The protein kinase SLK (LOSK) phosphorylates the 1A isoform of the p150Glued subunit of dynactin and targets it to the centrosome, where it maintains microtubule radial organization. In addition, dynactin phosphorylation is involved in Golgi reorientation in polarized cells.
Abstract
The microtubule- and centrosome-associated Ste20-like kinase (SLK; long Ste20-like kinase [LOSK]) regulates cytoskeleton organization and cell polarization and spreading. Its inhibition causes microtubule disorganization and release of centrosomal dynactin. The major function of dynactin is minus end–directed transport along microtubules in a complex with dynein motor. In addition, dynactin is required for maintenance of the microtubule radial array in interphase cells, and depletion of its centrosomal pool entails microtubule disorganization. Here we demonstrate that SLK (LOSK) phosphorylates the p150Glued subunit of dynactin and thus targets it to the centrosome, where it maintains microtubule radial organization. We show that phosphorylation is required only for centrosomal localization of p150Glued and does not affect its microtubule-organizing properties: artificial targeting of nonphosphorylatable p150Glued to the centrosome restores microtubule radial array in cells with inhibited SLK (LOSK). The phosphorylation site is located in a microtubule-binding region that is variable for two isoforms (1A and 1B) of p150Glued expressed in cultured fibroblast-like cells (isoform 1B lacks 20 amino acids in the basic microtubule-binding domain). The fact that SLK (LOSK) phosphorylates only a minor isoform 1A of p150Glued suggests that transport and microtubule-organizing functions of dynactin are distinctly divided between the two isoforms. We also show that dynactin phosphorylation is involved in Golgi reorientation in polarized cells.
INTRODUCTION
Microtubules (MTs) in interphase cells are organized into a radial array with the minus ends focused in the centrosome and plus ends directed toward cell's periphery. Such an array maintains polarized transport of molecules and organelles driven by motor proteins. The molecular mechanisms that regulate radial organization of microtubules are unknown. A typical microtubule-organizing center in fibroblast-like cultured cells is represented by the centrosome, where microtubules are nucleated and anchored. γ-Tubulin ring complexes nucleate microtubules and can remain bound to their minus ends further on (Wiese and Zheng, 2000, 2006; Anders and Sawin, 2011). γ-Tubulin, however, is not the only anchor of microtubules at the centrosome. Depletion of other centrosomal proteins—ninein (Mogensen et al., 2000; Dammermann and Merdes, 2002; Delgehyr et al., 2005; Ibi et al., 2011), 4.1R (Perez-Ferreiro et al., 2004), Nlp (Casenghi et al., 2005), CAP350 and FOP (Yan et al., 2006), and TACC3/maskin (Albee and Wiese, 2008)—causes randomization of microtubules in cells, although the centrosome retains its nucleating activity, which can be visualized by tracking plus end–binding proteins (EB1 and EB3) at growing ends of microtubules. Inhibition of some dynein-binding proteins, such as Bicaudal D and Nudel (Fumoto et al., 2006; Guo et al., 2006), and dynein–dynactin complex does not affect microtubule nucleation at the centrosome (Zhapparova et al., 2007; Burakov et al., 2008a) but does disturb anchoring. Rapid disorganization of microtubules after the inhibition of dynein-dependent transport might result from the compromised delivery of anchoring proteins to the centrosome (Fumoto et al., 2006). In case of dynactin inhibition, the centrosome still contains its major proteins—γ-tubulin, pericentrin, centrin, and ninein—but loses the ability to anchor microtubules (Burakov et al., 2008a, b). These data indicate that the role of dynactin in microtubule organization is not limited to its transport function.
Dynactin is considered as a multisubunit cofactor of dynein, which ensures cargo binding and processivity of the motor protein (King and Schroer, 2000; Schroer, 2004). One of the subunits, p150Glued, contains a MT-binding region (CAP-Gly and basic domains) and can interact with microtubules independently of dynein (Waterman-Storer et al., 1995; Culver-Hanlon et al., 2006; Weisbrich et al., 2007). Therefore dynactin might contribute to MT organization by direct or indirect MT anchoring at the centrosome and/or regulation of dynein activity and dynein-mediated transport of other anchoring proteins to the centrosome. In either case, depletion of centrosomal dynactin results in inevitable disorganization of microtubules (Quintyne et al., 1999; Kim et al., 2007). Two subunits of the complex (p50 and p150Glued) interact with centrosomal proteins (Cep135 and PCM1, respectively; Uetake et al., 2004; Kodani et al., 2010), but the regulation of dynactin localization at the centrosome is far from understood.
Previously we showed that depletion of centrosomal dynactin and randomization of microtubules were observed after the inhibition of the long Ste20-like kinase (LOSK; also called Ste20-like kinase [SLK]; Burakov et al., 2008b). SLK (LOSK) is a Ser/Thr-protein kinase associated with the centrosome and microtubules (Zinovkina et al., 1997, 1998; Itoh et al., 1997; Sabourin and Rudnicki, 1999; Yamada et al., 2000; Nadezhdina et al., 2001). Its inhibition results in disorganization of microtubules and disturbed polarization and motility of cells (Burakov et al., 2008b; Roovers et al., 2009). SLK phosphorylates several cytoskeletal proteins: human RhoA (Guilluy et al., 2008), paxillin (Quizi et al., 2013), and, presumably, ezrin (Viswanatha et al., 2012). None of them, however, is involved in centrosome-mediated microtubule organization. Given that inhibition of SLK results in a dramatic decrease of centrosomal dynactin (Burakov et al., 2008b), we suggested that dynactin might mediate the effect of SLK on microtubules.
RESULTS
SLK (LOSK) phosphorylates p150Glued
To test whether SLK can directly phosphorylate dynactin, we purified dynactin from bovine brain and incubated it with the recombinant kinase domain of SLK (SLKCD) in the presence of 32P-labeled ATP. The label was incorporated into a 150-kDa band (Figure 1A), also recognized by anti-p150Glued antibodies in Western blot (data not shown). We assume that it might have been a p150Glued subunit of the dynactin complex.
