Abstract
Sequential changes in glomerular filtration rate (GFR) during development of hypertension in the conscious Dahl salt-sensitive (SS) rat were determined using a new method for measurement. Utilizing a miniaturized device, disappearance curves of fluorescein isothiocyanate (FITC)-sinistrin were measured by transcutaneous excitation and real time detection of the emitted light through the skin. Rats with implanted femoral venous catheters (dye injection and sampling) and carotid catheters (mean arterial pressure (MAP) by telemetry) were studied while maintained on a 0.4% NaCl diet and on days 2,5,7,14 and 21 after switching to 4.0% (HS) diet. A separate group of rats were maintained on 0.4% for 21 days as a time control. MAP rose progressively from the last day of 0.4% (130±2 mmHg) reaching significance by day 5 of HS and averaged 162±7 mmHg by day 21. Urine albumin excretion was significantly elevated (3×) by day 7 of HS in SS rats. GFR became reduced on day 14 of HS falling from 1.53±0.06 ml/min/100g bwgt to 1.27±0.04. By day 21, GFR had fallen 28% to 1.1±0.04 ml/min/100g bwgt (t1/2 28.4±1.1 min.) No significant reductions of creatinine clearance (Ccre) were observed throughout the study in response to HS demonstrating the insensitivity of Ccre measurements even with creatinine measured using mass spectrometry. We conclude that the observed reduction of GFR was a consequence and not a cause of the hypertension and that this non-invasive approach could be used in these conscious SS rats for a longitudinal assessment of renal function.
Keywords: GFR, Dahl S rat, salt-sensitive hypertension, creatinine clearance
The present study had two goals: first, to evaluate the ability of a recently developed transcutaneous monitoring system to make sequential measurements of glomerular filtration rate (GFR) in conscious unrestrained rats over several weeks; and second, to determine the progression of changes of GFR that occur during the first 21 days as hypertension develops in Dahl salt-sensitive (SS) rats. Despite the central role of the kidneys in diseases of hypertension1, investigators have been severely hampered in obtaining such measurements for a number of technical reasons. To overcome the recognized limitations of making repetitive GFR measurements in the conscious state, we utilized a recently developed miniaturized device that enabled the transcutaneous determination of the elimination kinetics of FITC-sinistrin (fluorescein-isothiocyanate sinistrin), a fluorescently labeled inulin analog. The details and validation of this technique for determination of GFR in freely moving rats and mice have been reported by Schock-Kusch, Gretz and colleagues2,3,4. Sinistrinis a plant derived fructose polymer like inulin, but has much greater water solubility than inulin5. Excretion half-life of FITC-sinistrin, as determined using the single exponential excretion phase, was shown to provide a valid measurement of GFR as compared to sinistrin clearance measured enzymatically in 20 healthy awake SD rats2. Not only did this approach enable measurements in conscious rats, but also repetitive determination of GFR on sequential days.
We applied this novel technique in this study to determine the relationship of GFR and mean arterial pressure (MAP) during the development of salt-sensitive hypertension in the conscious SS rat following a step change in salt intake from 0.4% to 4.0% NaCl. Since creatinine clearance (Ccre) has become the accepted experimental and clinical indicator GFR and renal function, comparative measurements of Ccre were also determined, together with periodic measurements of urine albumin excretion. The results of these studies show the great utility and sensitivity of these new techniques and the significant limitations of creatinine as a useful marker of renal function.
Materials and Methods
Experimental animals
Male Dahl salt-sensitive rats (SS; SS/JrHsdMcwi) were obtained as weanlings from colonies maintained at the Medical College of Wisconsin since 19916. Rats were fed from weaning a purified AIN-76A rodent food (Dyets, Bethlehem, PA) containing 0.4% NaCl with ad libitum water. The high salt diet used was the same formulation and source but with 4% NaCl (high salt; HS). All experimental protocols were approved by the MCW Institutional Animal Care and Use Committee.
Phenotyping protocol
At 8-9 weeks of age, rats were surgically prepared (see online supplement) for measurement of mean arterial pressure (MAP) by telemetry7 and injection of indicator (FITC-sinistrin; ELS-525-892 EPIGAP Optoelektronik GmbH, Berlin, Germany). After 5-7 days of surgical recovery and 2-3 days of baseline control measurements of MAP and GFR, a venous blood sample (0.2 ml) was obtained to determine serum creatinine concentration and rats were placed in metabolic cages for an overnight (18 hr.) urine collection for the determination of urine volume (by weight), urine creatinine, protein and albumin. The rats were then switched from the 0.4% salt diet to HS and the measurements of MAP and GFR determined on days 2,5,7,14 and 21 of HS with urine and blood samples collected on a control day and days 7,14 and 21of HS for measurement of urine albumin, protein, creatinine and plasma creatinine (see online supplement methods).
