Abstract
Trials of immune therapies in new-onset type 1 diabetes (T1D) have shown success, but not all subjects respond, and the duration of response is limited. Our aim was to determine whether two courses of teplizumab, an Fc receptor–nonbinding anti-CD3 monoclonal antibody, reduces the decline in C-peptide levels in patients with T1D 2 years after disease onset. We also set out to identify characteristics of responders. We treated 52 subjects with new-onset T1D with teplizumab for 2 weeks at diagnosis and after 1 year in an open-label, randomized, controlled trial. In the intent to treat analysis of the primary end point, patients treated with teplizumab had a reduced decline in C-peptide at 2 years (mean −0.28 nmol/L [95% CI −0.36 to −0.20]) versus control (mean −0.46 nmol/L [95% CI −0.57 to −0.35]; P = 0.002), a 75% improvement. The most common adverse events were rash, transient upper respiratory infections, headache, and nausea. In a post hoc analysis we characterized clinical responders and found that metabolic (HbA1c and insulin use) and immunologic features distinguished this group from those who did not respond to teplizumab. We conclude that teplizumab treatment preserves insulin production and reduces the use of exogenous insulin in some patients with new-onset T1D. Metabolic and immunologic features at baseline can identify a subgroup with robust responses to immune therapy.
A number of trials have shown that the progression of type 1 diabetes (T1D) can be modulated by immune therapies. Cyclosporin A, azathioprine plus prednisone, and, more recently, CTLA4Ig, rituximab, and Fc receptor (FcR)–nonbinding anti-CD3 monoclonal antibody (mAb) treatments have reduced the fall in C-peptide responses that occurs in the first 2 years after disease onset (1–5). While the effects of therapy are not permanent, there is evidence that in at least some individuals, responses to immune therapy may persist for as long as 3 years after diagnosis, whereas in others there is no response to drug treatment (6,7). The reasons why immune therapies have not induced lasting remissions of the disease and why some individuals are more responsive to treatment than others are not known. One factor may involve the pharmacokinetics of the immune therapy in individual subjects (8). However, even drugs that have been given continuously, such as CTLA4Ig or cyclosporin A, have diminishing effects over time (3,9). Alternatively, there may be individual factors that affect escape from the effects of immune therapy, such as immune receptor signaling pathways or inflammatory mediators (10,11). Finally, there may be factors that affect β-cells, such as inflammatory cytokines (12). Glucose toxicity itself has been thought to affect these responses. In the Diabetes Control and Complications Trial (DCCT), individuals in the intensive control group showed less decline in stimulated C-peptide levels than those in the conventional control group (13).
Identifying individuals who are likely to respond to drug therapy would be valuable for the selection of patients for treatment. In previous studies, we and others showed that a single course of FcR-nonbinding anti-CD3 mAb given soon after the diagnosis of T1D improved C-peptide responses for 1 year after diagnosis, but the responses waned after that time (1,2,5). Therefore, we conducted a prospective, randomized, controlled trial of teplizumab in patients with new-onset T1D to test the effects of two courses of the drug, 1 year apart, on C-peptide responses 2 years after diagnosis. Using post hoc analyses, we also sought to identify the clinical and immunologic features of subjects who showed clinical responses to the drug. Our data show that treatment with an FcR-nonbinding anti-CD3 mAb can preserve insulin secretion in patients with new-onset T1D. Metabolic control and insulin use at the time of study enrollment were the strongest predictors of response as well as immunologic features. The lasting effect of metabolic features on responses to immune therapy has not been previously appreciated and deserves further study.
RESEARCH DESIGN AND METHODS
Study design and patients.
This was a randomized, open-label study performed at six medical centers conducted between 2005 and 2011. Eligible individuals were between 8 and 30 years of age, diagnosed with T1D within 8 weeks of study enrollment, and positive for anti-GAD65, anti-ICA512, or ICA. Written consent was obtained from all participants. The study was approved by the institutional review boards at each institution. A data and safety monitoring board reviewed safety data at least yearly. This study is registered with clinicaltrials.gov (NCT00129259). The complete protocol is available at www.immunetolerance.org.
Randomization and masking.
Subjects were randomized to drug treatment or a control group in a 2:1 ratio within randomly ordered blocks of six or three. The study was open label, but core laboratory personnel were masked to the treatment assignments.
Treatment and assessments.
The drug treatment group received a 14-day course of teplizumab (day 1, 51 μg/m2; day 2, 103 μg/m2; day 3, 206 μg/m2; day 4, 413 μg/m2; days 5–14, 826 μg/m2; median cumulative dose 11.6 mg; interquartile range 5.7 mg) diluted in normal saline solution and administered intravenously (14). The control group did not receive a placebo infusion. Subjects received ibuprofen, diphenhydramine, acetaminophen, or all three for infusion-related reactions. Drug treatment was discontinued during a treatment cycle if thrombocytopenia (<140,000/mm3 on day 1 or <100,000/mm3 on other days), anemia, neutropenia, fever (grade 3 or higher), liver function abnormalities (of two to three times the upper limit of the reference interval), hyperbilirubinemia, international normalized ratio >1.3, or other adverse events (AEs) grade 3 or higher occurred. Participants were not permitted to receive the second treatment cycle if they had an acute febrile illness within 4 weeks or developed anti-idiotype antibodies of >1:1000 titer or IgE isotype. Both groups were seen at regular intervals and received contact by a certified diabetes educator (CDE) every 2 weeks with a target treatment goal of a HbA1c <7.5% (58 mmol/mol).
