Abstract
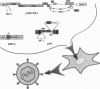
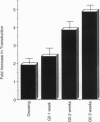
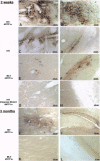
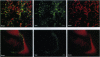
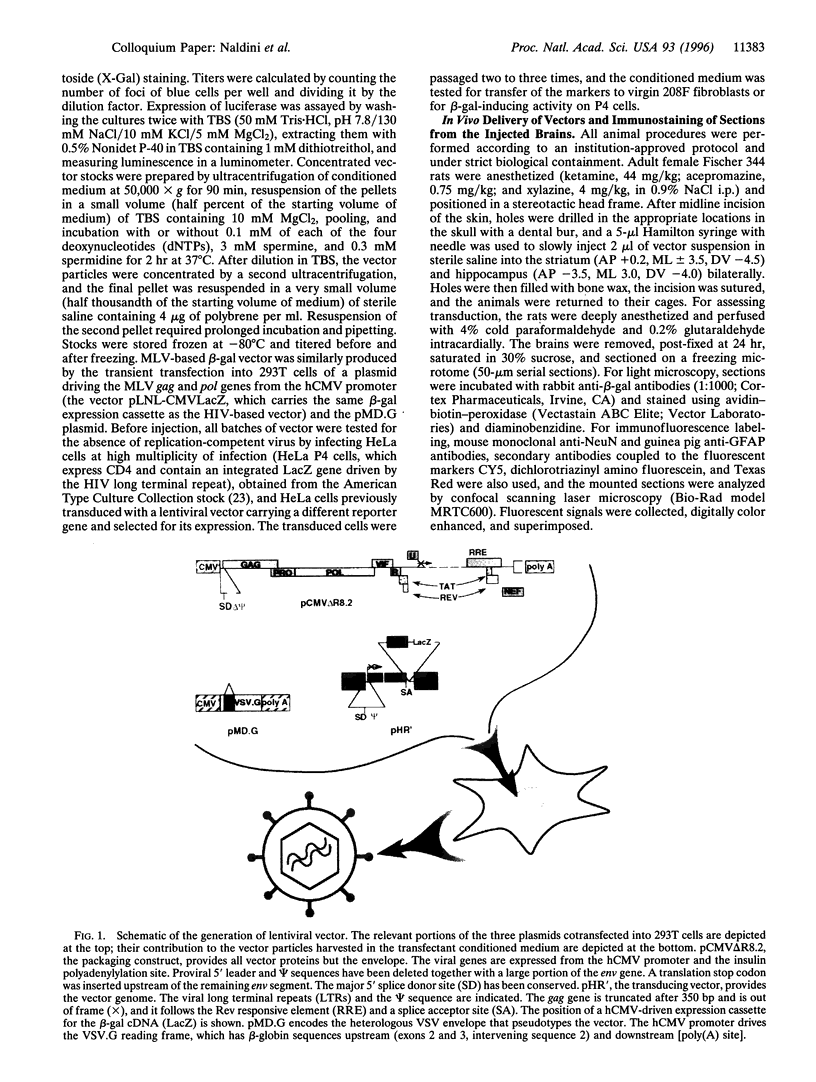
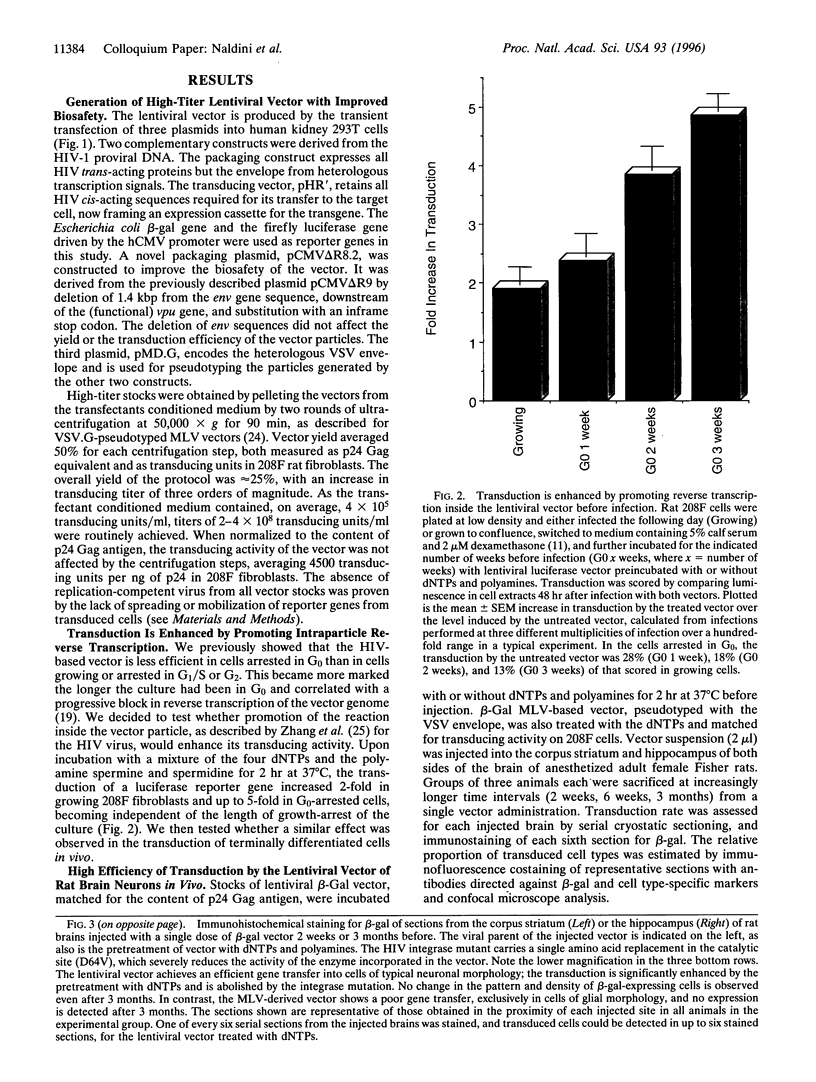
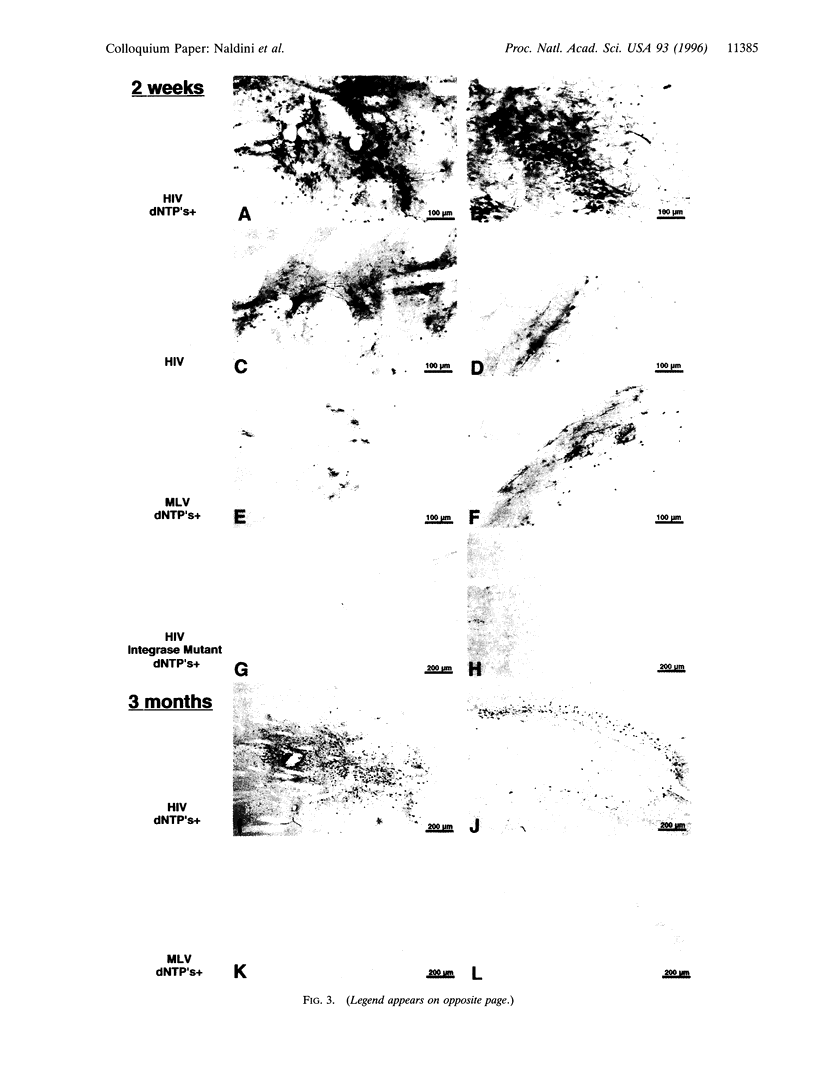
We describe the construction of a safe, replication-defective and efficient lentiviral vector suitable for in vivo gene delivery. The reverse transcription of the vector was found to be a rate-limiting step; therefore, promoting the reaction inside the vector particles before delivery significantly enhanced the efficiency of gene transfer. After injection into the brain of adult rats, sustained long-term expression of the transgene was obtained in the absence of detectable pathology. A high proportion of the neurons in the areas surrounding the injection sites of the vector expressed the transduced beta-galactosidase gene. This pattern was invariant in animals sacrificed several months after a single administration of the vector. Transduction occurs by integration of the vector genome, as it was abolished by a single amino acid substitution in the catalytic site of the integrase protein incorporated in the vector. Development of clinically acceptable derivatives of the lentiviral vector may thus enable the sustained delivery of significant amounts of a therapeutic gene product in a wide variety of somatic tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldovini A., Young R. A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol. 1990 May;64(5):1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskar J. F., Smith P. P., Nilaver G., Jupp R. A., Hoffmann S., Peffer N. J., Tenney D. J., Colberg-Poley A. M., Ghazal P., Nelson J. A. The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in transgenic mice. J Virol. 1996 May;70(5):3207–3214. doi: 10.1128/jvi.70.5.3207-3214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz R. D., Hammarskjöld M. L., Helga-Maria C., Rekosh D., Goff S. P. 5' regions of HIV-1 RNAs are not sufficient for encapsidation: implications for the HIV-1 packaging signal. Virology. 1995 Oct 1;212(2):718–723. doi: 10.1006/viro.1995.1530. [DOI] [PubMed] [Google Scholar]