Measurement GFR in conscious rats
For the measurement of GFR, a miniaturized device (NIC-Kidney, Mannheim Pharma & Diagnostics, Mannheim Germany) was utilized that was comprised of two light-emitting diodes that could transcutaneously excite FITC-sinistrin at 480 nm and a photodiode to detect the emitted light signal at 521 nm as described3. The device (batteries, diodes and microprocessor) was contained within a rodent jacket (Lomir Biomedical, Malone, NY) and the optical components affixed on a depilated region of the back using a double sided sticky patch (Lohmann GmbH KG, 56567, Neuwied, Germany) during a brief (< 5 min.), light anesthesia with 2% isofluorane. The rat was then placed in a polystyrene box residing on the receiver for the implanted transmitter collecting data from the carotid artery for the measurement of arterial blood pressure. The rat could move freely about this area as desired and measurements were initiated 30-40 minutes after affixing the device. The transcutaneous device contained a microprocessor for the amplification and digitization (10 bit) of the signal and the data was transferred by radio-telemetry to a remote computer for storage and analysis. After a resting baseline period of 10 min, a bolus of FITC-sinistrin (15 mg/100 g body weight; dissolved in 0.5 ml sterile isotonic saline) was injected through the femoral venous catheter. The excretion kinetics of FITC-sinistrin were determined using a sampling rate of 60 measurements/min with an excitation time of 10 ms/measurement. Excretion kinetics were determined following each injection over 120 min which resulted in 7200 data points for each GFR measurement as shown in Figure 1. Blood pressure was measured during this same period as well with data collected at 500 Hz for 10 sec/2 min. Elimination half-life (t1/2) determinations were calculated using an established one compartment model2,5,8 (see online supplement methods). Following each period of GFR and MAP measurement, the animal was returned to the home cage which was then placed on the same receiver.
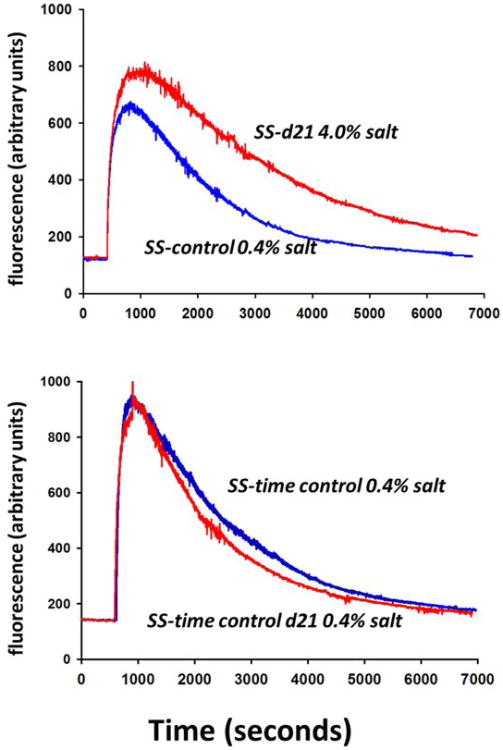
Figure 1.
Excretion kinetics for FITC-sinistrin in a representative SS rat from each group studied. Upper panel shows the measurement in an SS rat obtained on the last control day on 0.4% (blue line) and the measurement in the same SS rat on day 21 (d21) of 4.0% salt diet (high salt; red line). Lower panel shows the measurement in a SS rat from the time control group on the same days but with the animal maintained on 0.4% salt for the duration of the study.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). A two way ANOVA for repeated measures was used followed by a Holm-Sidak test for multiple comparisons to determine differences across and between groups over the time course of the study. A p <0.05 was considered significant.
Results
FITC-sinistrin excretion kinetics
Figure 1 illustrates the kinetics of FITC-sinistrin elimination determined in two SS rats; one from the HS group (upper panel) and one from the 0.4% time control group (lower panel). The prolonged rate of disappearance of the fluorescent signal is evident in the excretion kinetics (e.g. reduced slope) after 21 days of the HS. This is in marked contrast to the SS rat receiving the diet of 0.4% salt for the duration of the study. In this time control SS rat, the curves from the first time point and the last time point are superimposable indicating that the excretion kinetics remained constant over the 3 week period of study.