FIGURE 1:
SLK (LOSK) phosphorylates dynactin in vitro. (A) A catalytic domain of SLK (SLKCD) phosphorylates dynactin preparation from bovine brain. The 32P label is incorporated into a 150-kDa band. Autophosphorylation of the kinase domain is also visible and is likely to cause label incorporation into the lower bands (a result of truncated protein synthesis in E. coli or partial protein degradation). Myelin basic protein is used as positive control. Radioautograph (R/a) and Coomassie staining of the same gel are represented. (B) Identification of the phosphorylation site. SLKCD phosphorylates the N-terminal part of p150Glued-1A (aa 1–157) but does not phosphorylate the N-terminal part of p150Glued-1B (aa 1–137), ΔN-p150Glued-1A (aa 151–1281), or T145-147A mutant of p150Glued-1A (aa 1–157, T145-147A). Asterisks mark positions of recombinant proteins visualized by Ponceau staining before radioautography (Ponceau is not shown). (C) In vitro kinase reaction with GST-p150Glued-1A N-terminal fragment in the presence and absence of cold ATP. Western blot is stained with antiphosphothreonine antibodies. The reaction is inhibited in the absence of phosphatase inhibitors. (D) Threonines in all three positions are phosphorylated by SLK. In vitro kinase assay with the GST-fused N-terminal parts of wild-type p150Glued-1A and mutants (T145-147A, T145A, T146A, and T147A). 32P is incorporated in all three threonines (145–147; (lanes 3–5). Radioautograph and Ponceau-stained membrane. Kinase domain and all fragments of p150Glued shown were synthesized in E. coli as GST fusions and purified on glutathione-agarose. (E) Phosphorylation sites for SLK in human RhoA, paxillin, and the identified site in p150Glued-1A. Predicted site is represented according to Pike et al. (2008). Φ corresponds to aliphatic amino acid.
Dynactin phosphorylation was further studied with in vitro kinase assays using recombinant fragments of p150Glued. Two isoforms of p150Glued (1A and 1B) with molecular weight 150 kDa were described previously (Dixit et al., 2008; Zhapparova et al., 2009). They vary in the length of the basic microtubule-binding region. We tested glutathione S-transferase–fused recombinant fragments of the N-terminal parts of p150Glued-1A, with a full-length basic domain (amino acids [aa] 1–157), p150Glued-1B, without the basic domain (aa 1–137), and the ΔN-p150Glued fragment (aa 151–1281) shared by the two isoforms. 32P-phosphate was incorporated in only the N-terminal part of the 1A isoform (Figure 1B), indicating that the site of phosphorylation was located somewhere within the variable region. Phosphorylation of the N-terminal part of p150Glued-1A was also confirmed in a kinase assay with cold ATP and antiphosphothreonine antibodies (Figure 1C).
SLK belongs to the family of serine/threonine protein kinases. The variable region of p150Glued does not contain serine residues but has four threonines at positions 141 and 145–147. We obtained two mutants in which threonines were substituted for alanine, T141A and T145-147A, and tested them in a kinase assay. The label was still incorporated into the T141A mutant (data not shown) but not in the T145-147A mutant (Figure 1B), indicating that the phosphorylation site was located within threonines 145–147. To define which of three threonines was phosphorylated, we obtained a series of mutants, T145A, T146A, and T147A, and performed a kinase assay (Figure 1D). Phosphate incorporated in all three mutants, demonstrating that each of three threonines could be phosphorylated (Figure 1D). Simultaneous phosphorylation of adjacent amino acids is a rare phenomenon, which was described for a few viral proteins (Scheidtmann et al., 1982). It is intriguing whether SLK phosphorylates all three threonines or one or two of them and whether the phosphorylation status of dynactin varies under different conditions. We hope to elucidate this in further research. Here we studied the effect of dynactin phosphorylation using triple mutants of p150Glued (T145-147A and T145-147E). The identified phosphorylation site in p150Glued (140PTARKT*T*T*RRPKP) has common features with SLK sites in paxillin (Quizi et al., 2013) and RhoA (Guilluy et al., 2008; Figure 1E), although none of these sites coincides with the consensus site defined by peptide library analysis (X-X-X-Y-X-T*-Φ-R/K-X-X-X, where Φ is an aliphatic amino acid; Pike et al., 2008; Figure 1E).
p150Glued mutants vary in affinity for microtubules in vitro and in vivo
Because p150Glued was phosphorylated at the MT-binding domain, we suggested that this phosphorylation might affect the interaction with microtubules. To test this, we performed a copelleting assay. GST-fused N-terminal fragments (1–157 aa) of Ala-p150Glued (T145-147A) and Glu-p150Glued (T145-147E) mutants were incubated with Taxol-stabilized microtubules and pelleted through a glycerol cushion (Figure 2A). The Glu mutant exhibited slightly reduced affinity for microtubules compared with the Ala mutant: at 0.8 μM concentration Ala-p150Glued was found only in the microtubule pellet, whereas Glu-p150Glued was also detected in the supernatant (Figure 2A). At 2 μM concentration, Ala-p150Glued was found in the pellet and in the supernatant as well, indicating the saturation of microtubule binding. Wild-type p150Glued-1A exhibited similar affinity for microtubules as Ala-p150Glued: it bound microtubules tightly and was detected mostly in the pellet (Figure 2A and Supplemental Table S1; Zhapparova et al., 2009).
FIGURE 2:
p150Glued mutants vary in the affinity for microtubules in vitro and in vivo. (A) Co-pelleting of GST-fused N-terminal fragments (aa 1–157) of wild type (WT) and Ala- and Glu-p150Glued with microtubules. Fragments of p150Glued (0.4, 0.8, and 2 μM) were incubated with microtubules (16 μM) and pelleted through a 4 M glycerol cushion. In control (–MT), fragments of p150Glued were precipitated under the same sedimentation conditions in the absence of microtubules. Protein content in supernatant (S) and pellet (P) was analyzed by Western blot with anti-p150Glued antibodies. Relative content of p150Glued in microtubule pellets. Error bars represent SD. *p < 0.05 for Ala- and Glu-p150Glued. (B) Localization of full-length GFP-fused Ala- and Glu-p150Glued in Vero cells. Microtubules were stained with anti-tubulin antibodies. Ala-p150Glued decorates the entire microtubules, whereas Glu-p150Glued is concentrated mostly at distal ends. Line scans of microtubules indicated with arrowheads. Scale bar, 10 μm.
Then we analyzed the interaction of mutants with microtubules in vivo. We expressed green fluorescent protein (GFP)–fused full-length p150Glued mutants in Vero cells and analyzed their distribution along the microtubules (Figure 2B). Consistent with the in vitro results, the Ala mutant demonstrated higher affinity for microtubules and decorated them along the entire length (Figure 2B). The Glu mutant formed long comets at microtubule plus ends but rarely covered the entire microtubules. The localization pattern was independent of the level of recombinant protein expression (data not shown).
The Glu-mutant of p150Glued exhibits higher affinity for the centrosome
The activity of protein kinase SLK and the presence of dynein–dynactin complex at the centrosome are required for the maintenance of microtubule radial array (Quintyne et al., 1999; Burakov et al., 2008b). Considering p150Glued as a substrate of SLK, we studied its interaction with the centrosome regarding phosphorylation status. We expressed GFP-fused full-length Ala- and Glu-p150Glued mutants in Vero cells and visualized the centrosome with anti–γ-tubulin antibodies (Figure 3A). It is striking that Ala-p150Glued, which exhibited higher affinity for microtubules, was located at the centrosome in only 36% of cells. At the same time, Glu-p150Glued was concentrated at the centrosome in 64% of cells, indicating that centrosomal localization of p150Glued was regulated by SLK-dependent phosphorylation (Figure 3, A and B).
