Abstract
EPR oximetry using implantable resonators allow measurements at much deeper sites than are possible with surface resonators (> 80 mm vs. 10 mm) and have greater sensitivity at any depth. We report here the development of an improvement of the technique that now enables us to obtain the information from multiple sites and at a variety of depths. The measurements from the various sites are resolved using a simple magnetic field gradient. In the rat brain multi-probe implanted resonators measured pO2 at several sites simultaneously for over 6 months to record under normoxic, hypoxic and hyperoxic conditions. This technique also facilitates measurements in moving parts of the animal such as the heart, because the orientation of the paramagnetic material relative to the sensitive small loop is not altered by the motion. The measured response is very fast, enabling measurements in real time of physiological and pathological changes such as experimental cardiac ischemia in the mouse heart. The technique also is quite useful for following changes in tumor pO2, including applications with simultaneous measurements in tumors and adjacent normal tissues.
Keywords: Implantable resonator, L-band EPR, multiple sites, repeated pO2
1. INTRODUCTION
EPR has been developed for over several decades and applied successfully for detecting and monitoring radical levels in chemical and biological materials [1–3]. L-band EPR, in particular, has been used for measuring and monitoring tissue pO2 in rodents and in patients with subcutaneous tumors and skin cancer [4–7]. In spite of these successful applications, L-band EPR has some limitations such as being limited to measure at tissue depths of no more then 10 mm and considerable noise when measurements are made in organs that may move during the measurement, such as the beating heart. To overcome these limitations, we have developed improved implantable resonators for EPR to overcome these shortcomings, enhance the detective ability and to measure pO2 in diverse tissues/organs. The techniques also have applicability for other types of measurements by EPR, including biophysical parameters, free radicals, redox state, and dosimetry.
2. MATERIALS AND METHODS
2.1. Implantable resonators
The resonator has two sets of loops, a larger loop on one end and one or several small loops on the other end. The large loop is used to couple inductively to the L-band EPR spectrometer. The small loop(s) contains lithium phthalocyanine (LiPc) or other oxygen sensitive paramagnetic materials and is coated with a highly gas permeable, biocompatible material (I the studies reported here we used Teflon) [8]. The small loops are implanted in the sites of interest.
2.2. Animals and resonator implantation
All animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Dartmouth Medical School. The implantable resonators were placed surgically into different regions based on the experimental designs. To demonstrate measurements of pO2 in the heart, a short single probe resonator was used. The large coupling loop was placed under the skin close to the chest, while the small tip was carefully inserted into the myocardial muscle near the coronary artery of the mouse. After surgery, the animal was allowed to recover for a couple of days before the EPR measurements. To create temporary cardiac ischemia, a fine ligature was placed around the artery that could be tightened remotely to temporarily occlude the blood flow.
For brain pO2, either a single or a multi-probe resonator was inserted into the targeted sites while the large loop was placed beneath the scalp. Rats were allowed to recover for at least one day before hypoxic, normoxic, hyperoxic studies of brain tissue or measurements in tumors. For pO2 in the muscle, a single probe resonator was inserted into the thoracic muscle, and the coupling loop was buried subcutaneously, close to the tail of the animal. The tumors were established by injection in anesthetized (Isoflurane) rats of a suspension of F98 glioma cells (500,000 cells/10ul, 7ul/site) intracranially injected at a depth of 5~6 mm from the skull into the vicinity of the sensing tip.
2.3. EPR Oximetry
EPR measurements were carried out using an L-band EPR spectrometer constructed in our laboratory [9]. Rats or mice were anesthetized with ~2% isoflurane/air and were placed in the magnet with the resonator sensing tip in the center of the magnet. The external loop of the resonator was placed over the coupling loop of the implantable resonator. For multi-probe resonators, a single dimensional gradient power was applied to separate the EPR signals from each probe. For normoxia, the inhaled gas was 30% O2, to induce mild hypoxia the gas was changed to 15% O2, and for hyperoxia to 100% O2 or carbogen for a total of 35 ~ 40 minutes.
