Abstract
γ-Aminobutyric acid (GABA) has been shown to inhibit apoptosis of rodent β-cells in vitro. In this study, we show that activation of GABAA receptors (GABAA-Rs) or GABAB-Rs significantly inhibits oxidative stress–related β-cell apoptosis and preserves pancreatic β-cells in streptozotocin-rendered hyperglycemic mice. Moreover, treatment with GABA, or a GABAA-R– or GABAB-R–specific agonist, inhibited human β-cell apoptosis following islet transplantation into NOD/scid mice. Accordingly, activation of GABAA-Rs and/or GABAB-Rs may be a useful adjunct therapy for human islet transplantation. GABA-R agonists also promoted β-cell replication in hyperglycemic mice. While a number of agents can promote rodent β-cell replication, most fail to provide similar activities with human β-cells. In this study, we show that GABA administration promotes β-cell replication and functional recovery in human islets following implantation into NOD/scid mice. Human β-cell replication was induced by both GABAA-R and GABAB-R activation. Hence, GABA regulates both the survival and replication of human β-cells. These actions, together with the anti-inflammatory properties of GABA, suggest that modulation of peripheral GABA-Rs may represent a promising new therapeutic strategy for improving β-cell survival following human islet transplantation and increasing β-cells in patients with diabetes.
A central focus of research in the type 1 diabetes (T1D) field is to develop ways to safely improve β-cell survival and function and promote their replication. The addition of γ-aminobutyric acid (GABA) or the GABAB receptor (GABAB-R)–specific agonist baclofen to culture media has been shown to inhibit β-cell apoptosis in cultured rodent cell lines and islets (1,2). It remains to be determined whether GABA treatment can inhibit mouse β-cell apoptosis in vivo or, more importantly, whether it can protect human β-cells from stress-induced apoptosis. If GABA can inhibit human β-cell apoptosis, elucidating whether this effect is mediated through the G-protein–coupled GABAB-Rs, and/or the chloride channel GABAA-Rs will enable more specific drug targeting.
GABA can promote neurogenesis and neuronal proliferation and is a neuronal survival factor (3–8). GABA has also been shown to promote rodent β-cell replication (1,2). Those studies, however, differentially pointed to GABAA-Rs or GABAB-Rs as modulators of GABA’s effects, making it important to clarify whether one or both types of GABA receptors modulate rodent β-cell replication. While a number of mitogens and growth factors can promote rodent β-cell replication, most fail to promote human β-cell replication (reviewed in refs. 9,10). Therefore, a key question is whether GABA can promote human β-cell replication. Even a small amount of GABA-induced human β-cell replication may be clinically useful by lowering insulin requirements and reducing the risk for long-term complications in T1D patients (11).
RESEARCH DESIGN AND METHODS
Analysis of mouse and human β-cell apoptosis.
All experiments were approved by University of California Los Angeles’ Animal Research Committee. Male C57BL/6 mice (10 weeks old) received streptozotocin (STZ) (Sigma-Aldrich; 80 mg/kg/day for 2 days) intraperitoneally. Mildly hyperglycemic mice (blood glucose levels of 250–300 mg/dL) were given plain water or water containing GABA (2 or 6 mg/mL; Sigma-Aldrich), the GABAB-R–specific agonist baclofen (0.25 mg/mL; Sigma-Aldrich), or the GABAA-R–specific agonist muscimol (4.5 mg/mL; Bachem Bioscience). Forty-eight hours later, their pancreatic sections (4 μm) were subjected to TUNEL using a POD cell death detection kit (Boehringer Ingelheim). After development with diaminobenzidine, the sections were stained with guinea pig anti-insulin (Zymed Laboratories) at 4°C overnight and alkaline phosphatase–conjugated anti-guinea pig IgG (Piece) and visualized using the Valcan Fact Red Chromogen Kit 2 (Biocare Medical). The percentages of insulin+ β-cells or TUNEL+ apoptotic islet cells in total islet cells of at least 25 islets from individual mice were calculated in a blinded manner.