- Blaese R. M., Culver K. W., Miller A. D., Carter C. S., Fleisher T., Clerici M., Shearer G., Chang L., Chiang Y., Tolstoshev P. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science. 1995 Oct 20;270(5235):475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- Bordignon C., Notarangelo L. D., Nobili N., Ferrari G., Casorati G., Panina P., Mazzolari E., Maggioni D., Rossi C., Servida P. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients. Science. 1995 Oct 20;270(5235):470–475. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- Buchschacher G. L., Jr, Panganiban A. T. Human immunodeficiency virus vectors for inducible expression of foreign genes. J Virol. 1992 May;66(5):2731–2739. doi: 10.1128/jvi.66.5.2731-2739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. C., Friedmann T., Driever W., Burrascano M., Yee J. K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charneau P., Mirambeau G., Roux P., Paulous S., Buc H., Clavel F. HIV-1 reverse transcription. A termination step at the center of the genome. J Mol Biol. 1994 Sep 2;241(5):651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Barankiewicz J., Lederman H. M., Gelfand E. W. Purine and pyrimidine metabolism in human T lymphocytes. Regulation of deoxyribonucleotide metabolism. J Biol Chem. 1983 Oct 25;258(20):12334–12340. [PubMed] [Google Scholar]
- Crystal R. G. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995 Oct 20;270(5235):404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- Dai Y., Roman M., Naviaux R. K., Verma I. M. Gene therapy via primary myoblasts: long-term expression of factor IX protein following transplantation in vivo. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10892–10895. doi: 10.1073/pnas.89.22.10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Schwarz E. M., Gu D., Zhang W. W., Sarvetnick N., Verma I. M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. Y., Cara A., Gallo R. C., Lori F. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8925–8928. doi: 10.1073/pnas.90.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenmüller U., Linial M. L. Specific binding of human immunodeficiency virus type 1 (HIV-1) Gag-derived proteins to a 5' HIV-1 genomic RNA sequence. J Virol. 1996 Jan;70(1):667–671. doi: 10.1128/jvi.70.1.667-671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M., Rader D. J., Muller D. W., Kolansky D. M., Kozarsky K., Clark B. J., 3rd, Stein E. A., Lupien P. J., Brewer H. B., Jr, Raper S. E. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995 Nov;1(11):1148–1154. doi: 10.1038/nm1195-1148. [DOI] [PubMed] [Google Scholar]
