Abstract
Invariant natural killer T (iNKT) cells belong to the innate immune system and exercise a dual role as potent regulators of autoimmunity and participate in responses against different pathogens. They have been shown to prevent type 1 diabetes development and to promote antiviral responses. Many studies in the implication of environmental factors on the etiology of type 1 diabetes have suggested a link between enteroviral infections and the development of this disease. This study of the pancreatropic enterovirus Coxsackievirus B4 (CVB4) shows that although infection accelerated type 1 diabetes development in a subset of proinsulin 2–deficient NOD mice, the activation of iNKT cells by a specific agonist, α-galactosylceramide, at the time of infection inhibited the disease. Diabetes development was associated with the infiltration of pancreatic islets by inflammatory macrophages, producing high levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α and activation of anti-islet T cells. On the contrary, macrophages infiltrating the islets after CVB4 infection and iNKT-cell stimulation expressed a number of suppressive enzymes, among which indoleamine 2,3-dioxygenase was sufficient to inhibit anti-islet T-cell response and to prevent diabetes. This study highlights the critical interaction between virus and the immune system in the acceleration or prevention of type 1 diabetes.
Type 1 diabetes is characterized by the destruction of pancreatic islet β-cells by autoreactive CD4 and CD8 T cells, leading to low insulin production and incapacity to regulate blood glucose levels (1). Despite numerous studies, the etiology of type 1 diabetes remains elusive. Besides genetics (2–4), environmental factors such as viral infections have been suggested as triggers of type 1 diabetes (5–7). Most striking of these infections are the type B Coxsackieviruses belonging to the enterovirus genus whose genome and anti-Coxsackievirus antibodies were detected more frequently in the blood of recently diagnosed patients compared with healthy controls (8,9). Besides, enteroviral RNA or enteroviral particles were directly detected in the pancreas of type 1 diabetic patients, whereas they were undetectable in the pancreas of healthy donors (9,10). In a mouse model of type 1 diabetes, Serreze et al. (11) showed that diabetes can develop rapidly after Coxsackievirus B4 (CVB4) infection if mice had an advanced age and sufficient insulitis. Others have reported that inefficient islet β-cell response, viral dose, and replication rate as well as a lack of islet neogenesis could also promote accelerated diabetes development after CVB4 infection (12–14).
Natural killer T (NKT) cells are CD1d-restricted, nonconventional T cells recognizing self and exogenous glycolipids. Most NKT cells express an invariant T-cell receptor α chain, Vα14-Jα18 (Vα14) in mice and Vα24-Jα18 in humans, and are named invariant NKT (iNKT) cells. They can promptly secrete copious amounts of interferon-γ (IFN-γ) and interleukin (IL)-4 and provide maturation signals to dendritic cells (DCs) and lymphocytes, thereby contributing to both innate and acquired immunity (15,16). iNKT cells are potent regulatory cells that can inhibit autoimmunity and promote immune responses against pathogens (1,17). Diabetes can be prevented in NOD mice by increasing iNKT cell numbers and by iNKT-cell stimulation with exogenous ligands such as α-galactosylceramide (αGalCer) (15,18,19). NOD mice protected from diabetes by iNKT cells have weak T helper 1 anti-islet β-cell responses (20). Indeed, iNKT cells can impair the differentiation of anti-islet CD4 and CD8 T cells, which become hyporesponsive or anergic (21). Contrary to their suppressive role in type 1 diabetes, iNKT cells can enhance immune responses to pathogens such as parasites, bacteria, and viruses (22,23).
Our previous studies conducted in a murine model of type 1 diabetes with lymphocytic choriomeningitis virus infection revealed that iNKT cells could promote systemic antiviral CD8 T-cell responses while inhibiting deleterious anti-islet T-cell responses, thereby preventing type 1 diabetes (24,25). In the present study, we investigated the role of iNKT cells after CVB4 infection, revealing that diabetes development following CVB4 infection is associated with the infiltration of inflammatory macrophages into the pancreatic islets with subsequent activation of anti-islet T cells. However, the activation of iNKT cells during CVB4 infection results in the infiltration of suppressive macrophages into pancreatic islets. Indoleamine 2,3-dioxygenase (IDO) expressed by these macrophages was critical for the inhibition of diabetes development.
RESEARCH DESIGN AND METHODS
Mice.
Female proinsulin 2–deficient (Proins2−/−) NOD mice, Vα14 transgenic NOD mice expressing the Vα14-Jα18 T-cell receptor α chain, and BDC2.5 Cα−/− mice were previously described (15,21,25,26). NOD Vα14 were crossed with Proins2−/− NOD mice to generate Vα14 Proins2−/− NOD. Mice were bred and housed in specific pathogen-free conditions. This study was approved by the local ethics committee on animal experimentation (P2.AL.171.10).
In vivo treatments.
CVB4 Edwards strain 2 was injected intraperitoneally at the dose of 1 × 105 plaque-forming units (PFU)/mouse. When indicated, mice were treated with a single injection of αGalCer 2 µg/mouse i.p. (Alexis) diluted in PBS/Tween 0.05% at the time of CVB4 infection. For short-term blockade of IFN-γ and IL-4, mice were injected with purified anti-IFN-γ monoclonal antibody (mAb) (R46A2) 0.5 mg i.p., anti-IL-4 mAb (11B11) 0.5 mg i.p., or corresponding isotype controls on days −1 and +1 of virus infection for PCR analysis and on days −1, +1, +3 for diabetes incidence. IL-13 was blocked with soluble extracellular domain of IL-13 receptor 10 µg i.p. b.i.d. on days −1, 0, and +1 of infection. A selective inducible nitric oxide synthase (iNOS) inhibitor, 1400W 10 mg/kg/day i.p. (Calbiochem), and a selective arginase I inhibitor, Nω-hydroxy-nor-l-arginine (nor-NOHA) 20 mg/kg/day i.p. (Calbiochem), were injected starting from the day of infection and up to day 8. To inhibit IDO, mice were given 1-methyl-tryptophan (1MT) 4 mg/mL (Sigma) in drinking water 2 days before infection and for up to 8 days afterward. In some experiments, 2 × 105 macrophages were isolated from the pancreas of Proins2−/− mice treated with CVB4 or CVB4 + αGalCer 2 days earlier and transferred intravenously into recipient Proins2−/− mice infected with CVB4 1 day earlier.
Viral titration.
Pancreata were recovered and homogenized in liquid maintenance medium 199 (#11825-015; Gibco) complemented with distilled water (65%), sodium bicarbonate (2.7%), PBS (11.5%), penicillin/streptomycin (1.6%), and l-glutamine (1.6%) and centrifuged at 2,300 rpm for 20 min. Tenfold serial dilutions of the supernatant were overlaid on HeLa cell monolayers and incubated for 2 h at 37°C. The monolayers were washed with PBS and overlaid with mixed equal portions of maintenance medium containing FCS (PAA Laboratories) instead of PBS and 2.4% suspension of Avicel (RC581; BMC Biopolymer). Two days later, the overlay was removed, and the monolayers were fixed with formaldehyde and colored with crystal violet ammonium oxalate solution.
Diabetes diagnosis and histology.
Overt diabetes was defined as two positive urine glucose tests of glycemia >200 mg/dL 48 h apart (Glukotest kit; Roche). For histology analysis, paraffin-embedded sections were cut at three levels (200-µm intervals) and stained with hematoxylin-eosin. Insulitis severity was scored in a blinded fashion by two examiners with the following criteria: grade 0, no infiltration; grade 1, peri-islet lymphocytic infiltration; grade 2, <50% islet lymphocytic infiltration; and grade 3, >50% islet lymphocytic infiltration. At least 40 islets from each mouse were analyzed.
Preparation of single-cell suspensions from pancreas.
Pancreata were perfused with 5 mL Collagenase P solution (0.75 mg/mL) (Roche), dissected free from surrounding tissues, digested for 10 min at 37°C, and washed twice with RPMI medium-10% FCS. Islets were then purified on a Ficoll gradient and incubated with 1 mL nonenzymatic cell dissociation buffer (Invitrogen) for 10 min at 37°C and dissociated into a single-cell suspension by pipetting.
