Abstract
Like other members of the Herpesviridae family, human herpesvirus (HHV)-6A and HHV-6B have developed a wide variety of strategies to modulate or suppress host immune responses and, thereby, facilitate their own spread and persistence in vivo. Long considered two variants of the same virus, HHV-6A and HHV-6B have recently been reclassified as distinct viral species, although the established nomenclature has been maintained. In this review, we summarize the distinctive profiles of interaction of these two viruses with the human immune system. Both HHV-6A and HHV-6B display a tropism for CD4+ T lymphocytes, but they can also infect, in a productive or nonproductive fashion, other cells of the immune system. However, there are important differences regarding the ability of each virus to infect cytotoxic effector cells, as HHV-6A has been shown to productively infect several of these cells, whereas HHV-6B infects them inefficiently at best. In addition to direct cytopathic effects, both HHV-6A and HHV-6B can interfere with immunologic functions to varying degrees via cytokine modulation, including blockade of IL-12 production by professional antigen-presenting cells, modulation of cell-surface molecules essential for T-cell activation, and expression of viral chemokines and chemokine receptors. Some of these effects are related to signaling through and downregulation of the viral receptor, CD46, a key molecule linking innate and adaptive immune responses. Increasing attention has recently been focused on the importance of viral interactions with dendritic cells, which may serve both as targets of virus-mediated immunosuppression and as vehicles for viral transfer to CD4+ T cells. Our deepening knowledge of the mechanisms developed by HHV-6A and HHV-6B to evade immunologic control may lead to new strategies for the prevention and treatment of the diseases associated with these viruses. Moreover, elucidation of these viral mechanisms may uncover new avenues to therapeutically manipulate or modulate the immune system in immunologically mediated human diseases.
Keywords: antigen-presenting cells, CD46, chemokines, cytokines, HHV-6A, HHV-6B, immunomodulation, immunosuppression, receptors, T cells
In their millenial chess game with the host immune system, viruses have developed an extraordinary array of strategies aimed at escaping the host mechanisms of defense [1–3]. Herpesviruses are among the finest connoisseurs of the host immune system, having developed complex mechanisms to confound and eventually inactivate it. In particular, human herpesvirus (HHV)-6A and HHV-6B (Figure 1) are of great interest, as they display a peculiar complexity and a unique biology. Although these viruses have long been considered two variants of a single species, they have recently been recognized by the International Committee for Taxonomy of Viruses as two distinct viral species, despite maintaining the established designations of HHV-6A and HHV-6B [4]. Overall, these two viruses share more than 88% sequence homology; yet, they exhibit distinctive biological, epidemiological and clinical features [5]. Both species show a marked tropism for cells of the immune system, and for activated CD4+ T lymphocytes in particular, which sustain productive infection and undergo dramatic cytopathic effects. However, in line with the nearly ubiquitous distribution of their primary cellular receptor, CD46 [6], HHV-6A and HHV-6B have a much broader cellular tropism, including cells of diverse embryonic lineage origin, although in many cell types the viral cycle is restricted and does not proceed to completion. Nevertheless, major phenotypic and functional changes also occur in nonproductively infected cells, as observed in professional antigen-presenting cells such as mononuclear phagocytes and dendritic cells (DC) (Figure 2). Moreover, both HHV-6A and HHV-6B can indirectly influence the function of a broad range of immune cells via the aberrant induction or blockade of cytokines and chemokines, or by producing their own chemokines and chemokine receptors [1,7–11] (Figure 3). These experimental observations are in line with clinical evidence of immunosuppression associated with HHV-6A or HHV-6B infection in HIV-seronegative individuals. The present review summarizes the body of knowledge accrued over the past 25 years on the interaction between HHV-6A and HHV-6B and the immune system.
Figure 1. Electron micrographs of mature human herpesvirus 6A and human herpesvirus 6B virions.
Despite significant differences in their biological and epidemiological features, the two viruses are morphologically indistinguishable, exhibiting the typical features of HHV, with an electron-dense icosahedral core surrounded by a tegument and a lipoprotein envelope.
HHV: Human herpesvirus.
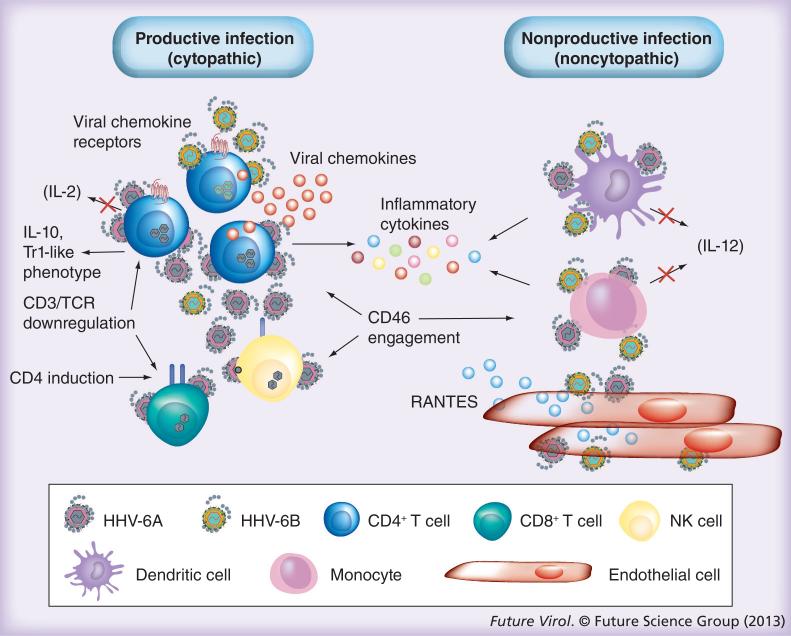
Figure 2. Schematic representation of the complex network of human herpesvirus 6A and human herpesvirus 6B interactions with lymphoid and nonlymphoid cells.
Some of the interactions have been documented primarily with HHV-6A and appear to occur less frequently, if at all, with HHV-6B.
HHV: Human herpesvirus; TCR: T-cell receptor.
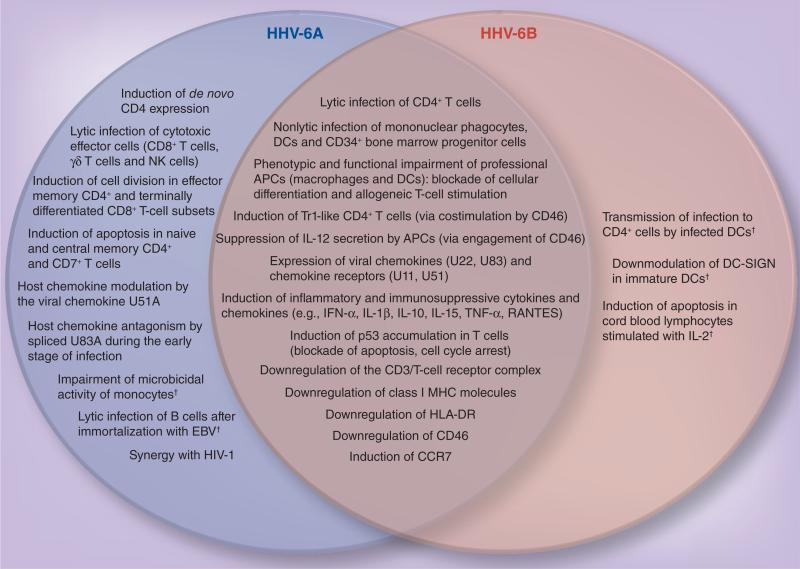
Figure 3. Common and species-specific mechanisms of immunomodulation by human herpesvirus 6A and human herpesvirus 6B.
Although the two viruses exert common effects on the immune system (Venn diagram intersection), they also exhibit specific activities (left and right panels for HHV-6A and -6B, respectively).
†It has to be emphasized that these mechanisms, listed under species-specific activities, were tested exclusively on a single viral species and therefore cannot be definitively considered specific.
DC: Dendritic cell; HHV: Human herpesvirus.
Mechanisms of immunomodulation & immunosuppression by HHV-6A & HHV-6B
Productive or nonproductive infection of cells of the immune system
The use of the CD46 receptor, a ubiquitously expressed regulator of complement activation, by both HHV-6A and HHV-6B, is indicative of a broad cellular tropism [6]. Productive infection, however, is limited to a relatively small range of cells, probably as a consequence of intracellular restriction factors acting beyond the viral entry step (Figure 2). Studies on the cellular tropism of HHV-6A and HHV-6B have suggested that these viruses infect various cells implicated in the generation of effective immune responses, thus affecting both cell-mediated and humoral immune responses. There are, however, notable differences in the cellular tropism of HHV-6A and HHV-6B.
Lytic infection
Both HHV-6A and HHV-6B efficiently infect and complete a productive lytic cycle in lymphoid cells. There is substantial consensus that primary targets for infection by both viruses in vivo and in vitro are activated CD4+ T cells [12,13]. Infection in CD4+ T cells is productive and cytopathic, leading to progressive increase in cell size, morphological changes with the adoption of an evenly rounded cell shape, formation of binucleated cells and, occasionally, syncytia, apoptosis and cell death within a variable period ranging from 5 to 10 days in culture [12–15]. These effects on CD4+ T cells represent the basis for the potential synergy between HHV-6 and HIV-1.
Unlike T cells, B cells are not typically infectable by HHV-6A or HHV-6B. However, it has been reported that B cells become susceptible to HHV-6A after their immortalization with EBV [16], suggesting that EBV infection induces the expression of membrane and/or soluble factors that make these cells permissive. To our knowledge, this has not been tested with HHV-6B. However, persistent nonproductive HHV-6 infection was reported in a B-cell line derived from Burkitt's lymphoma in the apparent absence of EBV coinfection [17]. Additional subsets of lymphoid cells can be productively infected by HHV-6A but inefficiently, if at all, by HHV-6B (see below).