To study apoptosis in human islets, freshly isolated human islets from the Integrated Islet Distribution Program were implanted under the kidney capsule (2,000 islets/mouse) of STZ-induced diabetic NOD/scid mice. The recipients were treated as described above. Their kidney sections (4 μm) were stained by TUNEL using a click-iT TUNEL Alexa Fluor 488 (Invitrogen) and costained with guinea pig anti-insulin. Subsequently, the sections were incubated with phycoerythrin (PE)–anti-guinea pig IgG and counterstained with DAPI. At least 2,500 human islet cells in 10 fields (original magnification ×400) from individual grafts were counted. The percentages of insulin+ β-cells or TUNEL+ apoptotic β-cells in total islet cells within the grafts of individual recipients were determined in a blinded manner.
Analysis of mouse β-cell replication.
C57BL/6 mice were STZ-rendered mildly hyperglycemic and randomly provided with water containing: 1) BrdU (0.8 mg/mL; Sigma-Aldrich), 2) BrdU plus GABA (2 or 6 mg/mL), or 3) BrdU plus baclofen (0.25 mg/mL) for 14 days. Other mice received a 14-day micro-osmotic pump (Alzet) subcutaneously containing 2 mg muscimol in 100 μL of PBS or vehicle alone and water containing BrdU. At the end of treatment, their pancreatic sections (5 μm) were stained with guinea pig anti-insulin and biotinylated anti-BrdU (AbD Serotec) followed by fluorescein isothiocyanate–anti-guinea pig IgG and PE-streptavidin and counterstaining with 4′,6-diamidino-2-phenylindole (DAPI). The percentages of BrdU+insulin+ β-cells in DAPI+ islet cells of 25 islets from individual mice were determined in a blinded manner.
Analysis of human β-cell replication.
Mildly hyperglycemic NOD/scid mice were implanted with adult human islets (2,000 islets/mouse) under the kidney capsule. The mice were treated for 14 days, as described above. At the end of treatment, the kidney tissue sections (5 μm) were subjected to immunofluorescent analysis of BrdU+insulin+ human β-cells, as described above. In another set of experiments, kidney tissue sections were subjected to immunofluorescent analysis of BrdU+insulin+ and Ki67+insulin+ human β-cells using PE–anti-human Ki67 (eBioscience). The percentages of BrdU+insulin+ or Ki67+insulin+ β-cells in 2,500 islet cells of 10 fields (original magnification ×400) of each islet graft were determined in a blinded manner.
Statistical analysis.
Data are expressed as the mean ± SEM of individual groups (N = 4–9/group) from two separate experiments. The difference between groups was determined by Student t test. A P value of <0.05 was considered statistically significant.
RESULTS
We treated C57BL/6 mice with STZ to induce β-cell oxidative stress and mild hyperglycemia and then randomized them into groups that received plain water, water containing GABA (2 or 6 mg/mL), the GABAB-R–specific agonist baclofen, or the GABAA-R–specific agonist muscimol for 48 h. The percentages of apoptotic islet cells and insulin+ β-cells in individual mice were characterized by TUNEL and immunohistochemistry assays (Fig. 1A and 2). The frequency of apoptotic islet cells in mice treated with GABA at 2 mg/mL was significantly reduced compared with that in control mice (Fig. 2A). The percentage of apoptotic islet cells in mice treated with GABA at 6 mg/mL was even lower (Fig. 2A). Thus, GABA treatment inhibited oxidative stress–related β-cell apoptosis, and its affects tended to be dose-dependent. Similarly, treatment with baclofen or muscimol decreased the frequency of apoptotic islet cells (Fig. 2A). Additionally, the percentages of insulin+ β-cells in the mice treated with GABA, baclofen, or muscimol were significantly higher than that in the control mice (Fig. 2B). Thus, activation of either GABAA-Rs or GABAB-Rs preserved β-cells from STZ-induced apoptosis.
