Abstract
Background/Aims
Hepatitis C virus (HCV) infection appears to contribute to the development of insulin resistance (IR). Among the multiple determinants of IR, body mass index (BMI) is the most important. We investigated the contribution of HCV to BMI-associated IR using a transgenic mouse model expressing HCV core protein.
Methods
Eight Tg and 5 non-Tg littermate controls were evaluated. Glucose and insulin tolerance tests (GTT and ITT) were performed on two separate occasions. Multivariate linear mixed modeling was used to evaluate and compare the effect of weight on IR between HCV core transgenic and non-transgenic controls.
Results
There were no statistically significant differences in GTT or ITT curves (P=0.58 and P=0.59, respectively) between the two groups and no difference in median weights between Tg and non-Tg mice (P=0.11). However, there was greater variance in the distributions of Tg as compared to non-transgenic mice for GTT and ITT. When evaluating this closely, a differential contribution of weight to insulin resistance curves between these groups was noted (P = 0.05). Among non-Tg mice insulin resistance curves for mice of different weights were comparable, however, for Tg mice, higher weights resulted in larger levels of insulin resistance curves with slower decay. In all animals, steatosis was absent or minimal.
Conclusion
Weight has a greater effect on IR in HCV Tg than non-Tg mice. HCV therefore synergizes with weight in the promotion of IR. Steatosis was not a prerequisite for the development of IR, implying that HCV’s effects on IR may be independent of steatosis.
Keywords: Hepatitis C, HCV, Diabetes, Insulin Resistance, Synergy
INTRODUCTION
Chronic hepatitis C virus (HCV) infection has been associated with a number of extrahepatic manifestations (1). An association between HCV infection and diabetes mellitus (DM) has also been found. A high prevalence of DM among HCV-infected individuals was initially reported by Allison et al in 1994 (2). This finding has been subsequently confirmed by others (3–10) and has been corroborated by evidence from the U.S. NHANES III (Third National Health and Nutrition Examination Survey) (6).
Recent investigations have demonstrated increased insulin resistance (IR) among persons infected with hepatitis C as compared with uninfected controls (11, 12). Insulin resistance is not only a precursor to diabetes, but is also independently associated with significant morbidity (13), including hypertension and coronary heart disease (14). The presence of insulin resistance in the setting of hepatitis C infection is of particular importance because it also plays a role in the progression of HCV-related liver disease (11, 15–19) and may be associated with suboptimal responses to antiviral therapy possibly mediated by an increase in tumor necrosis factor alpha (TNF-α) (20, 21).
Hepatitis C virus appears to contribute, directly or indirectly, to the development of IR. The mechanisms by which HCV increases IR are unknown. Aytug et al. demonstrated that HCV-infected subjects have defects in hepatic downstream signaling components such as insulin receptor substrate (IRS)-1, phosphatidylinositol 3-kinase (PI3-kinase), and Akt (22). Kawaguchi et al. found that HCV core-induced SOCS3 promotes proteasomal degradation of IRS1 and IRS2 through ubiquination (23). These findings support the observation that HCV induces insulin resistance. However, insulin resistance is a multifactorial condition, and it is likely that various factors contribute to its development in the setting of HCV infection.
Strong evidence indicates that overweight is associated with diabetes and insulin resistance in all genders and ethnic groups (24–26). Although the precise mechanism by which overweight induces insulin resistance remains to be more clearly elucidated, adipose tissue has recently been identified as an endocrine organ. Adipocytes actively secrete a complex array of proteins that regulate insulin sensitivity (27, 28). The significant role of overweight in the development of insulin resistance has been illustrated in clinical studies in which weight loss is accompanied by improvement in insulin sensitivity (29–31).
The most effective way to reduce complications of hepatitis C is eradication of the infection. However, approximately half of HCV-infected patients who are treated fail to achieve sustained virologic response (32) and many are not candidates for antiviral treatment because of contraindications. For many patients, the management of hepatitis C should focus not only on antiviral therapies but also on decreasing associated comorbidities, such as insulin resistance, that contribute to the progression of liver disease. Because body mass index (BMI) is the most important modifiable predictor of insulin resistance, the role of overweight in HCV infection merits careful attention. Thus far, a possible interaction between HCV and BMI-associated IR has not been characterized. The aim of this investigation was to evaluate the contribution of HCV to alterations in IR related to BMI using an animal model.