- Kaye J. F., Richardson J. H., Lever A. M. cis-acting sequences involved in human immunodeficiency virus type 1 RNA packaging. J Virol. 1995 Oct;69(10):6588–6592. doi: 10.1128/jvi.69.10.6588-6592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Lee K., O'Rear J. J. A short sequence upstream of the 5' major splice site is important for encapsidation of HIV-1 genomic RNA. Virology. 1994 Jan;198(1):336–340. doi: 10.1006/viro.1994.1037. [DOI] [PubMed] [Google Scholar]
- Knowles M. R., Hohneker K. W., Zhou Z., Olsen J. C., Noah T. L., Hu P. C., Leigh M. W., Engelhardt J. F., Edwards L. J., Jones K. R. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Engl J Med. 1995 Sep 28;333(13):823–831. doi: 10.1056/NEJM199509283331302. [DOI] [PubMed] [Google Scholar]
- Kohn D. B., Weinberg K. I., Nolta J. A., Heiss L. N., Lenarsky C., Crooks G. M., Hanley M. E., Annett G., Brooks J. S., el-Khoureiy A. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med. 1995 Oct;1(10):1017–1023. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E. J., Perricaudet M. Adenovirus and adeno-associated virus mediated gene transfer. Br Med Bull. 1995 Jan;51(1):31–44. doi: 10.1093/oxfordjournals.bmb.a072951. [DOI] [PubMed] [Google Scholar]
- Leavitt A. D., Robles G., Alesandro N., Varmus H. E. Human immunodeficiency virus type 1 integrase mutants retain in vitro integrase activity yet fail to integrate viral DNA efficiently during infection. J Virol. 1996 Feb;70(2):721–728. doi: 10.1128/jvi.70.2.721-728.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M. Gene therapy--promise, pitfalls, and prognosis. N Engl J Med. 1995 Sep 28;333(13):871–873. doi: 10.1056/NEJM199509283331310. [DOI] [PubMed] [Google Scholar]
- Lever A., Gottlinger H., Haseltine W., Sodroski J. Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. J Virol. 1989 Sep;63(9):4085–4087. doi: 10.1128/jvi.63.9.4085-4087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lori F., di Marzo Veronese F., de Vico A. L., Lusso P., Reitz M. S., Jr, Gallo R. C. Viral DNA carried by human immunodeficiency virus type 1 virions. J Virol. 1992 Aug;66(8):5067–5074. doi: 10.1128/jvi.66.8.5067-5074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luban J., Goff S. P. Mutational analysis of cis-acting packaging signals in human immunodeficiency virus type 1 RNA. J Virol. 1994 Jun;68(6):3784–3793. doi: 10.1128/jvi.68.6.3784-3793.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride M. S., Panganiban A. T. The human immunodeficiency virus type 1 encapsidation site is a multipartite RNA element composed of functional hairpin structures. J Virol. 