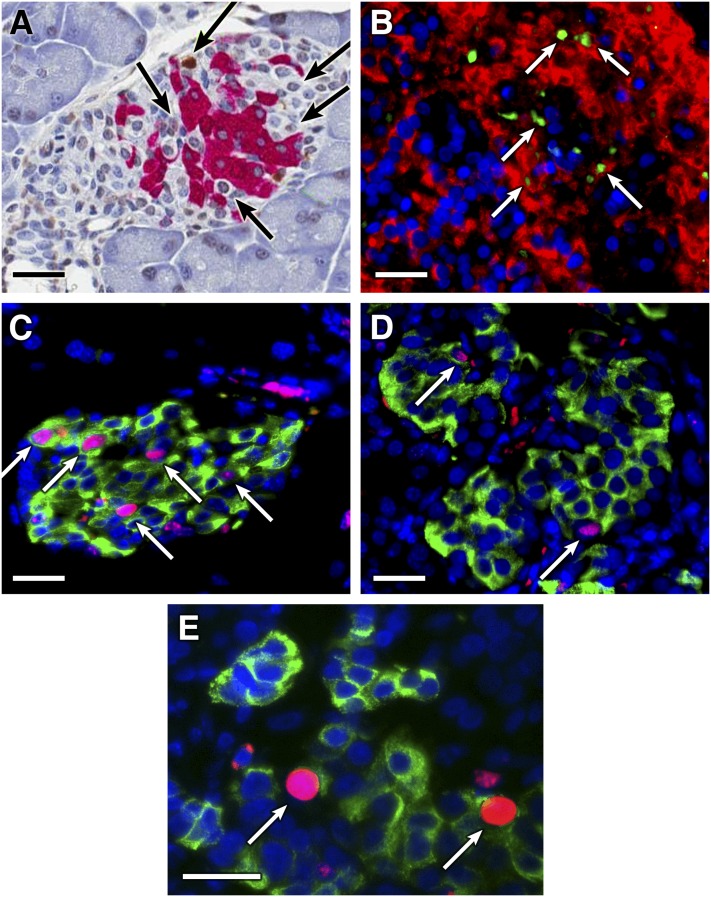
FIG. 1.
Analysis of β-cell apoptosis and replication in vivo. A and B: TUNEL analysis of mouse and human apoptotic β-cells in vivo. The apoptotic β-cells in mouse pancreas and human islet grafts in mice were characterized by immunohistochemistry- or immunofluorescent-based TUNEL, respectively, followed by anti-insulin staining. Data shown are representative images (original magnification ×400) of apoptotic β-cells in mouse islet (A) and human islet graft (B; green for TUNEL+, red for anti-insulin+). Arrows indicate TUNEL+ islet cells. The replication of mouse β-cells (C) and human β-cells within islet grafts (D) in hyperglycemic mice were characterized by immunofluorescent assays using anti-BrdU (red) and anti-insulin (green) staining. E: Representative image of anti-Ki67 (red) and anti-insulin (green) staining cells in a human islet graft. Data are representative images (original magnification ×400). Arrows indicate BrdU+Insulin+ or Ki67+insulin+ cells. Scale bars, 50 μm.
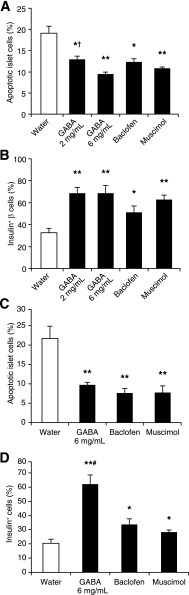
FIG. 2.
Oral GABA administration inhibits mouse and human islet cell apoptosis in vivo. C57BL/6 mice were STZ-rendered mildly hyperglycemic and given plain water or water containing GABA, baclofen, or muscimol for 48 h during which period all mice remained hyperglycemic. The percentages of apoptotic islet cells in mice were characterized by TUNEL and immunohistochemistry with anti-insulin. A: The percentages of apoptotic islet cells in hyperglycemic mice. B: The percentages of insulin+ β-cells. Mildly hyperglycemic NOD/scid mice were implanted with 2,000 human islets and treated as described in Research Design and Methods. The percentages of apoptotic islet cells were characterized using Alexa Fluor 488–based TUNEL, PE–anti-insulin, and DAPI. C: The percentages of apoptotic human islet cells. D: The percentages of human insulin+ β-cells. Data are expressed as the mean ± SEM of the percentages of apoptotic islet cells or insulin+ β-cells in different groups of mice (N = 4–8 mice/group) in two independent experiments. There were no obvious inflammatory infiltrates in pancreatic islets. *P < 0.05, **P < 0.01 vs. the control water group, †P < 0.05 vs. GABA 6 mg/mL, #P < 0.05 vs. baclofen and muscimol.