Subjects in both groups underwent a 4-h mixed-meal tolerance test (MMTT) every 6 months for 2 years, as described previously (2). After 1 year, subjects in the drug treatment group who had detectable C-peptide responses from MMTTs at all time points and who had not met stopping criteria for drug administration received a second course of teplizumab (n = 40) (median cumulative dose 12.4 mg, interquartile range 5.08 mg). A temporary hold in the protocol because of a drug manufacturing and safety review caused four of the first five subjects to receive their second course of drug later than the prescribed 1 year time point. These subjects had MMTTs within 100 days of 24 months after study entry. Insulin use was determined from patient logs and calculated as the average units per kilogram per day over the 3 days before the study visit.
Laboratory tests.
Autoantibodies were measured by radioimmunoassay at the Barbara Davis Center (Aurora, CO) and immunofluorescence was tested at the University of Florida. C-peptide and HbA1c levels were measured by two-site immunoenzymometric assay (Tosoh, San Francisco, CA) and ion exchange high-performance liquid chromatography (Bio-Rad Diagnostics) at the Northwest Lipid Research Laboratory (Seattle, WA) (3,15). The lower limit of detection in the C-peptide assay was 0.05 nmol/L. Serum teplizumab levels were measured in a subset (6) of drug-treated subjects using a previously defined flow cytometry–based assay (2). The peak teplizumab levels (mean ± SD) in serum collected before the last infusion of the first (day 13) and second cycles were 1095 ± 329 (n = 4) and 1097 ± 851 ng/ml (n = 2), respectively. The trough levels, calculated as the median of the values 1 and 6 h after the last infusion (where available), were 412 ± 215 and 514 ± 399 ng/ml, respectively.
Flow cytometry.
Peripheral mononuclear blood cells were isolated from whole blood at study entry and frozen at a core facility. Thawed cells were stained with 10-color panels, which included mAbs to CD2, CD4, CD8, CCR4, CCR5, CCR6, CCR7, CXCR3, CXCR5, CD25, CD28, CD38, CD39, CD45RA, CD45RO, CD127, Helios, Foxp3, TNFR2, PD-1, NKG2A (BD Pharmingen, San Jose, CA) and analyzed by flow cytometry on an LSRII cytometer. For intracellular cytokine staining, cells were stimulated for 6 h with or without phorbol myristic acid (500 ng/ml) and ionomycin (500 pg/ml) and stained with mAbs to interferon (IFN)-γ, tumor necrosis factor, interleukin (IL)-13, IL-17, and IL-10 (BD Pharmingen) and analyzed by flow cytometry.
Statistical analyses.
The 4-h C-peptide area under the curve (AUC) was calculated using the trapezoidal rule over the 4-h period (0–240 min). For this computation, the “time 0” C-peptide value was taken as the average of C-peptide values measured at time points −10 and 0 min. The AUC calculation was based on the time points available from the MMTT. Results reported as less than the lower limit of detection were imputed as zero for the MMTT at that time point. The primary end point was predefined as a comparison of the change in the mean 4-h C-peptide AUC from baseline, adjusted for the baseline C-peptide response, in the drug versus control groups at 24 months. Treated subjects who completed the MMTT at baseline and received at least 1 dose of study drug (n = 52) and subjects randomized to the control group with a baseline MMTT (n = 25) were used in the intent to treat (ITT) analysis. Seven subjects in the ITT population did not have an MMTT at month 24 (four in the control group, three in the teplizumab group). For the ITT analysis of the primary end point missing month 24 values were imputed using an algorithm designed to underestimate the true difference between arms. If the last available AUC value was zero, the missing month 24 AUC was imputed as zero. If the last observed AUC value was more than zero, the month 24 AUC values among subjects in the same arm were regressed on AUC values from the prior time point to yield a regression line and 95% CIs. Missing month 24 AUC values were imputed as predicted values from the upper 95% confidence band for control subjects and from the lower 95% confidence band for subjects in the teplizumab arm. A sensitivity analysis was conducted using the t statistic derived from the model with imputed data but reducing the degrees of freedom to account for the seven imputed values. The P values from both the primary and sensitivity analyses agreed to the fourth decimal place for the test of the treatment effect.
For group comparisons, C-peptide AUC values were transformed to ln(AUC + 1) values. An F test derived from an ANCOVA with baseline ln(AUC + 1) value as a covariate was used to compare treatment groups. Means and 95% CIs are presented on the untransformed scale. Percentage improvement was calculated as:
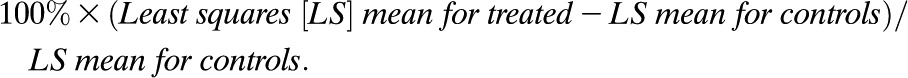 |
Similar methods, using a repeated measures analysis with baseline response as a covariate, were used for secondary clinical and mechanistic end points and for exploratory analyses using groups defined by responder status. Longitudinal analyses also were applied to test trends over time. For secondary and exploratory analyses, corrections were not made for multiple comparisons.
Baseline differences between treatment groups were evaluated using P values as descriptive measures of the strength of association; Mann-Whitney U tests and χ2 tests were used for continuous assessments and categorical characteristics, respectively. SAS software version 9.2 was used.
The sample size was based on a group comparison of ln(AUC + 1) after adjusting for baseline differences. On the basis of data from a prior study (1), we assumed that the ln(AUC + 1) after adjusting for baseline would be 0.309 in the teplizumab group. The current study was powered to detect a 50% worsening in adjusted 24-month C-peptide AUC in the control group compared with the treated group (i.e., assume mean AUC = [exp(0.31) − 1] = 0.36 for the teplizumab group compared with 0.181 for the control). With 2:1 randomization and a two-sided test of significance at α = 0.05, 72 subjects were needed for 92% power. The study accrued 77 subjects in the ITT population.