Nonlytic infection
Although certain cells of the immune system do not commonly sustain productive infection by HHV-6A or HHV-6B, nonlytic infection or even simply interaction of the cellular membrane with viral proteins may nonetheless cause important effects on their function. The cells of the mononuclear phagocytic system are a primary target for HHV-6A and HHV-6B infection both in vivo and in vitro, and have been suggested as a possible in vivo reservoir for the infection [18,19]. There is, however, some controversy on whether these cells can support productive infection by these agents. We have reported that, after exposure to either HHV-6A or HHV-6B, differentiated primary macrophages derived from peripheral blood monocytes do not exhibit any signs of productive infection, such as viral mRNA expression, viral DNA accumulation or cytopathic effects [20]. By contrast, others have reported that in vitro-differentiated macrophages are able to support at least transient replication of HHV-6B [19], and display some signs of cytopathic effects induced by both HHV-6A and HHV-6B [18]. In another study, freshly isolated blood monocytes were resistant to infection, but their differentiation with IL-15 resulted in a greater susceptibility to productive infection [21]. It is plausible that the reasons for these discrepancies could be related to the different experimental conditions used for growing and differentiating primary monocytes in vitro. High levels of HHV-6B DNA were found in circulating monocytes from children with primary infection, associated with expression of U79/80 mRNA, which is indicative of productive infection, and successful isolation of HHV-6B from these cells in culture [22]. During convalescence, HHV-6B DNA was detected in circulating monocytes in the absence of productive infection, leading to the suggestions that they may act as one of the in vivo reservoirs for the virus [19]. Thus, irrespective of the productive or nonproductive nature of infection, it is indisputable that mononuclear phagocytic cells are a major target for both HHV-6A and HHV-6B.
Similar to mononuclear phagocytes, peripheral blood-derived DCs are infectable by both HHV-6A and HHV-6B despite an ongoing controversy as to whether they can support productive infection (see below). Bone marrow progenitor cells (CD34+) are also susceptible to HHV-6B infection (no studies have been reported with HHV-6A), and can transfer latent virus to differentiated cells of multiple lineages, including monocytes/macrophages and DCs [23–25].
Strikingly, exposure to both HHV-6A and HHV-6B has been shown to dramatically suppress the differentiation and replication of different types of bone marrow precursor cells [23,26–28]. These findings are in line with reports of bone marrow graft failure in patients with active HHV-6 infection [29–33].
Immune system cells targeted by HHV-6A
HHV-6A efficiently and productively infects different types of cytotoxic effectors, such as CD8+ T cells, NK cells and γδT cells [34–36]. Considering the fact that these cell populations are mainly responsible for antiviral responses in vivo, this strategy may have a key role in counteracting the protective immune surveillance and determining a persistent infection in the host. In addition, it has recently been suggested that HHV-6A infection suppresses T-cell proliferation by inducing G2/M arrest in infected T cells [37]. Conversely, HHV-6A was shown to induce cell division in effector memory CD4+ T cells and terminally differentiated effector CD8+ T cells [38].
Immune system cells targeted by HHV-6B
The cellular tropism of HHV-6B seems to be more restricted than that of HHV-6A, at least among immune system cells, infecting cytotoxic effector cells rather inefficiently, if at all [39]. This observation has been confirmed using an ex vivo model of structured lymphoid tissue [40]. In children with acute primary infection, HHV-6B has been detected in circulating CD4+ T cells, which seem to represent the primary target both for virus replication and for subsequent latency [13].
Although the ability of HHV-6B to replicate in mononuclear phagocytes and DCs remains controversial, it has been shown that DCs exposed to the virus can effectively transmit infection to CD4+ T cells, resulting in productive infection, despite the lack of virion progeny release [41]. Treatment with phosphonoformic acid prevented virus transmission from DCs to CD4+ T cells, suggesting that DCs must be productively infected for virus transmission to occur. However, a ‘Trojan horse’ effect whereby DC-adsorbed live HHV-6B virions are passively transferred to CD4+ target cells cannot be excluded. Of note, similar studies have not been performed with HHV-6A.
Apoptosis
Apoptosis can lead to immune system modulation and dysfunction through a number of different pathways. Induction of CD4+ T-cell apoptosis by indirect pathways has been associated with both HHV-6A and HHV-6B infection [42], even though the mechanism and extent to which each virus controls the apoptotic event varies widely. In apparent contradiction with these observations, several reports have demonstrated that both HHV-6A and HHV-6B induce aberrant accumulation and phosphorylation of p53, a tumor-suppressor protein and a key regulator of cell cycle arrest and apoptosis, concomitant with blockade of apoptosis and cell cycle arrest [43–46]. Furthermore, inhibition of cellular proliferation was shown to occur by a pathway that is independent of p53 [43].
Apoptosis due to HHV-6A
HHV-6A was shown to induce apoptosis in a T-lymphoid cell line, HSB-2, in a mitochondria-mediated, caspase-dependent manner. In vitro studies indicate that increased levels of IL-6 and TNF-α promote apoptosis in HHV-6A-infected CD4+ T cells, and that the virus may accomplish this through the activation of JNK, c-jun and ATF-2, subsequently inducing upregulation of toll-like receptor 9 (TLR9) [47]. Furthermore, HHV-6A induces apoptosis primarily in naive and central memory CD4+ and CD8+ T cells, whereas effector memory and terminally differentiated effector T cells appear to be resistant to apoptosis induced by HHV-6A through a Bcl-2-independent pathway [38].
Apoptosis due to HHV-6B
While cells infected with HHV-6B almost invariably undergo cytopathic effects and die, it remains unclear whether cell death occurs primarily by necrosis or apoptosis [48]. One group has demonstrated that apoptosis is unlikely to be the major event during the early phase of HHV-6B infection in MT-4 cells, as the U95 early viral protein has high affinity for GRIM-19 and thus may mediate cell death via interference with interferon and retinoic acid signaling [48]. In addition, HHV-6B-infected cells have been shown to be nonapoptotic. In these cells, the virus may simply act as an inhibitor of host cell proliferation [44,45].
Although some groups have reported that the loss of CD14 in monocytes is accompanied by spontaneous apoptosis in uninfected cells, another study has demonstrated that downregulation of CD14 expression observed in monocytes exposed to HHV-6B does not result in apoptosis [49]. To the contrary, the same study concluded that HHV-6B may protect human monocytes from spontaneous apoptosis. However, apoptosis does occur in HHV-6B-infected cord blood lymphocytes stimulated with IL-2, suggesting that the activation signal induced by this cytokine is required for apoptosis [50]. Furthermore, a significantly higher proportion of CD4+ T lymphocytes obtained from the peripheral blood of patients with acute HHV-6B infection were shown to display an apoptotic phenotype as compared with matched controls, suggesting that HHV-6B renders CD4+ T lymphocytes susceptible to apoptosis in vivo [42].
CD46-mediated effects
In 1999, our group identified CD46, a complement-regulatory receptor also known as membrane cofactor protein (MCP), as the principal human cellular receptor for both HHV-6A and HHV-6B [6]. This conclusion was based on several complementary lines of evidence: CD46 is selectively downmodulated from the target cell surface during the course of infection; specific anti-CD46 monoclonal antibodies block the infectivity and fusogenic activity of both HHV-6A and HHV-6B; viral envelope-mediated fusion is inhibited by a soluble form of CD46; recombinant expression of human CD46 render otherwise insensitive non human cells susceptible to viral fusion and entry [6]. CD46 is a type-1 membrane glycoprotein expressed by all nucleated human cells, which plays a critical role in preventing spontaneous complement activation in vivo [51]. CD46 also serves as a receptor for other viral agents, including measles virus [52]. Although the existence of additional cellular receptors or coreceptors for HHV-6A or HHV-6B cannot be excluded, the fact that both viruses use a ubiquitous receptor like CD46 underlies their ability to bind a variety of cell types in vitro, and may help to explain the diverse clinical manifestations to which these viruses have been linked. The HHV-6-binding region was mapped within the short consensus repeat-2 and -3 domains of the CD46 extracellular region [53], whereas the gH glycoprotein was identified as the viral envelope component that interacts with CD46 [54]. However, gH alone is apparently unable to adopt the appropriate CD46-binding conformation and needs to be presented in the context of a tri-molecular complex formed by the gH, gL and gQ envelope glycoproteins [55].
Besides its complement-regulatory effects, CD46 has a key immunoregulatory role that bridges the innate and adaptive arms of the immune system. Indeed, it has been shown that HHV-6A or HHV-6B binding to CD46 dramatically and selectively suppresses the secretion of IL-12, a critical cytokine for the generation of Th1 cells, which play an essential role in the induction of effective antiviral immune responses. Differentiated primary macrophages, stimulated in vitro with IFN-γ and lipopolysaccharide and exposed to either HHV-6A or HHV-6B, fail to produce IL-12, while releasing normal amounts of other soluble factors such as TNF-α, RANTES and MIP-1β [20]. The synthesis of IL-12 is suppressed at the post-transcriptional level, and this effect does not require viral replication. Similar data were obtained with peripheral blood-derived DCs, where both HHV-6A and HHV-6B block cellular differentiation and IL-12 production, as well as the induction of allogeneic T-cell proliferation [56,57]. Again, productive infection is not required for this effect to occur. This in vitro effect seems to reflect what occurs in vivo during active infection with both HHV-6A and HHV-6B: it has been shown that the antigen-presenting capacity of DCs generated from a patient with severe HHV-6 reactivation was significantly lower than that of DCs generated from the same patient during the recovery phase [57].