We next examined the ability of GABA administration to limit β-cell apoptosis after human islet implantation. Transplanted islets undergo immediate hypoxic stress due to lack of vascularization, and a large percentage of β-cell mass is lost due to apoptosis within the first 3 days after implantation (12–14). Hyperglycemic NOD/scid mice received human islets under their kidney capsule and plain water or water containing GABA (6 mg/mL), baclofen, or muscimol. Two days later, their kidney sections were stained by TUNEL and anti-insulin antibodies (Fig. 1B). In comparison with the control group, there were significantly reduced percentages of apoptotic cells and increased frequencies of insulin+ β-cells in human islets that were implanted into mice that were treated with GABA, baclofen, or muscimol (Fig. 2C and D). Thus, activation of either GABAA-R or GABAB-R limits β-cell apoptosis in transplanted human islets. Accordingly, the inclusion of GABA in the treatment regimen following clinical human islet transplantation may reduce the number of islets required to achieve insulin independence.
To assess whether oral GABA promotes mouse β-cell replication in vivo, we STZ-rendered C57BL/6 mice mildly hyperglycemic (∼250–300 mg/dL) and provided them with water containing BrdU with, or without, GABA (2 or 6 mg/mL) for 14 days. Since: 1) low levels of β-cell replication would be difficult to detect as a change in β-cell mass and 2) GABA treatment can promote functional recovery of degranulated β-cells, leading to an increase in insulin+ islet cells and an apparent increase in β-cell mass, we first assessed β-cell replication using anti-insulin and anti-BrdU staining (Fig. 1C). While BrdU+insulin+ β-cells accounted for ∼1% of the islet cells in control mice, newly replicated β-cells reached ∼2.2 and 3.1% of the islets cells in mice that had been treated with 2 and 6 mg/mL of GABA, respectively (Fig. 3A). Since anti-BrdU staining can reflect DNA repair within damaged β-cells, we examined the frequency of BrdU+insulin+ cells that had a punctated BrdU+ staining pattern. There was no significant difference in the frequency of punctated BrdU+ islet cells among these groups of mice (data not shown), indicating that DNA repair in β-cells was similar in all groups. Additionally, we observed that the percentages of insulin+ cells in pancreatic islets significantly increased in both GABA-treated groups, relative to that in the control mice (Fig. 3B). Thus, oral GABA treatment promoted β-cell replication and functional recovery in hyperglycemic mice.
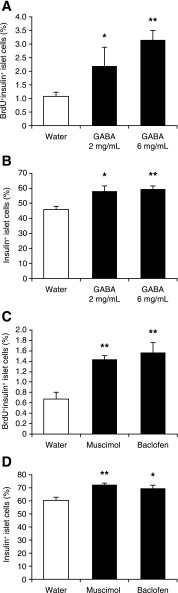
FIG. 3.
Oral GABA promotes mouse β-cell replication in vivo. C57BL/6 mice were STZ-rendered moderately hyperglycemic and given water containing BrdU with, or without, the indicated doses of GABA for 14 days. In another set of experiments, hyperglycemic mice were given water containing baclofen and BrdU or implanted subcutaneously with a 14-day micropump containing muscimol or vehicle (PBS) and fed with water containing BrdU for 14 days. None of the mice became normoglycemic during the 14-day observation period. The replication of β-cells in individual mice was characterized by immunofluorescent assays using anti-BrdU and anti-insulin and counterstaining with DAPI. A and C: The percentages of BrdU+insulin+ β-cells in mice. B and D: The percentages of insulin+ β-cells in mice. Data are expressed as the mean ± SEM of the percentages of BrdU+ and/or insulin+ islet cells in different groups of mice (N = 4–9 mice/group) from two independent experiments. *P < 0.05, **P < 0.01 vs. the control water group.
To assess whether GABAA-R and/or GABAB-R activation mediated GABA’s effect on β-cell replication, in another set of experiments, we treated hyperglycemic C57BL/6 mice with baclofen or muscimol together with BrdU for 14 days. We observed that treatment with either muscimol or baclofen elevated the percentages of BrdU+insulin+ β-cells in the pancreatic islets by more than twofold, as compared with that in the control group (Fig. 3C). Thus, activation of either GABAA-Rs or GABAB-Rs promotes mouse β-cell replication. We also observed a greater number of insulin+ cells in pancreatic islets from muscimol- or baclofen-treated mice, relative to that in the control mice (Fig. 3D). Collectively, our data indicate that GABA, through GABAA-Rs and GABAB-Rs, enhanced β-cell replication and functional recovery in hyperglycemic mice.