FIGURE 3:
Glu-p150Glued exhibits higher affinity for the centrosome compared with Ala-p150Glued. (A) Expression of full-length GFP-fused Ala- and Glu-p150Glued in Vero cells and immunostaining of fixed cells with anti–γ-tubulin antibodies to visualize centrosome. Boxed areas are shown at higher magnification. (B) Quantification of cells with centrosomal localization of p150Glued mutants. Ala, a full-length GFP-fused Ala-p150Glued (T145-147A; n = 349); Glu, a full-length GFP-fused Glu-p150Glued (T145-147E; n = 420); K63R/Ala, cotransfection of K63R (dsRed-fused dominant-negative mutant of SLKCD; aa 1–342) and Ala-p150Glued (n = 74); and K63R/Glu, cotransfection of K63R and Glu-p150Glued (n = 60). Experiments were performed in at least three repeats; n, total number of cells analyzed in all experiments. Error bars, SD; *p < 0.05 (t test).
The Glu mutant of p150Glued restores radial organization of microtubules in cells with inhibited SLK (LOSK)
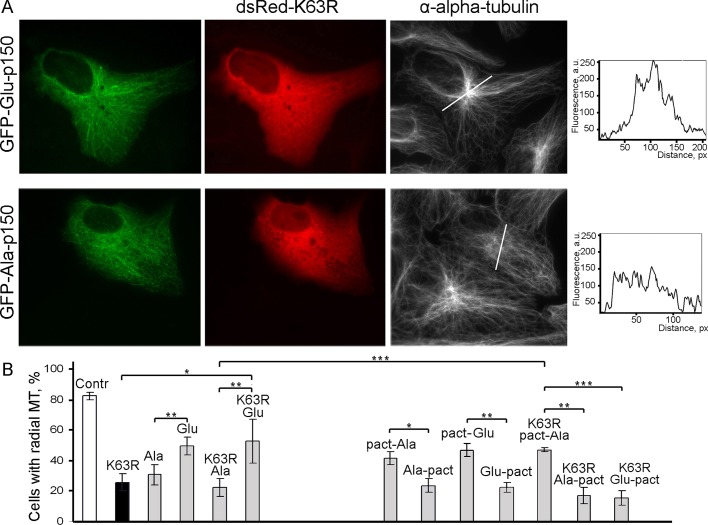
To determine the effect of p150Glued phosphorylation on the organization of microtubules, we examined the system of microtubules in cells with inhibited SLK. As we showed previously, expression of dominant-negative SLK (K63R) disrupts radial organization of microtubules in 80% of cells and entails marked depletion of centrosomal dynactin (Burakov et al., 2008b). Here we coexpressed dsRed-tagged dominant-negative SLK and GFP fusion of either Ala- or Glu-p150Glued. Glu-p150Glued markedly restored radial organization of microtubules in 53% of Vero cells, whereas Ala-p150Glued had no effect, and only 22% of Ala-p150Glued cells contained a radial array of microtubules (Figure 4, A and B). Note that the expression of full-length p150Glued itself induces randomization of microtubules in 58% of cells (Supplemental Figure S1). The effect might be caused by coiled-coil 1 region of p150Glued, which is responsible for dynein–dynactin interactions (Quintyne et al., 1999). Therefore the results of coexpression should be compared with corresponding controls of individual Ala- and Glu-p150Glued expression. Of note, Ala-p150Glued itself caused randomization of microtubules in 70% of cells (Figure 4B), indicating that nonphosphorylatable p150Glued could not maintain radial organization of microtubules.
FIGURE 4:
Glu-mutant of p150Glued restores radial organization of microtubules in cells with inhibited SLK. (A) Coexpression of dsRed-fused, dominant-negative kinase domain of SLK (K63R-ΔT) and full-length GFP-fused Ala- or Glu-p150Glued in Vero cells and immunostaining of fixed cells with anti-tubulin antibodies. Expression of Glu-p150Glued restores radial microtubule array in cells with inhibited SLK, whereas expression of Ala-p150Glued enhances microtubule randomization in such cells. Microtubule organization was estimated by measuring fluorescence intensity along lines drawn through the cell's center. Scans of fluorescence intensity along such lines are shown. (B) Quantification of cells with radial microtubule array. Contr, control nontransfected cells (n = 568); K63R, dsRed-fused dominant-negative mutant of SLK (aa 1–342; n = 948); Ala, full-length GFP-fused Ala-p150Glued (T145-147A; n = 389); Glu, full-length GFP-fused Glu-p150Glued (T145-147E; n = 225); K63R/Ala, cotransfection of K63R and Ala-p150Glued (n = 400); K63R/Glu, cotransfection of K63R and Glu-p150Glued (n = 862); pact-Ala, PACT-GFP-Ala-p150Glued (n = 104); Ala-pact, GFP-Ala-p150Glued-PACT (n = 77); pact-Glu, PACT-GFP-Glu-p150Glued (n = 68); Glu-pact, GFP-Glu-p150Glued-PACT (n = 273); K63R/pact-Ala, cotransfection of K63R and PACT-GFP-Ala-p150Glued (n = 374); K63R/Ala-pact, cotransfection of K63R and GFP-Ala-p150Glued-PACT (n = 179); K63R/Glu-pact, cotransfection of K63R and GFP-Glu-p150Glued-PACT (n = 273). Experiments were performed in at least three repeats; n, total number of cells analyzed in all experiments. Error bars, SD; *p < 0.05, **p < 0.01, ***p < 0.001 (t test).
In some cases, microtubule randomization in cells might result from the expression of microtubule-binding proteins, which stabilize microtubules. To test whether such an effect was occurring in our system, we expressed GFP-fused wild-type p150Glued-1A and p150Glued-1B. These isoforms vary in the length of microtubule-binding region and have different affinity for microtubules (Zhapparova et al., 2009). The 1A isoform, similarly to Ala-p150Glued, had higher affinity for microtubules but did not cause such dramatic randomization of microtubules as the mutant protein (Supplemental Table and Supplemental Figure S1). These data indicate that binding of dynactin to microtubules is not enough for their randomization.
SLK (LOSK) regulates microtubule organization by targeting p150Glued to the centrosome
The data raise a question about the exact function of dynactin phosphorylation. Phosphorylation 1) might be required only for targeting dynactin to the centrosome or 2) can affect interactions with microtubules and putative mediator proteins. If the first assumption is true, artificial targeting of nonphosphorylatable p150Glued to the centrosome would restore microtubule array in cells with inhibited SLK.