Costimulation of T cells with anti-CD46 antibodies was shown to induce a T-regulatory 1-like phenotype in human CD4+ T cells, associated with IL-10 production and suppression of bystander T-cell activation [58]. Since HHV-6A and HHV-6B can directly engage CD46, this could be another mechanism whereby these viruses can evade immunologic control. Wang and colleagues have demonstrated that infection with HHV-6A or HHV-6B leads to an expansion of IL-10-producing CD4+ T cells in vitro [59]. This observation has recently been confirmed and extended, showing that T-cell costimulation either with UV-inactivated HHV-6 virions or with anti-CD46 antibodies elicits a biased cytokine profile phenotype with mixed Th1-like and T-regulatory 1-like features [Lusso P et al., Unpublished Data]. If confirmed in additional in vitro and in vivo studies, the induction of T-regulatory 1-like cells by HHV-6A or HHV-6B may provide a further rationale for the evolutionary choice of these two viruses and other microbes to use CD46 as a cellular receptor.
CD46 usage by HHV-6B
Although the initial report by Santoro et al. clearly documented the use of CD46 by at least some strains of HHV-6B [6], Mori et al. have studied one Japanese HHV-6B strain, HST, which does not efficiently induce CD46-mediated syncytia formation after short-term virion binding to the external cell membrane in the absence of productive infection, an effect defined by some as ‘fusion-from-without’ [60]. This phenomenon may result from a lower CD46-binding affinity of this particular strain or, more generally, of HHV-6B, insufficient to mediate fusion under the specific conditions of this assay. Unfortunately, these experiments have not been cross-validated using the same strains studied by Santoro et al. or using strain HST in analogous gain-of-function experiments, making it difficult to draw any conclusion regarding the efficiency of CD46 usage by HHV-6B. Nevertheless, it cannot be excluded that certain HHV-6B strains may use another, still undefined, cellular receptor for entry.
Dysregulation of complement activation
As stated above, the short consensus repeat-2 and -3 domains of CD46 were identified as the HHV-6-binding domains of CD46 [53]. Since these domains are also involved in the complement-regulatory functions of CD46, it is likely that virus binding might interfere with the physiological role of CD46, which effectively suppresses spontaneous activation of autologous complement with its consequent tissue-damaging effects. This mechanism has been documented for measles virus using in vitro models [61]. Furthermore, productive infection with both HHV-6A and HHV-6B downmodulates the expression of membrane CD46, although the process is slower and less efficient in HHV-6B than in HHV-6A infection [6]. In an ex vivo model of structured lymphoid tissue, infection with HHV-6A caused a dramatic downmodulation of CD46 in both productively-infected cells and uninfected bystander cells [40]. Taken altogether, these data demonstrate that HHV-6A and HHV-6B have the potential to trigger complement activation, thereby inducing widespread cytolytic damage in infected lymphoid tissue.
Membrane receptor modulation
Both HHV-6A and HHV-6B have the ability to induce a profound alteration in the expression of crucial molecules on target cells. Early studies documented a dramatic reduction in the expression of the T-cell receptor (TCR) complex in CD4+ T cells infected with either HHV-6A or HHV-6B due to transcriptional downregulation of several CD3 chains [12,34]. Another receptor that is transcriptionally downmodulated by HHV-6A and HHV-6B is the lectin-like receptor DC-SIGN [62], an effect that may impair the ability of DCs to promote and maintain effective secondary immune responses. Conversely, both HHV-6A and HHV-6B are able to induce the expression of CCR7, a key chemokine receptor expressed in T cells and mature DCs, which regulates the homeostatic recirculation of lymphocytes to secondary lymphoid organs [63].
It has also been shown that both HHV-6A and HHV-6B downregulate class-I MHC molecules from the surface of infected DCs [64,65]. A possible mechanism underlying this observation has recently been suggested with the discovery that the U21 gene product of both viral species binds to and diverts class-I MHC molecules to the endolysosomal compartment, effectively removing them from the cell surface [65]. Although the two proteins share 89% amino acid identity, HHV-6A U21 appears to be more effective than HHV-6B U21 at removing class-I molecules from the cell surface [65]. It is nonetheless reasonable that additional mechanisms may be implicated in MHC class-I downregulation, as shown in other β-herpesvirus systems.
Membrane receptor modulation by HHV-6A
It has been shown that the U24 protein encoded by HHV-6A is responsible for the downregulation of the TCR/CD3 complex [66]: in the presence of pU24, the TCR/CD3 complex is endocytosed but not recycled back to the plasma membrane, accumulating in early and late endosomes. Interestingly, U24 was shown to downregulate CD3 independently of the T-cell activation status and to render infected/transfected T cells resistant to activation by antigen-presenting cells [66]. Considering the essential role of the TCR/CD3 complex in T-cell activation, the interference with the normal expression of CD3 and the TCR complex is likely to have a profound immunosuppressive effect, causing the impairment of adaptive immune responses.
Another unexpected effect observed upon the infection of primary human T cells with HHV-6A is coexpression of CD4 and CD8 [12]. This unique phenomenon is related to the ability of HHV-6A to induce expression of the CD4 glycoprotein in lymphoid cells that physiologically do not express it, including CD8+ T cells [39] and NK cells [35,36]. Subsequent studies have demonstrated that this effect is mediated by HHV-6A early gene products, which are able to activate the CD4 promoter [67]. De novo induction of CD4 expression has not been clearly established for HHV-6B.
Membrane receptor modulation by HHV-6B
It has been reported that in monocytes HHV-6B downmodulates the expression of CD14, CD64 (FcγRI) and HLA-DR; surface molecules crucial to the activation of antigen-presenting cells, whereas it doesn't affect the expression of CD32 (FcγRII) [49]. Although this specific phenomenon has not yet been specifically analyzed for HHV-6A, downregulation of HLA-DR expression has also been reported previously in DCs infected with HHV-6A [56].
Modulation of cytokine & chemokine responses
HHV-6A and HHV-6B infections can modulate the profile of cytokine and chemokine production by different cell types. This dysregulation can profoundly affect the initiation, polarization and normal functionality of effective immune responses. HHV-6A and HHV-6B have been shown to reduce the production of IL-2 by stimulated peripheral blood mononuclear cells or enriched T-cell cultures, resulting in a diminished cellular proliferation [68]. Conversely, the production of inflammatory cytokines such as TNF-α, IFN-α, IFN-γ, IL-1β, IL-6, IL-8, IL-15 and IL-18 is increased [7,9,11,38,68–71]. Production of IFN-γ and IL-10 has been documented as a marker of cell-mediated immune response to both HHV-6A and HHV-6B [72], while the production of TGF-β due to HHV-6A (not yet analyzed for HHV-6B) has been observed both in vivo in patients with glioma and in astrocyte cultures in vitro [71]. However, it has been observed that these viruses induce a cytokine imbalance with a switch from an antiviral Th1-polarized cytokine profile to a Th2 profile, since they have been shown to downregulate IL-12 and IFN-γ, while upregulating IL-10 [9]. This effect was not subsequently confirmed in microarray studies on a continuous CD4+ T-cell line, SupT1, where HHV-6B infection results in downregulation of IL-10, the IL-10 receptor and IL-14 [70]. In addition, Jacobson et al. have demonstrated an increase in IL-17 production as a result of a pro-inflammatory response due to the interaction between HHV-6 glycoproteins expressed on infected CNS cells and CD46 on responding CD4+ T lymphocytes [73]. Although the work did not differentiate between HHV-6A and HHV-6B, this phenomenon is supported by the frequent detection of HHV-6 (particularly HHV-6A) in patients with multiple sclerosis (MS), and further suggests a potential mechanism of HHV-6-induced CNS inflammation that is likely to occur in vivo.
Both HHV-6A and HHV-6B can significantly modulate the chemokine system both by affecting chemokine production and by expressing viral chemokines and chemokine receptors. In an ex vivo lymphoid tissue model, infection with HHV-6A was shown to induce a dramatic increase in the production of CCL5/RANTES, as well as, to a lesser extent, CCL4/MIP-1β and CCL3/MIP-1α, the ligands for CCR5 [8,40]. Similar data were obtained in primary endothelial cell cultures infected with HHV-6A, suggesting that these cells might be at least in part responsible for the increased RANTES production (HHV-6B was not analyzed in this study)[74]. Besides its implications for the interactions of HHV-6A with HIV-1 [8], which uses CCR5 as a cell surface coreceptor, induction of RANTES may lead to increased inflammatory responses as well as recruitment of new target cells for productive and nonproductive infection.
Another potential mechanism of immunomodulation was recently indicated by the discovery that the immediate-early (IE)-1 proteins of both HHV-6A and HHV-6B are powerful suppressants of IFN-β gene induction [75]. Whereas the stability of IFN-β mRNA does not seem to be affected by IE1, cells expressing IE1 show reduced expression of dimerized IRF3 and nucleus-translocated IRF3 in response to the activation of the cellular cascades normally associated with IFN-β induction. Intriguingly, IE1 is one of the first viral proteins synthesized upon viral entry. The prompt and potent inhibition of IFN-β production is likely to be a crucial contributor to the establishment and spread of both HHV-6A and HHV-6B infection.
Only few studies have attempted to correlate the in vitro findings with in vivo studies in infected patients. One study showed increased frequencies of IL-10- and IL-4-producing T cells and reduced IFN-γ-producing T cells in the peripheral blood of infected individuals compared with uninfected controls [59]. Unfortunately, the authors of this study did not investigate whether HHV-6 infection was active, nor did they determine the viral species responsible for the infection. It is also important to consider that the specific anatomic environment may significantly influence the specific clinical manifestations of HHV-6-induced immunomodulation via cytokine/chemokine response. In patients with HHV-6 encephalopathy, Kawabe et al. reported significantly elevated levels of IL-10 and IL-8 in cerebrospinal fluid (CSF) as compared with serum, while they observed no significant difference in CSF versus serum IL-6 levels [76].