We next examined whether oral GABA promotes human β-cell replication in mice following islet transplantation. Adult human islets were implanted under the kidney capsule of hyperglycemic NOD/scid mice. These mice were randomized and provided with water containing BrdU with, or without, GABA or baclofen for 14 days. Other groups of mice received PBS or PBS plus muscimol via an osmotic mini-pump for 14 days, along with water containing BrdU. At the end of treatment, kidneys containing the islet grafts were removed, and the percentages of BrdU+insulin+ β-cells in total islet cells within the islet grafts of individual recipients were determined by immunofluorescent assays (Fig. 1D). We observed that while ∼0.8% of human islet cells were BrdU+insulin+ in the control group, ∼2% of islet cells were BrdU+insulin+ in mice that had been treated with GABA, baclofen, or muscimol (Fig. 4A). Additionally, we observed that GABA, baclofen, and muscimol treatment significantly increased the percentages of total insulin+ cells within the islet grafts, as compared with that in the control grafts (Fig. 4B). In an additional set of human islet transplantation studies, we again observed that GABA treatment increased the frequency of BrdU+insulin+ β-cells (data not shown), as well as Ki67+insulin+ β-cells, compared with the control group (0.89 vs. 0.4%; P < 0.02; [Figs. 1E and 4C]). Thus, both anti-BrdU and anti-Ki67 staining demonstrate that GABA treatment enhanced human β-cell replication. Conceivably, GABA-R promoted β-cell replication may reduce exogenous insulin requirements and limit the development of hyperglycemia-related complications in T1D patients.
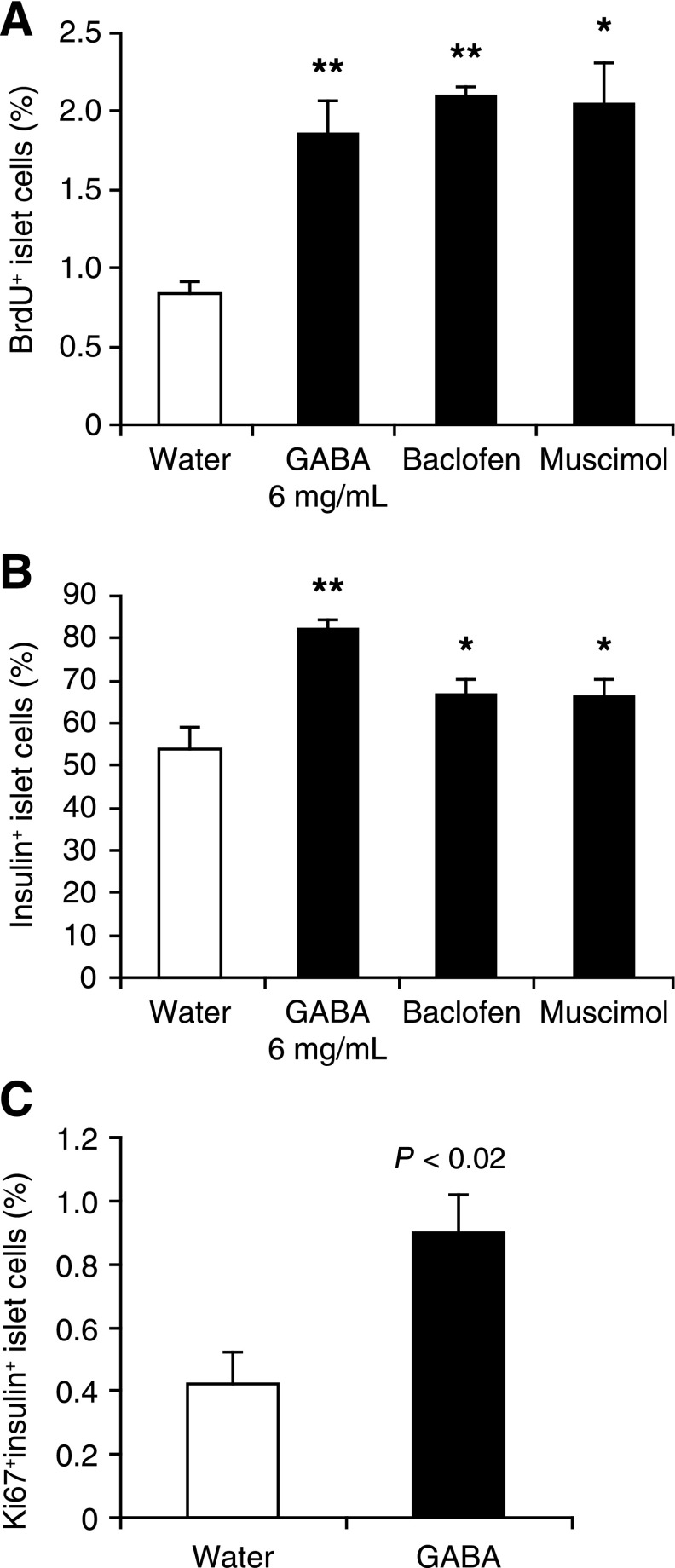
FIG. 4.