To verify the hypothesis, we fused Ala-p150Glued to the PACT domain of AKAP450 protein. The PACT domain is responsible for centrosomal localization of AKAP450 and pericentrin, and its fusion to GFP results in clear centrosomal localization of the latter (Gillingham and Munro, 2000). We expressed full-length GFP-fused Ala-p150Glued carrying the PACT domain at the N- or C-terminus (Figure 5A). Both proteins accumulated at the centrosome of >90% cells (compare to 34% cells in the case of Ala-p150Glued without PACT domain; Figure 5A). Then we coexpressed the described constructs and dominant-negative SLK and examined microtubule organization (Figure 5B). PACT-Ala-p150Glued restored microtubule array in 47% cells (compare to 50 and 23% in the case of Glu-p150Glued and Ala-p150Glued, respectively; Figure 4B). Expression of GFP-fused PACT had no effect on microtubule organization (data not shown). The obtained data indicate that phosphorylation itself is required only for dynactin targeting to the centrosome and not for its interactions with microtubules or microtubule-interacting proteins there.
FIGURE 5:
SLK (LOSK) regulates microtubule organization by targeting p150Glued to the centrosome. (A) Expression of PACT fusions in Vero cells and immunostaining of fixed cells with anti–γ-tubulin antibodies to visualize centrosome. Boxed areas are shown at higher magnification. PACT at either end of Ala-p150Glued ensures centrosomal localization of the protein in >90% of cells. (B) Coexpression of dsRed-fused, dominant-negative kinase domain of SLK (K63R) and p150Glued mutants fused with PACT domain and immunostaining of fixed cells with anti-tubulin antibodies. PACT at the N-terminus of Ala-p150Glued rescues radial microtubule array in cells with inhibited SLK. PACT at the C-terminus of Ala-p150Glued or Glu-p150Glued does not restore radial microtubule array in cells with inhibited SLK.
We obtained unexpected results with Ala-p150 carrying the PACT domain at the C-terminus (Figure 5, A and B). It was unable to restore a microtubule array in cells with inhibited SLK, although the protein exhibited clear centrosomal localization (Figures 5A and 4B). Moreover, microtubules were disorganized in 80% of cells expressing the Ala-p150-PACT chimera, indicating either involvement of p150Glued C-tail in the interaction with some proteins or the importance of proper orientation of dynactin in the pericentriolar matrix.
To verify the importance of C-terminal interactions for microtubule organization, we expressed Glu-p150Glued fused to the PACT domain at the C-terminus. As we showed in previous experiments, expression of Glu-p150Glued successfully restored microtubule array in cells with inhibited SLK. Expression of Glu-p150Glued-PACT, however, caused microtubule randomization and did not restore the radial array in cells with inhibited SLK (Figures 5B and 4B).
In cells, p150Glued functions as a part of dynactin complex, and inhibition of SLK results in the release of all dynactin subunits from the centrosome (Burakov et al., 2008). Here we demonstrated that phosphorylation of p150Glued is required for its centrosomal localization and for the maintenance of microtubule radial array. Next we studied whether other dynactin subunits were implicated in this process. The ability of p150Glued mutants to interact with other dynactin subunits was verified by immunoprecipitation (Supplemental Figure S2). Then we immunostained cells expressing dominant-negative SLK and Glu-p150Glued for the dynamitin (p50) subunit. Dynamitin was recruited to the centrosome in only half of cells with centrosomal localization of Glu-p150Glued (data not shown). The absence of other dynactin subunits seems to have no effect on microtubule radial organization once Glu-p150Glued is located at the centrosome.
Glu-p150Glued rescues Golgi reorientation in polarized cells with inhibited SLK (LOSK)
Together with the maintenance of microtubule array, SLK was shown to be required for Golgi reorientation in polarized cells (Burakov et al., 2008b). We examined whether this effect depended on dynactin phosphorylation. Polarized cells were obtained at the edge of an experimental wound scratched in a monolayer of Vero cells. Normally, compact Golgi cisternae localize in the perinuclear region, and during polarization they are translocated toward the leading edge. Inhibition of SLK results in Golgi mislocalization in 80% of cells (Burakov et al., 2008b; Figure 6, A and B). Coexpression of dominant-negative SLK and Glu-p150Glued restored Golgi orientation in 60% of cells. The observed orientation of Golgi was independent of microtubule organization: only 33% of cells expressing Ala-p150Glued had radial array of microtubules (Figure 4B), but >60% of such cells contained normally oriented Golgi. The p150Glued mutants themselves had almost no effect on Golgi orientation.
FIGURE 6:

Glu-p150Glued rescues Golgi reorientation in polarized cells with inhibited SLK (LOSK). (A) Coexpression of dsRed-fused, dominant-negative kinase domain of SLK (K63R) and GFP-fused Ala- or Glu-p150Glued in polarized Vero cells and immunostaining of fixed cells with anti–mannosidase II antibodies to visualize the Golgi. Polarized cells were obtained at the edge of an experimental wound in a monolayer of Vero cells. Expression of Glu-p150Glued restores normal reorientation of the Golgi toward the leading edge of the cell with inhibited SLK. (B) Quantification of cells with polarized Golgi. Contr, control nontransfected cells (n = 157); K63R, dsRed-fused, dominant-negative mutant of SLK (aa 1–342; n = 75); Ala, full-length GFP-fused Ala-p150Glued (T145-147A; n = 66); Glu, full-length GFP-fused Glu-p150Glued (T145-147E; n = 70); K63R/Ala, cotransfection of K63R and Ala-p150Glued (n = 69); K63R/Glu, cotransfection of K63R and Glu-p150Glued (n = 81). Experiments were performed in at least three repeats; n, total number of cells analyzed in all experiments. Error bars, SD. ***p < 0.001 (t test).
Together with reoriented Golgi, polarized migrating fibroblast-like cells usually have their centrosomes translocated toward the leading edge (Euteneuer and Schliwa, 1992). Such localization facilitates microtubule growth toward the leading edge. We examined centrosome positioning in cells coexpressing dominant-negative SLK and a p150Glued mutant. In all cases the centrosome was oriented as in the nontransfected control (data not shown). The result indicates that Golgi and the centrosome, which usually migrate together, are uncoupled in cells with inhibited SLK.
DISCUSSION
Here we unraveled a new regulatory mechanism of microtubule anchoring at the centrosome. We demonstrated that the protein kinase SLK (LOSK), required for the maintenance of microtubule array, phosphorylates the p150Glued subunit of the dynactin complex. A phosphomimicking mutant of p150Glued exhibits high affinity for the centrosome and restores microtubule array in cells with inhibited SLK. This mutant also restores Golgi polarization in cells with inhibited SLK at the edge of an experimental wound.