Cytokine & chemokine response impairment by HHV-6B
It has recently been shown that alterations in the Th1/Th2 balance caused by HHV-6B are mediated primarily through TLR9 (by inducing IFN-λ1 responses) and IFN-α in cord plasma-cytoid DCs. In addition, although the expression levels of TLRs are unchanged following HHV-6B infection of DCs, the level of cytokines produced following stimulation with TLR ligands was significantly decreased in HHV-6B-infected DCs, indicating that infection may impair intracellular signaling through this TLR-mediated cytokine modulation [77].
A study of patients with primary HHV-6B infection demonstrated that, during the acute phase of the disease, three cytokines (IFN-γ, IL-2 and IL-4) and one chemokine (MCP-1) were elevated in patients compared with controls; in the convalescent stage of infection, IL-5 was significantly elevated compared with controls [78]. This has not been analyzed for HHV-6A, as little is currently known about the infrequently observed primary HHV-6A infection.
Viral chemokines & chemokine receptors
Both HHV-6 species encode viral chemokine and chemokine receptor homologs, which can significantly modulate immune responses. Both HHV-6A and HHV-6B encode two viral chemokines (U22 and U83) and two viral chemokine receptors (U12 and U51) [79–82]. U12, expressed in the late stages of both HHV-6A and HHV-6B infection, is a β-chemokine receptor homolog related to CCR-1, -3 and -5, which can be activated by CCL5/RANTES, CCL3/MIP-1α, CCL4/MIP-1β and CCL2/MCP-1, but not by CXCL8/IL-8, suggesting a chemokine selectivity distinct from that of other known mammalian chemokine receptors [22,81].
Unlike U12, U51 is transcribed at an early time postinfection and, when expressed on epithelial cells, it specifically binds CCL2, CCL5, CCL7, CCL11 and CCL13, but fails to transduce intracellular signaling upon binding [79,83]. pU51 has the ability to constitutively activate phospholipase C, inhibiting gene expression mediated by the cAMP response element (CRE) [84]. This activity is modulated by various chemokines. In epithelial cells, U51 induces transcriptional downregulation and reduced extracellular release of CCL5/RANTES [79]. Some chemokine regulation specific to U51A has been described (see below). Altogether, these observations support the hypothesis that U51 is a pivotal factor in viral dissemination and host transmission by chemotaxis of infected cells to sites of chemokine secretion specific for U51, as well as in immune evasion via chemokine diversion and downregulation. Moreover, U51 may also act as a positive regulator of virus replication, by promoting virus-induced membrane fusion and cell-to-cell spread [85].
HHV-6A chemokine U83A & chemokine receptor U51A
U83A, encoded by HHV-6A, is a highly selective and effective CCR1, 4, 5, 6 and 8 agonist that can induce calcium mobilization and chemotaxis in monocytoid cells [82,86,87]. Interestingly, full-length U83A is expressed only late in the infection cycle, but its spliced transcript (U83A-Npep) is expressed early. U83A-Npep lacks agonistic activity, but it has antagonistic activity on CCR1, 5 and 8. This sequence of events is consistent with an early antagonistic activity exerted by the spliced chemokine, which would protect infected cells from immune recognition, while during the late stages of infection the agonistic full-length chemokine would foster spread of the infection and the establishment of latency by attracting new target cells for the virus [88].
The chemokine receptor homolog U51A was recently shown to possess an inducible calcium signaling activity in response to CCL2, CCL5 and CCL11 stimulation. Moreover, it was also shown to bind XCL1 and CCL19, acting as an antagonist of their human receptors, XCR1 and CCR7, on lymphocytes, NK cells and DCs [83]. U51A-expressing cell lines and ex vivo infected leukocytes showed migration towards chemokine gradients and displayed chemokine internalization capacity [83].
HHV-6B chemokine U83B
Unlike HHV-6A, HHV-6B does not express two differentially spliced isoforms of its viral chemokine. Its only chemokine homolog, U83B (vCCL4), was shown to act as a weak CCR2-specific agonist [86].
Role of HHV-6A & HHV-6B in autoimmune disorders
A role for HHV-6A and HHV-6B has been proposed in several autoimmune disorders, including autoimmune hemolytic anemia/neutropenia [89], autoimmune acute hepatitis [90], MS [91], scleroderma [92] and Hashimoto's thyroiditis [93], among others. Early observations documented a higher rate of viral isolation in patients with collagen vascular diseases compared with healthy subjects [94]. Our group has documented significant rates of active infection in patients with autoimmune connective tissue diseases, particularly in scleroderma [92]. More recently, we have extended these observations by showing frequent reactivation of HHV-6, but not of other herpesviruses, including hCMV, EBV and HHV-8, in autoimmune connective tissue disease patients, with the highest rate (>30%) in patients with systemic lupus erythematosus [Broccolo F et al., Unpublished Data]. These studies did not discriminate between HHV-6A and HHV-6B. Although the potential mechanism of autoimmune pathogenesis by HHV-6A or HHV-6B remains unknown, molecular mimicry may play a role in the initiation of self-reactive immune responses. Possible additional pathogenic links between infection and autoimmune diseases include general dysregulation of the immune responses, induction of cell damage in a pro-inflammatory context and superantigen stimulation [95].
A large number of reports have linked HHV-6A, and to some extent also HHV-6B, to MS, even though the evidence so far accumulated remains insufficient to conclude that these viruses play a direct role in MS pathogenesis. Specific viral antigens have been identified in MS plaques, and viral DNA has been detected in neurons and oligodendrocytes of MS patients [96–99]. Furthermore, active HHV-6A infection has been detected both in blood [96,100,101] and in CSF [102] of patients with relapsing/remitting MS. MS patients were also shown to have increased serum anti-HHV-6 IgG titers [103,104], with a high proportion (50–70%) simultaneously positive for anti-HHV-6 IgM antibodies [103,105,106]. Although antibodies to whole HHV-6 virus were not significantly correlated with MS, antibodies to the HHV-6 U94/REP protein – which is associated with latency – were found to be significantly increased among MS patients [107,108]. Furthermore, it has been suggested that HHV-6 proteins may cause the transactivation of other cellular or viral genes contributing to the immune derangement of MS [108]. Simpson et al. reported a dose-dependent relationship between baseline anti-HHV-6 IgG and subsequent MS relapse [109]. Due to the absence of similar trends for EBV, a specific anti-HHV-6 response may be involved in the etiology of MS, rather than a nonspecific immune response against herpesviruses in general. Molecular mimicry based on similarity between HHV-6-specific antigens and basic myelin in the CNS has been proposed as a possible mechanism of pathogenesis [110]. Some reports have suggested the possibility of genetic control associated with HHV-6 replication among MS patients, most notably tied to polymorphisms of the MHC2TA and CD46 genes [111–114], which may also determine a poor response to IFN-β treatment [115]. Increased levels of soluble CD46 receptor have been detected in the serum of MS patients [116]. As previously discussed, binding of HHV-6 to CD46 can bias the cytokine profile of specific T-cell responses, which may in turn contribute to the CNS tissue damage in MS [73].
Infection with HHV-6A has recently been associated with Hashimoto's thyroiditis, a common autoimmune thyroid disease [93]. Caselli et al. documented significantly increased levels of HHV-6 DNA in thyroid tissue from active autoimmune thyroiditis patients, as well as features of low-grade HHV-6 acute infection in the tissue. Specific cell-mediated immune responses directed to the U94/REP protein were detected. In all the subjects where the specific viral species were tested, HHV-6A was consistently detected. In addition, an in vitro model for the induction of thyroiditis was presented with the demonstration that the Nthy-ori3–1 thyroid follicular epithelial cell line becomes a target for NK-mediated killing upon infection with HHV-6A [93].
Other mechanisms of immunoevasion
It has also been suggested that HHV-6A may reduce the capacity of monocytes to destroy internalized Cryptococcus neoformans micro-organisms, thereby facilitating the intracellular survival replication of these fungi [117]. However, these data were obtained only using a monocytoid cell line (THP-1) in vitro, and await validation in more physiological models based on primary monocytic cells.
Conclusion
HHV-6A and HHV-6B are two closely related β-herpesviruses that entertain a very intimate relationship with the immune system. Although multiple mechanisms of immunomodulation and immunosuppression by these viruses have been defined using in vitro and ex vivo study models, the clinical relevance of these phenomena is still uncertain because systematic large-scale prospective studies associating active infection with clinical immunodeficiency are lacking. The increasing awareness of HHV-6 diseases among physicians coupled with the availability of specific markers for the diagnosis of active infection and for the unambiguous discrimination between HHV-6A and HHV-6B will be essential to establish the role of these viruses in various conditions of natural or iatrogenic human immunologic dysregulation or overt immunodeficiency.
Future perspective
Both HHV-6A and HHV-6B have been established as causative agents of severe, often life-threatening, opportunistic infections in immunocompromised individuals, such as patients with AIDS and solid organ or bone marrow graft recipients. The spectrum of diseases associated with these viruses is broad, including encephalitis and meningo-encephalitis, interstitial pneumonia, hepatitis, retinitis and bone marrow graft failure. Moreover, HHV-6A and possibly HHV-6B have been postulated to play a cofactor role in AIDS and in at least a subset of cases of MS. Unfortunately, the therapeutic options currently available for the treatment of HHV-6 infection are limited, emphasizing the need for the identification of novel therapeutic targets. Deepening our knowledge of the mechanisms used by these closely related viruses to escape from immunologic control may eventually lead to the development of new effective strategies for the prevention and treatment of their associated diseases.