RESULTS
Recruitment and treatment of patients with new-onset T1D.
We screened 125 subjects <8 weeks from diagnosis of T1D and enrolled 83 (Fig. 1). Six subjects left the study before the first MMTT and are not included in the ITT analysis (n = 77). Fifty-two of the subjects randomized to drug treatment received all or part of the first drug course and are included in the ITT analysis; 15 discontinued teplizumab treatment after receiving some of cycle 1 or 2 (Supplementary Table 1). Twelve of the 15 subjects developed laboratory abnormalities or experienced AEs, leading to drug discontinuation. There were no significant differences between the study groups at entry (Table 1). The majority of subjects (94%) were <18 years old; 64% were ≤12 years old.
FIG. 1.
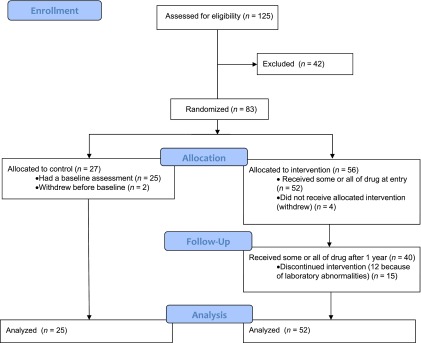
Enrollment, randomization, and participation. Subjects (n = 125) were screened and 83 were eligible for enrollment. The majority of subjects who were excluded failed to meet entry criteria. Of 83 subjects randomized, 25 in the control group and 52 in the teplizumab group underwent an MMTT at baseline, received the first dose of study drug (teplizumab group), and are included in the ITT analysis. Of the subjects randomized to the teplizumab group, 12 did not receive the second course of drug because of AEs leading to discontinuation during cycle 1 (n = 6), had predefined laboratory abnormalities that precluded readministration (n = 4), or were withdrawn (n = 2). In addition, 3 of 40 subjects who started cycle 2 discontinued treatment because of AEs. The reasons for drug discontinuation are listed in Supplementary Table 1.
TABLE 1.
Baseline characteristics
Effects of teplizumab treatment on C-peptide and clinical responses.
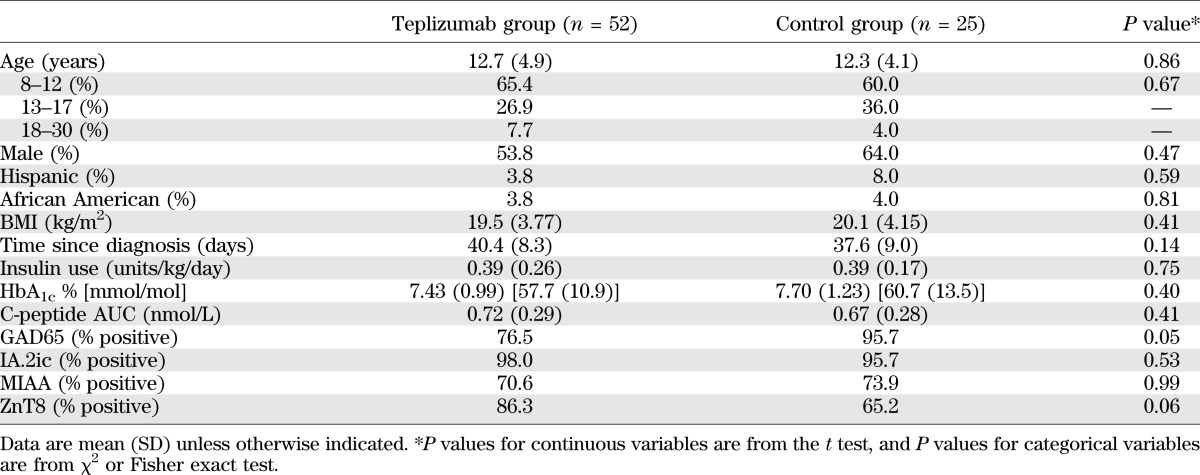
Teplizumab treatment significantly reduced the loss of C-peptide 2 years after study entry (P = 0.002; Table 2, Fig. 2A). After adjusting for the baseline imbalance, the mean C-peptide AUC level at year 2 was 75% higher in the teplizumab arm compared with controls. Likewise, the drop in C-peptide AUC from baseline to year 2, adjusting for baseline C-peptide AUC, was on average smaller in patients treated with teplizumab compared with the untreated controls as an absolute (P = 0.002) or percentage change (P < 0.001) from baseline (Table 2). Treated subjects were estimated to reach 6-month control group values 15.9 months later than the control group (i.e., month 21.9) (Fig. 2B). At the month 24 visit, more subjects treated with teplizumab had detectable levels of C-peptide compared with control subjects (P = 0.002; Fig. 2C).
TABLE 2.