GFR, elimination half-life (t1/2), MAP and albumin excretion
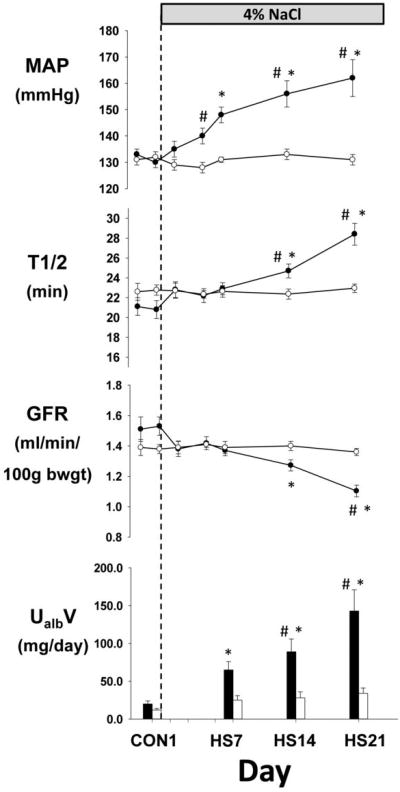
Figure 2 summarizes the GFR, t1/2, MAP and urinary albumin excretion in the two groups of SS rats studied. In each group, baseline measurements were obtained while the animals were on 0.4% salt diet. Following the measurements on the last day of baseline, the diet was switched in one group of SS rats to HS (4.0% NaCl; n=9) while the other group was maintained on the 0.4% salt diet for the duration of the study as a time control (n=6). Measurements were obtained on days 2,5,7,14 and 21 after switching to the 4.0% salt diet and on the same days for the time control group maintained on 0.4% salt. Since urine could not be collected simultaneously with the hemodynamic data, time points for urine albumin correspond to the next morning following the experimental period of control day 1 and HS day 7, 14, and 21.
Figure 2.
Comparison of the time control group of SS rats (open circle; n=6) maintained on 0.4% salt diet to a group of SS rats fed a high salt diet of 4.0% (HS) for 21 days after control measurements (closed circle; n=9). Mean arterial pressure (MAP), FITC-sinistrin elimination half-life (t1/2), glomerular filtration rate (GFR), and urinary excretion of albumin (UalbV) are summarized. * indicates significant (p < 0.05) within group difference from control days. # indicates significant (p < 0.05) between group differences. Values are mean±SEM.
In SS rats switched from 0.4% to HS, MAP rose progressively from the last control day (130±2 mmHg) reaching significance by day 5 of HS and averaged 162±7 mmHg by day 21. Although there tended to be a small reduction of GFR by the second day of HS, significant changes from control were not observed until day 14 when GFR was reduced from 1.53±0.06ml/min/100g bwgt (t1/2=20.8±0.9 min) to 1.27±0.04 (t1/2=24.7±0.7). By day 21, GFR had fallen by 28% to an average of 1.1±0.04 ml/min/100g bwgt (t1/228.4±1.1 min.). Subsequent histological analysis of the kidneys removed on day 21 quantified glomerular injury as we have described9 and found significantly greater injury in the SS rats receiving the 4.0% salt diet than the time control SS rats fed the 0.4% diet (data not shown; see Table S1 in online supplement).
Urinary excretion of albumin, however, changed significantly from a control of 20±4 to 65±11 by HS day 7. This change clearly preceded any measurable reductions of GFR and was significantly different from the time control group by HS day 14 with a nearly 7-fold increase above control values by HS day 21.
In contrast to rats fed the high salt diet, SS rats maintained on 0.4% salt diet throughout the study (time control) exhibited no significant changes in MAP, t1/2, GFR or UalbV throughout the study. Although SS rats eventually develop hypertension even when maintained on low salt diets9, MAP remained constant through the entire period of experimental monitoring (131±2 mmHg on first day of measurement and 131±2 on the last day of measurement). Importantly, this time control group of rats maintained on 0.4% salt diet provide the first assessment of the reproducibility of the transcutaneous FITC-sinistrin GFR and t1/2 measurements obtained at 7 different time points over a total period of nearly 25 days (including control measurements). Specifically, the average coefficient of variation of GFR over these 25 days within the rats in this group averaged 5.3%.
Creatinine and urine indices of renal injury
Table 1 summarizes the creatinine measurements and the calculated clearance (Ccre) values as well as urinary protein excretion. The goal was to utilize the sensitive and reliable mass spectrometry method of measurement of creatinine10 then compare the commonly used index of Ccre to the transcutaneous method. Urine and plasma collections were matched to four of the seven days of measurements of MAP and GFR. In contrast to the reductions of GFR detected by FITC-sinistrin excretion kinetics, no significant decrease of Ccre from the control measurement was observed in response to HS. There was not even a tendency for a decrease of Ccre by HS day14 even though GFR, determined by FITC-sinistrin, had significantly fallen by this time (Figure 2). The increases of total urine protein excretion in SS rats fed HS mirrored that of albumin, rising significantly from control values of 53±18 to levels of 130±18 by day 7 while no significant changes were observed in SS rats maintained on 0.4% salt diet. Body weight, used for the calculation of GFR, was not different between the two groups of SS rats but increased progressively over the 3-4 weeks of study appropriate for the growth pattern for this young age.
Table 1.