A possible therapeutic target is undoubtedly the viral receptor, CD46, which is essential for both HHV-6A and HHV-6B entry into target cells and most likely mediates some of the immunosuppressive effects of each virus. The major complication potentially associated with targeting CD46 is the risk of reducing the complement-regulatory activity of this receptor, thus exposing tissues to the damaging effects of autologous complement activation. In addition, new evidence has identified HHV-6B-specific CD8+ T-cell responses. With this new development in mind, future active or passive immunotherapies could be aimed at reconstituting virus-specific T-cell responses.
Among the other potential targets so far identified are the viral chemokines U22 and U83, and the viral chemokine receptors U12 and U51. Although dispensable for replication in vitro, these molecules are likely to play essential roles in viral spread and persistence in vivo. Furthermore, one should consider the viral protein encoded by U24, which has been associated with CD3/TCR down-regulation, and the IE-1 protein, which seems to be involved in IFN-β suppression. However, it has to be emphasized that we are still at the beginning of our understanding of the fine molecular mechanisms of immune modulation by HHV-6A and HHV-6B. Further species-specific studies will be essential for elucidating the viral factors responsible for each of these mechanisms and, thereby, for indicating novel molecular targets for the development of specific HHV-6 inhibitors.
Finally, novel immunomodulatory or immunosuppressive strategies may be designed by mimicking the mechanisms used by HHV-6 to contrast the immune system, such as the therapeutic usage of cytokine/chemokine analogs or nonsignaling (soluble) receptors. In this respect, a better comprehension of the viral mechanisms of immunomodulation and immunosuppression might contribute to novel treatments for diseases characterized by a dysregulated or hyperactive immune system.
Executive summary.
Infection of cells of the immune system
■ CD4+ T cells are primary targets of both human herpesvirus (HHV)-6A and HHV-6B, both of which induce cytopathic effects and apoptosis.
■ HHV-6A efficiently and productively infects CD8+ T cells, NK cells and γδ T cells.
■ HHV-6A suppresses T-cell proliferation by inducing G2/M arrest.
■ Monocytes and dendritic cells (DCs) are primary targets of HHV-6A and HHV-6B, which can induce significant alterations in these cells irrespective of completion of the viral lytic cycle.
■ DCs exposed to HHV-6B can effectively transmit infection to CD4+ cells.
■ Exposure to HHV-6A and HHV-6B suppresses maturation and growth of bone marrow precursors.
Apoptosis
■ HHV-6A induces apoptosis primarily in naive and central memory CD4+ and CD8+ T cells.
■ Monocytes exposed to HHV-6B seem to be protected from apoptosis.
CD46-mediated immunomodulation & immunosuppression
■ CD46 binding by HHV-6A or HHV-6B suppresses the secretion of IL-12 by professional antigen-presenting cells.
■ Both viruses block DC differentiation and induction of allogeneic T-cell proliferation.
■ CD46-mediated costimulation induces a T-regulatory 1-like phenotype in CD4+ T cells.
Dysregulation of complement activation
■ Both HHV-6A and HHV-6B induce downmodulation of membrane CD46 expression, although the process is less efficient with HHV-6B.
■ Loss of CD46 facilitates spontaneous autologous complement activation and cellular damage in both infected and bystander cells.
Modulation of other membrane receptors
■ HHV-6A and HHV-6B downregulate the CD3/T-cell receptor complex from the cell membrane; the U24 protein of HHV-6A has been linked with this effect.
■ HHV-6A transcriptionally activates CD4 in lymphoid cells (including CD8, NK and γδ T cells).
■ HHV-6B downmodulates the expression of DC-SIGN in immature DCs and of CD14, CD64 and HLA-DR in antigen presenting cells, whereas it does not affect the expression of CD32.
■ HHV-6A and HHV-6B downregulate class-I MHC from the surface of infected DCs.
■ Both HHV-6A and HHV-6B induce the expression of CCR7.
Cytokine & chemokine responses
■ Both HHV-6A and HHV-6B reduce the production of IL-2 and induce the production of several inflammatory cytokines and chemokines.
■ Infection with both viruses may induce a cytokine imbalance leading to a Th2-polarized response.
Viral chemokine & chemokine receptor homologs
■ Both HHV-6A and HHV-6B encode two viral chemokines (U22 and U83) and two viral chemokine receptors (U12 and U51) that may affect the activation and effectiveness of physiological immune responses.
Role of HHV-6A & HHV-6B in autoimmune disorders
■ A role for HHV-6A and HHV-6B has been proposed in several autoimmune disorders.
■ HHV-6A, in particular, has been associated with multiple sclerosis and Hashimoto's thyroiditis.
Other mechanisms of immunoevasion
■ HHV-6A infection may impair the microbicidal activity of monocytes.
Future perspective
■ We need to better understand the mechanisms exploited by HHV-6A and HHV-6B in order to develop more effective therapeutic strategies for the prevention and treatment of diseases associated with these agents.
■ Possible future targets for specific therapy include the HHV-6 cellular receptor, CD46, or several cytokines and chemokines elicited or encoded by the virus during infection.
■ A fine comprehension of the mechanisms of immunomodulation and immunosuppression by these viruses may pave the way toward novel strategies to therapeutically manipulate or modulate the immune system in immunologically-mediated human diseases.
Acknowledgements
The authors would like to thank D Ablashi of the HHV-6 Foundation for critical reading of the manuscript.
This work was supported in part by the Intramural Research Program of the NIAID, NIH.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■of interest
■■ of considerable interest
- 1.Dagna L, Lusso P. Virus-encoded chemokines, chemokine receptors and chemokine-binding proteins: new paradigms for future therapy. Future Virol. 2007;2(4):353–368. [Google Scholar]
- 2.Alcami A, Koszinowski UH. Viral mechanisms of immune evasion. Immunol. Today. 2000;21(9):447–455. doi: 10.1016/S0167-5699(00)01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu. Rev. Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 4.Adams MJ, Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2012). Arch. Virol. 157(7):1411–1422 (2012. doi: 10.1007/s00705-012-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ablashi DV, Balachandran N, Josephs SF, et al. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology. 1991;184(2):545–552. doi: 10.1016/0042-6822(91)90424-a. [DOI] [PubMed] [Google Scholar]
- 6■■.Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99(7):817–827. doi: 10.1016/s0092-8674(00)81678-5. [Identified the principal cellular receptor for human herpesvirus (HHV)-6A and HHV-6B, CD46, whose engagement by the viral envelope mediates several mechanisms of immunomodulation by these agents.] [DOI] [PubMed] [Google Scholar]
- 7.Kikuta H, Nakane A, Lu H, Taguchi Y, Minagawa T, Matsumoto S. Interferon induction by human herpesvirus 6 in human mononuclear cells. J. Infect. Dis. 1990;162(1):35–38. doi: 10.1093/infdis/162.1.35. [DOI] [PubMed] [Google Scholar]
- 8.Grivel JC, Ito Y, Faga G, et al. Suppression of CCR5- but not CXCR4-tropic HIV-1 in lymphoid tissue by human herpesvirus 6. Nat. Med. 2001;7(11):1232–1235. doi: 10.1038/nm1101-1232. [DOI] [PubMed] [Google Scholar]
- 9.Arena A, Liberto MC, Iannello D, Capozza AB, Foca A. Altered cytokine production after human herpes virus type 6 infection. New Microbiol. 1999;22(4):293–300. [PubMed] [Google Scholar]
- 10.Flamand L, Stefanescu I, Menezes J. Human herpesvirus-6 enhances natural killer cell cytotoxicity via IL-15. J. Clin. Invest. 1996;97(6):1373–1381. doi: 10.1172/JCI118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flamand L, Gosselin J, D'Addario M, et al. Human herpesvirus 6 induces interleukin-1 beta and tumor necrosis factor alpha, but not interleukin-6, in peripheral blood mononuclear cell cultures. J. Virol. 1991;65(9):5105–5110. doi: 10.1128/jvi.65.9.5105-5110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12■.Lusso P, Markham PD, Tschachler E, et al. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6). J. Exp. Med. 1988;167(5):1659–1670. doi: 10.1084/jem.167.5.1659. [Established for the first time the primary T-cell tropism of HHV-6A, which was initially considered to be a B-lymphotropic virus.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13■.Takahashi K, Sonoda S, Higashi K, et al. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J. Virol. 1989;63(7):3161–3163. doi: 10.1128/jvi.63.7.3161-3163.1989. [Established for the first time the primary T-cell tropism of HHV-6B.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14■■.Lopez C, Pellett P, Stewart J, et al. Characteristics of human herpesvirus-6. J. Infect. Dis. 1988;157(6):1271–1273. doi: 10.1093/infdis/157.6.1271. [First characterization of HHV-6B.] [DOI] [PubMed] [Google Scholar]