GABAA-R and GABAB-R activation promotes human β-cell replication in transplanted islets. Hyperglycemic NOD/scid mice were transplanted with human islets under the kidney capsule and randomly treated as described in the research design and methods. All islets recipients became normoglycemic within 2 days after transplantation and remained so during the observation period. The replication of human β-cells in the islet grafts was characterized by immunofluorescent assays using PE–anti-BrdU and fluorescein isothiocyanate–anti-insulin, followed by counterstaining with DAPI. A: The percentages of BrdU+insulin+ β-cells in the islet grafts. B: The percentages of insulin+ β-cells in the grafts. In another set of experiments, the kidney tissue sections (5 μm) were subjected to immunofluorescent analysis of BrdU+insulin+ and Ki67+insulin+ human β-cells using PE–anti-human Ki67. C: The percentages of Ki67+insulin+ β-cells. Data are expressed as the mean ± SEM of the percentages of BrdU+insulin+, Ki67+insulin+, or insulin+ islet cells in different groups of mice (N = 4–9 mice/group) from three separate experiments. Significantly higher percentages of BrdU+insulin+ or insulin+ islet cells were observed in the GABA-treated grafts (data not shown). *P < 0.05, **P < 0.01 vs. the water group.
In summary, we observed that activation of GABAA-R or GABAB-R inhibited oxidative stress–related β-cell apoptosis and preserved pancreatic β-cells in hyperglycemic mice. Similarly, treatment with either a GABAA-R– or GABAB-R–specific agonist inhibited human islet cell apoptosis in mice following islet transplantation. Furthermore, treatment with either a GABAA-R– or GABAB-R–specific agonist promoted mouse and human β-cell replication in mice. Hence, GABA acts as a growth factor that regulates the survival and replication of islet β-cells.
GABA can inhibit autoreactive Th1 cell responses directly ex vivo (15–17), increase regulatory T cells (2,18), inhibit antigen-presenting cell function (2,19), and inhibit inflammation in mouse models of T1D (2,16,20), rheumatoid arthritis (17), multiple sclerosis (19), and type 2 diabetes (18). Twenty-six weeks of GABA treatment did not significantly alter the numbers or percentages of splenic CD4+, CD8+, T, and B lymphocytes (16), nor did long-term GABA treatment desensitize T cells to GABA-mediated inhibition (16,17). Several studies have suggested that local inflammation helps promote β-cell replication (21 and references therein). Accordingly, although inflammation should be very limited in the C57Bl/6 and NOD/scid mouse models that we have studied, GABA’s anti-inflammatory activity may have partially counteracted its pro–β-cell replication activity.
GABA’s anti-inflammatory properties, together with its ability to promote β-cell replication and functional recovery, suggest that modulation of peripheral GABA-Rs may be a promising strategy for preserving and increasing islet β-cells. GABA has little capacity to pass through the blood–brain barrier and is safe for human consumption (22–25). Therefore, our findings may provide a basis for the design of new therapies for patients with type I and II diabetes.
ACKNOWLEDGMENTS
This work was supported by grants from JDRF (40-2009-710 and 17-2012-30) and the National Institutes of Health (DK-092480 to D.L.K.). J.T. and D.L.K. are inventors of GABA-related patents. D.L.K. serves on the Scientific Advisory Board of Diamyd Medical. No other potential conflicts of interest relevant to this article were reported.
J.T. conceived, designed, and performed the experiments; analyzed the data; and wrote the manuscript. H.D. performed the experiments and analyzed the data. Z.C., A.G., Y.J., and M.A.A. performed the experiments. D.L.K. conceived and designed the experiments and wrote the manuscript. J.T. and D.L.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors regret that space limitations preclude referencing many previous studies that provided important contributions to the field. The authors thank Peter Butler for comments on the manuscript and the Integrated Islet Distribution Program for providing isolated human islets.