Summary of body weight (BWGT), urine flow (UV), urinary excretion of protein (UprotV), urinary excretion of creatinine (UcreaV), plasma creatinine (Pcrea) and creatinine clearance (Ccrea) during the control period (CON1; 0.4% salt diet) and on day 7, 14, and 21 of 4.0% high salt diet (HS).
| Day | Group | BWGT (gm) | UV (mL/day) | UprotV (mg/day) | UcreV (mg/day) | Pcre (mg/dL) | Ccre (ml/min/100g BWGT) |
|---|---|---|---|---|---|---|---|
| CON1 | SS-0.4% (N=8) | 289±8 | 0.22±.02 | 53±18 | 8.4±.4 | .25±.01# | .84±.06 |
| TC-SS; 0.4% (N=6) | 291±4 | 0.2±.02 | 42±5 | 7.3±.6 | .21±.01 | .85±.06 | |
| HS7 | SS-4.0% (N=8) | 315±6* | 1.5±.1* # | 130±18* | 10.6±.6* | .23±.02 | 1.08±.06* |
| TC-SS; 0.4% (N=6) | 316±8* | 0.3±.03 | 68±11 | 8.8±.7 | .22±.01 | .90±.06 | |
| HS14 | SS-4.0% (N=8) | 339±8* | 1.7±2* # | 173±26* # | 12.2±.8* | .26±.01 | .98±.05 |
| TC-SS; 0.4% (N=6) | 335±6* | 0.57±.2 | 81±17 | 10.9±.2* | .24±.02 | .92±.12 | |
| HS21 | SS-4.0% (N=8) | 353±8* | 2.1±2* # | 237±37* # | 14.0±.9* | .29±.02* | .98±.06 |
| TC-SS; 0.4% (N=6) | 350±9* | 0.67±.2* | 83±10 | 12.0±.4* | .27±.02* | .91±.04 |
indicates significant difference from LS control p< 0.05 ;
indicates significant difference from the time control group on the same day p < 0.05
Discussion
There are several novel aspects of the present study. First, sequential changes of GFR have not been previously measured in unanesthetized rodents during the development of any form of hypertension. Second, the progressive changes of GFR were uniquely quantified during the critical period of hypertension development in the most commonly used model of salt-sensitive hypertension, the Dahl S rat. Third, increases of albumin excretion preceded any observed reductions of GFR and represented the most sensitive index of renal injury measured in this study followed by GFR measurements using FITC-sinistrin. The least sensitive index was Ccre which did not detect relatively large reductions of GFR (e.g. 28%) even when using mass spectrometry to measure creatinine.
The results show that MAP rose progressively after SS rats were switched from 0.4% salt diet to HS reaching significance by day 5 of HS. Lagging by nearly 10 days, a significant decrease of GFR was observed by day 14 of HS with an average decrease of 28% by day 21. Given the delay in the reduction of GFR relative to the increase in blood pressure, it is likely that this reduction was a consequence of the glomeruli being subjected to injurious levels of elevated renal perfusion pressures. The concept of pressure-induced injury is consistent with results obtained from studies of SS rats fed a high salt diet in which protection of the kidneys from hypertension greatly reduced glomerular injury. Specifically, the impact of renal perfusion pressure to the left kidney was prevented by continuous servo-control inflation of an aortic balloon implanted between the renal arteries which maintained the pressure to the kidney at normal control levels as hypertension developed in response to 4% salt diet for two weeks9. Comparison of injury between the servo-controlled left kidney to the uncontrolled right kidney showed that the glomeruli of the kidneys exposed to hypertension exhibited significantly greater levels of injury than the pressure-controlled kidneys demonstrating that elevations of renal perfusion pressure contribute significantly to the renal injury seen in SS rats during the early phase of salt-induced hypertension.
We have reported that SS rats of the same age used in the present study (9 weeks), exhibit glomerular injury even when fed a lower salt diet (0.1%) than the present study and prior to the development of hypertension11. When fed 0.1% NaCl diet since weaning, glomerulosclerosis was apparent in 10% of glomeruli of the SS rats with about 8% showing evidence of glomerular ischemia (collapse)11. Mild tubulointerstitial injury was also observed as evidenced by tubular dilation, cast formation, interstitial widening and mononuclear cell infiltration11. In this same study, even at 3 weeks of age in SS rats, 1.6% of glomeruli showed evidence of ischemia (glomeruli collapsed) compared to an absence of injury in the Dahl salt-resistant rats11. Other studies have found that GFR (measured under anesthesia) was nearly 15% lower in SS rats (3H-inulin clearance) at 9 weeks of age when fed a 0.1 to 0.4% sodium diet as compared to salt-resistant Dahl (DR) or SS.13BN consomic rats12,13. These observations indicate that kidneys of SS rats begin to develop glomerular injury at a very early age prior to hypertension. The present study shows the sequence of progression of GFR changes over the 3 week period during the rapid development of hypertension after switching to a high salt diet.