3. RESULTS
3.1. Myocardial pO2 under normal and ischemic conditions
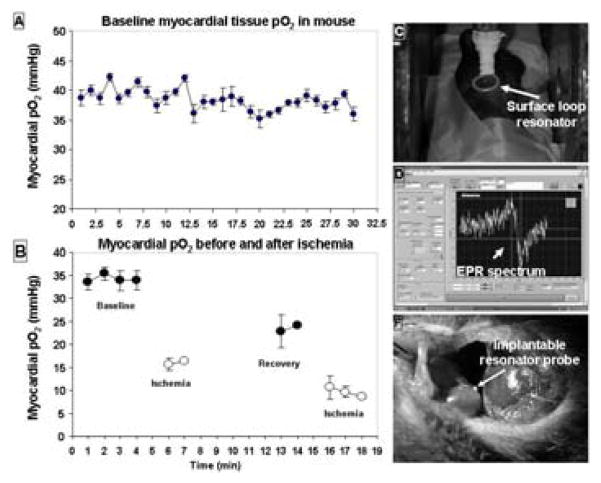
Figure 1 shows the myocardial pO2 during baseline and ischemic conditions. Under normoxic conditions, the mouse had a very stable myocardial pO2 around 40 mm Hg. With occlusion, the pO2 immediately decreased to 15 mm Hg. Upon reperfusion, the pO2 increased but did not reach the baseline level.
Figure 1.
Measurement of myocardial pO2 using a short single probe resonator. A: baseline pO2 of a mouse heart. B: pO2 under several different conditions: baseline, ischemia and reperfusion. C: positioning of the external resonator over the large loop of the implantable resonator that was under the skin. D: a typical EPR spectrum (baseline pO2). E: location of the resonator probe.
3.2. Long term, repeated measurements of brain pO2 under normal, hypoxic, and hyperoxic conditions
For repeated brain pO2 measurements, a 3-probe resonator was placed into a rat brain with the following coordinates: Left, 3.5 mm with depth at 3.5 mm, 1.5 mm with depth at 2.5 mm to the midline; right 1.5 mm with depth at 2.5 mm to the midline. The pO2 measurement was started the second day after the surgical implantation of the resonator and continued for up to 180 days, Fig. 2. In the first several days, tissue pO2 showed a large variation, which is likely due to the trauma associated with the insertion of the probes.
Figure 2.
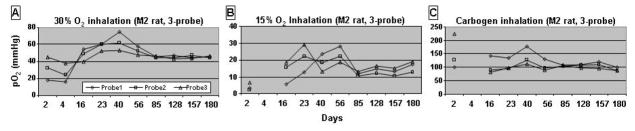
Repeated, long term pO2 measurements with a 3-probe resonator implanted in a rat brain. The measurements were started the second day after implantation and continued for up to 180 days. A: 30% O2 for baseline, B: 15% O2 for hypoxia and C: carbogen for hyperoxia.
After two weeks, the tissue pO2 stabilized and could be measured with a good signal to noise ratio in all measurements, under each gas condition. The tissue pO2 of a rat breathing 30% O2 was 45 mm Hg (Fig. 2A). Under 15% O2, the rat had a pO2 of 15 mm Hg (Fig. 2B). When the rats were allowed to breathe carbogen, a robust increase in tissue pO2 was observed with the pO2 increasing to around 100 mm Hg, (Fig. 2C). Importantly, there was no large variation in brain tissue pO2 while breathing 30% O2 from Day 56 to day 180. These results demonstrate the ability of this technique to monitor brain tissue oxygenation in both normal and pathologic conditions for prolonged periods of time.
3.3. Brain pO2 dynamic changes under different oxygen concentrations
For this experiment, we used a 4-probe resonator implanted in indicated in the figure legend. When the oxygen concentration was changed from 30% to room air, the brain pO2 decreased gradually and reached a stable level after 10 minutes. Although all four sites did not have exactly the same pO2 values, the dynamic patterns were very similar. When the oxygen level was changed from room air to 15% O2, the tissue pO2 in all sites reduced to much lower values (17~22 mm Hg). During a carbogen challenge, the two lateral sites had a much larger pO2 response, compared to the two medial sites.