MATERIALS and METHODS
Animal Studies
All animal experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of the Massachusetts General Hospital.
A transgenic (Tg) mouse model expressing HCV core cDNA (genotype 1b), under the control of the hepatitis B virus (HBV) promoter, was created using a construct kindly provided by Moriya K. et al. (33). Transgenic mice were developed on a hybrid C57BL/6/FVB background as previously described (34). As demonstrated previously, this model fails to develop a histologic phenotype (34). Non-transgenic littermates of the HCV core Tg mice on the C57BL/6/FVB background were used as control. Control mice were exposed to the same diet and environmental factors as transgenic mice.
Enzyme-linked immunsorbent assays
Reagents for mouse insulin enzyme-linked immunosorbent assay (ELISA) were purchased from Linco Research, Inc. (Missouri, USA) and used according to manufacturer’s instructions. Ascensia ELITE Glucometer and test strips from Bayer (Philadelphia, USA) were used to monitor plasma glucose concentrations.
Glucose Tolerance Tests
Mice fasted for 8 hours were injected intraperitoneally with 3 mg of glucose per gram of body weight. Blood glucose was measured from tail-vein blood collected at the designated times (0, 15, 30, 60 and 90 minutes post injection). Each animal underwent two glucose tolerance tests (GTT) on separate occasions (occasion 1 and 2) taken 4 days apart.
Insulin Tolerance Tests
Mice fasted for 8 hours were injected intraperitoneally with 1 IU of recombinant regular human insulin (Humulin-R) (Eli Lilly, Indianapolis, IN) per kilogram of body weight. Blood glucose was measured from tail-vein blood collected at the designated times (0, 15, 30, 45, 60, 90 minutes post injection). Each animal underwent two insulin tolerance tests (ITT) on separate occasions (occasion 1 and 2) taken 4 days apart.
Histologic Analysis
Liver tissue from the animals was isolated and immediately placed in 10% formalin for histological analysis. Histological evaluation of the formalin-fixed liver tissues by H&E staining was also carried out by a pathologist (L.Z.) in a blinded manner.
Statistical Analysis
Mouse ages and weights by transgenic group are presented in Table 1. The observed GTT and ITT curves for each mice were plotted with geometric means at each time point, i.e., the exponential of the mean logarithm transformed GTT and ITT. The distribution of GTT and ITT curves (natural logarithm transformed) were compared between Tg and non-Tg using multivariate linear mixed models. In models for GTT, the fixed effect of time was included as a cubic polynomial (time + time2 + time3) and in models for ITT, the fixed effect of time was included as a quadratic polynomial (time + time2). To account for correlations within mice measured on two occasions across repeated observations over time, multilevel random effects were fitted, i.e., random effects for mice (intercept and for time2) and for occasion of the test (1 or 2) within mice (intercept and for time). By including these random effects, we allowed the overall pattern to vary between mice in terms of GTT and ITT at time 0 and in terms of the curvature of the peak. In addition, differences between the pattern of the two occasions of the same mice were assumed to be a shift in GTT and ITT at time 0 and the location of the peak (but common curvature across occasions). Our choice of model with the fixed and random effects was based on model parsimony, model fitness (inspection of fitted vs. observed and residuals), AIC, log-likelihood (P < 0.05 for the fixed effects), and restricted log-likelihood ratio tests (P < 0.05 for the random effects). To compare curves between Tg and non-Tg an interaction between time terms and transgenic was tested via maximum likelihood ratio test (LRT). To compare curves of mice with varying weights at time 0 an interaction between time terms and weight was tested via LRT. To assess the differential effect of weight on ITT between Tg and non-Tg an interaction term between time, weight and transgenic was used. (Detail of the final models are presented in the Appendix).
TABLE 1.
Mouse Characteristics
| Non-Tg (n=10*) |
Tg (n=16*) |
p Value1 | |
|---|---|---|---|
| Gender | Male | Male | NS |
| Median Age (months) (range) | 17 (16–18) | 17 (16–18) | NS |
| Median Weight (g) (range) | 48 (39–53) | 45 (40–47) | 0.11 |
5 Non-Tg mice in 2 occasions and 8 Tg mice in two occasions
Wilcoxon test
All analyses were performed using S-plus 8 (Insightful Inc., Seattle, USA).