Flow cytometry.
The following mAbs were used: CD45 (30F11), CD11b (M1/70), Ly-6G (RB6–8C5), F4/80 (BM8), CD115 (AFS98), IL-4 (11B11), and IL-13 (eBio13A) (all from eBiosciences) and CD11c (HL3), 120G8, Ly-6C (AL21), IFN-γ (XMG1.2), CD62L (Mel-14), CD4 (GK1.5), CD8 (53-6.7), and anti-human Ki-67 (B56) (all from BD Biosciences). Stainings were performed in 5% FCS in PBS for 20 min at 4°C. Nonspecific Fc binding was blocked with an anti-CD16/CD32 antibody (24G2). Allophycocyanin–conjugated αGalCer-loaded CD1d tetramer was prepared in our laboratory. For cytokine stainings, cells were stimulated with phorbol myristic acid 10 ng/mL and ionomycine 1 µg/mL in the presence of Brefeldin A 1 mg/mL for 4 h at 37°C (all from Sigma). After the surface staining, cells were fixed, permeabilized for 30 min with cytofix-cytoperm kit (BD Biosciences), and incubated with intracellular mAbs for 30 min. Cells were either analyzed with a BD Fortessa flow cytometer or sorted with a BD ARIA II sorter.
Quantitative RT-PCR.
RNA was extracted with an RNeasy mini kit (Qiagen) and reverse transcribed with Superscript III (Invitrogen). Quantitative RT-PCR was performed with SYBR Green (Roche) and analyzed with a LightCycler 480 (Roche). Relative expression was calculated by the 2–∆∆Ct method and normalized to the expression of the housekeeping gene GAPDH. The stability of GAPDH expression was confirmed by comparison with HPRT mRNA (Supplementary Fig. 1).
In vitro T-cell responses.
Single-cell suspensions of pancreatic islets were cultured in the presence of islet-specific glucose-6-phosphatase catalytic subunit–related protein (IGRP)206–214 peptide (VYLKTNVFL) 10 μmol/L for 4 h 30 min at 37°C in the presence of Brefeldin A 1 mg/mL. After surface staining, cells were fixed and permeabilized, and intracellular IFN-γ staining was performed. The proliferation was assessed in a thymidine incorporation assay. Sorted naïve BDC2.5+ CD4 T cells (3 × 104) were cultured with anti-CD3/CD28 beads (Invitrogen) and 3 × 104 macrophages isolated from the pancreas of mice either infected with CVB4 alone or infected and treated with αGalCer. After 48-h culture, wells were pulsed with 1-μCi tritiated thymidine ([3H]-TdR) overnight. [3H]-TdR incorporation was measured with a TopCount counter (PerkinElmer) at the Cochin cytometry and immunobiology facility. 1MT was prepared as a 20 mmol/L stock solution in 0.1 mol/L NaOH and added to the T-cell culture at a final concentration of 200 μmol/L.
Statistical analysis.
Diabetes incidence was plotted according to the Kaplan-Meier method. Incidences between groups were compared with the log-rank test. For other experiments, comparison between means was performed with the nonparametric Mann-Whitney U test. P < 0.05 was considered statistically significant. All data were analyzed with Prism version 5 software (GraphPad Software).
RESULTS
iNKT-cell activation inhibits diabetes development after CVB4 infection.
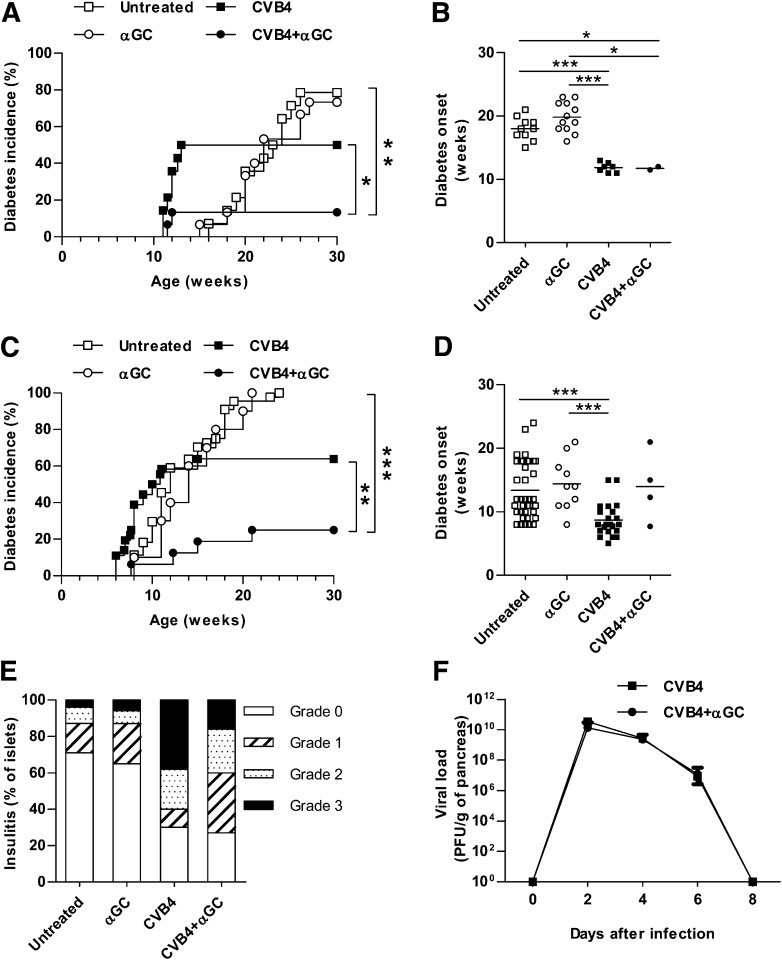
As previously described by Serreze et al. (11), 50% of NOD mice developed diabetes after CVB4 infection at 10 weeks of age, whereas the remaining mice stayed diabetes free for up to 30 weeks of age (Fig. 1A). Analysis of a subset of diabetic mice showed that diabetes acceleration after CVB4 infection was significant compared with noninfected mice (Fig. 1B). Similar data were obtained with Proins2−/− mice (Fig. 1C and D), which were used because they develop accelerated spontaneous type 1 diabetes compared with wild-type NOD mice, thus shortening the duration of experiments. Proins2−/− mice were used at 5–6 weeks of age when they already exhibited moderate insulitis. Of note, one single injection of the iNKT-cell agonist αGalCer at the time of infection strongly decreased diabetes incidence in both NOD and Proins2−/− mice (13 and 25%, respectively). Insulitis was also reduced in CVB4 + αGalCer Proins2−/− mice compared with CVB4 infection only (Fig. 1E). Because islet β-cell infection could promote diabetes development (12,27), we analyzed CVB4 pancreatic and islet β-cell infection. Viral titers determined by PFU were similar in the pancreas from infected mice treated or not with αGalCer (Fig. 1F and Supplementary Fig. 2A). Although in situ hybridization showed CVB4 infection of exocrine tissue, it did not reveal islet β-cell infection on day 3 and 7 postinfection (data not shown). Altogether, the data show that iNKT-cell activation decreased the incidence of diabetes following CVB4 infection in NOD and Proins2−/− mice.
FIG. 1.
iNKT-cell activation prevents diabetes development after CVB4 infection. A: Diabetes incidence of female NOD mice (10 weeks old) inoculated intraperitoneally with CVB4 1 × 105 PFU/mouse or PBS and treated with αGalCer (αGC) or control vehicle (untreated n = 14, αGC n = 15, CVB4 n = 14, CVB4 + αGC n = 15). *P < 0.05; **P < 0.005 by log-rank test. Data represent two pooled independent experiments. B: Age of diabetes onset of NOD mice that became diabetic. *P < 0.05; ***P < 0.0005 by Mann-Whitney U test. C: Diabetes incidence of female Proins2−/− mice inoculated intraperitoneally with CVB4 1 × 105 PFU/mouse or PBS and treated with αGC or control vehicle at 5–6 weeks of age (untreated n = 44, αGC n = 10, CVB4 n = 36, CVB4 + αGC n = 17). **P < 0.005; ***P < 0.0005 by log-rank test. Data represent two to four pooled independent experiments. D: Age of diabetes onset of Proins2−/− mice that became diabetic. ***P < 0.0005 by Mann-Whitney U test. E: Histological scoring of insulitis was performed on pancreatic sections of Proins2−/− mice from days 7 to 10 postinfection and stained with hematoxylin-eosin (n = 6 mice/group). Grade 0, no infiltration; grade 1, peri-islet lymphocytic infiltration; grade 2, <50% islet lymphocytic infiltration, and grade 3, >50% islet lymphocytic infiltration. F: Pancreata were isolated from Proins2−/− mice on different days postinfection and weighed, and viral titers were determined on HeLa cell monolayers with a plaque assay technique. Mean ± SD viremia titers are expressed as PFU per gram of pancreas (n = 6 mice/group for each day).
iNKT-cell activation dampens pancreatic inflammatory response and promotes the expression of suppressive enzymes.
