Edited by Helaine E. Resnick, PhD, MPH
Rhein Inhibits Apoptosis in Pancreatic β-Cells by Blocking Hyperglycemia-Induced Drp1 Expression
A new report by Liu et al. (p. 3927) may help to explain the protective effect of rhein, an anthraquinone compound isolated from rhubarb. Rhein (4,5-dihydroxyanthraquinone-2-carboxylic acid) has been used medicinally in China for more than 2,000 years. Previous work has shown that rhein can improve glucose metabolism and ameliorate fatty liver disease in diabetic mice, as well as inhibit apoptosis of islet cells. However, the mechanism by which rhein plays a protective role has remained unclear. Hyperglycemia acts as a major factor causing β-cell apoptosis in type 2 diabetes, and prior studies have shown a key role for dynamin-related protein 1 (Drp1) in promoting hyperglycemia-induced β-cell apoptosis. In the study reported in this issue of Diabetes, Liu et al. administered 120 mg/kg rhein or vehicle for 8 weeks in db/db mice. Investigators also made use of the mouse pancreatic β-cell line NIT-1. Rhein significantly improved glucose tolerance and reduced fasting glucose. In vivo and in vitro cell apoptosis assays showed that rhein inhibited β-cell apoptosis, and morphological analysis found that rhein treatment prevented hyperglycemia-induced mitochondrial fission in pancreatic β-cells. Hyperglycemia-induced Drp1 expression was largely abolished by rhein treatment. In addition, rhein greatly decreased the induction of reactive oxygen species (ROS) in both the NIT-1 cells and isolated islets. Taken together, these findings suggest that rhein inhibits apoptosis in pancreatic β-cells by blocking hyperglycemia-induced Drp1 expression and that it may have potential as a therapeutic agent for the treatment of hyperglycemia associated with β-cell failure. — Laura Gehl, PhD
Liu et al. Rhein protects pancreatic β-cells from dynamin-related protein-1–mediated mitochondrial fission and cell apoptosis under hyperglycemia. Diabetes 2013;62:3927–3935
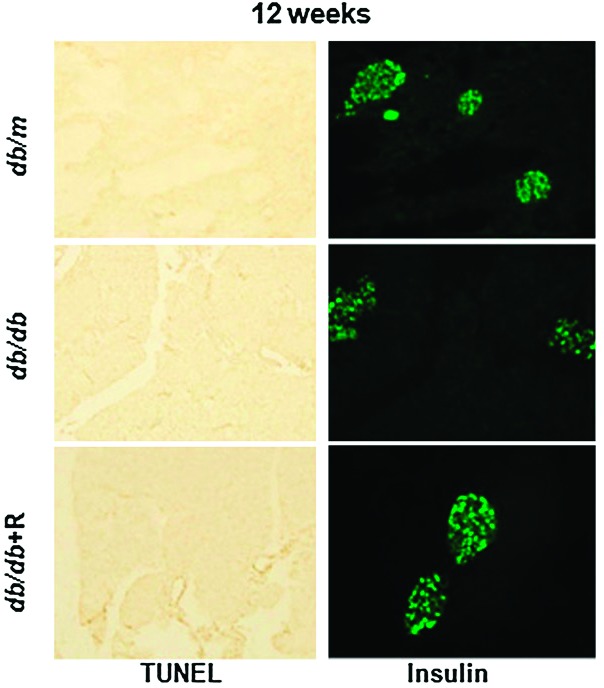
Protective effect of rhein on pancreatic β-cell apoptosis and function. Pancreatic tissues were collected from db/db mice at 12 weeks of age. To identify β-cells, the consecutive pancreatic sections were stained with anti-insulin antibody (green).
Teplizumab Treatment Prevents Loss of C-Peptide in Patients With New-Onset Type 1 Diabetes
In this issue of Diabetes, Herold et al. (p. 3766) explore the effect of teplizumab on C-peptide levels in individuals with new-onset type 1 diabetes. Although previous studies have shown the success of some immune therapies in type 1 diabetes, these frequently have limited response duration and some patients respond more robustly than others. The reasons why cyclosporin A, azathioprine plus prednisone, CTLA41g, rituximab, and Fc receptor–nonbinding anti-CD3 monoclonal antibody (teplizumab) treatments have not resulted in the persistent remission of diabetes, and why some individuals fail to respond to these treatments, remains unclear. In this new work, the investigators conducted a randomized, controlled trial of teplizumab in patients with new-onset type 1 diabetes. One hundred twenty-five subjects were screened less than eight weeks from diagnosis with type 1 diabetes, and eighty-three enrolled in the study. Of these, 6 dropped out, leaving 77 individuals on whom results were based. Participants in the intervention group received two courses of teplizumab. The first 14-day course was given at enrollment, and the second course was administered at 1 year. Participants in the intervention group showed reduced loss of C-peptide at 2 years after the first treatment. As in previous studies, response to the drug varied considerably. The investigators conducted post hoc analyses to identify immunologic and metabolic features that distinguished responders from nonresponders. These analyses showed that responders had lower levels of HbA1c and insulin use at baseline, as well as reduced numbers of Th1-like cells. These results may suggest that the response to immune treatment is influenced by the degree of metabolic control prior to initiating therapy. The new study underscores how drug actions interact with patient characteristics in ways that may determine the clinical value of a given therapy. — Laura Gehl, PhD
Herold et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 2013;62:3766–3774
Deletion of IRS Genes in Murine Models Leads to Heart Failure and Point to a Molecular Mechanism for Insulin Resistance