RESULTS
Mouse characteristics
Characteristics of the mice studied are summarized in Table 1. A total of 8 male Tg and 5 male non-Tg littermates were evaluated in this investigation. The age range of mice from both groups was between 16 and 18 months. There were no statistically significant differences between weights of Tg and non-Tg mice (P t-test= 0.30). The Tg model used in this investigation has been previously used for other investigations from our group and was not associated with a discernible histologic phenotype, including inflammation, fibrosis, or steatosis (34) up to 21 months of age. In our study, mild steatosis was observed in less than 5% of the hepatocytes in all mice studied. No fibrosis or inflammation was observed in any of the mice studied (data not shown).
Effect of weight on insulin resistance for Tg and non-Tg mice
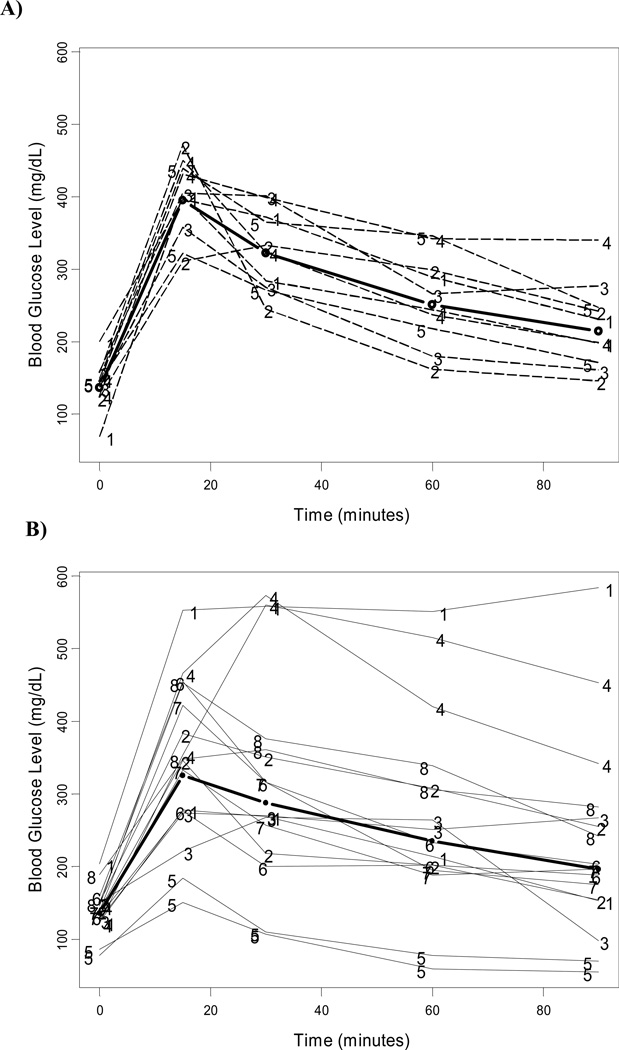
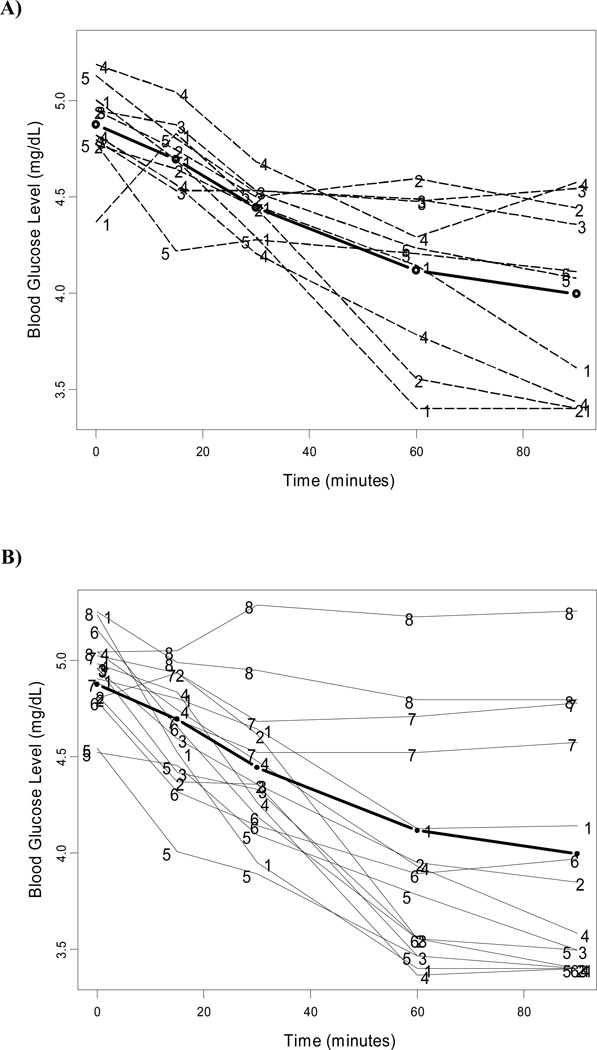
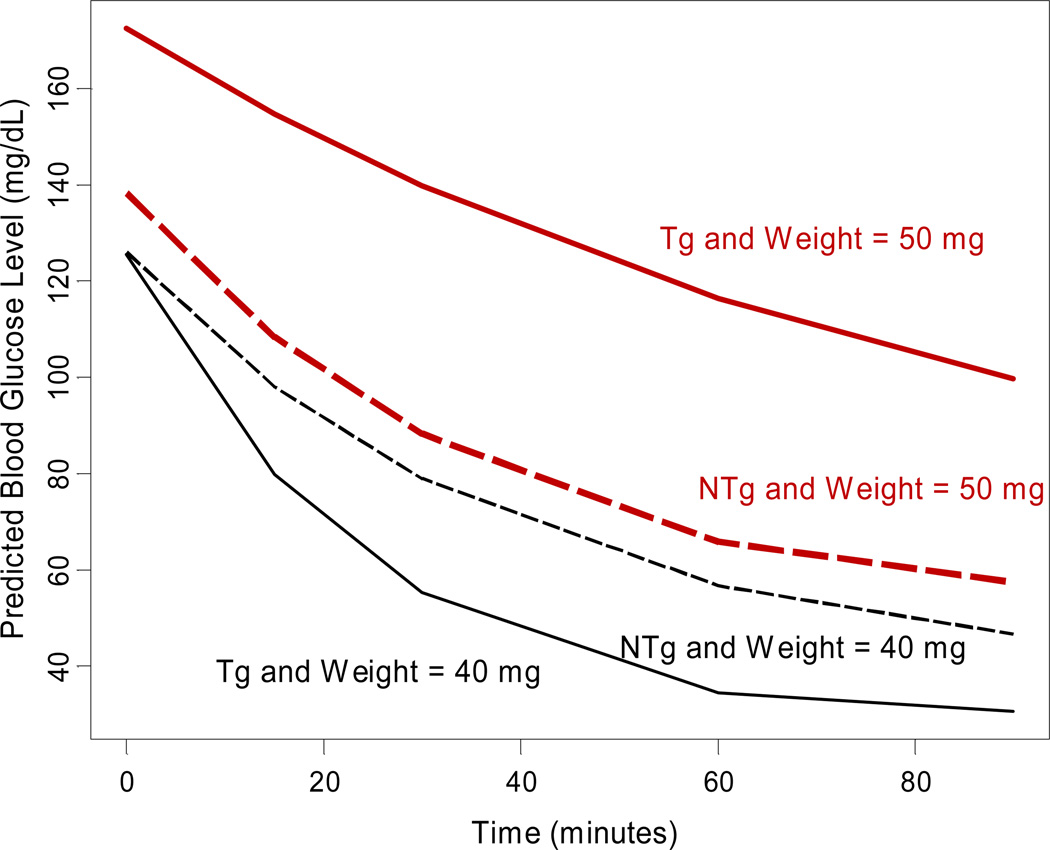
The overall model estimates for the mean of the natural logarithm transformed GTT and ITT over time are presented in Table 2. There was no statistically significant difference in the curve of glucose tolerance tests between Tg and non-Tg mice (P = 0.58). Also, weight at baseline did not have any effect on glucose tolerance tests (P = 0.27). No significant difference was noted in mean curves of insulin tolerance tests between Tg and non-Tg mice (P = 0.59) and across animal weight (P = 0.106). However, there appeared to be greater variability in the distribution of glucose and insulin tolerance tests for Tg mice than non-Tg mice (Figures 1, 2). We then sought to evaluate factors that contributed to the development of elevated insulin resistance among some mice in the Tg group that could explain the greater variability noted in this group. We found that, although weights between the two groups of interest were comparable, there was a differential contribution of weight to insulin resistance curves between these groups (P for interaction between weight and transgenic category on insulin resistance = 0.05). Among non-Tg mice, insulin resistance curves for mice of different weights were not statistically different; however, for Tg mice, higher weights resulted in larger levels of insulin resistance curves with slower decay (Figure 3). Figure 3 represents the expected insulin resistance curve for mice of 40 and 50 mg (approximately the minimum and maximum weights of mice in our sample).