3.4. Development of hypoxia during growth of a brain tumor
We inserted one probe implantable resonators into the rat brain, at a depth of 5~6 mm from the brain surface, for monitoring the pO2 during tumor growth. Fig. 4A shows the MRI images of a sham injected rat 7 days before and 19 days after an equal volume of culture media injection (upper row images). There was no obvious morphological change in the MRI images. The pO2 stayed at a very similar level (40~55 mm Hg) across the measurements (Fig. 4B). The changes in the MRI for the rats with tumors are shown in Fig. 4A, the tumor grew around the resonator tip). There was a decrease of the pO2 in the tumor sites; the pattern of the decrease differed in different rats showed different pO2 decline rates (Fig. 4B). On day 25 after cell inoculation, all rats received a carbogen challenge. The brains in the sham injected rats had a robust response to the challenge while the tumors had almost no response.
Figure 4.
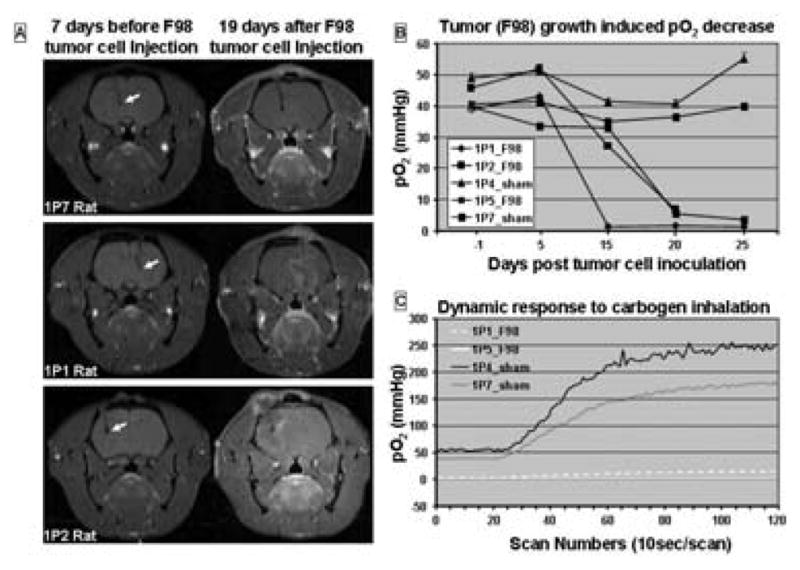
pO2 in rat F98 brain tumor measured with a single probe resonator. A: MRI images before and 19 days after F98 inoculation. Arrows indicate the probe locations in the brain. B: pO2 measured the day before, 5, 15, 20 and 25 days after F98 inoculation in 3 rats with tumors and 2 rats with sham injections. C: Carbogen challenge at day 25 after baseline 30% pO2.
3.5. Muscle pO2 in rats breathing different oxygen concentrations
For convenience we used a 1-probe resonator in the thoracic muscle. We observed response patterns similar to those observed in the brain and the heart. Four days after the implantation, we studied the dynamic changes of the muscle pO2 by switching the breathing gas from 30% to 15% and back, and from 30% to carbogen. A baseline pO2 of 45 mm Hg was observed in rats breathing 30%; breathing 15% resulted in a gradual decline of the pO2 to 15 mm Hg in approximately 10 minutes (Fig. 5A). Changing the gas to 30% resulted in an increase in tissue pO2 back to baseline in 20 minutes (Fig. 5B). Carbogen produced a significant increase of the muscle pO2 that reached a plateau in ~ 20 minutes (Fig. 5C).
Figure 5.
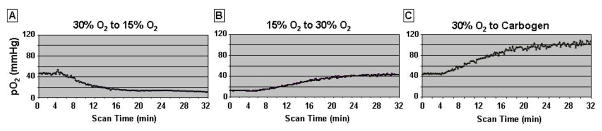
pO2 in skeletal muscle measured with a single probe resonator. The measurement was done four days after the implantation. As shown, the resonator measured the gradual changes of the pO2 when oxygen in the breathing gas was changed from 30% to 15% (A), from 15% to 30% (B) and from 30% to Carbogen (C).