Phosphorylation status of p150Glued depends on cell signaling pathways (Farshori and Holzbaur, 1997). Although Farshori and Holzbaur (1997) detected only phosphoserine residues in the phosphorylated dynactin, p150Glued phosphorylated at threonines is associated with the centrosome and thus might comprise a minor fraction of the whole dynactin pool. Phosphorylation at Ser-19 (presumably by cAMP-dependent protein kinase) regulates p150Glued interaction with microtubules (Vaughan et al., 2002). In prophase mammalian cells, polo-like kinase Plk1 phosphorylates p150Glued at Ser-179 located distally to the basic domain (Li et al., 2010) and thus regulates nuclear envelope breakdown. In mitotic Drosophila cells, the dynamics of p150Glued is regulated by Aurora A, which phosphorylates serines in the N-terminal microtubule-binding domain (Romé et al., 2010). Note that Drosophila p150Glued contains a short basic domain, which lacks the variable region with threonines (Zhapparova et al., 2009).
Dynactin can fulfill two possible functions: 1) deliver microtubule-anchoring proteins to the centrosome within the dynein–dynactin complex (transport function) and 2) anchor microtubules directly via its MT-binding subunit p150Glued or indirectly via some dynactin-binding proteins or even dynein (anchoring function). SLK does not affect the transport function of dynactin, and the major proteins involved in microtubule anchoring retain their centrosomal localization—γ-tubulin, pericentrin, ninein (Burakov et al., 2008b), and EB1 (data not shown). Our results, however, indicate that dynactin phosphorylation is definitely required for its microtubule-anchoring function. It seems that the cell contains at least two pools of dynactin with distinct functions. Such functional diversity might be explained by structural differences of p150Glued isoforms that vary in the microtubule-binding region (Dixit et al., 2008; Zhapparova et al., 2009). Fibroblasts and other nonneuronal cells express mostly p150Glued-1B, which lacks a 20-aa region in the basic MT-binding domain. A full-length 1A isoform is expressed in such cells in trace amounts and is located mostly in the centrosomal area, where its functions are unknown (Zhapparova et al., 2009). The fact that the phosphorylation site is within the variable region of two isoforms suggests that they might fulfill different functions in cells. The dominant isoform (p150Glued-1B) might be responsible for intracellular transport, and the minor one (1A) might be implicated in microtubule organization.
The precise role of dynactin in microtubule anchoring at the centrosome is unknown: dynactin can directly hold microtubules or engage mediator proteins. We demonstrate here that phosphorylation is essential for targeting p150Glued to the centrosome, although it does not affect centrosomal function. It seems that SLK phosphorylates dynactin and recruits it to the centrosome, where the increased amount of dynactin anchors more microtubules and maintains microtubule radial organization more efficiently. Centrosomal proteins that interact with the phosphorylated basic domain of p150Glued are unknown, and further experiments are required for their identification.
Experiments with overexpression of Glu-p150Glued suggest that centrosomal localization of other dynactin subunits is not essential for microtubule radial organization when p150Glued is targeted to the centrosome. In vivo, however, the integrity of the dynactin complex might be required for its transport to the centrosome and its anchoring there. The mechanism of dynactin retention at the centrosome is debatable. Until recently, a “shoulder” part of dynactin was considered to be responsible for binding to the centrosome. Dynamitin (p50) was shown to interact with centrosomal protein Cep135 (Uetake et al., 2004), and separation of “shoulder” and “arm” induced by overexpression of the p24 subunit resulted in the retention of Arp1 filament and release of p150Glued from the centrosome (Quintyne et al., 1999). Recent data (Kodani et al., 2010) indicate that p150Glued interacts with PCM-1 and thus can bind the centrosome. Alternatively, p150Glued might have some other partners at the centrosome besides PCM-1.
Although p150Glued can directly interact with microtubules, our experiments masking the C-terminus of p150Glued with the PACT domain indicate that dynactin requires some additional proteins to hold a microtubule at the centrosome. The C-tail of p150Glued interacts with cargo proteins during vesicular transport: Rab7-interacting lysosomal protein (Johansson et al., 2007), transport protein particle TRAPPC9 (Zong et al., 2012), and COPII proteins Sec23/24 (Watson et al., 2005). It is unlikely, however, that these proteins are involved in microtubule organization at the centrosome. The C-terminus of p150Glued also might be important for proper positioning of dynactin in a multicomponent and multilayer pericentriolar material (Lawo et al., 2012).
Note that not only is phosphorylation of p150Glued required for microtubule organization, but it is also involved in cell polarization. In nonneuronal cells the 1A isoform of p150Glued usually accumulates at the centrosome, whereas during cell polarization it binds to microtubules directed toward the leading edge (Zhapparova et al., 2009). Our results demonstrate that SLK-dependent phosphorylation is required for proper orientation of the Golgi in polarized cells. The observed phenomenon might be explained by the fact that formation of the Golgi and its positioning in polarized cells depend on microtubules that grow from the centrosome (Vinogradova et al., 2012). Cells with inhibited SLK activity contain few such microtubules. Alternatively, the amount of phosphorylated p150Glued might be increased at microtubules directed toward the leading edge, since isoform 1A is actively recruited there during cell polarization (Zhapparova et al., 2009). Phosphorylated dynactin might specifically interact with Golgi proteins and thus ensure reorientation of the organelle. We could not, however, detect any differences in the localization of phosphomimicking p150Glued at microtubules in the leading and rear parts of the cell, which might result from high levels of protein expression.
Overall our data indicate that SLK regulates the organization of microtubules and cell polarization through phosphorylation of p150Glued-1A, which does not affect the transport functions of dynactin.
MATERIALS AND METHODS
DNA constructs
Full-length human cDNA encoding p150Glued-1B and mouse cDNA encoding p150Glued-1A (described in Zhapparova et al., 2009) were cloned into a pEGFP-C1 vector (Clontech, Mountain View, CA). N-terminal fragments encoding aa 1–157 of p150Glued-1A, 1–137 of p150Glued-1B, and 151–1281 of a ΔN fragment of p150Glued-1A were cloned into pGEX-4T3 (GE Healthcare, Piscataway, NJ). High-fidelity PCR Enzyme Mix, restriction endonucleases, and other enzymes used for molecular cloning were from Fermentas (Vilnius, Lithuania). Primers were purchased from Syntol (Moscow, Russia). Site-directed mutagenesis was performed with a QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA). In GST-fused N-terminal fragments of p150Glued-1A, each threonine in positions 145–147 was substituted for an alanine residue to obtain T145A, T146A, and T147A mutants; threonine in position 141 was substituted for alanine to obtain the T141A mutant; and all threonines in positions 145–147 were substituted for alanines to obtain the T145-147A mutant. In GFP-fused full-length p150Glued-1A, threonines in positions 145–147 were simultaneously substituted for three alanine or three glutamate residues to obtain Ala-p150Glued and Glu-p150Glued mutants, respectively. GFP-fused catalytic domain of SLK (SLKCD, aa 1–342) and a dominant-negative mutant (K63R, aa 1-342) were described in Burakov et al. (2008b). Dominant-negative mutant fused to dsRed was obtained by subcloning into a dsRed-C1 vector (Clontech).