Footnotes
See accompanying commentary, p. 3674.
REFERENCES
- 1.Ligon B, Yang J, Morin SB, Ruberti MF, Steer ML. Regulation of pancreatic islet cell survival and replication by gamma-aminobutyric acid. Diabetologia 2007;50:764–773 [DOI] [PubMed] [Google Scholar]
- 2.Soltani N, Qiu H, Aleksic M, et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci USA 2011;108:11692–11697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci 2007;30:1–8 [DOI] [PubMed] [Google Scholar]
- 4.Yuan TF. GABA effects on neurogenesis: an arsenal of regulation. Sci Signal 2008;1:jc1. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda Y, Nishiyama N, Saito H, Katsuki H. GABAA receptor stimulation promotes survival of embryonic rat striatal neurons in culture. Brain Res Dev Brain Res 1997;98:253–258 [DOI] [PubMed] [Google Scholar]
- 6.Fiszman ML, Borodinsky LN, Neale JH. GABA induces proliferation of immature cerebellar granule cells grown in vitro. Brain Res Dev Brain Res 1999;115:1–8 [DOI] [PubMed] [Google Scholar]
- 7.Nakamichi N, Takarada T, Yoneda Y. Neurogenesis mediated by gamma-aminobutyric acid and glutamate signaling. J Pharmacol Sci 2009;110:133–149 [DOI] [PubMed] [Google Scholar]
- 8.Cesetti T, Fila T, Obernier K, et al. GABAA receptor signaling induces osmotic swelling and cell cycle activation of neonatal prominin+ precursors. Stem Cells 2011;29:307–319 [DOI] [PubMed] [Google Scholar]
- 9.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. Beta-cell growth and regeneration: replication is only part of the story. Diabetes 2010;59:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 2012;61:2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biarnés M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 2002;51:66–72 [DOI] [PubMed] [Google Scholar]
- 13.Emamaullee JA, Shapiro AM. Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant 2007;16:1–8 [PubMed] [Google Scholar]
- 14.Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner-Weir S, Weir GC. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation 1995;59:817–820 [PubMed] [Google Scholar]
- 15.Tian J, Chau C, Hales TG, Kaufman DL. GABA(A) receptors mediate inhibition of T cell responses. J Neuroimmunol 1999;96:21–28 [DOI] [PubMed] [Google Scholar]
- 16.Tian J, Lu Y, Zhang H, Chau CH, Dang HN, Kaufman DL. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol 2004;173:5298–5304 [DOI] [PubMed] [Google Scholar]
- 17.Tian J, Yong J, Dang H, Kaufman DL. Oral GABA treatment downregulates inflammatory responses in a mouse model of rheumatoid arthritis. Autoimmunity 2011;44:465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian J, Dang H, Yong J, et al. Oral treatment with γ-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PloS One 2011;6:e25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat R, Axtell R, Mitra A, et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci USA 2010;107:2580–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian J, Dang H, Kaufman DL. Combining antigen-based therapy with GABA treatment synergistically prolongs survival of transplanted ß-cells in diabetic NOD mice. PLoS One 2011;6:e25337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes 2006;55:3238–3245 [DOI] [PubMed] [Google Scholar]
- 22.Otomo E, Araki G, Mori A, Kurihara M. Clinical evaluation of GABA in the treatment of cerebrovascular disorders. Multi-center double-blind study in comparison with pyrithioxine and placebo. Arzneimittelforschung 1981;31:1511–1523 [PubMed] [Google Scholar]
- 23.Loeb C, Benassi E, Bo GP, Cocito L, Maffini M, Scotto P. Preliminary evaluation of the effect of GABA and phosphatidylserine in epileptic patients. Epilepsy Res 1987;1:209–212 [DOI] [PubMed] [Google Scholar]
- 24.Tower DB, Roberts E. Inhibition in the Nervous System and GABA. New York, Pergamon Press, 1960, p. 562–578 [Google Scholar]
- 25.Kuriyama K, Sze PY. Blood-brain barrier to H3-gamma-aminobutyric acid in normal and amino oxyacetic acid-treated animals. Neuropharmacology 1971;10:103–108 [DOI] [PubMed] [Google Scholar]