Measurement of GFR Inulin (and its analog sinistrin) is a biologically inert fructose polymer which is freely filtered in the glomerulus and neither secreted, reabsorbed nor metabolized in the renal tubules. This makes inulin an ideal substance for the determination of GFR14,15. The amount of filtered plasma needed to provide the inulin excreted in the urine (i.e., inulin clearance) accurately reflects the GFR in humans and rodents. This clearance is determined reliably either by infusing inulin continuously to achieve a steady-state plasma concentration with accurately timed urine and plasma samples or by measurement of the disappearance rate of radiolabeled or fluorescently labeled inulin from the circulation. So, although the basic techniques to accurately determine GFR are well established, the gold standard of measurement as determined by the renal clearance of inulin is not simple and requires considerable time and inconvenience to the experimental animal or patient as well as precise chemical quantification of inulin concentrations in both plasma and urine15,16,17,18. The practical limitations of these techniques are magnified when applied to rodent models. Especially relevant is the importance of repeated sampling of blood in the absence of anesthesia which is recognized to reduce GFR19. Although a light dose of isoflurane was briefly administered to depilate the region of the skin under the cutaneous sensor and secure the jacket in place, this procedure took less than five minutes and was followed by a 30-40 minute period before baseline measurements were initiated. Animals were quite alert and ambulatory as baseline pressure measurements were begun and the MAP recorded was similar to those levels measured in other groups of conscious SS rats on 0.4% salt diet. While we cannot account for circadian variations in this study, all measurements were made between 9 a.m. and noon during the light cycle and should reflect the resting phase.
One of the major challenges for GFR measurements in rodents has been the necessary and repeated sampling of blood which can result in hypovolemia triggering increasesof sympathetic nerve activity and renin secretion thereby altering GFR20. Added to this is the stress that conscious rodents undergo as they are generally restrained for these procedures as urine is collected and blood is sampled. Blood sampling during bolus clearance requires implantation of an arterial catheter for repeated sampling at least seven times within 75 minutes21. Even in large animals or humans, inulin clearance is not a viable option for either routine or, especially, repeated clinical assessment of GFR15,22. The non-invasive techniques used in the present study clearly demonstrate the feasibility of now carrying out such measurements in conscious, unrestrained rats over a period of many weeks.
Albumin excretion The key measurements generally used to define chronic kidney disease are a reduced GFR and increased albuminuria, both of which generally coexist with hypertension and other recognized cardiovascular risk factors23. In the present study, a threefold increase of urinary albumin excretion was observed as early as 6 days after the start of the HS diet, preceding by one week any significant reductions of GFR. It was indeed a sensitive predictor of the reductions of GFR that followed. This observation is consistent with recent epidemiological evidence from the multicenter ONTARGET/TRANSCEND studies of 23,480 patients which found changes in albuminuria were significantly associated with and a useful predictor of risk of cardiovascular death and renal disease 24,25.
Creatinine clearance Determination of endogenous Ccre has been the most commonly used indicator of GFR in many experimental and clinical studies. Although it is generally conceded that serum creatinine and Ccre measurements lack sufficient sensitivity to meaningfully assess moderate reduction of renal function, eGFR, as extrapolated from serum creatinine measurements, is most often used as an estimate of GFR and to classify the contribution of renal function to population risk of cardiovascular events and mortality26. Although lower eGFR has been found to predict the composite cardiovascular end point and stroke, this index of renal function appears to be a weaker predictor of outcome than 24-hour systolic blood pressure27. A number of studies in various species indicate that from 30 to 60% of total Ccre is due to tubular secretion which therefore overestimates the real GFR21,28. In addition, the Jaffe colorimetric method to determine plasma creatinine concentrations is not accurate at low concentration ranges28 and even when accurately measured by using high-performance liquid chromatography (HPLC) or by mass spectrometry, the GFR is influenced by the serum creatinine dependency on muscular tissue mass and dietary habits. The results in the present study comparing GFR measured by FITC–sinistrin kinetics to Ccre measurements confirm limitations described by others and demonstrate that creatinine is an unreliable surrogate marker of GFR. It has been estimated by others to reflect reductions of kidney function only after the destruction of about half of the functional renal tissue22,29,30.