The need for understanding the link between diabetes and cardiac dysfunction is obvious: roughly two-thirds of patients with type 2 diabetes die of heart failure. Work by Qi et al. in this issue of Diabetes (p. 3887) highlights the crucial role of insulin receptor substrate (IRS) proteins in mediating cardiac function and suggests a molecular mechanism by which hyperinsulinemia induces insulin resistance in myocardial tissue. Earlier work has shown that the deletion of IRS genes disrupts insulin action in the liver and results in diabetes. In this new study, deletion of both IRS1 and IRS2 genes in the hearts of mice resulted in severe cardiac dysfunction compared with controls. Abnormalities included increased apoptosis, disruption of cardiac insulin signaling via Foxo1, decreased cardiac metabolic gene expression, and reduced cardiac ATP content, as well as sudden death beginning at 6–8 weeks of age. When one allele each of IRS1 and IRS2 was removed from the heart, mice developed cardiac dysfunction and showed a 50% reduction in myocardial IRS1 and IRS2 protein levels, indicating downregulation of these genes. Qi et al. conducted parallel experiments involving control mice and two treatments: diabetic dyslipidemic mice and mice given 4 months of a high-fat diet (HFD). Relative to controls, both diabetic and HFD mice exhibited significantly impaired cardiac function, downregulation of IRS1 and IRS2 genes, and decreased heart IRS1 and IRS2 protein levels. Further, p38 phosphorylation, a marker of metabolic stress, was increased in the hearts of these mice. In combination with the in vivo experiments, investigators found that in neonatal rats, prolonged (24-h) insulin exposure in vitro impaired the cardiac Akt→Foxo1 signaling cascade and decreased IRS1 and IRS2 protein levels relative to controls. Interestingly, when the 24-h insulin treatment was immediately followed by a repeated dose for 0.5 h, the effect of the 0.5-h dose on Akt→Foxo1 signaling was greatly attenuated. The investigators determined that overexpression of IRS1 and IRS2 compensated for the decreased Akt phosphorylation. In addition, chronic insulin exposure induced degradation of IRS1 and IRS2 proteins while increasing p38 phosphorylation, thereby revealing a molecular mechanism for the development of insulin resistance. The authors suggest that resensitizing Akt →Foxo1 signaling may be a promising direction for future investigations aimed at reducing the risk of heart failure in the setting of insulin resistance and type 2 diabetes. — Wendy Chou, PhD
Qi et al. Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38α MAPK during insulin resistance. Diabetes 2013;62:3887–3900
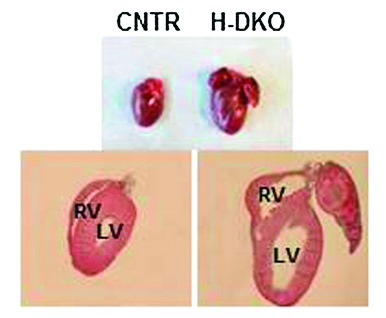
Cardiac morphology in ventricular chamber sections in heart-specific IRS1 and IRS2 gene double-knockout (H-DKO) and control (CNTR) mice at 5 weeks. LV, left ventricle; RV, right ventricle.
New Tools for Studying Insulin Granule Turnover
Impaired release of insulin from secretory granules (SGs) is a key feature of type 2 diabetes. However, the controls on insulin SG turnover are currently poorly understood, and progress has been impeded by an inability to precisely determine SG age. In this issue of Diabetes, Ivanova et al. (p. 3687) describe a new method for quantifying SG age, and they provide new data that improve our understanding of the release of newly synthesized insulin. The new report includes the first images of SGs of defined age through the use of genetically encoded fluorescent tags—SNAP tags—fused to human insulin. Applied in vitro and in vivo in mouse islets, this technique assessed insulin SGs’ spatial and age distribution with greater detail than has been possible with currently available methods. Among the key findings of the new report is the observation that the mean speed of processive SGs at the cell cortex decreases linearly over time from 3 to 6 h. In addition, older SGs exhibited decreased motility, while greater proximity to the cell surface also played a role in limiting displacement. It was important that the investigators were able to follow age-distinct pools of SGs in response to glucose stimulation, and they showed that newly synthesized insulin was released preferentially relative to older insulin. These new methods pave the way for further exploration of the underlying mechanisms regulating insulin SG mobilization, and they may therefore provide important new tools in understanding fundamental defects in insulin secretion in individuals with type 2 diabetes. — Wendy Chou, PhD
Ivanova et al. Age-dependent labeling and imaging of insulin secretory granules. Diabetes 2013;62:3687–3696
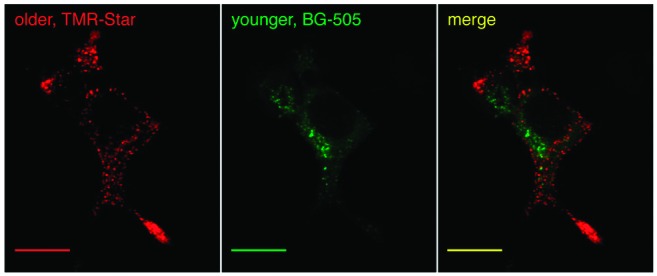
Simultaneous detection of age-distinct insulin SGs.