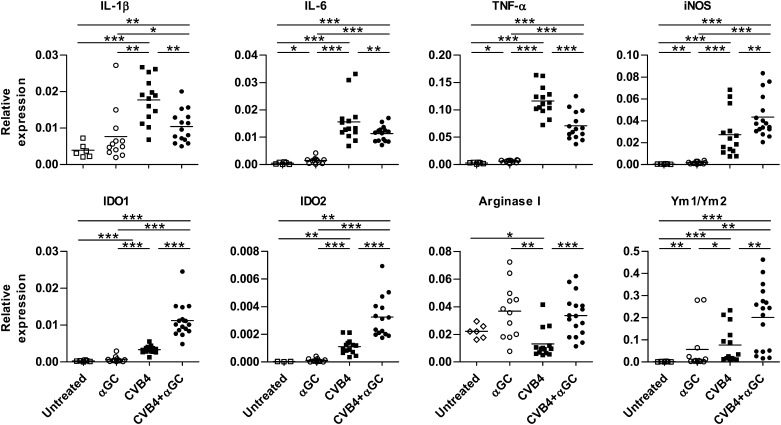
To study the mechanism of diabetes prevention by activated iNKT cells, the inflammatory response was analyzed in pancreatic islets of CVB4-infected mice. At day 2 postinfection, the expression of proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, was significantly decreased in Proins2−/− and NOD mice from the CVB4 + αGalCer group compared with the untreated infected mice (Fig. 2 and Supplementary Fig. 2B). No differences were found in the mRNA level of the suppressive cytokines IL-10 and transforming growth factor-β (data not shown). Strikingly, αGalCer treatment at the time of infection induced a strong upregulation of the suppressive enzymes iNOS, IDO1 and IDO2 (hereafter referred as IDO), and arginase I. Although arginase I was induced by αGalCer alone, the strongest upregulation of iNOS and IDO required both αGalCer and CVB4 infection. Of note, Ym1/Ym2 mRNA was also upregulated in the islets from CVB4 + αGalCer mice, suggesting the presence of alternatively activated macrophages (28,29). The expression of these molecules was transitory, with a peak on day 2 postinfection (Supplementary Fig. 3). Thus, the activation of iNKT cells during CVB4 infection favors the establishment of a less inflammatory and more immunosuppressive environment in pancreatic islets compared with CVB4 infection only.
FIG. 2.
iNKT-cell activation dampens pancreatic inflammatory response and promotes the expression of suppressive enzymes. Female Proins2−/− mice at 5–6 weeks of age were inoculated intraperitoneally with either CVB4 1 × 105 PFU/mouse or PBS and treated with αGalCer (αGC) or control vehicle. On day 2 postinfection, pancreatic islets were harvested, total RNA was extracted, and mRNA levels were measured by quantitative RT-PCR. Data are the specific gene expression relative to GAPDH. Each symbol represents pooled islets of an individual mouse (untreated n = 6, αGC n = 12, CVB4 n = 16, CVB4 + αGC n = 16). *P < 0.05; **P < 0.005; ***P < 0.0005 by Mann-Whitney U test.
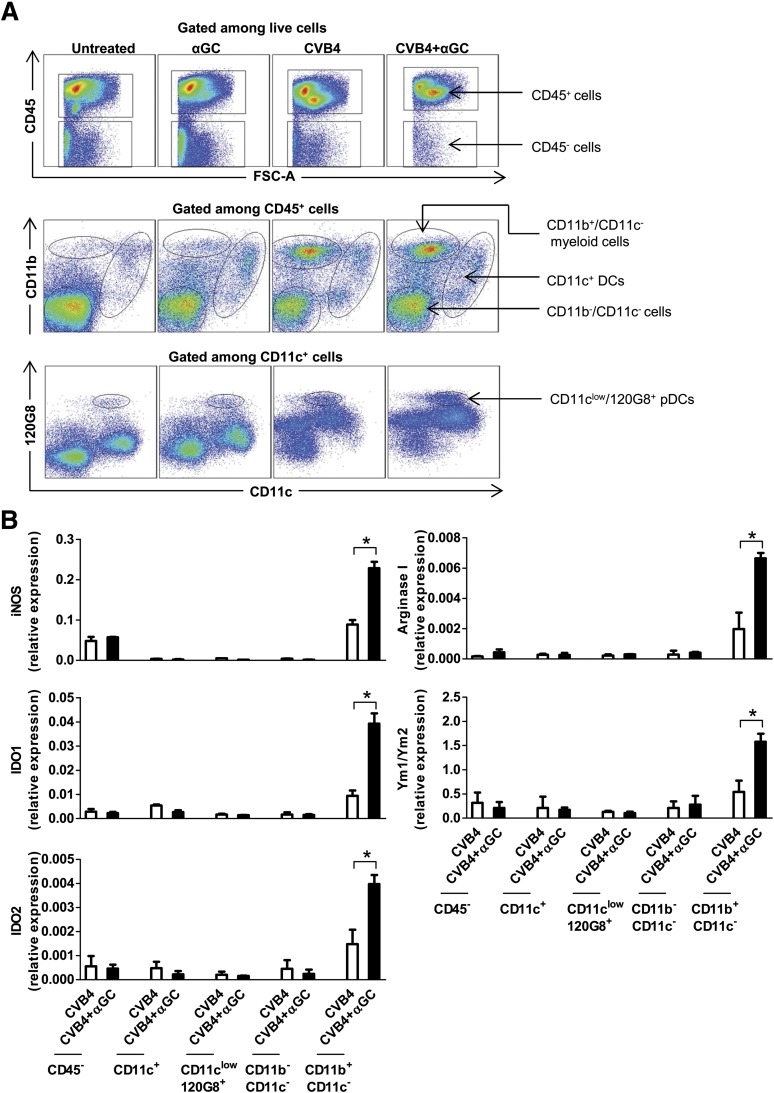
CD11b+/Ly-6C+ macrophages are the main population expressing suppressive enzymes and Ym1/Ym2.
We next investigated which cell populations expressed the immunosuppressive enzymes iNOS, IDO, arginase I, and Ym1/Ym2. On day 2 postinfection, the highest expression of these molecules was detected in myeloid cells isolated from pancreatic islets (islet myeloid cells) (CD11b+/CD11c–) of Proins2−/− mice from the CVB4 + αGalCer group compared with CD45+ DCs (CD11c+), plasmacytoid DCs (CD11clow/120G8+), remaining CD11b–/CD11c–, and CD45– cells (Fig. 3A and B). Of note, these enzymes were not detected in sorted populations of pancreatic lymph node (PLN) and spleen from the same mice tested up to 10 days posttreatment (data not shown).
FIG. 3.
CD11b+/CD11c– cells are the main population expressing the suppressive enzymes and Ym1/Ym2. Female Proins2−/− mice at 5–6 weeks of age were inoculated intraperitoneally with a single dose of either CVB4 or PBS and treated with αGalCer (αGC) or control vehicle. On day 2 postinfection, pancreatic islets were harvested and dissociated into a single-cell suspension, and cells were stained with surface antibodies directed against CD45, CD11c, 120G8, and CD11b. Cells were then sorted with a BD ARIA II sorter. A: Dot plots correspond to a representative staining in pancreatic islets with gates used for sorting. B: Total RNA was isolated from sorted populations from CVB4- and CVB4 + αGC–treated mice, and mRNA levels were measured by quantitative RT-PCR. Data are the mean ± SD of specific gene expression relative to GAPDH. Data were obtained from three independent experiments with three mice in each group. *P < 0.05 by Mann-Whitney U test. pDC, plasmacytoid DC.