It is now 50 years since Lewis K. Dahl developed the salt-sensitive (SS) model of hypertension from the outbred Sprague Dawley rat strain31. Numerous studies have characterized the abnormalities of this model as most recently reviewed by Zicha et al.32 Renal function in SS rats fed a high salt diet is characterized by a variety of abnormalities including blunted pressure-diuresis and natriuresis responses13,33,34. GFR obtained in anesthetized SS rats has been found to be reduced compared to salt-resistant rats maintained on either a normal or high salt diet11,12,13,35. There have been no GFR data obtained in conscious rats and certainly none characterizing the progression of changes in response to a high salt diet. The present data show that the reduction of GFR occurs well after the development of the hypertension following an increase of sodium intake. MAP was significantly elevated above control levels by day 5 of the high salt diet while significant reductions of GFR or the t1/2were not observed until day 14. This sequence of events is consistent with what appears to be a gradual impairment of renal blood flow myogenic autoregulation in SS rats, a response which follows only after the appearance of morphological changes36. No evidence of reduced tubuloglomerular feedback has been found in SS rats fed a high salt diet36,37. It should be recognized that the sequence of events observed in SS rats in the present study does not imply that the same sequence occurs in other forms of hypertension.
Perspectives
The use of the novel non-invasive method with FITC-sinistrin has enabled repetitive tracking of GFR in freely moving rats during disease progression. We have shown that this method is far more sensitive than standard measurements of Ccre. Importantly, it has enabled us to determine that the reduction of GFR in SS rats follows the development of hypertension by nearly two weeks and is therefore a consequence of the hypertension and not the cause. Given the ease of such measurements in even small rodent model systems, it would seem logical to apply this non-invasive approach to assess renal function in human subjects for routine clinical evaluations of patients at risk and to refine risk stratification assessments.
Supplementary Material
Table S1: Glomerular injury and tubular necrosis evaluation in kidneys collected in SS rats on day 21 of 4% salt and in SS rats in the time control (TC) group fed 0.4% salt for the same period. Gomoritrichrome stained sections were examined at 20X magnification and 60 superficial and 30 juxtamedullary glomeruli evaluated and scored from 0 (no injury) to 4 as we have described7,8. Tubular necrosis was determined by a threshold method as we have described that quantified the % of the outer medullary area with positive stained protein cast indicating tubular necrosis8.
Novelty and Significance.
What is new? There are several novel aspects of the present study. First, sequential changes of GFR have never been previously measured in unanesthetized rodents during the development of any form of hypertension. Second, GFR was uniquely quantified during the critical period of hypertension development in the most commonly used model of salt-sensitive hypertension, the Dahl salt sensitive rat.
What is relevant? The data show that reduction of GFR was a consequence and not a cause of the hypertension. The results also demonstrate the inability of creatinine to track even relatively large reductions of GFR (e.g. 28%) showing how misleading creatinine clearance measurements can be even when accurately measured using mass spectrometry.
Summary of the conclusions of the study. The use of the novel non-invasive method with FITC-sinistrin has enabled repetitive tracking of GFR in freely moving rats during disease progression. We have shown reduction of GFR in Dahl salt-sensitive rats follows the development of hypertension by nearly two weeks and is therefore a consequence of the hypertension and not the cause. Given the ease of such measurements in even small rodent model systems, it would seem logical to apply this non-invasive approach to assess renal function in human subjects for routine clinical evaluations of patients at risk and to refine risk stratification assessments.
Acknowledgments
The authors wish to thank Jenifer Phillips and Camille Taylor for the measurement of albumin and protein.
Source of funding: The work was supported by National Heart, Lung, and Blood Institute Grants HL-28587, HL-82798, GM-94503 (A.W.C., R.P.R., T.K., M.S.). Urine and plasma creatinine levels were measured by the UAB-UCSD O'Brien Core Center (NIH P30 DK-079337).