For cloning of the PACT domain, total RNA was isolated from cultured HeLa cells using an RNeasy Kit (Qiagen, Hilden, Germany). First-strand cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen) and random hexanucleotide primers (Syntol). PACT domain (aa 3702–3789) of AKAP450 (AJ131693.1) was amplified using the primers 5′- TATGGTAAATACTTGAGGGCAGAAAG-3′ and 5′-TGACTCGATGCCACCGTCGAAC-3′. The obtained PACT-domain DNA was amplified with corresponding primers and subcloned into pEGFPC1 vector at EcoRI/SalI sites (GFP-PACT). PACT-domain DNA was subcloned into pEGFPC1 vector carrying Ala-p150Glued either upstream of GFP (at AgeI site, PACT-Ala-p150Glued) or downstream of Ala-p150Glued (Ala-p150Glued-PACT). Glu-p150Glued-PACT was obtained as a result of PACT subcloning into pEGFPC1 vector carrying Glu-p150Glued downstream of dynactin. All constructs were verified with automated sequencing, and the length of corresponding protein products was confirmed with SDS–PAGE and Western blot.
Mammalian cell growth, transfection, fixation, immunostaining, and wound-healing assay
Green monkey kidney Vero epithelia-like cells were cultured in DMEM/F12 (1:1) medium (Paneco, Moscow, Russia) supplemented with 7.5% fetal bovine serum (Paneco) at 37°C and 5% CO2.
Transfection was performed using TransIT-LT1 reagent (Mirus Bio, Madison WI) or Lipofectamine 2000 (Invitrogen, Carlsbad, CA).
For immunostaining, cells were fixed with methanol for 10 min at −20°C, followed by 3% paraformaldehyde for 20 min at 4°C. The following antibodies were used: mouse monoclonal antibody to p150Glued (610473; BD Biosciences, Franklin Lakes, NJ), p50/dynamitin (611002; BD Biosciences), tubulin DM-1A (Sigma-Aldrich, St. Louis, MO); rat monoclonal anti–tubulin YOL1/34 antibody (Abcam, Cambridge, United Kingdom); and rabbit polyclonal antibodies to mannosidase II (12277; Abcam), phosphothreonine (71-8200; Invitrogen), and γ-tubulin (kindly provided by R. Uzbekov, Lomonosov Moscow State University, Russia). Fluorochrome-conjugated (fluorescein isothiocyanate, tetramethylrhodamine isothiocyanate, pentamethincyanine) secondary antibodies (MultiLabeling type) were obtained from Jackson ImmunoResearch Laboratories (Newmarket, United Kingdom). Images of immunostained cells were taken using a Carl Zeiss Axiovert 200M microscope supplied with a 12-bit AxioCamHR camera and AxioVision software (Carl Zeiss, Jena, Germany). The data were analyzed using ImageJ (National Institutes of Health, Bethesda, MD) and Excel software (Microsoft, Redmond, WA.
For wound-healing assay, Vero cells were grown to a monolayer, then scratched with a pipet tip, incubated at 37°C for 2 h, and fixed as described.
Tubulin purification and microtubule copelleting assay
Tubulin was isolated from rat brains as described in Zhapparova et al. (2009). For copelleting experiments, 6 mg/ml rat tubulin was incubated in BRB buffer (80 mM 1,4-piperazinediethanesulfonic acid [PIPES], pH 6.8, 1 mM MgCl2, 1 mM ethylene glycol tetraacetic acid) with 1 mM GTP for 20 min at 37°C; then 2 μM Taxol (Sigma-Aldrich) was added. The mixture was incubated at 37°C for 15 min, after which the Taxol concentration was increased to 20 μM and the mixture was incubated at 37°C for another 15 min. We mixed 16 μM microtubules, 1 mM GTP, and 15 μM Taxol in BRB buffer with GST-dynactin fragments, incubated at 37°C for 30 min, and applied over a warm 4 M glycerol cushion with 1 mM GTP and 5 μM Taxol in BRB. Microtubules were pelleted in a TLS55 rotor (Beckman Coulter, Brea, CA) at 50,000 rpm and 25°C for 30 min. Supernatants were collected and mixed with 4× sample buffer (SB), and cushions were washed three times with BRB and discarded. The pellets (mostly invisible) were resuspended in an equal volume of 2× SB.
Immunoprecipitation, recombinant protein production, SDS–PAGE, and Western blot analysis
For immunoprecipitation, human embryonic kidney HEK293T cells were transfected and harvested in PHEM buffer (50 mM PIPES, 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES], 1 mM EDTA, 2 mM MgSO4, pH 7.0) supplemented with 0.5% Nonidet P-40, 0.5% Triton X-100, and 0.25% sodium deoxycholate. After centrifugation (TLS55 rotor [Beckman Coulter], 32,000 rpm, 4°C, 20 min), supernatant was incubated with protein A–Sepharose (P3391; Sigma-Aldrich) or MabSelect-Sepharose (GE Healthcare) and antibodies for immunoprecipitation against p50/dynamitin (sc-135135; Santa Cruz Biotechnology, Santa Cruz, CA) or GFP (GMA0311; Protein Synthesis, Moscow, Russia) for 3 h at 4°C. The results were analyzed with Western blot using mouse monoclonal anti-p150Glued (610473; BD Biosciences), anti-dynamitin/p50 (611002; BD Biosciences), anti-GFP (AMA 0311; Protein Synthesis) antibodies and rabbit polyclonal anti-GFP (AB121; Evrogen, Moscow, Russia) and anti-ACTR1B (ab84809, Abcam) antibodies.
Fragments of GST-fused p150Glued and SLKCD were expressed in Escherichia coli and purified using a glutathione-agarose column (Sigma-Aldrich) according to the manufacturer's instructions. Purified N-terminal fragments of p150Glued were used in microtubule copelleting and in vitro phosphorylation experiments. SDS–PAGE was performed in 7.5, 10, or 6–12% gradient gels. Nitrocellulose membranes (Bio-Rad, Hercules, CA) were blocked in Tris-buffered saline supplemented with 0.05% Tween-20 and 5% fat-free milk overnight and subsequently incubated with primary antibodies and goat anti-rabbit or anti-mouse immunoglobulin G antibodies conjugated to alkaline phosphatase (KPL, Gaithersburg, MD). The results were visualized with 5-bromo-4-chloro-3’-indolyphosphate/nitro-blue tetrazolium solution (KPL).
Preparation of dynactin isolated from bovine brain was kindly provided by S. Kuznetsov (University of Rostock, Rostock, Germany).