- 15■■.Salahuddin SZ, Ablashi DV, Markham PD, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234(4776):596–601. doi: 10.1126/science.2876520. [Landmark report describing the discovery of HHV-6.] [DOI] [PubMed] [Google Scholar]
- 16.Ablashi DV, Lusso P, Hung CL, et al. Utilization of human hematopoietic cell lines for the propagation and characterization of HBLV (human herpesvirus 6). Int. J. Cancer. 1988;42(5):787–791. doi: 10.1002/ijc.2910420526. [DOI] [PubMed] [Google Scholar]
- 17.Bandobashi K, Daibata M, Kamioka M, et al. Human herpesvirus 6 (HHV-6)-positive Burkitt's lymphoma: establishment of a novel cell line infected with HHV-6. Blood. 1997;90(3):1200–1207. [PubMed] [Google Scholar]
- 18.Burd EM, Carrigan DR. Human herpesvirus 6 (HHV-6)-associated dysfunction of blood monocytes. Virus Res. 1993;29(1):79–90. doi: 10.1016/0168-1702(93)90127-9. [DOI] [PubMed] [Google Scholar]
- 19.Kondo K, Kondo T, Okuno T, Takahashi M, Yamanishi K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J. Gen. Virol. 1991;72(Pt 6):1401–1408. doi: 10.1099/0022-1317-72-6-1401. [DOI] [PubMed] [Google Scholar]
- 20■.Smith A, Santoro F, Di Lullo G, Dagna L, Verani A, Lusso P. Selective suppression of IL-12 production by human herpesvirus 6. Blood. 2003;102(8):2877–2884. doi: 10.1182/blood-2002-10-3152. [Illustrated a major mechanism of immunosuppression by HHV-6A and -6B, showing its independence from viral replication.] [DOI] [PubMed] [Google Scholar]
- 21.Arena A, Merendino RA, Bonina L, Iannello D, Stassi G, Mastroeni P. Role of IL-15 on monocytic resistance to human herpesvirus 6 infection. New Microbiol. 2000;23(2):105–112. [PubMed] [Google Scholar]
- 22.Kondo K, Kondo T, Shimada K, Amo K, Miyagawa H, Yamanishi K. Strong interaction between human herpesvirus 6 and peripheral blood monocytes/macrophages during acute infection. J. Med. Virol. 2002;67(3):364–369. doi: 10.1002/jmv.10082. [DOI] [PubMed] [Google Scholar]
- 23.Luppi M, Barozzi P, Morris C, et al. Human herpesvirus 6 latently infects early bone marrow progenitors in vivo. J. Virol. 1999;73(1):754–759. doi: 10.1128/jvi.73.1.754-759.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isomura H, Yoshida M, Namba H, Yamada M. Interaction of human herpesvirus 6 with human CD34 positive cells. J. Med. Virol. 2003;70(3):444–450. doi: 10.1002/jmv.10415. [DOI] [PubMed] [Google Scholar]
- 25.Isomura H, Yoshida M, Namba H, et al. Suppressive effects of human herpesvirus-6 on thrombopoietin-inducible megakaryocytic colony formation in vitro. J. Gen. Virol. 2000;81(Pt 3):663–673. doi: 10.1099/0022-1317-81-3-663. [DOI] [PubMed] [Google Scholar]
- 26.Knox KK, Carrigan DR. In vitro suppression of bone marrow progenitor cell differentiation by human herpesvirus 6 infection. J. Infect. Dis. 1992;165(5):925–929. doi: 10.1093/infdis/165.5.925. [DOI] [PubMed] [Google Scholar]
- 27.Isomura H, Yamada M, Yoshida M, et al. Suppressive effects of human herpesvirus 6 on in vitro colony formation of hematopoietic progenitor cells. J. Med. Virol. 1997;52(4):406–412. doi: 10.1002/(sici)1096-9071(199708)52:4<406::aid-jmv11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Yasukawa M, Ohminami H, Sada E, et al. Latent infection and reactivation of human herpesvirus 6 in two novel myeloid cell lines. Blood. 1999;93(3):991–999. [PubMed] [Google Scholar]
- 29.Drobyski WR, Dunne WM, Burd EM, et al. Human herpesvirus-6 (HHV-6) infection in allogeneic bone marrow transplant recipients: evidence of a marrow-suppressive role for HHV-6 in vivo. J. Infect. Dis. 1993;167(3):735–739. doi: 10.1093/infdis/167.3.735. [DOI] [PubMed] [Google Scholar]
- 30.Johnston RE, Geretti AM, Prentice HG, et al. HHV-6-related secondary graft failure following allogeneic bone marrow transplantation. Br. J. Haematol. 1999;105(4):1041–1043. doi: 10.1046/j.1365-2141.1999.01443.x. [DOI] [PubMed] [Google Scholar]
- 31.Dulery R, Salleron J, Dewilde A, et al. Early human herpesvirus type 6 reactivation after allogeneic stem cell transplantation: a large-scale clinical study. Biol. Blood Marrow Transplant. 2012;18(7):1080–1089. doi: 10.1016/j.bbmt.2011.12.579. [DOI] [PubMed] [Google Scholar]
- 32.Pichereau C, Desseaux K, Janin A, et al. The complex relationship between human herpesvirus 6 and acute graft-versus-host disease. Biol. Blood Marrow Transplant. 2012;18(1):141–144. doi: 10.1016/j.bbmt.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Zerr DM, Boeckh M, Delaney C, et al. HHV-6 reactivation and associated sequelae after hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2012;18(11):1700–1708. doi: 10.1016/j.bbmt.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lusso P, Malnati M, De Maria A, et al. Productive infection of CD4+ and CD8+ mature human T cell populations and clones by human herpesvirus 6. Transcriptional down-regulation of CD3. J. Immunol. 1991;147(2):685–691. [PubMed] [Google Scholar]
- 35.Lusso P, Malnati MS, Garzino-Demo A, Crowley RW, Long EO, Gallo RC. Infection of natural killer cells by human herpesvirus 6. Nature. 1993;362(6419):458–462. doi: 10.1038/362458a0. [DOI] [PubMed] [Google Scholar]
- 36.Lusso P, Garzino-Demo A, Crowley RW, Malnati MS. Infection of gamma/delta T lymphocytes by human herpesvirus 6: transcriptional induction of CD4 and susceptibility to HIV infection. J. Exp. Med. 1995;181(4):1303–1310. doi: 10.1084/jem.181.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Gu B, Zhou F, et al. Human herpesvirus 6A infects human embryonic fibroblasts and induces G2/M arrest and cell death. J. Med. Virol. 2012;84(4):657–663. doi: 10.1002/jmv.23226. [DOI] [PubMed] [Google Scholar]
- 38.Gupta S, Agrawal S, Gollapudi S. Differential effect of human herpesvirus 6A on cell division and apoptosis among naive and central and effector memory CD4+ and CD8+ T-cell subsets. J. Virol. 2009;83(11):5442–5450. doi: 10.1128/JVI.00106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39■.Lusso P, De Maria A, Malnati M, et al. Induction of CD4 and susceptibility to HIV-1 infection in human CD8+ T lymphocytes by human herpesvirus 6. Nature. 1991;349(6309):533–535. doi: 10.1038/349533a0. [Reported the ability of HHV-6A to productively infect cytotoxic effector T cells and induce de novo expression of CD4.] [DOI] [PubMed] [Google Scholar]
- 40.Grivel JC, Santoro F, Chen S, et al. Pathogenic effects of human herpesvirus 6 in human lymphoid tissue ex vivo. J. Virol. 2003;77(15):8280–8289. doi: 10.1128/JVI.77.15.8280-8289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41■.Takemoto M, Imasawa T, Yamanishi K, Mori Y. Role of dendritic cells infected with human herpesvirus 6 in virus transmission to CD4+ T cells. Virology. 2009;385(2):294–302. doi: 10.1016/j.virol.2008.11.049. [Described for the first time the role of dendritic cells as promoters of T-cell infection by HHV-6B.] [DOI] [PubMed] [Google Scholar]
- 42.Yasukawa M, Inoue Y, Ohminami H, Terada K, Fujita S. Apoptosis of CD4+ T lymphocytes in human herpesvirus-6 infection. J. Gen. Virol. 1998;79(Pt 1):143–147. doi: 10.1099/0022-1317-79-1-143. [DOI] [PubMed] [Google Scholar]
- 43.Oster B, Kaspersen MD, Kofod-Olsen E, Bundgaard B, Hollsberg P. Human herpesvirus 6B inhibits cell proliferation by a p53-independent pathway. J. Clin. Virol. 2006;37(Suppl. 1):S63–68. doi: 10.1016/S1386-6532(06)70014-2. [DOI] [PubMed] [Google Scholar]
- 44.Oster B, Bundgaard B, Hollsberg P. Human herpesvirus 6B induces cell cycle arrest concomitant with p53 phosphorylation and accumulation in T cells. J. Virol. 2005;79(3):1961–1965. doi: 10.1128/JVI.79.3.1961-1965.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takemoto M, Mori Y, Ueda K, Kondo K, Yamanishi K. Productive human herpesvirus 6 infection causes aberrant accumulation of p53 and prevents apoptosis. J. Gen. Virol. 2004;85(Pt 4):869–879. doi: 10.1099/vir.0.19626-0. [DOI] [PubMed] [Google Scholar]