Primary end point analysis of MMTT-stimulated 4-h C-peptide AUC (nanomoles per liter)
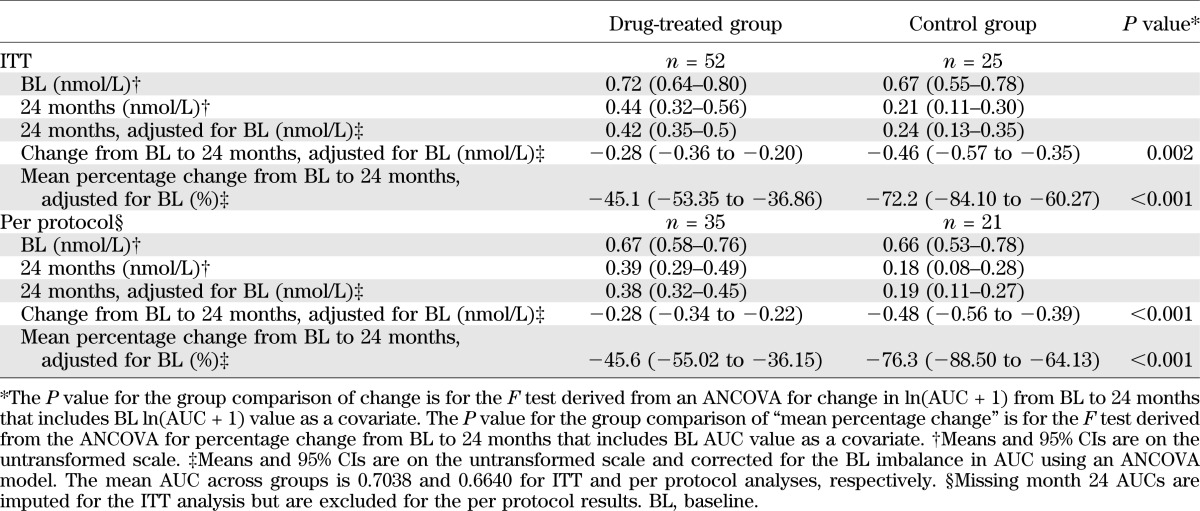
FIG. 2.
C-peptide (C-pep) responses in drug-treated and control subjects. A: The mean ± 25th and 75th percentiles of the C-pep AUC (nanomoles per liter) are shown for the drug and control groups (***P = 0.002; **P < 0.02, ANCOVA for each time point). B: Estimates and 95% CIs from a mixed effects model, with fixed effects for treatment group and linear and quadratic trends over time and random subject-level effects for intercepts and linear trends over time. Drug-treated subjects are estimated to have a delay of decline in C-pep by 15.9 months (i.e., would reach control 6-month values 21.9 months after study entry). C: Proportion of subjects with detectable C-pep (i.e., >0.05 nmol/L). The actual study dates when the MMTTs were performed are shown. There was a significantly greater loss of detectable C-pep secretion in the control group at month 24 (P = 0.002, χ2 test).
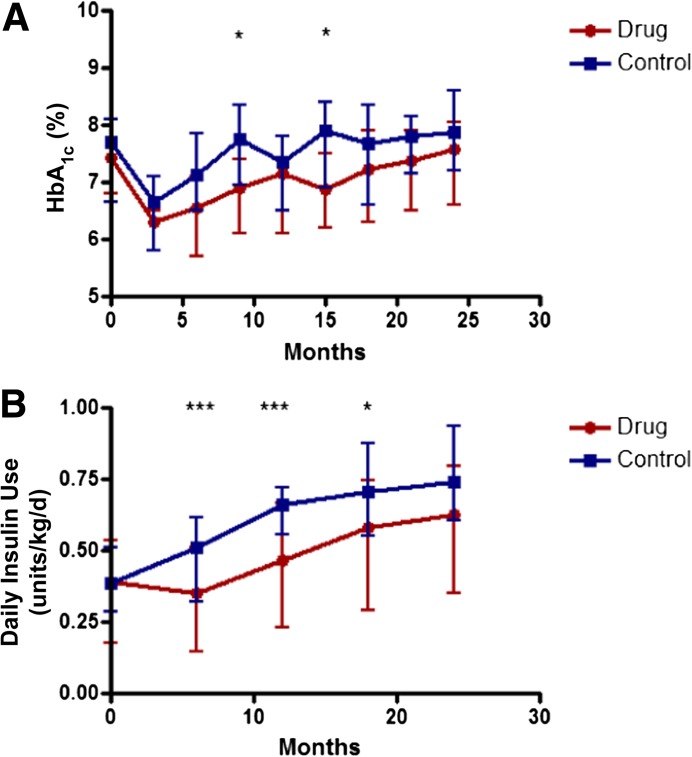
Intensive diabetes care with contact with a CDE was provided to all subjects to “treat to target” of <7.5% (58 mmol/mol). There was not a significant difference in the HbA1c levels in the drug and control groups (P = 0.093) over the 24-month study, although at 9 and 15 months the change from baseline HbA1c levels was significantly higher in the control group (P = 0.035 and 0.011, respectively; Fig. 3A). The drug-treated group used significantly less insulin to achieve this level of glycemic control (P = 0.036; Fig. 3B).
FIG. 3.
Insulin use and HbA1c in drug and control groups. A: HbA1c levels in the drug and control groups (means ± 25th and 75th percentiles are shown; *P < 0.05). B: Average insulin use in the 3 days before the visit was calculated (group means ± 25th and 75th percentiles; *P < 0.05, ***P < 0.005; P < 0.001 for overall trend at month 12, and P = 0.022 at month 18 using ANCOVA).
Adverse events.
Drug-related events were transient and resolved (Supplementary Table 2–5). There were a total of 11 serious AEs (SAEs) in 10 drug-treated subjects and 2 SAEs in 1 control subject (Supplementary Table 2). The AEs and those occurring in >15% of all subjects are shown in Supplementary Tables 3–5. Cytokine release syndrome was identified in five treated subjects. The rates of severe hypoglycemia were similar in the drug and control groups.
Of the patients in the drug-treated group who were seropositive for Epstein-Barr virus (EBV) (n = 21) and cytomegalovirus (n = 16), nine and two patients, respectively, had a transient increase in the viral load detected by PCR 1 month after the first course of drug treatment, and three and one, respectively, had an increase after the second course of the drug (range 100–5,500 copies/ml; median 200). Five subjects with an increase in EBV viral load had possible symptoms of transient EBV infection. In all subjects the viral loads were undetectable 2 months after treatment.