In vitro kinase assay
GST-fused SLKCD and N-terminal fragments of p150Glued were expressed in E. coli and purified on glutathione-agarose. For in vitro kinase assay, 5 μg of kinase and dynactin fragments or 2.5 μg of myelin basic protein (Sigma-Aldrich) were mixed with γ-32P-ATP (10 μCi; FEI Research Center, Obninsk, Russia) in kinase buffer (50 mM HEPES, pH 7.4, 10 mM MgCl2, 100 mM KCl, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol) and incubated at 25°C for 1 h. For the experiments with brain dynactin, 0.2 mM heparin was added to inhibit contaminant endogenous casein kinase activity in dynactin preparation. The results were analyzed with SDS–PAGE and autoradiography. In vitro kinase assay with cold ATP was performed with the same amount of proteins; the reaction was incubated overnight at 4°C. The results were analyzed with Western blot stained with antiphosphothreonine antibodies (Invitrogen).
Supplementary Material
Acknowledgments
We are grateful to colleagues who provided reagents and especially thank Rustem Uzbekov and Sergei Kuznetsov. We thank Irina Tsimbarevich for help in experiments and Anton Burakov for fruitful discussions. Some preliminary experiments were performed by the late Olga Kovalenko. The work was financially supported by Russian Foundation of Basic Research Grants 11-04-01022, 12-04-31380, and 12-04-33178. N.V. was supported by RF Presidential Program in Support of Young Scientists Grant MK-5961.2012.4 and the Dmitry Zimin Dynasty Foundation.
Abbreviations used:
- LOSK
long Ste20-like protein kinase
- MT
microtubules
- SLK
Ste20-like protein kinase
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E13-03-0137) on August 28, 2013.
REFERENCES
- Albee AJ, Wiese C. Xenopus TACC3/maskin is not required for microtubule stability but is required for anchoring microtubules at the centrosome. Mol Biol Cell. 2008;19:3347–3356. doi: 10.1091/mbc.E07-11-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders A, Sawin KE. Microtubule stabilization in vivo by nucleation-incompetent γ-tubulin complex. J Cell Sci. 2011;124:1207–1213. doi: 10.1242/jcs.083741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burakov A, Kovalenko O, Semenova I, Zhapparova O, Nadezhdina E, Rodionov V. Cytoplasmic dynein is involved in the retention of microtubules at the centrosome in interphase cells. Traffic. 2008a;4:472–480. doi: 10.1111/j.1600-0854.2007.00698.x. [DOI] [PubMed] [Google Scholar]
- Burakov A, Zhapparova O, Kovalenko O, Zinovkina L, Potekhina E, Shanina N, Weiss D, Kuznetsov S, Nadezhdina E. Ste20-related protein kinase LOSK (SLK) controls microtubule radial array in interphase. Mol Biol Cell. 2008b;19:1952–1961. doi: 10.1091/mbc.E06-12-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casenghi M, Barr F, Nigg E. Phosphorylation of Nlp by Plk1 negatively regulates its dynein-dynactin-dependent targeting to the centrosome. J Cell Sci. 2005;118:5101–5108. doi: 10.1242/jcs.02622. [DOI] [PubMed] [Google Scholar]
- Culver-Hanlon T, Lex S, Stephens A, Quintyne N, King S. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nat Cell Biol. 2006;8:264–270. doi: 10.1038/ncb1370. [DOI] [PubMed] [Google Scholar]
- Dammermann A, Merdes A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J Cell Biol. 2002;159:255–266. doi: 10.1083/jcb.200204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgehyr N, Silllibourne J, Bornens M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J Cell Sci. 2005;118:1565–1575. doi: 10.1242/jcs.02302. [DOI] [PubMed] [Google Scholar]
- Dixit R, Levy J, Tokito M, Ligon L, Holzbaur E. Regulation of dynactin through the differential expression of p150Glued isoforms. J Cell Biol. 2008;283:33611–33619. doi: 10.1074/jbc.M804840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U, Schliwa M. Mechanism of centrosome positioning during the wound response in BSC-1 cells. J Cell Biol. 1992;116:1157–1166. doi: 10.1083/jcb.116.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farshori P, Holzbaur EL. Dynactin phosphorylation is modulated in response to cellular effectors. Biochem Biophys Res Commun. 1997;232:810–816. doi: 10.1006/bbrc.1997.6379. [DOI] [PubMed] [Google Scholar]
- Fumoto K, Hoogenraad CC, Kikuchi A. GSK-3beta-regulated interaction of BICD with dynein is involved in microtubule anchorage at centrosome. EMBO J. 2006;25:5670–5682. doi: 10.1038/sj.emboj.7601459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 2000;1:524–529. doi: 10.1093/embo-reports/kvd105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilluy C, Rolli-Derkinderen M, Loufrani L, Bourge A, Henrion D, Sabourin L, Loirand G, Pacaud P. Ste20-related kinase SLK phosphorylates Ser188 of RhoA to induce vasodilation in response to angiotensin II type 2 receptor activation. Circ Res. 2008;102:1265–1274. doi: 10.1161/CIRCRESAHA.107.164764. [DOI] [PubMed] [Google Scholar]
- Guo J, Yang Z, Song W, Chen Q, Wang F, Zhang Q, Zhu X. Nudel contributes to microtubule anchoring at the mother centriole and is involved in both dynein-dependent and -independent centrosomal protein assembly. Mol Biol Cell. 2006;17:680–689. doi: 10.1091/mbc.E05-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi M, et al. Trichoplein controls microtubule anchoring at the centrosome by binding to Odf2 and ninein. J Cell Sci. 2011;124:857–864. doi: 10.1242/jcs.075705. [DOI] [PubMed] [Google Scholar]
- Itoh S, Kameda Y, Yamada E, Tsujikawa K, Mimura T, Kohama Y. Molecular cloning and characterization of a novel putative STE20-like kinase in guinea pigs. Arch Biochem Biophys. 1997;340:201–207. doi: 10.1006/abbi.1997.9893. [DOI] [PubMed] [Google Scholar]
- Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ling S, Rogers G, Kural C, Selvin P, Rogers S, Gelfand V. Microtubule binding by dynactin is required for microtubule organization but not cargo transport. J Cell Biol. 2007;176:641–651. doi: 10.1083/jcb.200608128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S, Schroer T. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- Kodani A, Tonthat V, Wu B, Sutterlin C. Par6 alpha interacts with the dynactin subunit p150Glued and is a critical regulator of centrosomal protein recruitment. Mol Biol Cell. 2010;21:3376–3385. doi: 10.1091/mbc.E10-05-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawo S, Hasegan M, Gupta GD, Pelletier L. Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material. Nat Cell Biol. 2012;14:1148–1158. doi: 10.1038/ncb2591. [DOI] [PubMed] [Google Scholar]