1996 May;70(5):2963–2973. doi: 10.1128/jvi.70.5.2963-2973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick P. J., Danhauser L. L., Rustum Y. M., Bertram J. S. Changes in ribo- and deoxyribonucleoside triphosphate pools within the cell cycle of a synchronized mouse fibroblast cell line. Biochim Biophys Acta. 1983 Mar 15;756(1):36–40. doi: 10.1016/0304-4165(83)90021-1. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Vartanian J. P., Hultgren C., Plikat U., Karlsson A., Wang L., Eriksson S., Wain-Hobson S. Restriction and enhancement of human immunodeficiency virus type 1 replication by modulation of intracellular deoxynucleoside triphosphate pools. J Virol. 1994 Jan;68(1):535–540. doi: 10.1128/jvi.68.1.535-540.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Miller D. G., Garcia J. V., Lynch C. M. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- Miller D. G., Adam M. A., Miller A. D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990 Aug;10(8):4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen R. J., Buck C. R., Smith A. M. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992 Sep;116(1):201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F. H., Verma I. M., Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996 Apr 12;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Paillart J. C., Skripkin E., Ehresmann B., Ehresmann C., Marquet R. A loop-loop "kissing" complex is the essential part of the dimer linkage of genomic HIV-1 RNA. Proc Natl Acad Sci U S A. 1996 May 28;93(11):5572–5577. doi: 10.1073/pnas.93.11.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T. D., Rosman G. J., Osborne W. R., Miller A. D. Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1330–1334. doi: 10.1073/pnas.88.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin C., Dorfman T., Palú G., Göttlinger H., Sodroski J. Analysis in human immunodeficiency virus type 1 vectors of cis-acting sequences that affect gene transfer into human lymphocytes. J Virol. 1994 Jun;68(6):3888–3895. doi: 10.1128/jvi.68.6.3888-3895.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Scharfmann R., Axelrod J. H., Verma I. M. Long-term in vivo expression of retrovirus-mediated gene transfer in mouse fibroblast implants. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4626–4630. doi: 10.1073/pnas.88.11.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S. K., Black H. B., Goldwasser E., Leiden J. M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996 May;2(5):545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- Trono D., Feinberg M. B., Baltimore D. HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989 Oct 6;59(1):113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- Trono D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J Virol. 