Effects of teplizumab treatment on autoantibodies.
The titers of biochemical autoantibodies were similar in the treatment groups at baseline. There was a significant reduction in the titer of anti–zinc transporter 8 antibodies but not the other biochemical autoantibodies in the drug-treated group at the end of year 1 (P < 0.05). The differences at 2 years were not statistically significant (Supplementary Fig. 1).
Identification of responders.
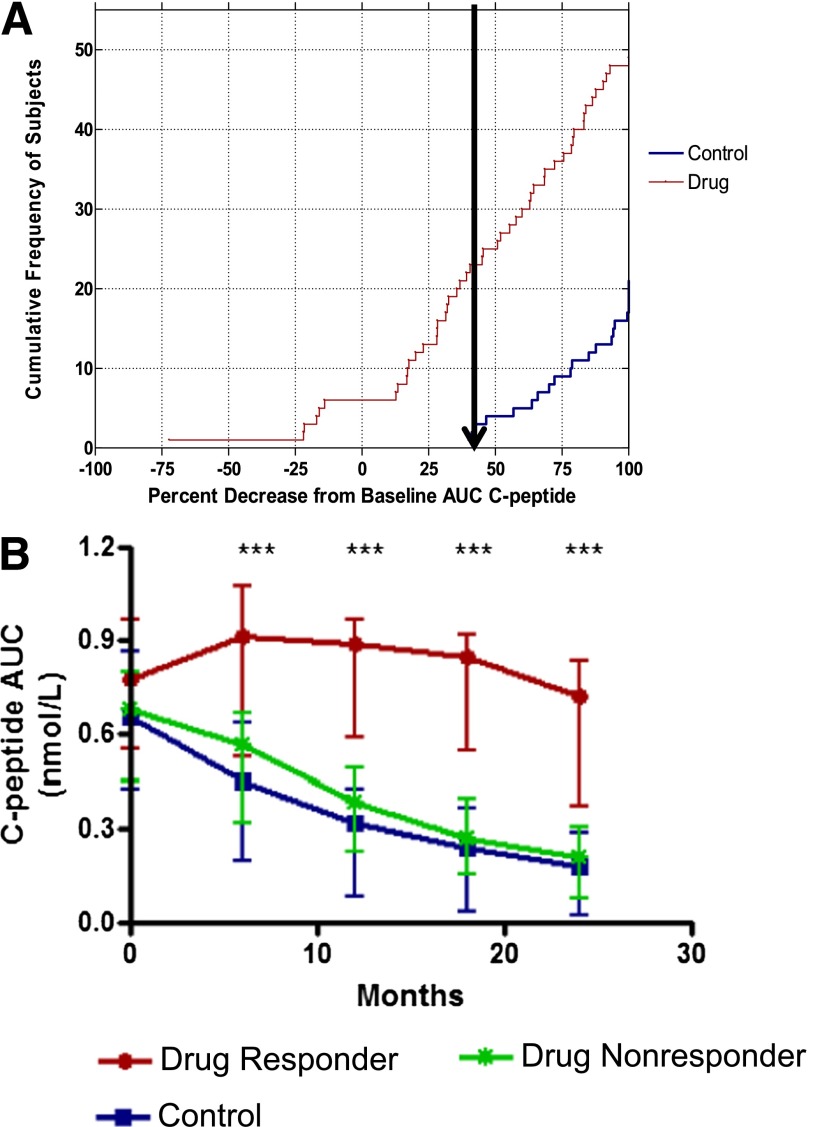
The C-peptide responses varied widely among drug-treated subjects, suggesting there was a spectrum of responses. In a post hoc analysis we sought to identify clinical and immunologic features that differentiated those individuals who did and did not show a response to teplizumab treatment. For the purpose of generating a hypothesis, we differentiated the responses of the drug-treated subjects based on changes in C-peptide secretion in the untreated control group at 24 months (Fig. 4A). All of the 25 control subjects lost ≥40% of the baseline C-peptide response by 24 months, and 27 of 49 drug-treated subjects showed a similar loss of ≥40%. Conversely, 22 of 49 of the drug-treated subjects (45%) lost <40% of baseline C-peptide; we refer to them as “responders.” The three subjects with missing month 24 C-peptide results were not evaluated for responder status.
FIG. 4.
Identification of clinical responders to teplizumab. A: The cumulative frequency of subjects and distribution of percentage decrease from baseline C-peptide AUC at month 24. The arrow shows the smallest percentage loss of C-peptide AUC in the control group. B: The C-peptide AUC at each time point (means ± 25th and 75th percentiles) is shown for the responders (red line) and nonresponders (green line) in the drug-treated group and for the control subjects (blue line). ***P < 0.001 between responders and nonresponders at each time point based on ANCOVAs.
Our analysis of the percentage loss of C-peptide, shown in Fig. 4A, did not indicate whether the pattern of the response to drug treatment reflected a true qualitative difference, in which the curves describing the change in the C-peptide responses were different from the untreated controls, or a quantitative difference, in which the curves describing the change in C-peptide responses were similar to the untreated control group but shifted over time, as suggested by our ITT analysis of all of the drug-treated subjects (Fig. 2A). Therefore, we compared the C-peptide AUC over time in the responders, nonresponders, and control subjects (Fig. 4B). This analysis suggested that the responders showed both quantitative and qualitative differences in their response to teplizumab. Even after 18 months, the average C-peptide level for responders was 113% of the baseline response and decreased from baseline by an average of only 6% at month 24. After adjusting for baseline differences, the C-peptide levels were nearly threefold (on average, 199%) higher among responders versus control subjects at month 24 but were not different in the nonresponders versus controls.