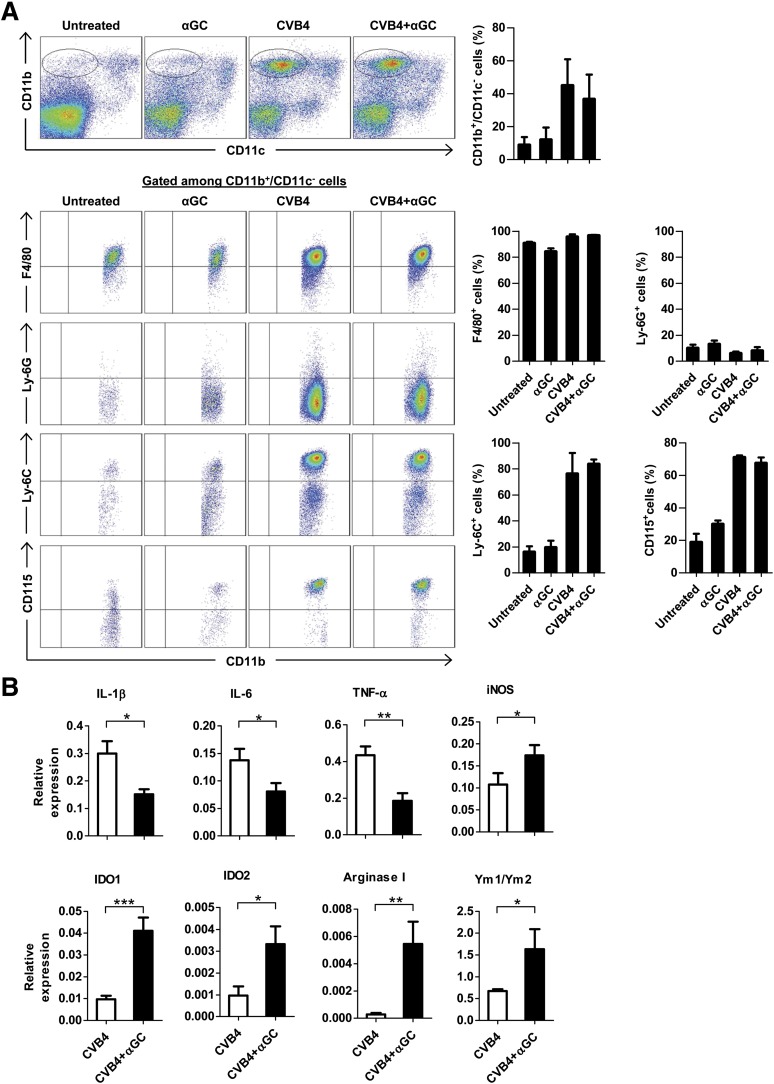
Further characterization of islet CD11b+/CD11c– myeloid cells showed that they were predominantly F4/80+ and Ly-6G– macrophages and that their frequency did not differ between CVB4- and CVB4 + αGalCer–treated mice (Fig. 4A). Of note, the kinetics of their infiltration into islets correlated with the pattern of expression of inflammatory and suppressive molecules in total islets (Supplementary Fig. 4A). Macrophages were further stained with Ly-6C and CD115 mAbs because these molecules can be upregulated on suppressive macrophages (30,31). However, after infection, Ly-6C and CD115 were upregulated to the same extent independently of αGalCer treatment. Similar data were obtained in NOD mice (data not shown). Analysis of sorted Ly-6C+/CD115+ and Ly-6C–/CD115– pancreatic infiltrating cells revealed that the inflammatory cytokines and suppressive enzymes were mainly expressed by Ly-6C+/CD115+ macrophages (Fig. 4B and data not shown). Of note, Ly-6C+ macrophages from spleen and PLN did not express these suppressive enzymes (Supplementary Fig. 4B). Altogether, these results indicate that CVB4 infection induces the infiltration of Ly-6C+/CD115+ macrophages into pancreatic islets, which resemble classically activated macrophages (CAMϕ) strongly expressing inflammatory cytokines. However, when iNKT cells are activated, macrophages express suppressive enzymes characteristic of myeloid-derived suppressor cells (MDSCs).
FIG. 4.
Inflammatory and suppressive molecules are expressed by CD11b+, F4/80+, Ly-6C+, and CD115+ cells. Female Proins2−/− mice at 5–6 weeks of age were inoculated intraperitoneally with CVB4 1 ×105 PFU/mouse or PBS and treated with αGalCer (αGC) or control vehicle. On day 2 postinfection, pancreatic islets were harvested, dissociated, stained with different surface antibodies, and analyzed by flow cytometry. A: Dot plots correspond to a representative staining in pancreatic islets. Summary data (right) were obtained from three independent experiments with three mice in each group ± SD. B: At day 2 postinfection, pancreatic islet CD45+/CD11b+/F4/80+/Ly-6C+/CD115+ cells were sorted from CVB4-infected mice treated or not with αGC. Total RNA was extracted, and mRNA levels were measured by quantitative RT-PCR. Data are the mean ± SD of specific gene expression relative to GAPDH. Data were obtained from three independent experiments with three mice in each group. *P < 0.05; **P < 0.005; ***P < 0.0005 by Mann-Whitney U test.
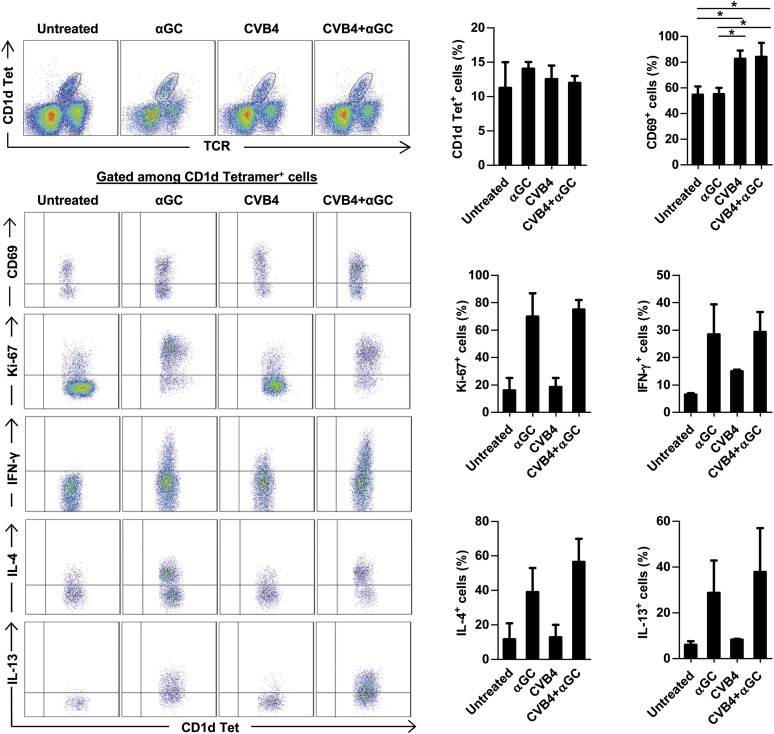
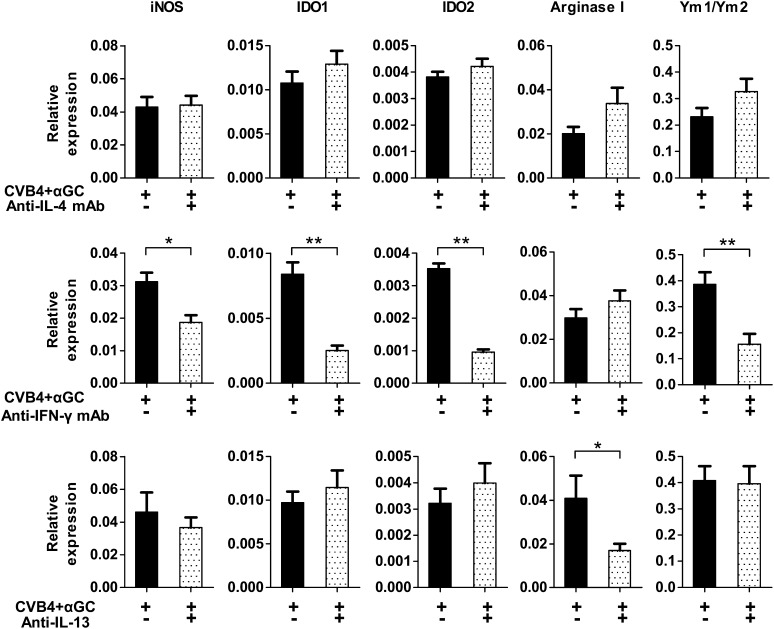
iNKT-cell activation and the critical role of IFN-γ and IL-13 in the expression of suppressive enzymes.