- 46.Li L, Gu B, Zhou F, et al. Human herpesvirus 6 suppresses T cell proliferation through induction of cell cycle arrest in infected cells in the G2/M phase. J. Virol. 2011;85(13):6774–6783. doi: 10.1128/JVI.02577-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chi J, Wang F, Li L, et al. The role of MAPK in CD4+ T cells Toll-like receptor 9-mediated signaling following HHV-6 infection. Virology. 2012;422(1):92–98. doi: 10.1016/j.virol.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 48.Yeo WM, Isegawa Y, Chow VT. The U95 protein of human herpesvirus 6B interacts with human GRIM-19: silencing of U95 expression reduces viral load and abrogates loss of mitochondrial membrane potential. J. Virol. 2008;82(2):1011–1020. doi: 10.1128/JVI.01156-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janelle ME, Flamand L. Phenotypic alterations and survival of monocytes following infection by human herpesvirus-6. Arch. Virol. 2006;151(8):1603–1614. doi: 10.1007/s00705-005-0715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ichimi R, Jin-No T, Ito M. Induction of apoptosis in cord blood lymphocytes by HHV-6. J. Med. Virol. 1999;58(1):63–68. doi: 10.1002/(sici)1096-9071(199905)58:1<63::aid-jmv10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 51.Liszewski MK, Post TW, Atkinson JP. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 52.Naniche D, Varior-Krishnan G, Cervoni F, et al. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 1993;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenstone HL, Santoro F, Lusso P, Berger EA. Human Herpesvirus 6 and measles virus employ distinct CD46 domains for receptor function. J. Bio. Chem. 2002;277(42):39112–39118. doi: 10.1074/jbc.M206488200. [DOI] [PubMed] [Google Scholar]
- 54.Santoro F, Greenstone HL, Insinga A, et al. Interaction of glycoprotein H of human herpesvirus 6 with the cellular receptor CD46. J. Bio. Chem. 2003;278(28):25964–25969. doi: 10.1074/jbc.M302373200. [DOI] [PubMed] [Google Scholar]
- 55.Mori Y, Akkapaiboon P, Yang X, Yamanishi K. The human herpesvirus 6 U100 gene product is the third component of the gH-gL glycoprotein complex on the viral envelope. J. Virol. 2003;77(4):2452–2458. doi: 10.1128/JVI.77.4.2452-2458.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith AP, Paolucci C, Di Lullo G, Burastero SE, Santoro F, Lusso P. Viral replication-independent blockade of dendritic cell maturation and interleukin-12 production by human herpesvirus 6. J. Virol. 2005;79(5):2807–2813. doi: 10.1128/JVI.79.5.2807-2813.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niiya H, Lei J, Guo Y, et al. Human herpesvirus 6 impairs differentiation of monocytes to dendritic cells. Exp. Hematol. 2006;34(5):642–653. doi: 10.1016/j.exphem.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421(6921):388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 59.Wang F, Yao K, Yin QZ, et al. Human herpesvirus-6-specific interleukin 10-producing CD4+ T cells suppress the CD4+ T-cell response in infected individuals. Microbiol. Immunol. 2006;50(10):787–803. doi: 10.1111/j.1348-0421.2006.tb03855.x. [DOI] [PubMed] [Google Scholar]
- 60.Mori Y, Seya T, Huang HL, Akkapaiboon P, Dhepakson P, Yamanishi K. Human herpesvirus 6 variant A but not variant B induces fusion from without in a variety of human cells through a human herpesvirus 6 entry receptor, CD46. J. Virol. 2002;76(13):6750–6761. doi: 10.1128/JVI.76.13.6750-6761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schnorr JJ, Dunster LM, Nanan R, Schneider-Schaulies J, Schneider-Schaulies S, Ter Meulen V. Measles virus-induced down-regulation of CD46 is associated with enhanced sensitivity to complement-mediated lysis of infected cells. Eur. J. Immunol. 1995;25(4):976–984. doi: 10.1002/eji.1830250418. [DOI] [PubMed] [Google Scholar]
- 62.Niiya H, Azuma T, Jin L, et al. Transcriptional downregulation of DC-SIGN in human herpesvirus 6-infected dendritic cells. J. Gen. Virol. 2004;85(Pt 9):2639–2642. doi: 10.1099/vir.0.80095-0. [DOI] [PubMed] [Google Scholar]
- 63.Hasegawa H, Utsunomiya Y, Yasukawa M, Yanagisawa K, Fujita S. Induction of G protein-coupled peptide receptor EBI 1 by human herpesvirus 6 and 7 infection in CD4+ T cells. J. Virol. 1994;68(8):5326–5329. doi: 10.1128/jvi.68.8.5326-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirata Y, Kondo K, Yamanishi K. Human herpesvirus 6 downregulates major histocompatibility complex class I in dendritic cells. J. Med. Virol. 2001;65(3):576–583. [PubMed] [Google Scholar]
- 65.Glosson NL, Hudson AW. Human herpesvirus-6A and -6B encode viral immunoevasins that downregulate class I MHC molecules. Virology. 2007;365(1):125–135. doi: 10.1016/j.virol.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 66.Sullivan BM, Coscoy L. Downregulation of the T-cell receptor complex and impairment of T-cell activation by human herpesvirus 6 u24 protein. J. Virol. 2008;82(2):602–608. doi: 10.1128/JVI.01571-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flamand L, Romerio F, Reitz MS, Gallo RC. CD4 promoter transactivation by human herpesvirus 6. J. Virology. 1998;72(11):8797–8805. doi: 10.1128/jvi.72.11.8797-8805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flamand L, Gosselin J, Stefanescu I, Ablashi D, Menezes J. Immunosuppressive effect of human herpesvirus 6 on T-cell functions: suppression of interleukin-2 synthesis and cell proliferation. Blood. 1995;85(5):1263–1271. [PubMed] [Google Scholar]
- 69.Inagi R, Guntapong R, Nakao M, et al. Human herpesvirus 6 induces IL-8 gene expression in human hepatoma cell line, Hep G2. J. Med. Virol. 1996;49(1):34–40. doi: 10.1002/(SICI)1096-9071(199605)49:1<34::AID-JMV6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 70.Mayne M, Cheadle C, Soldan SS, et al. Gene expression profile of herpesvirus-infected T cells obtained using immunomicroarrays: induction of proinflammatory mechanisms. J. Virol. 2001;75(23):11641–11650. doi: 10.1128/JVI.75.23.11641-11650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chi J, Gu B, Zhang C, et al. Human herpesvirus 6 latent infection in patients with glioma. J. Infect. Dis. 2012;206(9):1394–1398. doi: 10.1093/infdis/jis513. [DOI] [PubMed] [Google Scholar]
- 72.Nastke MD, Becerra A, Yin L, et al. Human CD4+ T cell response to human herpesvirus 6. J. Virol. 2012;86(9):4776–4792. doi: 10.1128/JVI.06573-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao K, Graham J, Akahata Y, Oh U, Jacobson S. Mechanism of neuroinflammation: enhanced cytotoxicity and IL-17 production via CD46 binding. J. Neuroimmune Pharmacol. 2010;5(3):469–478. doi: 10.1007/s11481-010-9232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caruso A, Favilli F, Rotola A, et al. Human herpesvirus-6 modulates RANTES production in primary human endothelial cell cultures. J. Med. Virol. 2003;70(3):451–458. doi: 10.1002/jmv.10416. [DOI] [PubMed] [Google Scholar]
- 75.Jaworska J, Gravel A, Fink K, Grandvaux N, Flamand L. Inhibition of transcription of the beta interferon gene by the human herpesvirus 6 immediate-early 1 protein. J. Virol. 2007;81(11):5737–5748. doi: 10.1128/JVI.02443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawabe S, Ito Y, Ohta R, et al. Comparison of the levels of human herpesvirus 6 (HHV-6) DNA and cytokines in the cerebrospinal fluid and serum of children with HHV-6 encephalopathy. J. Med. Virol. 2010;82(8):1410–1415. doi: 10.1002/jmv.21808. [DOI] [PubMed] [Google Scholar]
- 77.Martin LK, Schub A, Dillinger S, Moosmann A. Specific CD8+ T cells recognize human herpesvirus 6B. Eur. J. Immunol. 2012;42(11):2901–2912. doi: 10.1002/eji.201242439. [DOI] [PubMed] [Google Scholar]
- 78.Yoshikawa T, Kato Y, Ihira M, et al. Kinetics of cytokine and chemokine responses in patients with primary human herpesvirus 6 infection. J. Clin. Virol. 2011;50(1):65–68. doi: 10.1016/j.jcv.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 79■.Milne RS, Mattick C, Nicholson L, Devaraj P, Alcami A, Gompels UA. RANTES binding and down-regulation by a novel human herpesvirus-6 beta chemokine receptor. J. Immunol. 2000;164(5):2396–2404. doi: 10.4049/jimmunol.164.5.2396. [Described for the first time the biological relevance of a virally encoded HHV-6 chemokine receptor.] [DOI] [PubMed] [Google Scholar]
- 80.French C, Menegazzi P, Nicholson L, Macaulay H, Diluca D, Gompels UA. Novel, nonconsensus cellular splicing regulates expression of a gene encoding a chemokine-like protein that shows high variation and is specific for human herpesvirus 6. Virology. 1999;262(1):139–151. doi: 10.1006/viro.1999.9875. [DOI] [PubMed] [Google Scholar]
- 81.Isegawa Y, Ping Z, Nakano K, Sugimoto N, Yamanishi K. Human herpesvirus 6 open reading frame U12 encodes a functional beta-chemokine receptor. J. Virol. 1998;72(7):6104–6112. doi: 10.1128/jvi.72.7.6104-6112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zou P, Isegawa Y, Nakano K, Haque M, Horiguchi Y, Yamanishi K. Human herpesvirus 6 open reading frame U83 encodes a functional chemokine. J. Virol. 1999;73(7):5926–5933. doi: 10.1128/jvi.73.7.5926-5933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Catusse J, Spinks J, Mattick C, et al. Immunomodulation by herpesvirus U51A chemokine receptor via CCL5 and FOG-2 down-regulation plus XCR1 and CCR7 mimicry in human leukocytes. Eur. J. Immunol. 2008;38(3):763–777. doi: 10.1002/eji.200737618. [DOI] [PubMed] [Google Scholar]