Metabolic and immunologic features of responders at baseline.
The patients’ age, sex, BMI, duration of disease, and baseline autoantibody titers were not predictors of the response to teplizumab treatment (Fig. 5A). The responders and nonresponders did not receive significantly different amounts of drug. However, clinical responders had lower HbA1c levels (mean 7.05% [95% CI 6.689–7.411%] vs. 7.78% [7.360–8.203%]; mean 53.54 mmol/mol [95% CI 49.59–57.49 mmol/mol] vs. 61.54 mmol/mol [56.93–66.14 mmol/mol]; P = 0.011) and used less insulin (mean 0.28 units/kg/day [95% CI 0.204–0.357 units/kg/day] vs. 0.49 units/kg/day [0.376–0.606 units/kg/day]; P = 0.004) at baseline (Fig. 5B) even after correcting for baseline C-peptide AUC (P = 0.018 and 0.008, respectively). The baseline C-peptide AUC responses in the responders (mean 0.78 nmol/L [95% CI 0.65–0.91 nmol/L]) and nonresponders (0.68 pmolM/L [0.56–0.79 pmolM/L]) were not significantly different (P = 0.245).
FIG. 5.
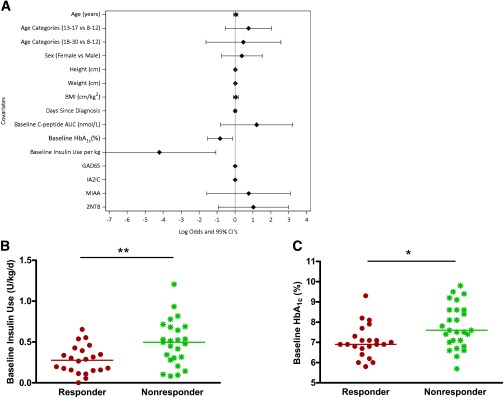
Baseline clinical characteristics in responders versus nonresponders. A: Ladder plot of covariates and clinical response. The effects of the indicated covariates on the ratio of log odds of responder status and 95% CIs are shown. The baseline HbA1c (P = 0.011) and insulin use (P = 0.004) were inversely associated with response. Baseline insulin use (B) and HbA1c (C) in the drug-treated responders (red) and nonresponders (green) (**P = 0.004, *P = 0.011).
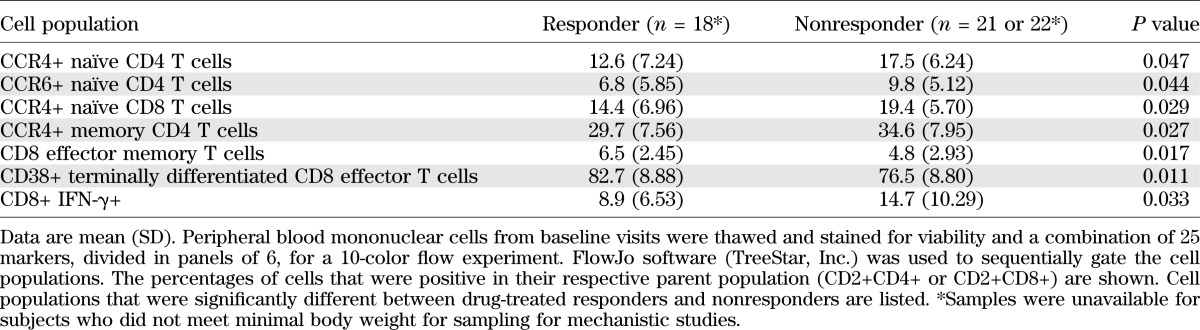
There was a lower frequency of CD4+CCR4+ memory and naïve T cells (P = 0.027 and 0.047), CD4+CCR6+ naïve CD4+ T cells (P = 0.044), naïve CCR4+ CD8+ T cells (P = 0.029), and IFN-γ-producing CD8+ T cells (P = 0.03) at baseline. There was a higher number of activated CD8+ terminally differentiated effector and CD8+ effector memory T cells (P = 0.011 and 0.017) in responders versus nonresponders (Table 3).
TABLE 3.
Immunologic features of responders at baseline
DISCUSSION
In this randomized controlled trial we show that two courses of treatment with FcR-nonbinding anti-CD3 mAb (teplizumab) reduced the loss of C-peptide 2 years after the first treatment in patients with new-onset T1D. Fewer drug-treated subjects lost detectable insulin production. Less exogenous insulin was needed to maintain equivalent or lower levels of HbA1c (3,4). However, the responses to the drug varied and, therefore, in a post hoc analysis, we sought to identify individuals who were clinical responders and distinct from the nonresponders and to compare the immunologic and metabolic features of these subgroups. The strongest differences were in metabolic features: responders had lower levels of HbA1c and insulin use at baseline. In addition, responders had reduced numbers of Th1-like cells and other differences in T-cell subsets. These findings suggest lasting effects of metabolic control before treatment on responses to immune therapy since the end point of the study was 2 years after enrollment and the glucose control during the study period was not significantly different.
The results of this trial suggest a more robust effect on the course of T1D (75% higher C-peptide responses vs. control) than was recently reported by the Protégé Study, a phase III trial that also used teplizumab (approximately 33% higher C-peptide responses vs. placebo) (14). A number of design differences in these two trials may account for this. Although the control arm did not receive placebo, subjects were randomized to treatment arms and balanced at baseline, and there were few dropouts. Our study was restricted to North American sites where the diagnosis of disease was made relatively early, there were fewer insulin requirements and lower HbA1c levels, and the frequency of diabetic ketoacidosis was low. Although we did not find a significant effect of age on treatment efficacy, unlike the Protégé trial, our subjects were younger, and the rate of decline in C-peptide responses is greater in younger subjects (16).