Because iNKT-cell manipulation leads to infiltration of suppressive macrophages and has a major impact on the development of diabetes after CVB4 infection, iNKT cells were analyzed in pancreatic islets (Fig. 5). Vα14 transgenic Proins2−/− mice were used for this study because they exhibit a 10-fold increased frequency and number of iNKT cells, making them easier to detect. CVB4 infection induced the activation of pancreatic iNKT cells that upregulated CD69 without increasing their proliferation (no Ki-67 upregulation) and their cytokine production. In contrast, αGalCer injection led to a strong iNKT-cell proliferation and massive production of IFN-γ, IL-4, and IL-13. We next investigated the role of these cytokines in the induction of MDSC and alternatively activated macrophages in mice from the CVB4 + αGalCer group because IFN-γ can induce the expression of iNOS and IDO, whereas IL-4 and IL-13 can induce arginase I and Ym1/Ym2 expression. The blockade of IL-4 by specific mAb did not alter the expression of any of these enzymes at day 2 postinfection (Fig. 6). In contrast, blocking IFN-γ by a specific mAb significantly reduced the expression of iNOS, IDO, and Ym1/Ym2, whereas blocking IL-13 significantly decreased the expression of arginase I. Thus, cytokines produced by iNKT cells might be the key mediators in the induction of pancreatic suppressive macrophages after CVB4 infection.
FIG. 5.
iNKT-cell activation and cytokine production. iNKT cells were analyzed in Vα14 transgenic Proins2−/− female mice inoculated with CVB4 or PBS and treated with αGalCer (αGC) or control vehicle. Pancreatic islets were harvested on day 2 of the treatment and dissociated into a single-cell suspension. Cells were stimulated with phorbol myristic acid/ionomycin in the presence of Brefeldin A for 4 h, and intracellular staining was performed. Dot plots correspond to a representative staining in pancreatic islets. Summary data (right) were obtained from three independent experiments with three mice in each group ± SD. *P < 0.05 by Mann-Whitney U test. Tet, tetramer.
FIG. 6.
Critical role of IFN-γ and IL-13 in the induction of suppressive enzymes. CVB4-infected and αGalCer (αGC)-treated female Proins2−/− mice were injected with blocking anti-IL-4 mAb (11B11), anti-IFN-γ mAb (R46A2), or respective isotype control antibodies on days −1 and +1 of infection. To block IL-13, mice were injected with IL-13 inhibitor twice a day on days −1, 0, and +1 of infection. On day 2 of infection, pancreatic islets were isolated, and quantitative RT-PCR was performed with total islet RNA. Data are the mean ± SD of specific gene expression relative to GAPDH. Data represent two independent experiments with three mice per group. *P < 0.05; **P < 0.005 by Mann-Whitney U test.
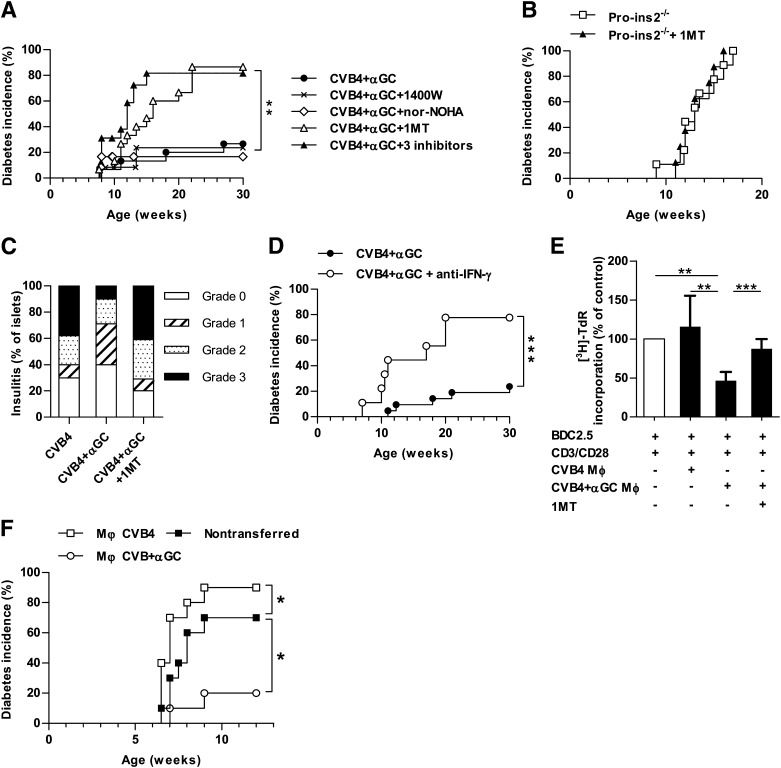
IDO is required for the inhibition of diabetes onset.
To determine the role of suppressive enzymes in the protection against diabetes, Proins2−/− mice from the CVB4 + αGalCer group were treated with specific inhibitors of iNOS, IDO, and arginase I or control vehicles. Treatment with the iNOS inhibitor 1400W or the arginase I inhibitor nor-NOHA did not abrogate the protection against diabetes (Fig. 7A). However, although the IDO inhibitor 1MT did not affect diabetes incidence of control Proins2−/− mice (Fig. 7B), it abolished the protection of mice from the CVB4 + αGalCer group because 86% became diabetic compared with only 27% from the CVB4 + αGalCer group treated with vehicle. Of importance, combined treatment with the three inhibitors resulted in an incidence of diabetes similar to that of the IDO inhibitor only, suggesting that iNOS and arginase I did not play a significant role in the protection against diabetes in this setting. Consistent with the incidence of diabetes, 1MT treatment increased the severity of insulitis in mice from the CVB4 + αGalCer group (Fig. 7C). Because IDO expression in islets was induced by IFN-γ, we next tested the role of IFN-γ in the protection against diabetes. Short-term IFN-γ blockade during the period when IDO is detected in islets increased diabetes incidence of CVB4 + αGalCer–treated mice (Fig. 7D). Of note, both 1MT treatment and IFN-γ blockade induced a moderate increase of diabetes incidence in mice from the CVB4 group (Supplementary Fig. 5). Altogether, these results show that IDO plays a critical role in the inhibition of diabetes development during CVB4 infection and iNKT-cell activation.
FIG. 7.