TABLE 2.
| Term | Coefficient | Standard Error | P-value |
|---|---|---|---|
| Modeling ln (GTT)* | |||
| Intercept | 4.97 | 0.7 × 10−1 | |
| Time | 7.07 × 10−2 | 0.5 × 10−2 | |
| Time2 | −1.67 × 10−2 | 0.1 × 10−3 | |
| Time3 | 1.03 × 10−5 | 0.1 × 10−5 | P < 0.0001 |
| Modeling ln (ITT):** | |||
| Crude | |||
| Intercept | 4.93 | 0.5 × 10−1 | |
| Time | −2.11 × 10−2 | 0.2 × 10−2 | |
| Time2 | 1.09 × 10−4 | 0.2 × 10−4 | P < 0.0001 |
Figure 1. Greater variance in glucose tolerance tests for HCV core transgenic compared to non-transgenic controls.
Plot of individual and mean (thicker line) with 95% confidence interval glucose tolerance testing profiles in non-transgenic (A) and HCV core transgenic mice (B). Numbers represent mouse identification at each trial.
Figure 2. Greater variance in insulin tolerance tests for HCV core transgenic compared to non-transgenic controls.
Plot of individual and mean (thicker line) with 95% confidence interval insulin tolerance testing profile in non-transgenic (A) and HCV core transgenic mice (B). Numbers represent mouse identification at each trial.
Figure 3. Greater effect of weight on insulin resistance for HCV core transgenic than non-transgenic mice.
The effect of weight on IR profile on HCV-core transgenic (Tg) was greater than non-transgenic (NTg) mice and differences were statistically significant (P for ITT = 0.05). In the figure, among non-transgenic (dashed curves), the predicted mean glucose response over time of low weight (black), represented by 40 grams mice, nearly overlaps the curve of high weight (red and bold), represented by 50 grams mice. In contrast, transgenic (solid curves) low weight (black) mouse had a faster glucose intake than transgenic high weight (red) mouse. Predicted curves represented the exponential of the predicted fixed effects in the model: (ITT) = time + time2 + transgenic + weight + transgenic × weight + weight × (time + time2)+ transgenic × (time + time2)+ transgenic × weight × (time + time2) (see Appendix for statistical details)
DISCUSSION
In an effort to gain a deeper understanding of the effects of HCV infection on glucose homeostasis, we evaluated the contribution of HCV to alterations in IR as a result of weight using an animal model. Our choice of a transgenic mouse model was intended to minimize other potential confounders present in human studies, especially in a complex multifactorial condition such as insulin resistance. These counfounders include diabetes, alcohol use, age, diet, and level of activity. We found that the independent effect of increasing weight on insulin resistance was significantly greater for Tg mice than for their non-Tg littermates. Our results demonstrate that HCV infection has a synergistic effect with body weight on the development of insulin resistance.