1992 Aug;66(8):4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M. Gene therapy: hopes, hypes, and hurdles. Mol Med. 1994 Nov;1(1):2–3. [PMC free article] [PubMed] [Google Scholar]
- Vicenzi E., Dimitrov D. S., Engelman A., Migone T. S., Purcell D. F., Leonard J., Englund G., Martin M. A. An integration-defective U5 deletion mutant of human immunodeficiency virus type 1 reverts by eliminating additional long terminal repeat sequences. J Virol. 1994 Dec;68(12):7879–7890. doi: 10.1128/jvi.68.12.7879-7890.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Ertl H. C., Wilson J. M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994 Aug;1(5):433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack J. A., Haislip A. M., Krogstad P., Chen I. S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992 Mar;66(3):1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Dornadula G., Pomerantz R. J. Endogenous reverse transcription of human immunodeficiency virus type 1 in physiological microenviroments: an important stage for viral infection of nondividing cells. J Virol. 1996 May;70(5):2809–2824. doi: 10.1128/jvi.70.5.2809-2824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Duan L. X., Dornadula G., Pomerantz R. J. Increasing transduction efficiency of recombinant murine retrovirus vectors by initiation of endogenous reverse transcription: potential utility for genetic therapies. J Virol. 1995 Jun;69(6):3929–3932. doi: 10.1128/jvi.69.6.3929-3932.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang Y., Spicer T. P., Abbott L. Z., Abbott M., Poiesz B. J. Reverse transcription takes place within extracellular HIV-1 virions: potential biological significance. AIDS Res Hum Retroviruses. 1993 Dec;9(12):1287–1296. doi: 10.1089/aid.1993.9.1287. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang Y., Spicer T., Henrard D., Poiesz B. J. Nascent human immunodeficiency virus type 1 reverse transcription occurs within an enveloped particle. J Virol. 1995 Jun;69(6):3675–3682. doi: 10.1128/jvi.69.6.3675-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Cunningham J. M. Minus-strand DNA is present within murine type C ecotropic retroviruses prior to infection. J Virol. 1993 Apr;67(4):2385–2388. doi: 10.1128/jvi.67.4.2385-2388.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]