Critical role of IDO in diabetes prevention. Female Proins2−/− mice infected with CVB4 and injected with αGalCer (αGC) received treatments as indicated (1400W, 1MT, and nor-NOHA to inhibit iNOS; IDO; and arginase I or control vehicles). A: Incidence of diabetes following different treatments (CVB4 + αGC n = 15, CVB4 + αGC + 1400W n = 12, CVB4 + αGC + nor-NOHA n = 12, CVB4 + αGC + 1MT n = 15, CVB4 + αGC + 1400W + nor-NOHA + 1MT n = 12). **P < 0.005 by log-rank test. Data represent two pooled independent experiments. B: Incidence of diabetes of control female Proins2−/− mice treated or not with 1MT (n = 9 for each group). C: Histological scoring of insulitis was performed on pancreatic sections of female Proins2−/− mice from days 7 to 10 postinfection and stained with hematoxylin-eosin (n = 6 mice). D: Incidence of diabetes of female Proins2−/− mice infected with CVB4 and injected with αGC and treated with an anti-IFN-γ mAb (R46A2) (n = 9) or control isotype mAb (n = 15). E and F: CD11b+/F4/80+/Ly-6C+ were sorted from pancreatic islets of female Proins2−/− mice from the CVB4 or CVB4 + αGC groups on the second day of infection. E: Macrophages were cultured (3 × 104/well) with sorted CD62L+ BDC2.5 CD4 T cells (3 × 104/well) in the presence of anti-CD3/CD28 beads ± 1MT (200 µmol/L). After 48 h of culture, [3H]-TdR was added to wells overnight. Results are expressed as percentage of [3H]-TdR uptake compared with control BDC2.5 CD4 T cells cultured with anti-CD3/CD28 beads only, which was considered to be 100%. Data represent mean ± SD for three independent experiments. **P < 0.005; ***P < 0.005 by Mann-Whitney U test. F: Sorted macrophages were injected intravenously (2 × 105/mouse) into female Proins2−/− mice infected with a single dose of CVB4 (1 × 105 PFU/mouse) 1 day earlier. Data represent the incidence of diabetes in the three groups (n = 10 for each) from two independent experiments. *P < 0.05 by log-rank test.
The suppressive capacity of macrophages was then assessed in vitro in a T-cell proliferation assay (Fig. 7E). Macrophages were isolated from the pancreas of Proins2−/− mice from the CVB4 and CVB4 + αGalCer groups on day 2 postinfection and cultured with naïve BDC2.5 CD4 T cells stimulated with anti-CD3/CD28 beads. Macrophages from CVB4-infected mice enhanced BDC2.5 CD4 T-cell proliferation, even though this increase did not reach statistical significance. On the contrary, macrophages from mice of the CVB4 + αGalCer group significantly suppressed T-cell proliferation, and the suppression was reversed when 1MT was added to the culture. To demonstrate the role of pancreatic islet–infiltrating macrophages in vivo, macrophages were sorted from pancreatic islets of either CVB4- or CVB4 + αGalCer–treated Proins2−/− mice on day 2 postinfection and transferred into CVB4 recipients infected the day before. The transfer of macrophages from the CVB4 group significantly increased diabetes incidence of the CVB4-infected recipient mice, whereas the transfer of macrophages from the CVB4 + αGalCer group significantly reduced diabetes incidence (Fig. 7F). Altogether, these results highlight the dual role of pancreatic macrophages after CVB4 infection.
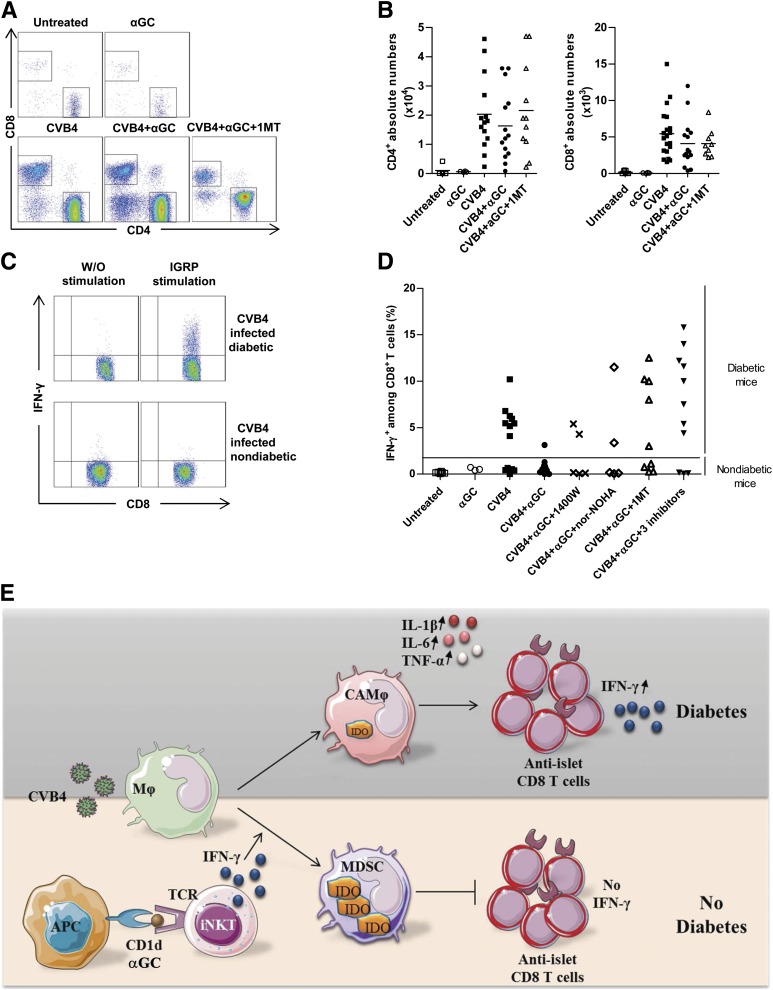
Strong pancreatic anti-islet T-cell response is associated with diabetes induction by CVB4.
To further decipher the immune mechanisms involved in the development of diabetes after CVB4 infection and its inhibition by iNKT-cell activation, we evaluated specific anti-islet T-cell responses. CVB4-infected Proins2−/− and NOD mice were analyzed 1–3 weeks postinfection when diabetes was clearly established. Untreated, αGalCer-treated, or CVB4-infected nondiabetic mice were tested in parallel. CVB4 infection dramatically increased the total numbers of both CD4 and CD8 T cells infiltrating the pancreas of Proins2−/− and NOD mice compared with untreated and mice treated only with αGalCer (Fig. 8A and B and Supplementary Fig. 6A). IGRP-specific IFN-γ–producing CD8 T cells were detected in all Proins2−/− and NOD mice that became diabetic after CVB4 infection, regardless of the treatment received. In contrast, the frequency of IGRP-specific IFN-γ–producing CD8 T cells remained low in untreated or nondiabetic infected mice (Fig. 8C and D and Supplementary Fig. 6B). Treatment with IDO inhibitor, which abolished diabetes protection, induced a strong anti-IGRP effector CD8 T-cell response only in diabetic mice. Altogether, the results strongly suggest that diabetes development after CVB4 infection is caused by anti-islet T-cell responses.
FIG. 8.
Anti-islet T-cell response in the development of diabetes. A and B: Representative CD8 vs. CD4 dot plots of T cells from islets of female Proins2−/− mice 2 weeks after treatment as indicated (A) and summary of CD8 and CD4 T-cell counts in the islets of female Proins2−/− mice from day 7 to 3 weeks post-CVB4 infection of several independent experiments (B). C: Representative dot plots of intracellular IFN-γ staining among islet CD8 T cells from CVB4-infected diabetic and nondiabetic female Proins2−/− mice 2 weeks postinfection after 4 h 30 min stimulation with IGRP206–214 peptide. D: The percentage of islet CD8 T cells secreting IFN-γ after IGRP206–214 peptide stimulation of diabetic and nondiabetic mice receiving different treatments as indicated. The horizontal line shows the limit between diabetic and nondiabetic mice. Each symbol represents a single mouse. E: Schematic view of the immune cell interplay in the islets after infection. After CVB4 infection, macrophages strongly infiltrate pancreatic islets and resemble CAMϕ with low IDO expression and high secretion of proinflammatory cytokines IL-1β, IL-6, and TNF-α. In this setting, anti-islet T cells strongly infiltrate the islets and produce IFN-γ, which can kill islet β-cells. However, when iNKT cells are activated by αGalCer at the time of infection, they produce a large amount of IFN-γ, inducing a strong upregulation of IDO expression in islet-infiltrating suppressive macrophages (MDSCs). IDO-expressing MDSCs suppress IFN-γ production by anti-islet T cells in the pancreas, thereby preventing type 1 diabetes onset. αGC, αGalCer; W/O, without.