Footnotes
Disclosures: No disclosures for A.W.C., R.P.R., T.K., M.S. D.S.K. and N.G. own patents for the production of FITC-sinistrin and the transcutaneous device. D.S.K. is co-founder of Mannheim Pharma & Diagnostics GmbH, supplier of FITC-Sinistrin and the NIC-Kidney devices.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cowley AW., Jr Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- 2.Schock-Kusch D, Sadick M, Henninger N, Kraenzlin B, Claus G, Kloetzer HM, Weiss C, Pill J, Gretz N. Transcutaneous measurement of glomerular filtration rate using FITC-sinistrin in rats. Nephrol Dial Transplant. 2009;24:2997–3001. doi: 10.1093/ndt/gfp225. [DOI] [PubMed] [Google Scholar]
- 3.Schock-Kusch D, Zie Q, Shulhevich Y, Hesser J, Stsepankou D, Sadick M, Koenig s, Hoecklin F, Pill J, Gretz N. Transcutaneous assessment of renal function in conscious rats with a device for measuring FITC-sinistrin disappearance curves. Kidney Int. 2011;79:1254–1258. doi: 10.1038/ki.2011.31. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D, Neudecker S, Koenig s, Heinrich R, Hoechlin F, Pill J, Friedemann J, Schweda F, Gretz N, Schock-Kusch D. Transcutaneous measurement of renal function in conscious mice. Am J Physiol. 2012;303:F783–F788. doi: 10.1152/ajprenal.00279.2012. [DOI] [PubMed] [Google Scholar]
- 5.Pill J, Kraenzlin B, Jander J, Sattelkau T, Sadick M, Kloetzer HM, Deus C, Kraemer U, Gretz N. Fluorescein-labeled sinistrin as marker of glomerular filtration rate. Eur J Med Chem. 2005;40:1056–1061. doi: 10.1016/j.ejmech.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 6.http://rgd.mcw.edu/rgdweb/report/strain/main.html?id=2308886[accessdate2-4-13]
- 7.Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O'Connor, Cowley AW., Jr Increased expression of NADPH oxidase subunit p67phox in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metabolism. 2012;15:1–8. doi: 10.1016/j.cmet.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pill J, Issaeva O, Woderer S, Sadick M, Kranzlin B, Fiedler F, Klotzer HM, Kramer U, Gretz N. Pharmacological profile and toxicity of fluorescein-labeled sinistrin, a novel marker for GFR measurements. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:204–211. doi: 10.1007/s00210-006-0067-0. [DOI] [PubMed] [Google Scholar]
- 9.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW., Jr High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol. 2008;19:1472–1480. doi: 10.1681/ASN.2007121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young A, Struys E, Wood T. Quantification of creatine and guanidioacetate using GC-MS and LC-MS/MS for the detection of cerebral creatine deficiency syndromes. Curr Protoc Hum Genet. 2007;54:17.3.1–17.3.18. doi: 10.1002/0471142905.hg1703s54. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RJ, Gordon KL, Giachelli C, Kurth T, Skelton MM, Cowley AW., Jr Tubulointerstitial injury and loss of nitric oxide synthases parallel the development of hypertension in the Dahl-SS rat. J Hypertens. 2000;18:1497–1505. doi: 10.1097/00004872-200018100-00019. [DOI] [PubMed] [Google Scholar]
- 12.Cowley AW, Jr, roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromsome 13 confers protection from high salt to consomic Dahl S rat. Hypertension. 2001;37:456–461. doi: 10.1161/01.hyp.37.2.456. [DOI] [PubMed] [Google Scholar]
- 13.Roman RJ, Kaldunski M. Pressure natriuresis and cortical and papillary blood flow in inbred Dahl rats. Am J Physiol. 1991;261:R595–R602. doi: 10.1152/ajpregu.1991.261.3.R595. [DOI] [PubMed] [Google Scholar]
- 14.Aurell M. Accurate and feasible measurements of GFR—is the iohexol clearance the answer? Nephrol Dial Transpl. 1994;9:1222–1224. [PubMed] [Google Scholar]
- 15.Tett ES, Kirkpatrick CM, Gross AS, McLachlan AJ. Principles and clinical application of assessing alterations in renal elimination pathways. Clin Phamacokinet. 2003;42:1193–1211. doi: 10.2165/00003088-200342140-00002. [DOI] [PubMed] [Google Scholar]
- 16.Gretz N, Ecker-Tschimer KH, Kuhnle HR, von Dahl K, Kirschfink M, Drescher P, Lasserre JJ, Strauch M. Practicability of the inulin plasma single-shot clearance. Contrib Nephrol. 1990;81:220–228. doi: 10.1159/000418757. [DOI] [PubMed] [Google Scholar]
- 17.Jung K, Henke W, Schulze BD, Sydow K, Precht K, Klotzek S. Practical approach for determining glomerular filtration rate by single-injection inulin clearance. Clin Chem. 1992;38:403–407. [PubMed] [Google Scholar]
- 18.Swinkels DW, Hendriks JC, Nauta J, de Jong MC. Glomerular filtration rate by single-injection inulin clearance: definition of a workable protocol for children. Ann Clin Biochem. 2000;37:60–66. doi: 10.1258/0004563001901533. [DOI] [PubMed] [Google Scholar]
- 19.Fusellier M, Desfontis JC, Madec S, Gautier F, Debailleaul M, Gogny M. Influence of three anesthetic protocols on glomerular filtration rate in dogs. Am J Vet Res. 2007;68:807–811. doi: 10.2460/ajvr.68.8.807. [DOI] [PubMed] [Google Scholar]
