or remained with IGT, ISR increased significantly yet plasma insulin response declined by 40–50%. In insulin-resistant states, i.e., T2DM and obesity, insulin MCR is reduced (26,27). Although not directly measured, it is most likely that the MCR of insulin increased markedly after PGZ treatment. Consistent with this, rosiglitazone also has been shown to increase the MCR of insulin (27). Elevated plasma FFA levels have been shown to reduce the MCR of insulin (38). However, we did not observe a decrease in the plasma FFA concentration in the current study in PGZ-treated subjects (Supplementary Table 3). This has important implications for interpreting the exponential relationship between insulin sensitivity and plasma insulin response (i.e., IS/IR or disposition index), which becomes linear with ln transformation. The plasma insulin response should be equated not with IS (which goes in the opposite direction in PGZ-treated subjects) but, rather, with the sum of IS plus insulin clearance.
Lipotoxicity (12,14,39) has been implicated in β-cell failure and IR in T2DM. Thiazolidinediones mobilize fat out of muscle/liver and β-cells (12,14), leading to enhanced insulin sensitivity (13,16,21,22) and improved β-cell function (11,19,28). The improved insulin sensitivity and IS observed with thiazolidinedione treatment are correlated with weight gain (11,21,40). In PGZ-treated subjects in the current study, improved insulin sensitivity (r = 0.20, P < 0.005) was correlated with the increase in body weight. Increased insulin sensitivity and β-cell function were correlated with the declines in fasting plasma FFA levels (r = −0.135, P < 0.05; r = −0.27, P < 0.001, respectively) and plasma FFA AUC during OGTT (r = −0.08, P < 0.10; r = −0.19, P < 0.02). Such correlations do not prove causality but suggest that improved IR and β-cell function with PGZ may, in part, be related to the reversal of lipotoxicity. Alternatively, since PPAR-γ is present in muscle/liver and β-cells (10,13), simultaneous PPAR-γ activation in these tissues could explain the strong association between improved insulin sensitivity and enhanced β-cell function. According to this scenario, amelioration of IR would be not the primary driver of improvement in β-cell function but, rather, a beneficial effect that occurs in parallel with enhanced β-cell function. Last, enhanced insulin sensitivity after PGZ treatment could be the primary effect, leading to “unloading” of the β-cell and enhanced β-cell function (19).
Glucotoxicity has been implicated in progressive β-cell failure as individuals move from NGT to IGT to T2DM (8). The reduction in both FPG levels (r = −0.47, P < 0.0001) and the glucose AUC during OGTTs (r = −0.46, P < 0.0001) correlated with improved insulin sensitivity at study end. Reduced glucose AUC during OGTTs also correlated with improved β-cell function (r = −0.35, P < 0.0001). These correlations, although moderate, do not allow one to determine whether improved glycemic control preceded and accounted for the improvement in β-cell function or resulted from improved β-cell function.
There are several limitations regarding the present results. First, the NGT control group had some high-risk characteristics that led to screening. Thus, they may not be completely representative of NGT individuals. Second, insulin MCR was not measured directly, but was inferred from the marked difference in plasma insulin and plasma C-peptide responses. Third, correlations between fasting/post-OGTT PG and FFA concentrations versus insulin sensitivity and IS are consistent with lipotoxicity and glucotoxicity, but by no means establish causality. Fourth, only 441 of the original 602 IGT subjects were available for follow-up. However, the clinical/anthropometric/metabolic characteristics of the groups were very similar (Supplementary Tables 1 and 2). Fifth, the presence of IGT at baseline was established by a single OGTT, which has some inherent day-to-day variability. However, such variability would be expected, if anything, to diminish the likelihood of obtaining statistically significant results.
In conclusion, both improved insulin sensitivity and enhanced IS were strongly related to final glucose tolerance status in IGT subjects treated with PGZ or placebo. However, the strongest factor associated with final glucose tolerance status was improvement in IS/IR index. Not surprisingly, the addition of changes in insulin sensitivity and plasma insulin response to hyperglycemia—which are contained in the IS/IR measure of β-cell function—did not further increase the predictive value. Preservation/improvement in β-cell function is the main mechanism by which PGZ prevents diabetes and improves glucose tolerance.
ACKNOWLEDGMENTS
This study was supported in part by National Institutes of Health General Clinical Research Center grant MO1-RR00221 to the University of Tennessee Health Science Center and National Institutes of Health Clinical and Translational Science Award UL1TR000130 to the University of Southern California and the South Texas Veterans Health Care System—Audie Murphy Division.
This study was also supported by an investigator-initiated and unrestricted research grant from Takeda. R.A.D. has received grants from Amylin and Takeda. D.C.S. has received funding from the Phoenix Data Coordinating Center by means of a grant from Takeda. M.B. has received grants from Takeda and Merck. T.A.B. has received grant support from Allergan and Takeda. A.G. has received grant support from Amylin and Roche. R.R.H. has received grant support from AstraZeneca, Bristol-Myers Squibb (BMS), Eli Lilly, Sanofi, and Medtronics. R.E.R. has received research support from Takeda. P.D.R. has received research grants from BMS and Novo Nordisk. R.A.D. serves on advisory boards for Amylin, Takeda, BMS, Novo Nordisk, Janssen, and Boehringer Ingelheim, and is on the Speakers Bureau for Novo Nordisk. D.T. has received consulting fees from HDL Diagnostics Inc. M.B. has received consulting fees from Sanofi, Merck, Roche, and Boehringer Ingelheim and fees for participation in review activities from Novartis and BMS. T.A.B. is on an advisory panel and the Speakers Bureau of Takeda, and holds stock options from Tethys Bioscience. S.C.C. is a full-time employee of Merck. A.G. is a consultant for Roche. R.R.H. is a consultant to Boehringer Ingelheim, Gilead, Intarcia, Isis, Eli Lilly, Novo Nordisk, Roche, and Medtronics and is on advisory boards for Amgen, AstraZeneca, BMS, Gilead, Intarcia, Johnson & Johnson/Janssen, Eli Lilly, Merck, Novo Nordisk, Roche, Sanofi, Daiichi Sankyo, and Elcelyx. S.M. has been a speaker for Takeda. P.D.R. has received support as a speaker for Amylin and is a consultant for BMS. No other potential conflicts of interest relevant to this article were reported.