4. DISCUSSION
We have successfully used implantable resonators to measure tissue pO2 in the heart, brain, and muscle under diverse conditions. The use of the implantable resonators in the beating heart enabled us to overcome the problems usually encountered when trying to make measurements of pO2 in a moving organ. Previously, to measure myocardial pO2, we had to carry out a large amount of data averaging to reduce the noise induced by the relative motion between the paramagnetic material and the probe. This precluded measuring dynamic changes such as those associated with the acute induction of ischemia. Using single or multi-probe resonator for long term brain pO2 measurements confirmed the very stable pO2 in the targeted sites, and we were able to measure this for days and months. Such measurements would be very difficult to do with polarographic electrodes or the OxyLite system.
Another very important advantage of this technique is the feasibility of carrying out oximetry at any depth. This extends the advantages of EPR oximetry to almost any site where the resonator can be implanted. The implantable resonators, once inserted, allow repeated pO2 measurements with excellent time resolution and can be repeated across days or weeks.
Figure 3.
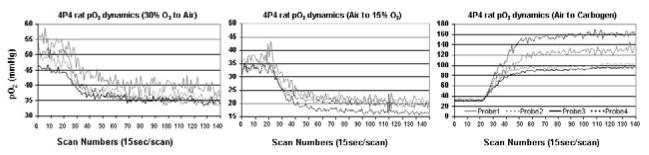
A four-probe implantable resonator used to measure dynamic responses in the rat brain to changes in inhaled oxygen levels. The resonator was inserted at the following coordinates: AP, 0 mm; ML, 2.0 mm and 3.5 mm to the left and right of midline, DV, 5.5 mm. Oxygen was delivered through a nose cone to the rat. Plots from left to right: brain pO2 during change from 30% O2 to air, from air to 15% O2, and from air to carbogen.
Acknowledgments
This work was supported by NIH grant PO1EB2180 and a Dartmouth NCCC Prouty Pilot Grant, and used the facilities of the EPR Center for Viable Systems (P41EB002032).
References
- 1.Swartz HM, Walczak T. Developing in vivo EPR oximetry for clinical use. Adv Exp Med Biol. 1998;454:243–52. doi: 10.1007/978-1-4615-4863-8_29. [DOI] [PubMed] [Google Scholar]
- 2.Swartz HM, Reichling BA. The safety of X-ray examination of radioisotope scan. JAMA. 1978;239:2031–2. doi: 10.1001/jama.239.19.2031. [DOI] [PubMed] [Google Scholar]
- 3.Hou H, et al. The effect of oxygen therapy on brain damage and cerebral pO(2) in transient focal cerebral ischemia in the rat. Physiol Meas. 2007;28:963–76. doi: 10.1088/0967-3334/28/8/017. [DOI] [PubMed] [Google Scholar]
- 4.Som S, et al. EPR oximetry in three spatial dimensions using sparse spin distribution. J Magn Reson. 2008 doi: 10.1016/j.jmr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumoto S, et al. Low-field paramagnetic resonance imaging of tumor oxygenation and glycolytic activity in mice. J Clin Invest. 2008;118:1965–73. doi: 10.1172/JCI34928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou H, et al. Cerebral PtO2, acute hypoxia, and volatile anesthetics in the rat brain. Adv Exp Med Biol. 2005;566:179–85. doi: 10.1007/0-387-26206-7_25. [DOI] [PubMed] [Google Scholar]
- 7.Khan N, et al. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9:1169–82. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinguizli M, et al. Development and evaluation of biocompatible films of polytetrafluoroethylene polymers holding lithium phthalocyanine crystals for their use in EPR oximetry. Biosens Bioelectron. 2006;21:1015–22. doi: 10.1016/j.bios.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Hou H, et al. Increased oxygenation of intracranial tumors by efaproxyn (efaproxiral), an allosteric hemoglobin modifier: In vivo EPR oximetry study. Int J Radiat Oncol Biol Phys. 2005;61:1503–9. doi: 10.1016/j.ijrobp.2004.12.077. [DOI] [PubMed] [Google Scholar]