- 84.Fitzsimons CP, Gompels UA, Verzijl D, et al. Chemokine-directed trafficking of receptor stimulus to different g proteins: selective inducible and constitutive signaling by human herpesvirus 6-encoded chemokine receptor U51. Mol. Pharmacol. 2006;69(3):888–898. doi: 10.1124/mol.105.015222. [DOI] [PubMed] [Google Scholar]
- 85.Zhen Z, Bradel-Tretheway B, Sumagin S, Bidlack JM, Dewhurst S. The human herpesvirus 6 G protein-coupled receptor homolog U51 positively regulates virus replication and enhances cell-cell fusion in vitro. J. Virol. 2005;79(18):11914–11924. doi: 10.1128/JVI.79.18.11914-11924.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luttichau HR, Clark-Lewis I, Jensen PO, Moser C, Gerstoft J, Schwartz TW. A highly selective CCR2 chemokine agonist encoded by human herpesvirus 6. J. Biol. Chem. 2003;278(13):10928–10933. doi: 10.1074/jbc.M211329200. [DOI] [PubMed] [Google Scholar]
- 87.Catusse J, Parry CM, Dewin DR, Gompels UA. Inhibition of HIV-1 infection by viral chemokine U83A via high-affinity CCR5 interactions that block human chemokine-induced leukocyte chemotaxis and receptor internalization. Blood. 2007;109(9):3633–3639. doi: 10.1182/blood-2006-08-042622. [DOI] [PubMed] [Google Scholar]
- 88■.Dewin DR, Catusse J, Gompels UA. Identification and characterization of U83A viral chemokine, a broad and potent beta-chemokine agonist for human CCRs with unique selectivity and inhibition by spliced isoform. J. Immunol. 2006;176(1):544–556. doi: 10.4049/jimmunol.176.1.544. [Described the biological activity of the virally encoded U83 chemokine of HHV-6A.] [DOI] [PubMed] [Google Scholar]
- 89.Yagasaki H, Kato M, Shimizu N, Shichino H, Chin M, Mugishima H. Autoimmune hemolytic anemia and autoimmune neutropenia in a child with erythroblastopenia of childhood (TEC) caused by human herpesvirus-6 (HHV-6). Ann. Hematol. 2011;90(7):851–852. doi: 10.1007/s00277-010-1095-x. [DOI] [PubMed] [Google Scholar]
- 90.Grima P, Chiavaroli R, Calabrese P, Tundo P, Grima P. Severe hepatitis with autoimmune features following a HHV-6: a case report. Cases J. 2008;1(1):110. doi: 10.1186/1757-1626-1-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tejada-Simon MV, Zang YC, Hong J, Rivera VM, Zhang JZ. Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann. Neurol. 2003;53(2):189–197. doi: 10.1002/ana.10425. [DOI] [PubMed] [Google Scholar]
- 92.Broccolo F, Drago F, Paolino S, et al. Reactivation of human herpesvirus 6 (HHV-6) infection in patients with connective tissue diseases. J. Clin. Virol. 2009;46(1):43–46. doi: 10.1016/j.jcv.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 93■.Caselli E, Zatelli MC, Rizzo R, et al. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto's thyroiditis. PLoS Pathog. 2012;8(10):e1002951. doi: 10.1371/journal.ppat.1002951. [Established the first connection between HHV-6A and Hashimoto's thyroiditis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krueger GR, Sander C, Hoffmann A, Barth A, Koch B, Braun M. Isolation of human herpesvirus-6 (HHV-6) from patients with collagen vascular diseases. In Vivo. 1991;5(3):217–225. [PubMed] [Google Scholar]
- 95.Lusso P. HHV-6 and the immune system: mechanisms of immunomodulation and viral escape. J. Clin. Virol. 2006;37(Suppl. 1):S4–10. doi: 10.1016/S1386-6532(06)70004-X. [DOI] [PubMed] [Google Scholar]
- 96.Alvarez-Lafuente R, Garcia-Montojo M, De Las Heras V, Bartolome M, Arroyo R. Clinical parameters and HHV-6 active replication in relapsing-remitting multiple sclerosis patients. J. Clin. Virol. 2006;37(Suppl. 1):S24–S26. doi: 10.1016/S1386-6532(06)70007-5. [DOI] [PubMed] [Google Scholar]
- 97.Virtanen JO, Zabriskie JB, Siren V, et al. Co-localization of human herpes virus 6 and tissue plasminogen activator in multiple sclerosis brain tissue. Med. Sci. Monit. 2005;11(3):BR84–BR87. [PubMed] [Google Scholar]
- 98.Friedman JE, Lyons MJ, Cu G, et al. The association of the human herpesvirus-6 and MS. Mult. Scler. 1999;5(5):355–362. doi: 10.1177/135245859900500509. [DOI] [PubMed] [Google Scholar]
- 99■.Challoner PB, Smith KT, Parker JD, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc. Natl Acad. Sci. USA. 1995;92(16):7440–7444. doi: 10.1073/pnas.92.16.7440. [Established the first connection between HHV-6 and multiple sclerosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Akhyani N, Berti R, Brennan MB, et al. Tissue distribution and variant characterization of human herpesvirus (HHV)-6: increased prevalence of HHV-6A in patients with multiple sclerosis. J. Infect. Dis. 2000;182(5):1321–1325. doi: 10.1086/315893. [DOI] [PubMed] [Google Scholar]
- 101.Alvarez-Lafuente R, De Las Heras V, Bartolome M, Picazo JJ, Arroyo R. Relapsingremitting multiple sclerosis and human herpesvirus 6 active infection. Arch. Neurol. 2004;61(10):1523–1527. doi: 10.1001/archneur.61.10.1523. [DOI] [PubMed] [Google Scholar]
- 102.Rotola A, Merlotti I, Caniatti L, et al. Human herpesvirus 6 infects the central nervous system of multiple sclerosis patients in the early stages of the disease. Mult. Scler. 2004;10(4):348–354. doi: 10.1191/1352458504ms1045oa. [DOI] [PubMed] [Google Scholar]
- 103.Ablashi DV, Eastman HB, Owen CB, et al. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. J. Clin. Virol. 2000;16(3):179–191. doi: 10.1016/s1386-6532(99)00079-7. [DOI] [PubMed] [Google Scholar]
- 104.Sola P, Merelli E, Marasca R, et al. Human herpesvirus 6 and multiple sclerosis: survey of anti-HHV-6 antibodies by immunofluorescence analysis and of viral sequences by polymerase chain reaction. J. Neurol. Neurosurg. Psychiatry. 1993;56(8):917–919. doi: 10.1136/jnnp.56.8.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ablashi DV, Lapps W, Kaplan M, Whitman JE, Richert JR, Pearson GR. Human Herpesvirus-6 (HHV-6) infection in multiple sclerosis: a preliminary report. Mult. Scler. 1998;4(6):490–496. doi: 10.1177/135245859800400606. [DOI] [PubMed] [Google Scholar]
- 106.Soldan SS, Berti R, Salem N, et al. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat. Med. 1997;3(12):1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- 107.Caselli E, Boni M, Bracci A, et al. Detection of antibodies directed against human herpesvirus 6 U94/REP in sera of patients affected by multiple sclerosis. J. Clin. Microbiol. 2002;40(11):4131–4137. doi: 10.1128/JCM.40.11.4131-4137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ben-Fredj N, Ben-Selma W, Rotola A, et al. Prevalence of human herpesvirus U94/REP antibodies and DNA in Tunisian multiple sclerosis patients. J. Neurovirol. 2013;19(1):42–47. doi: 10.1007/s13365-012-0138-6. [DOI] [PubMed] [Google Scholar]
- 109.Simpson S, Jr., Taylor B, Dwyer DE, et al. Anti-HHV-6 IgG titer significantly predicts subsequent relapse risk in multiple sclerosis. Mult. Scler. 2012;18(6):799–806. doi: 10.1177/1352458511428081. [DOI] [PubMed] [Google Scholar]
- 110.Cirone M, Cuomo L, Zompetta C, et al. Human herpesvirus 6 and multiple sclerosis: a study of T cell cross-reactivity to viral and myelin basic protein antigens. J. Med. Virol. 2002;68(2):268–272. doi: 10.1002/jmv.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garcia-Montojo M, Martinez A, De Las Heras V, et al. Herpesvirus active replication in multiple sclerosis: a genetic control? J. Neurol. Sci. 2011;311(1–2):98–102. doi: 10.1016/j.jns.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Dominguez-Mozo MI, Garcia-Montojo M, De Las Heras V, et al. MHC2TA mRNA levels and human herpesvirus 6 in multiple sclerosis patients treated with interferon beta along two-year follow-up. BMC Neurol. 2012;12(1):107. doi: 10.1186/1471-2377-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Soldan SS, Fogdell-Hahn A, Brennan MB, et al. Elevated serum and cerebrospinal fluid levels of soluble human herpesvirus type 6 cellular receptor, membrane cofactor protein, in patients with multiple sclerosis. Ann. Neurol. 2001;50(4):486–493. doi: 10.1002/ana.1135. [DOI] [PubMed] [Google Scholar]
- 114.Astier AL, Hafler DA. Abnormal Tr1 differentiation in multiple sclerosis. J. Neuroimmunol. 2007;191(1–2):70–78. doi: 10.1016/j.jneuroim.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alvarez-Lafuente R, Blanco-Kelly F, Garcia-Montojo M, et al. CD46 in a Spanish cohort of multiple sclerosis patients: genetics, mRNA expression and response to interferon-beta treatment. Mult. Scler. 2011;17(5):513–520. doi: 10.1177/1352458510393263. [DOI] [PubMed] [Google Scholar]
- 116.Fogdell-Hahn A, Soldan SS, Shue S, et al. Co-purification of soluble membrane cofactor protein (CD46) and human herpesvirus 6 variant A genome in serum from multiple sclerosis patients. Virus Res. 2005;110(1–2):57–63. doi: 10.1016/j.virusres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 117.Cermelli C, Cenacchi V, Beretti F, Pezzini F, Luca DD, Blasi E. Human herpesvirus-6 dysregulates monocyte-mediated anticryptococcal defences. J. Med. Microbial. 2006;55(Pt 6):695–702. doi: 10.1099/jmm.0.46496-0. [DOI] [PubMed] [Google Scholar]