DISCUSSION
The current study shows that although CVB4 infection can rapidly induce diabetes in a subset of Proins2−/− and NOD mice, the concomitant activation of iNKT cells inhibited diabetes development after CVB4 infection very efficiently. This prevention was associated with the reduction of inflammatory cytokine expression and the induction of suppressive enzymes in pancreatic infiltrating macrophages. Pancreatic iNKT cells produced IFN-γ, which was required for the induction of high levels of IDO that prevented diabetes onset. The development of diabetes was associated with an increased anti-islet T-cell response, whereas the frequency of these IFN-γ–producing auto-reactive T cells remained very low in nondiabetic mice.
Numerous studies have shown the pathogenic role of macrophages in type 1 diabetes (32–35). CD11b+, F4/80+, Ly-6C+, and CD115+ inflammatory macrophages (36) highly infiltrated pancreatic islets after CVB4 infection in both Proins2−/− and NOD mice but differed in their regulatory functions between mice treated and mice not treated with αGalCer. Pancreas of mice that developed diabetes after CVB4 infection harbored macrophages with a classically activated phenotype expressing high levels of IL-1β, IL-6, and TNF-α, which can favor diabetes development (37–39). According to these previous articles, it is not surprising that as a consequence of macrophage depletion and low expression of inflammatory cytokines, fewer mice developed diabetes after CVB4 infection (Supplementary Fig. 7).
In contrast to the production of proinflammatory cytokines by the pancreatic macrophages from CVB4-infected mice, in the CVB4 + αGalCer group, macrophages produced suppressive enzymes, including IDO, which was required for the decrease of diabetes incidence in these mice. The high IDO expression was a result of IFN-γ, a cytokine highly produced by αGalCer-activated iNKT cells. IFN-γ alone was not sufficient for diabetes protection because αGalCer-treated mice had diabetes incidence similar to that of untreated mice despite high levels of IFN-γ. Of importance, although CVB4 infection induced some IDO, αGalCer treatment alone did not induce any, suggesting that viral infection might be the initiator of IDO induction. Of note, previous studies have suggested the role of IDO in the control of diabetes development. The poor induction of IDO in DCs from prediabetic NOD mice was shown to favor diabetes development (40), whereas transplanted pancreatic islets are protected by IDO-expressing neighboring fibroblasts (41).
CVB4-induced diabetes depends on lymphocytes because NOD Scid, NOD Igµ−/− (11), and NOD Cα−/− (data not shown) mice do not develop diabetes after CVB4 infection. Similarly, CVB4-infected Proins2−/− Scid mice did not become diabetic (data not shown). Diabetes development only in a subset of CVB4-infected Proins2−/− mice could result from differences in insulitis level before infection, as suggested in NOD mice (11). Although CD8 T-cell numbers greatly increased in islets of all infected mice, IFN-γ–producing anti-IGRP CD8 T cells were only detected in the pancreas of diabetic mice, therefore strengthening the role of T cells in diabetes induced by CVB4 as previously suggested (11,42). The inhibition of IDO in CVB4 + αGalCer–treated mice resulted in a strong anti-IGRP CD8 T-cell effector response and a high incidence of diabetes, showing the protective role of IDO in diabetes. Most often, IDO induces tolerance by suppressing T-cell proliferation through tryptophan depletion or by inducing apoptosis through by-products of tryptophan degradation, such as kynurenines (43,44). However, the numbers of T cells did not differ in mice expressing a low or high level of IDO, suggesting that IDO does not affect the proliferation of T cells but, rather, induces their local inhibition in the pancreas. The present data show that IDO inhibiting the function of CD8 T cells is reminiscent of a previous study in the allograft setting (45). Moreover, local regulation by IDO has been previously observed in other infectious contexts as well as in cancer (46).
Of note, the present study suggests that IFN-γ can play a protective or deleterious role in diabetes development, depending on the setting (Fig. 8E). We hypothesize that a strong burst of IFN-γ early after viral infection upregulates IDO expression, which is initially induced by CVB4 infection to downregulate the inflammation after viral infection. In contrast, in the absence of iNKT-cell activation, the production of inflammatory cytokines could promote the recruitment and activation of pathogenic T cells, which would accumulate and produce IFN-γ. At this later point, IDO is no longer expressed in the pancreas, and IFN-γ production would lead to islet β-cell death rather than to IDO upregulation. Of note, we did not detect any difference of viral load in the pancreas of infected mice treated or not with αGalCer. However, we cannot rule out that IFN-γ, produced by αGalCer treatment, might contribute to the prevention of diabetes by dampening CVB4 infection specifically in islet β-cells. The infection of islet β-cells could lead to type 1 diabetes development because it would favor the engulfment of infected islet β-cells by allophycocyanin, presentation of islet β-cell autoantigens to autoreactive T cells, and initiation of diabetes (27,47,48). Indeed, studies in mouse models and human samples have shown that IFNs, including IFN-γ, are important in regulating islet β-cell permissiveness of infection and replication in islet β-cells (12,49,50). Of note, some reports have described islet β-cell infection by enterovirus in recent type 1 diabetic patients (51,52). Therefore, IFN-γ production in the islets of CVB4 + αGalCer–treated mice could prevent diabetes not only by inducing IDO but also by limiting islet β-cell infection.
IDO-mediated suppression could involve several mechanisms, including regulatory FoxP3+ T-cell induction in pancreatic islets (53). However, in the present study, the percentages of FoxP3+ CD4 T cells; their expression of CTLA-4, CD103, GITR, and OX40; and their production of IL-10 and transforming growth factor-β were similar in the islets, PLN, and spleen of infected Proins2−/− and NOD mice with or without αGalCer treatment (Supplementary Fig. 8 and data not shown). Tryptophan metabolites can also render DCs tolerogenic with intermediate expression of stimulatory major histocompatibility complex II, CD86, and upregulation of the suppressive programmed death ligands 1 and 2 (54). These molecules were highly upregulated by DCs in the pancreas, PLN, and spleen after the infection. However, the expression of these molecules was similar between CVB4-infected mice treated or not with αGalCer. Similarly, the production of IL-10 and IL-12 by DCs did not differ in any of the organs studied (data not shown).
In conclusion, this study shows how the manipulation of iNKT cells can lead to the induction of suppressive macrophages in the pancreas of CVB4-infected mice and highlights the role of macrophages in the development or prevention of diabetes after CVB4 infection. The outcome of this study can help in the design of novel therapeutic approaches to manipulating iNKT cells and suppressive macrophages.
ACKNOWLEDGMENTS
L.G. was supported by doctoral fellowships from the Ministère de l’Education Nationale. This work was supported by funds from INSERM and CNRS and grant ANR-09-GENO-023 and Laboratoire d’Excellence INFLAMEX to A.L. A.L. is the recipient of an Assistance Publique-Hôpitaux de Paris-CNRS Contrat Hospitalier de Recherche Translationnelle.
No potential conflicts of interest relevant to this article were reported.
L.G. researched data, performed experiments, and wrote the manuscript. J.D. researched data and reviewed the manuscript. L.B. and P.G.L. performed experiments. R.K.P. provided the IL-13 inhibitor and reviewed and edited the manuscript. N.v.R. provided clodronate liposomes and reviewed and edited the manuscript. M.F.-T. provided CVB4 and reviewed and edited the manuscript. A.L. designed and supervised the study and wrote the manuscript. L.G. and A.L. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank R. Mallone from INSERM U1016, for IGRP peptide and the staff of the mouse facility for animal care.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0958/-/DC1.