- Li H, Liu XS, Yang X, Song B, Wang Y, Liu X. Polo-like kinase 1 phosphorylation of p150Glued facilitates nuclear envelope breakdown during prophase. Proc Natl Acad Sci USA. 2010;107:14633–14638. doi: 10.1073/pnas.1006615107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen M, Malik A, Piel M, Bouckson-Castaing V, Bornens M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J Cell Sci. 2000;113:3013–3023. doi: 10.1242/jcs.113.17.3013. [DOI] [PubMed] [Google Scholar]
- Nadezhdina ES, Zinovkina LA, Fais D, Chentsov Iu S. Spermatozoa of the loach Misgurnus fossilis as a test system for identification of new centrosome proteins [in Russian] Ontogenez. 2001;32:41–50. [PubMed] [Google Scholar]
- Perez-Ferreiro C, Vernos I, Correas I. Protein 4.1R regulates interphase microtubule organization at the centrosome. J Cell Sci. 2004;117:6197–6206. doi: 10.1242/jcs.01544. [DOI] [PubMed] [Google Scholar]
- Pike AC, Rellos P, Niesen FH, Turnbull A, Oliver AW, Parker SA, Turk BE, Pearl LH, Knapp S. Activation segment dimerization: a mechanism for kinase autophosphorylation of non-consensus sites. EMBO J. 2008;27:704–714. doi: 10.1038/emboj.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer TA. Dynactin is required for microtubule anchoring at centrosomes. J Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quizi JL, Baron K, Al-Zahrani KN, O'Reilly P, Sriram RK, Conway J, Laurin AA, Sabourin LA. SLK-mediated phosphorylation of paxillin is required for focal adhesion turnover and cell migration. Oncogene. 2013;32:4656–4663. doi: 10.1038/onc.2012.488. [DOI] [PubMed] [Google Scholar]
- Romé P, Montembault E, Franck N, Pascal A, Glover DM, Giet R. Aurora A contributes to p150(Glued) phosphorylation and function during mitosis. J Cell Biol. 2010;189:651–659. doi: 10.1083/jcb.201001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers K, Wagner S, Storbeck CJ, O'Reilly P, Lo V, Northey JJ, Chmielecki J, Muller WJ, Siegel PM, Sabourin LA. The Ste20-like kinase SLK is required for ErbB2-driven breast cancer cell motility. Oncogene. 2009;28:2839–2848. doi: 10.1038/onc.2009.146. [DOI] [PubMed] [Google Scholar]
- Sabourin L, Rudnicki M. Induction of apoptosis by SLK, a Ste20-like kinase. Oncogene. 1999;18:7566–7575. doi: 10.1038/sj.onc.1203119. [DOI] [PubMed] [Google Scholar]
- Scheidtmann KH, Echle B, Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982;44:116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- Uetake Y, Terada Y, Matuliene J, Kuriyama R. Interaction of Cep135 with a p50 dynactin subunit in mammalian centrosomes. Cell Motil Cytoskeleton. 2004;58:53–66. doi: 10.1002/cm.10175. [DOI] [PubMed] [Google Scholar]
- Vaughan PS, Miura P, Henderson M, Byrne B, Vaughan KT. A role for regulated binding of p150(Glued) to microtubule plus ends in organelle transport. J Cell Biol. 2002;158:305–319. doi: 10.1083/jcb.200201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova T, Paul R, Grimaldi AD, Loncarek J, Miller PM, Yampolsky D, Magidson V, Khodjakov A, Mogilner A, Kaverina I. Concerted effort of centrosomal and Golgi-derived microtubules is required for proper Golgi complex assembly but not for maintenance. Mol Biol Cell. 2012;23:820–833. doi: 10.1091/mbc.E11-06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanatha R, Ohouo PY, Smolka MB, Bretscher A. Local phosphocycling mediated by LOK/SLK restricts ezrin function to the apical aspect of epithelial cells. J Cell Biol. 2012;199:969–984. doi: 10.1083/jcb.201207047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki S, Holzbaur EL. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc Natl Acad Sci USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Forster R, Palmer KJ, Pepperkok R, Stephens DJ. Coupling of ER exit to microtubules through direct interaction of COPII with dynactin. Nat Cell Biol. 2005;7:48–55. doi: 10.1038/ncb1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrich A, Honnappa S, Jaussi R, Okhrimenko O, Frey D, Jelesarov I, Akhmanova A, Steinmetz M. Structure-function relationship of CAP-Gly domains. Nat Struct Mol Biol. 2007;14:959–967. doi: 10.1038/nsmb1291. [DOI] [PubMed] [Google Scholar]
- Wiese C, Zheng Y. A new function for the gamma-tubulin ring complex as a microtubule minus-end cap. Nat Cell Biol. 2000;2:358–364. doi: 10.1038/35014051. [DOI] [PubMed] [Google Scholar]
- Wiese C, Zheng Y. Microtubule nucleation: gamma-tubulin and beyond. J Cell Sci. 2006;119:4143–4153. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]
- Yamada E, Tsujikawa K, Itoh S, Kameda Y, Kohama Y, Yamamoto H. Molecular cloning and characterization of a novel human Ste20-like kinase, hSLK. Biochim Biophys Acta. 2000;1495:250–262. doi: 10.1016/s0167-4889(99)00164-0. [DOI] [PubMed] [Google Scholar]
- Yan X, Habedanck R, Nigg E. A complex of two centrosomal proteins, CAP350 and FOP, cooperates with EB1 in microtubule anchoring. Mol Biol Cell. 2006;17:634–644. doi: 10.1091/mbc.E05-08-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhapparova ON, Bryantseva SA, Dergunova LV, Raevskaya NM, Burakov AV, Bantysh OB, Shanina NA, Nadezhdina ES. Dynactin subunit p150Glued isoforms notable for differential interaction with microtubules. Traffic. 2009;10:1635–1646. doi: 10.1111/j.1600-0854.2009.00976.x. [DOI] [PubMed] [Google Scholar]
- Zhapparova ON, Burakov AV, Nadezhdina ES. The centrosome keeps nucleating microtubules but looses the ability to anchor them after the inhibition of dynein-dynactin complex. Biochemistry (Mosc) 2007;72:1233–1240. doi: 10.1134/s0006297907110090. [DOI] [PubMed] [Google Scholar]
- Zinovkina L, Poltaraus A, Solovyanova O, Nadezhdina E. Chinese hamster protein homologous to human putative protein kinase KIAA0204 is associated with nuclei, microtubules and centrosomes in CHO-K1 cells. FEBS Lett. 1997;414:135–139. doi: 10.1016/s0014-5793(97)00952-6. [DOI] [PubMed] [Google Scholar]
- Zinovkina LA, Poltaraus AB, Solov'ianova OB, Nadezhdina ES. A proposed new mammalian cell protein kinase, associated with microtubules. Mol Biol (Mosk) 1998;32:341–348. [PubMed] [Google Scholar]
- Zong M, Satoh A, Yu MK, Siu KY, Ng WY, Chan HC, Tanner JA, Yu S. TRAPPC9 mediates the interaction between p150 and COPII vesicles at the target membrane. PLoS One. 2012;7:e29995. doi: 10.1371/journal.pone.0029995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.