- 20.DiBona GF. Peripheral and central interactions between the renin-angiotensin system and the renal sympathetic nerves in control of renal function. Ann NY Acad Sci. 2001;940:395–406. doi: 10.1111/j.1749-6632.2001.tb03693.x. [DOI] [PubMed] [Google Scholar]
- 21.Eisner C, Faulhaber-Water R, Wang Y, Leelahavanichkul A, Yuen PS, Mizel D, Star RA, Briggs JP, Levine M, Schnermann J. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int. 2010;77:519–526. doi: 10.1038/ki.2009.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolton WK, Kliger AS. Chronic renal insufficiency: current understandings and their implications. Am J Kidney Dis. 2000;36:S4–S12. doi: 10.1053/ajkd.2000.19926. [DOI] [PubMed] [Google Scholar]
- 23.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Dis. 2011;59:e1–420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Schmieder RE, Mann JRE, Schumacher H, Cao P, Mancia G, Weber MA, McQueen M, Koon T, Yusuf S, on behalf of the ONTARGET Investigators Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol. 2011;22:1353–1364. doi: 10.1681/ASN.2010091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redon J, Martinez F. Microalbuminuria as surrogate endpoint in therapeutic trials. Curr Hypertens Rep. 2012;14:345–349. doi: 10.1007/s11906-012-0270-y. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boggia J, Thijs L, Li Y, Hansen TW, Kikuya M, Björklund-Bodegård K, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Schwedt E, Sandoya E, Kawecka-Jaszcz K, Filipovsky J, Imai Y, Wang J, Ibsen H, O'Brien E, Staessen JA, on behalf of the International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators Risk stratification by 24-hour ambulatory blood pressure and estimated glomerular filtration rate in 5322 subjects from 11 populations. Hypertension. 2013;61:18–26. doi: 10.1161/HYPERTENSIONAHA.112.197376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keppler A, Gretz N, Schmidt R, Loetzer HM, Groene HJ, Lelongt B, Meyer M, Sadick M, Pill J. Plasma creatinine determination in mice and rats: an enzymatic methods compares favorably with a high-performance liquid chromatography assay. Kidney Int. 2007;71:74–78. doi: 10.1038/sj.ki.5001988. [DOI] [PubMed] [Google Scholar]
- 29.Burkhardt H, Bojarsky G, Gretz N, Gladisch R. Creatinine clearance, Cockcroft-Gault formula and cystatin C: estimators of true glomerular filtration rate in the elderly. Gerontology. 2002;48:140–146. doi: 10.1159/000052832. [DOI] [PubMed] [Google Scholar]
- 30.Fliser D, Bischoff I, Hanses A, Block S, Joest M, Ritz E, Mutschler E. Renal handling of drugs in the healthy elderly. Creatinine clearance underestimates renal function and pharmacokinetics remain virtually unchanged. Eur J Clin Pharmacol. 1999;55:205–211. doi: 10.1007/s002280050619. [DOI] [PubMed] [Google Scholar]
- 31.Dahl LK. Salt and hypertension. Am J Clin Nutr. 1972;25:231–244. doi: 10.1093/ajcn/25.2.231. [DOI] [PubMed] [Google Scholar]
- 32.Zicha J, Dobesova Z, Vokurkova M, Rauchova H, Hojna S, Kadlecova M, Behuliak M, Vaneckova I, Kunes J. Age-dependent salt hypertension in Dahl rats: fifty years of research. Physiological Res. 2012;61:S35–S87. doi: 10.33549/physiolres.932363. [DOI] [PubMed] [Google Scholar]
- 33.Roman RJ. Abnormal renal hemodynamics and pressure-natriuresis relationship in Dahl salt-sensitive rats. Am J Physiol. 1986;251:F57–F65. doi: 10.1152/ajprenal.1986.251.1.F57. [DOI] [PubMed] [Google Scholar]
- 34.Tobian L, lang J, Iwai J, Hiller K, Johnson MA, Goossens P. Prevention with thiazide of NaCl-induced hypertension in Dahl “S” rats. Evidence for a Na-retaining humoral agent in “S” rats. Hypertension. 1979;1:316–323. doi: 10.1161/01.hyp.1.3.316. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Wang DH. Role of TRPV1 channels in renal haemodynamics and function in Dahl salt-sensitive hypertensive rats. Exp Physiol. 2008;93:945–953. doi: 10.1113/expphysiol.2008.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlsen FM, Andersen CB, Leyssac PP, Holstein-Rathlou NH. Dynamic autoregulation and renal injury in Dahl rats. Hypertension. 1997;30:975–983. doi: 10.1161/01.hyp.30.4.975. [DOI] [PubMed] [Google Scholar]
- 37.Wilcox CS, Welch WJ. TGF and nitric oxide: effects of salt intake and salt-sensitive hypertension. Kidney Int Suppl. 1996;55:S9–S13. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Glomerular injury and tubular necrosis evaluation in kidneys collected in SS rats on day 21 of 4% salt and in SS rats in the time control (TC) group fed 0.4% salt for the same period. Gomoritrichrome stained sections were examined at 20X magnification and 60 superficial and 30 juxtamedullary glomeruli evaluated and scored from 0 (no injury) to 4 as we have described7,8. Tubular necrosis was determined by a threshold method as we have described that quantified the % of the outer medullary area with positive stained protein cast indicating tubular necrosis8.