REFERENCES
- 1.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol 2010;10:501–513 [DOI] [PubMed] [Google Scholar]
- 2.Eurodiab Ace Study Group; Eurodiab Ace Substudy 2 Study Group. Familial risk of type I diabetes in European children. Diabetologia 1998;41:1151–1156 [DOI] [PubMed] [Google Scholar]
- 3.Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 2008;359:2849–2850 [DOI] [PubMed] [Google Scholar]
- 4.Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes 2003;52:1052–1055 [DOI] [PubMed] [Google Scholar]
- 5.Hober D, Sauter P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat Rev Endocrinol 2010;6:279–289 [DOI] [PubMed] [Google Scholar]
- 6.von Herrath M. Diabetes: A virus-gene collaboration. Nature 2009;459:518–519 [DOI] [PubMed] [Google Scholar]
- 7.Ghazarian L, Diana J, Simoni Y, Beaudoin L, Lehuen A. Prevention or acceleration of type 1 diabetes by viruses. Cell Mol Life Sci 2013;70:239–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lind K, Hühn MH, Flodström-Tullberg M. Immunology in the clinic review series; focus on type 1 diabetes and viruses: the innate immune response to enteroviruses and its possible role in regulating type 1 diabetes. Clin Exp Immunol 2012;168:30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 2011;342:d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dotta F, Censini S, van Halteren AG, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 2007;104:5115–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serreze DV, Ottendorfer EW, Ellis TM, Gauntt CJ, Atkinson MA. Acceleration of type 1 diabetes by a Coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes 2000;49:708–711 [DOI] [PubMed] [Google Scholar]
- 12.Flodström M, Maday A, Balakrishna D, Cleary MM, Yoshimura A, Sarvetnick N. Target cell defense prevents the development of diabetes after viral infection. Nat Immunol 2002;3:373–382 [DOI] [PubMed] [Google Scholar]
- 13.Kanno T, Kim K, Kono K, Drescher KM, Chapman NM, Tracy S. Group B coxsackievirus diabetogenic phenotype correlates with replication efficiency. J Virol 2006;80:5637–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yap IS, Giddings G, Pocock E, Chantler JK. Lack of islet neogenesis plays a key role in beta-cell depletion in mice infected with a diabetogenic variant of coxsackievirus B4. J Gen Virol 2003;84:3051–3068 [DOI] [PubMed] [Google Scholar]
- 15.Lehuen A, Lantz O, Beaudoin L, et al. Overexpression of natural killer T cells protects Valpha14- Jalpha281 transgenic nonobese diabetic mice against diabetes. J Exp Med 1998;188:1831–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol 2009;9:28–38 [DOI] [PubMed] [Google Scholar]
- 17.Diana J, Lehuen A. NKT cells: friend or foe during viral infections? Eur J Immunol 2009;39:3283–3291 [DOI] [PubMed] [Google Scholar]
- 18.Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune type 1 diabetes. Nat Med 2001;7:1057–1062 [DOI] [PubMed] [Google Scholar]
- 19.Simoni Y, Diana J, Ghazarian L, Beaudoin L, Lehuen A. Therapeutic manipulation of natural killer (NK) T cells in autoimmunity: are we close to reality? Clin Exp Immunol 2013;171:8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laloux V, Beaudoin L, Ronet C, Lehuen A. Phenotypic and functional differences between NKT cells colonizing splanchnic and peripheral lymph nodes. J Immunol 2002;168:3251–3258 [DOI] [PubMed] [Google Scholar]
- 21.Beaudoin L, Laloux V, Novak J, Lucas B, Lehuen A. NKT cells inhibit the onset of diabetes by impairing the development of pathogenic T cells specific for pancreatic beta cells. Immunity 2002;17:725–736 [DOI] [PubMed] [Google Scholar]
- 22.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 2005;434:525–529 [DOI] [PubMed] [Google Scholar]
- 23.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol 2007;5:405–417 [DOI] [PubMed] [Google Scholar]
- 24.Diana J, Griseri T, Lagaye S, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity 2009;30:289–299 [DOI] [PubMed] [Google Scholar]
- 25.Diana J, Brezar V, Beaudoin L, et al. Viral infection prevents diabetes by inducing regulatory T cells through NKT cell-plasmacytoid dendritic cell interplay. J Exp Med 2011;208:729–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thébault-Baumont K, Dubois-Laforgue D, Krief P, et al. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest 2003;111:851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz MS, Fine C, Ilic A, Sarvetnick N. Requirements for viral-mediated autoimmune diabetes: beta-cell damage and immune infiltration. J Autoimmun 2001;16:211–217 [DOI] [PubMed] [Google Scholar]
- 28.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol 2002;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsa R, Andresen P, Gillett A, et al. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes 2012;61:2881–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006;66:1123–1131 [DOI] [PubMed] [Google Scholar]
- 31.Zhu B, Bando Y, Xiao S, et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol 2007;179:5228–5237 [DOI] [PubMed] [Google Scholar]
- 32.Mathis D, Vence L, Benoist C. Beta-cell death during progression to diabetes. Nature 2001;414:792–798 [DOI] [PubMed] [Google Scholar]
- 33.Calderon B, Suri A, Unanue ER. In CD4+ T-cell-induced diabetes, macrophages are the final effector cells that mediate islet beta-cell killing: studies from an acute model. Am J Pathol 2006;169:2137–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin AP, Rankin S, Pitchford S, Charo IF, Furtado GC, Lira SA. Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes 2008;57:3025–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jun HS, Yoon CS, Zbytnuik L, van Rooijen N, Yoon JW. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med 1999;189:347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geissmann F, Auffray C, Palframan R, et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol 2008;86:398–408 [DOI] [PubMed] [Google Scholar]
- 37.Eizirik DL, Mandrup-Poulsen T. A choice of death—the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001;44:2115–2133 [DOI] [PubMed] [Google Scholar]
- 38.Uno S, Imagawa A, Okita K, et al. Macrophages and dendritic cells infiltrating islets with or without beta cells produce tumour necrosis factor-alpha in patients with recent-onset type 1 diabetes. Diabetologia 2007;50:596–601 [DOI] [PubMed] [Google Scholar]
- 39.Cantor J, Haskins K. Recruitment and activation of macrophages by pathogenic CD4 T cells in type 1 diabetes: evidence for involvement of CCR8 and CCL1. J Immunol 2007;179:5760–5767 [DOI] [PubMed] [Google Scholar]
- 40.Grohmann U, Fallarino F, Bianchi R, et al. A defect in tryptophan catabolism impairs tolerance in nonobese diabetic mice. J Exp Med 2003;198:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander AM, Crawford M, Bertera S, et al. Indoleamine 2,3-dioxygenase expression in transplanted NOD islets prolongs graft survival after adoptive transfer of diabetogenic splenocytes. Diabetes 2002;51:356–365 [DOI] [PubMed] [Google Scholar]
- 42.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med 1998;4:781–785 [DOI] [PubMed] [Google Scholar]
- 43.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 1999;189:1363–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by kynurenines. Adv Exp Med Biol 2003;527:183–190 [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Liu L, Liu K, Bizargity P, Hancock WW, Visner GA. Reduced cytotoxic function of effector CD8+ T cells is responsible for indoleamine 2,3-dioxygenase-dependent immune suppression. J Immunol 2009;183:1022–1031 [DOI] [PubMed] [Google Scholar]
- 46.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 2013;34:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horwitz MS, Krahl T, Fine C, Lee J, Sarvetnick N. Protection from lethal Coxsackievirus-induced pancreatitis by expression of gamma interferon. J Virol 1999;73:1756–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horwitz MS, Ilic A, Fine C, Balasa B, Sarvetnick N. Coxsackieviral-mediated diabetes: induction requires antigen-presenting cells and is accompanied by phagocytosis of beta cells. Clin Immunol 2004;110:134–144 [DOI] [PubMed] [Google Scholar]
- 49.Hultcrantz M, Hühn MH, Wolf M, et al. Interferons induce an antiviral state in human pancreatic islet cells. Virology 2007;367:92–101 [DOI] [PubMed] [Google Scholar]
- 50.Lind K, Richardson SJ, Leete P, Morgan NG, Korsgren O, Flodström-Tullberg M. Induction of an antiviral state and attenuated Coxsackievirus replication in type III interferon-treated primary human pancreatic islets. J Virol 2013;87:7646–7654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 2009;52:1143–1151 [DOI] [PubMed] [Google Scholar]
- 52.Ylipaasto P, Klingel K, Lindberg AM, et al. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 2004;47:225–239 [DOI] [PubMed] [Google Scholar]
- 53.Mellor AL, Chandler P, Baban B, et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol 2004;16:1391–1401 [DOI] [PubMed] [Google Scholar]
- 54.Belladonna ML, Grohmann U, Guidetti P, et al. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol 2006;177:130–137 [DOI] [PubMed] [Google Scholar]