Takeda played no role in the study design, data collection/analysis, or manuscript preparation/review.
R.A.D., D.T., T.A.B., A.G., and P.D.R. participated in writing and reviewing the first draft of the manuscript. All authors reviewed the manuscript prior to submission. R.A.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the nurses and other technical staff for their enormous and expert help. The authors also thank the 602 impaired glucose tolerance patients who participated in this study. The authors thank Lorrie Albarado of the University of Texas Health Care System at San Antonio for providing expert secretarial assistance in the preparation of the manuscript.
Footnotes
Clinical trial reg. no. NCT00220961, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0265/-/DC1.
See accompanying commentary, p. 3663.
REFERENCES
- 1.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care 2006;29:1263–1268 [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–497 [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78:305–312 [DOI] [PubMed] [Google Scholar]
- 4.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA, San Antonio metabolism study Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 2004;47:31–39 [DOI] [PubMed] [Google Scholar]
- 5.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 6.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 7.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37:667–687 [DOI] [PubMed] [Google Scholar]
- 9.Xiang AH, Peters RK, Kjos SL, et al. Pharmacological treatment of insulin resistance at two different stages in the evolution of type 2 diabetes: impact on glucose tolerance and beta-cell function. J Clin Endocrinol Metab 2004;89:2846–2851 [DOI] [PubMed] [Google Scholar]
- 10.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007;292:E871–E883 [DOI] [PubMed] [Google Scholar]
- 12.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab 2004;89:463–478 [DOI] [PubMed] [Google Scholar]
- 13.Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004;351:1106–1118 [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010;53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiegelman BM. PPAR-γ: adipogenic regulator and thiazolidinedione receptor. Diabetes 1998;47:507–514 [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki Y, Mahankali A, Matsuda M, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2002;87:2784–2791 [DOI] [PubMed] [Google Scholar]
- 17.Defronzo RA, Banerji M, Bray GA, et al. Actos Now for the prevention of diabetes (ACT NOW) study. BMC Endocr Disord 2009;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Tripathy D, Schwenke DC, et al. ACT NOW Study Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed] [Google Scholar]
- 19.Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic β-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 2006;55:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazaki Y, Mahankali A, Wajcberg E, Bajaj M, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2004;89:4312–4319 [DOI] [PubMed] [Google Scholar]
- 21.Bajaj M, Baig R, Suraamornkul S, et al. Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2010;95:1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297–2307 [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 24.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Ghani MA, Molina-Carrion M, Jani R, Jenkinson CP, Defronzo RA. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the anti-lipolytic effect of insulin. Acta Diabetol 2008;45:147–150 [DOI] [PubMed] [Google Scholar]
- 26.Mittelman SD, Van Citters GW, Kim SP, et al. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced β-cell response. Diabetes 2000;49:2116–2125 [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Abbasi F, Chu JW, et al. Rosiglitazone reduces glucose-stimulated insulin secretion rate and increases insulin clearance in nondiabetic, insulin-resistant individuals. Diabetes 2005;54:2447–2452 [DOI] [PubMed] [Google Scholar]
- 28.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 29.Knowler WC, Hamman RF, Edelstein SL, et al. Diabetes Prevention Program Research Group Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 2005;54:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerstein HC, Yusuf S, Bosch J, et al. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 31.Dormandy JA, Charbonnel B, Eckland DJ, et al. PROactive investigators Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 32.Betteridge DJ, DeFronzo RA, Chilton RJ. PROactive: time for a critical appraisal. Eur Heart J 2008;29:969–983 [DOI] [PubMed] [Google Scholar]
- 33.Kitabchi AE, Temprosa M, Knowler WC, et al. Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 1999;48:2197–2203 [DOI] [PubMed] [Google Scholar]
- 35.Cavaghan MK, Ehrmann DA, Polonsky KS. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J Clin Invest 2000;106:329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sumner AE, Farmer NM, Cochran CS, et al. Obese premenopausal African-American women with normal and impaired glucose tolerance have a similar degree of insulin resistance but differ in β-cell function. Diabetes Care 2001;24:1978–1983 [DOI] [PubMed] [Google Scholar]
- 37.Ferrannini E, Gastaldelli A, Miyazaki Y, et al. Predominant role of reduced beta-cell sensitivity to glucose over insulin resistance in impaired glucose tolerance. Diabetologia 2003;46:1211–1219 [DOI] [PubMed] [Google Scholar]
- 38.Bergman RN. Non-esterified fatty acids and the liver: why is insulin secreted into the portal vein? Diabetologia 2000;43:946–952 [DOI] [PubMed] [Google Scholar]
- 39.Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis 2009;14:1484–1495 [DOI] [PubMed] [Google Scholar]
- 40.Miyazaki YDFE, Bajaj M, Wajcberg E, et al. Predictors of improved glycaemic control with rosiglitazone therapy in type 2 diabetic patients: a practical approach for the primary care physician. Br J Diabetes Vasc Dis 2005;5:28–35 [Google Scholar]