The differences in the rates of AEs and SAEs were greater in the drug-treated versus the control group in this trial. In addition to teplizumab-associated events, the drug-treated group was seen more frequently, which may have contributed to further imbalance in reporting AEs. The SAE most closely related to the study drug, cytokine release syndrome, was transient. Of the 52 subjects randomized to drug treatment, 12 were unable to receive the second course of the drug because of protocol-defined rules for stopping treatment. The rules were based on prior experience to prevent more significant AEs with continued drug administration (6), but the AEs were generally of low grade at the time of discontinuation.
We used a definition for responders based on the characteristics of the randomized control group in this study. Previous studies used definitions of responders based on the absence of a decline in C-peptide or features of the C-peptide assay (1,17), which, when applied to our dataset, identified a smaller subgroup. It is important to note that our designation of drug-treated subjects as responders and nonresponders did not reflect a clear bimodal division but rather was based on overall changes in the C-peptide responses in the drug-treated group that were different from the untreated controls, all of whom had levels at least 40% less than the baseline responses. Nevertheless, when analyzed in this way the responders and nonresponders had quantitatively and qualitatively different responses, which did not simply reflect a delay in the decline in the C-peptide response, as suggested by our ITT analysis, or as reported with other successful immune therapies such as abatacept or rituximab (3,4). Instead, in the responder group the effects of teplizumab were robust and durable, with C-peptide values above the baseline level for at least 18 months on average and a C-peptide response of almost three times that of the untreated group after 2 years, whereas in the nonresponders even the initial effect was modest. Within the responder group identified in this way, we did not find a clear bimodal distribution; there were subjects in whom the C-peptide response did not change or even increased after 2 years and others in whom the decline in C-peptide responses were slightly less than 40% of the baseline response. Our original objective in this trial was to test whether a second course of drug treatment at 1 year would improve the clinical responses that appeared to wane during the second year after a single course of teplizumab. The second course of teplizumab did not seem to have an effect on the drug-treated group as a whole, but the robust pattern of response in the responders raises the possibility that the second course was effective in maintaining C-peptide levels in this subset. However, we cannot directly assess this since we did not have a comparison group receiving a single treatment course.
A surprising finding was the differences in metabolic and immunologic features of the drug-treated responder group compared with the drug-treated nonresponders. HbA1c and insulin use at the time of study enrollment were significantly lower among responders compared with nonresponders. These parameters are thought to reflect endogenous β-cell function, yet baseline C-peptide AUC measurements were not significantly different between the groups. An effect of tight glucose control on preservation of C-peptide responses was shown in the DCCT, and for that reason all subjects kept in close contact with a CDE with the intent to maintain HbA1c ≤7.5% (58 mmol/mol) (13). The mechanisms whereby glucose control and insulin sensitivity might lead to improved responses are unknown. Metabolic pathways can affect effector and memory T-cell differentiation and function (18,19). Glucose can stimulate IL-1β production by β-cells, which may impair the effects of the FcR-nonbinding anti-CD3 mAb (20,21). Chronic exposure to elevated glucose causes a deterioration of β-cell function as a consequence of oxidative stress (22). The damage inflicted on β-cells before the immune therapy may render the cells incapable of recovery and repair. However, even more striking was the difference in insulin use between responders and nonresponders. Since we did not find a significant difference in C-peptide responses between the responders and nonresponders, this finding suggests differences in insulin sensitivity: chronic insulin resistance may cause metabolic stress of residual β-cells, rendering them susceptible to immune-mediated damage. The contribution of metabolic factors to immune therapeutic responses has not been reported within the drug-treatment groups in earlier immune therapy trials, but we observed an interesting similar trend in an analysis of two previous trials of teplizumab that we conducted (data not reported) (1,15). Although a trend for lower HbA1c (<6.0%), but not lower insulin usage, favoring response to abatacept, was suggested previously (3), that analysis compared drug- and placebo-treated subjects, whereas our analysis involved only subjects within the drug-treated group.
In addition, we found differences in immune parameters in the responder versus nonresponder groups. The decreased IFN-γ-producing CD8+ and CCR4+CD4+ T cells at baseline in responders suggest a lower frequency of Th1-like T cells, which may be contributory since a Th1 phenotype has been associated with pathogenic T cells (23–25). In this exploratory analysis we did not correct for multiple comparisons; in addition, longitudinal studies of these subsets and others that have been implicated as mediators of the effects of teplizumab will be necessary to understand the role of cellular responses in the clinical response (26–31).
In summary, we found a robust effect of an FcR-nonbinding anti-CD3 mAb in a subset of patients with new-onset T1D. Within this subgroup, we found differences in metabolic and immunologic parameters, suggesting that both contribute to the efficacy that we observed. Our studies support the potential value of this immunotherapeutic approach. The results highlight the interactions between host factors and drug action, which ultimately determine the clinical value of treatment.
ACKNOWLEDGMENTS
This research was performed as a project of the Immune Tolerance Network (National Institutes of Health contract no. N01 AI15416), an international clinical research consortium headquartered at the Benaroya Research Institute and supported by the National Institute of Allergy and Infectious Diseases and the Juvenile Diabetes Research Foundation. The work also was supported by grants UL1 RR024131 from the National Center for Research Resources and UL1 RR024139 from the National Center for Advancing Translational Science.