The finding of synergy between HCV and weight on insulin resistance has important implications for the pathogenetic mechanism of HCV-associated insulin resistance. The multiplicative effect of HCV and body weight likely reflects the complexity of the systems responsible for glucose and insulin homeostasis. Our results suggest that the pathway mediating HCV-induced IR interacts at some level with that of obesity-induced IR. It is well established that obesity leads to an altered expression of several adipokines that in turn contribute to insulin resistance (28). It is possible, for instance, that further disruption of the balance between pro-inflammatory and anti-inflammatory adipokines (e.g. TNF-α and adiponectin, respectively) by HCV could explain the synergistic effects observed in this study. Additional investigations are necessary to evaluate this hypothesis. Of note, the paucity of steatosis observed in this study implies that HCV’s effects on IR may be independent of steatosis. In this study, we did not find statistically significant differences in GTT or ITT between the two groups of interest, but rather, found differences in the degree of variability or variance of the HCV Tg group. It is possible that more significant differences may have been noted with greater number of mice. Of note, the lack of statistically significant difference in insulin sensitivity between non-transgenic mice of varying weights does not contradict established notions about the effect of weight on insulin resistance, since there was a trend towards greater insulin resistance for the 50 g as compared to the 40 g mouse. More importantly, it would not be possible to extrapolate actual effect sizes from this investigation to humans. What is clearly shown in our results, however, is the differential effect of weight on insulin resistance depending on transgenic status.
In addition to the implications of our findings for HCV pathogenesis, these results may have important clinical implications. We have demonstrated that weight has a significantly greater effect on insulin resistance in HCV infection than in HCV-negative control groups. From these results it may be inferred that overweight and obesity likely pose a significantly greater risk of increasing insulin resistance for HCV-infected individuals as compared to their uninfected counterparts. Development of insulin resistance is a serious risk that not only leads to diabetes but also to significant cardiovascular morbidity and increased mortality. Moreover, development of insulin resistance in the setting of HCV infection is of particular significance. Insulin resistance is thought to contribute to fibrosis progression in HCV infection indirectly through the development of steatosis (18, 19, 35–38). In addition, a direct contribution of hyperinsulinemia to fibrosis progression in HCV infection has been demonstrated in recent clinical studies (11, 15) and has been further supported by investigations at the cellular level (16) (17). Furthermore, increasing evidence suggests that insulin resistance, mediated by tumor necrosis factor (TNF-α), may be associated with decreased response to antiviral therapy in HCV infection (39–46).
At present, HCV-infected patients are not routinely screened for insulin resistance or diabetes. Based on the results of this investigation, it is possible that weight control among HCV infected subjects may have a more substantial effect on insulin resistance than would be expected in uninfected individuals. This will need to be confirmed in clinical studies.
Acknowledgements
We are grateful to Dr. Kazuhiko Koike (University of Tokyo) for his gift of the HCV core experimental construct.
This work was supported by: NIH T32 DK07477 (ADB), NIH K08 DK070022(ADB), NIH DK57857 (RTC), and DK78772 (RTC)
List of Abbreviations
- HCV
Hepatitis C virus
- DM
diabetes mellitus
- NHANES III
Third National Health and Nutrition Examination Survey
- IR
insulin resistance
- TNF-α
tumor necrosis factor alpha
- IRS-1
insulin receptor substrate
- PI3-kinase
phosphatidylinositol 3-kinase
- BMI
body mass index
- IACUC
Institutional Animal Care and Use Committee
- Tg
transgenic
- ELISA
enzyme-linked immunosorbent assay
- GTT
glucose tolerance tests
- ITT
insulin tolerance tests
- AUC
area under the curve
- SD
standard deviations
APPENDIX
Mixed model for ITT, crude and used in Figure 3
where:
Also, the fixed effects or mean of ln (ITT) used in Figure 3 (including the interaction term) was: ln̅(ITT) = 4.46 − 1.9 × 10−2time + 3.17 × 10−5time2 − 9.11 × 10−1Tg(vs.non − Tg) − 9.32 × 10−3Weight + 2.25 × 10−2Tg × Weight −1.17 × 10−1time × Tg + 2.83 × 10−4time × Weight + 8.74 × 10−4time2 × Tg + 1.10 × 10−6time2 × Weight + 2.53 × 10−3time × Tg × Weight − 1.89 × 10−3time2 × Tg × Weight
References
- 1.Sene D, Limal N, Cacoub P. Hepatitis C virus-associated extrahepatic manifestations: a review. Metab Brain Dis. 2004;19:357–381. doi: 10.1023/b:mebr.0000043982.17294.9b. [DOI] [PubMed] [Google Scholar]
- 2.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 3.Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O'Rahilly S, et al. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059–1063. doi: 10.1002/hep.510300416. [DOI] [PubMed] [Google Scholar]
- 4.Durand JM. Extrahepatic manifestations of hepatitis C virus infection. Am J Gastroenterol. 1997;92:1402. [PubMed] [Google Scholar]
- 5.Fraser GM, Harman I, Meller N, Niv Y, Porath A. Diabetes mellitus is associated with chronic hepatitis C but not chronic hepatitis B infection. Isr J Med Sci. 1996;32:526–530. [PubMed] [Google Scholar]
- 6.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 7.Knobler H, Stagnaro-Green A, Wallenstein S, Schwartz M, Roman SH. Higher incidence of diabetes in liver transplant recipients with hepatitis C. J Clin Gastroenterol. 1998;26:30–33. doi: 10.1097/00004836-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, Feng S, Chung RT, Tolkoff-Rubin N, et al. Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001;72:1066–1072. doi: 10.1097/00007890-200109270-00015. [DOI] [PubMed] [Google Scholar]
- 9.Rudoni S, Petit JM, Bour JB, Aho LS, Castaneda A, Vaillant G, Verges B, et al. HCV infection and diabetes mellitus: influence of the use of finger stick devices on nosocomial transmission. Diabetes Metab. 1999;25:502–505. [PubMed] [Google Scholar]
- 10.Ozyilkan E, Erbas T, Simsek H, Telatar F, Kayhan B, Telatar H. Increased prevalence of hepatitis C virus antibodies in patients with diabetes mellitus. J Intern Med. 1994;235:283–284. doi: 10.1111/j.1365-2796.1994.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 11.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, et al. Insulin resistance is associated with chronic hepatitis C and virus infection fibrosis progression. Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Delgado-Borrego A, Casson D, Schoenfeld D, Somsouk M, Terella A, Jordan SH, Bhan A, et al. Hepatitis C virus is independently associated with increased insulin resistance after liver transplantation. Transplantation. 2004;77:703–710. doi: 10.1097/01.tp.0000114283.04840.3a. [DOI] [PubMed] [Google Scholar]
- 13.Baron AD. Impaired glucose tolerance as a disease. Am J Cardiol. 2001;88:16H–19H. doi: 10.1016/s0002-9149(01)01832-x. [DOI] [PubMed] [Google Scholar]
- 14.Zavaroni I, Bonini L, Gasparini P, Barilli AL, Zuccarelli A, Dall'Aglio E, Delsignore R, et al. Hyperinsulinemia in a normal population as a predictor of non-insulin-dependent diabetes mellitus, hypertension, and coronary heart disease: the Barilla factory revisited. Metabolism. 1999;48:989–994. doi: 10.1016/s0026-0495(99)90195-6. [DOI] [PubMed] [Google Scholar]
- 15.Hickman IJ, Powell EE, Prins JB, Clouston AD, Ash S, Purdie DM, Jonsson JR. In overweight patients with chronic hepatitis C, circulating insulin is associated with hepatic fibrosis: implications for therapy. J Hepatol. 2003;39:1042–1048. doi: 10.1016/s0168-8278(03)00463-x. [DOI] [PubMed] [Google Scholar]
- 16.Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 17.Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751. doi: 10.1002/hep.510290632. [DOI] [PubMed] [Google Scholar]
- 18.Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: Mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586–597. doi: 10.1053/j.gastro.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- 20.Mbow ML, Sarisky RT. What is disrupting IFN-alpha's antiviral activity? Trends Biotechnol. 2004;22:395–399. doi: 10.1016/j.tibtech.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Larrea E, Garcia N, Qian C, Civeira MP, Prieto J. Tumor necrosis factor alpha gene expression and the response to interferon in chronic hepatitis C. Hepatology. 1996;23:210–217. doi: 10.1002/hep.510230203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384–1392. doi: 10.1016/j.hep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 26.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 27.Jazet IM, Pijl H, Meinders AE. Adipose tissue as an endocrine organ: impact on insulin resistance. Neth J Med. 2003;61:194–212. [PubMed] [Google Scholar]
- 28.Kadowaki T, Hara K, Yamauchi T, Terauchi Y, Tobe K, Nagai R. Molecular mechanism of insulin resistance and obesity. Exp Biol Med (Maywood) 2003;228:1111–1117. doi: 10.1177/153537020322801003. [DOI] [PubMed] [Google Scholar]
- 29.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–419. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider R, Golzman B, Turkot S, Kogan J, Oren S. Effect of weight loss on blood pressure, arterial compliance, and insulin resistance in normotensive obese subjects. Am J Med Sci. 2005;330:157–160. doi: 10.1097/00000441-200510000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Racette SB, Weiss EP, Hickner RC, Holloszy JO. Modest weight loss improves insulin action in obese African Americans. Metabolism. 2005;54:960–965. doi: 10.1016/j.metabol.2005.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keeffe EB. Chronic hepatitis C: management of treatment failures. Clin Gastroenterol Hepatol. 2005;3:S102–S105. doi: 10.1016/s1542-3565(05)00698-1. [DOI] [PubMed] [Google Scholar]
- 33.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 34.Kamegaya Y, Hiasa Y, Zukerberg L, Fowler N, Blackard JT, Lin W, Choe WH, et al. Hepatitis C virus acts as a tumor accelerator by blocking apoptosis in a mouse model of hepatocarcinogenesis. Hepatology. 2005;41:660–667. doi: 10.1002/hep.20621. [DOI] [PubMed] [Google Scholar]
- 35.Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:729–736. doi: 10.1053/jhep.2002.35064. [DOI] [PubMed] [Google Scholar]
- 36.Monto A. Hepatitis C and steatosis. Semin Gastrointest Dis. 2002;13:40–46. [PubMed] [Google Scholar]
- 37.Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A, Powell EE. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. 1999;29:1215–1219. doi: 10.1002/hep.510290401. [DOI] [PubMed] [Google Scholar]
- 38.Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstal R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol. 2002;37:837–842. doi: 10.1016/s0168-8278(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 39.Nelson DR, Lim HL, Marousis CG, Fang JW, Davis GL, Shen L, Urdea MS, et al. Activation of tumor necrosis factor-alpha system in chronic hepatitis C virus infection. Dig Dis Sci. 1997;42:2487–2494. doi: 10.1023/a:1018804426724. [DOI] [PubMed] [Google Scholar]
- 40.Kasprzak A, Zabel M, Biczysko W, Wysocki J, Adamek A, Spachacz R, Surdyk-Zasada J. Expression of cytokines (TNF-alpha, IL-1alpha, and IL-2) in chronic hepatitis C: comparative hybridocytochemical and immunocytochemical study in children and adult patients. J Histochem Cytochem. 2004;52:29–38. doi: 10.1177/002215540405200104. [DOI] [PubMed] [Google Scholar]
- 41.Jia HY, Du J, Zhu SH, Ma YJ, Chen HY, Yang BS, Cai HF. The roles of serum IL-18, IL-10, TNF-alpha and sIL-2R in patients with chronic hepatitis C. Hepatobiliary Pancreat Dis Int. 2002;1:378–382. [PubMed] [Google Scholar]
- 42.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, et al. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 43.Maeno T, Okumura A, Ishikawa T, Kato K, Sakakibara F, Sato K, Ayada M, et al. Mechanisms of increased insulin resistance in non-cirrhotic patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2003;18:1358–1363. doi: 10.1046/j.1440-1746.2003.03179.x. [DOI] [PubMed] [Google Scholar]
- 44.Neuman MG, Benhamou JP, Martinot M, Boyer N, Shear NH, Malkiewicz I, Katz GG, et al. Predictors of sustained response to alpha interferon therapy in chronic hepatitis C. Clin Biochem. 1999;32:537–545. doi: 10.1016/s0009-9120(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 45.Neuman MG, Benhamou JP, Malkiewicz IM, Akremi R, Shear NH, Asselah T, Ibrahim A, et al. Cytokines as predictors for sustained response and as markers for immunomodulation in patients with chronic hepatitis C. Clin Biochem. 2001;34:173–182. doi: 10.1016/s0009-9120(01)00212-0. [DOI] [PubMed] [Google Scholar]
- 46.Dumoulin FL, Wennrich U, Nischalke HD, Leifeld L, Fischer HP, Sauerbruch T, Spengler U. Intrahepatic mRNA levels of interferon gamma and tumor necrosis factor alpha and response to antiviral treatment of chronic hepatitis C. J Hum Virol. 2001;4:195–199. [PubMed] [Google Scholar]