MacroGenics provided teplizumab. LifeScan Division of Johnson & Johnson provided blood glucose monitoring meters and strips to participants.
K.C.H. has received grant support from MacroGenics, Inc. J.A.B. has a patent on the teplizumab molecule. No other potential conflicts of interest relevant to this article were reported.
The sponsor (Immune Tolerance Network and the National Institute of Allergy and Infectious Diseases) participated in study design, analysis, and writing of the manuscript.
K.C.H., S.E.G., M.R.E., P.A.G., C.J.G., and W.H. carried out the clinical trial and wrote the manuscript. K.D.B. and L.K.-E. performed statistical analysis and wrote the manuscript. S.A., D.P., and J.A.B. analyzed mechanistic data and wrote the manuscript. P.H.S. served as clinical monitor, designed the protocol, and wrote the manuscript. J.M. designed the protocol, analyzed the data, and wrote the manuscript. K.C.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Special thanks is given to Margaret Lund Fitzgibbon, RN (National Institute of Allergy and Infectious Diseases).
APPENDIX
Other members of the AbATE Study Team include Melissa Johnson, Preeti Chugha, PhD, and Tracia Debnam (Immune Tolerance Network); Jennifer Sherr, MD, Frank Waldron-Lynch, MD, PhD, Linda Rink, RN, Kimberly Kunze, and Anna Wurtz (Yale University); Jennifer Bollyky, MD, Deborah Hefty, RN, CDE, Marli McCulloch-Olson, and Srinath Sanda, MD (Benaroya Research Institute); Stephen Rosenthal, MD, Saleh Adi, MD, Christine Torok, RN, and Rebecca Wesch (University of California, San Francisco); Jenna Lungaro, Allison Proto, RN, Amy Wallace, and Kimber Westbrook (Barbara Davis Center); and Rachel Hervey (Pacific Northwest Research Institute).
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0345/-/DC1.
Clinical trial reg. no. NCT00129259, clinicaltrials.gov.
A list of other members of the AbATE Study Team can be found in the appendix.
See accompanying commentary, p. 3656.
REFERENCES
- 1.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005;54:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 3.Orban T, Bundy B, Becker DJ, et al. Type 1 Diabetes TrialNet Abatacept Study Group Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011;378:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 6.Herold KC, Gitelman S, Greenbaum C, et al. Immune Tolerance Network ITN007AI Study Group Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol 2009;132:166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia 2010;53:614–623 [DOI] [PubMed] [Google Scholar]
- 8.Press RR, de Fijter JW, Guchelaar HJ. Individualizing calcineurin inhibitor therapy in renal transplantation—current limitations and perspectives. Curr Pharm Des 2010;16:176–186 [DOI] [PubMed] [Google Scholar]
- 9.Bougnères PF, Landais P, Boisson C, et al. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes 1990;39:1264–1272 [DOI] [PubMed] [Google Scholar]
- 10.Bertin-Maghit S, Pang D, O’Sullivan B, et al. Interleukin-1β produced in response to islet autoantigen presentation differentiates T-helper 17 cells at the expense of regulatory T-cells: Implications for the timing of tolerizing immunotherapy. Diabetes 2011;60:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 2008;29:44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 13.Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med 1998;128:517–523 [DOI] [PubMed] [Google Scholar]
- 14.Sherry N, Hagopian W, Ludvigsson J, et al. Protégé Trial Investigators Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 2011;378:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herold KC, Gitelman SE, Willi SM, et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia 2013;56:391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenbaum CJ, Beam CA, Boulware D, et al. Type 1 Diabetes TrialNet Study Group Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes 2012;61:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herold KC, Pescovitz MD, McGee P, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J Immunol 2011;187:1998–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerriets VA, Rathmell JC. Metabolic pathways in T cell fate and function. Trends Immunol 2012;33:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol 2005;174:4670–4677 [DOI] [PubMed] [Google Scholar]
- 20.Mandrup-Poulsen T, Spinas GA, Prowse SJ, et al. Islet cytotoxicity of interleukin 1. Influence of culture conditions and islet donor characteristics. Diabetes 1987;36:641–647 [DOI] [PubMed] [Google Scholar]
- 21.Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002;110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 2003;52:581–587 [DOI] [PubMed] [Google Scholar]
- 23.Antonelli A, Fallahi P, Ferrari SM, et al. Serum Th1 (CXCL10) and Th2 (CCL2) chemokine levels in children with newly diagnosed type 1 diabetes: a longitudinal study. Diabet Med 2008;25:1349–1353 [DOI] [PubMed] [Google Scholar]
- 24.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh N, Hanafusa T, Miyazaki A, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest 1993;92:2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ablamunits V, Bisikirska B, Herold KC. Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF. Eur J Immunol 2010;40:2891–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ablamunits V, Henegariu O, Preston-Hurlburt P, Herold KC. NKG2A is a marker for acquisition of regulatory function by human CD8+ T cells activated with anti-CD3 antibody. Eur J Immunol 2011;41:1832–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest 2005;115:2904–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belghith M, Bluestone JA, Barriot S, Mégret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 2003;9:1202–1208 [DOI] [PubMed] [Google Scholar]
- 30.You S, Leforban B, Garcia C, Bach JF, Bluestone JA, Chatenoud L. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc Natl Acad Sci U S A 2007;104:6335–6340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldron-Lynch F, Henegariu O, Deng S, Preston-Hurlburt P, Tooley J, Flavell R, Herold KC. Teplizumab induces human gut-tropic regulatory cells in humanized mice and patients. Sci Transl Med 2012;4:118ra112. [DOI] [PMC free article] [PubMed] [Google Scholar]