Abstract
Pancreatic tumors are rare in children and adolescents. Here, we report the case of a 15-year-old boy who presented with a mixed acinar cell carcinoma/ductal adenocarcinoma with blastomatous components. He received multimodal treatment including various chemotherapy regimens and multistep surgery including liver transplantation. Introduction of FOLFIRINOX after relapse repeatedly achieved a durable metabolic and clinical response with good quality of life.
Key words: FOLFIRINOX, Acinar cell carcinoma, Ductal adenocarcinoma, Pancreatoblastoma, Pancreatic cancer, Autologous stem cell transplantation, Multimodal treatment
Introduction
Malignant tumors of the exocrine pancreas occur mainly in adults in their sixth or seventh decade of life. They commonly present with late-stage disease, and have poor prognosis, even in cases with primary resectable disease. Most exocrine pancreatic tumors (∼85%) are ductal adenocarcinomas. Less frequent histologies include intraductal papillary mucinous neoplasms with transition to an invasive carcinoma, serous cystadenocarcinomas or acinar cell carcinomas. In children, malignant pancreatic tumors are exceedingly rare, and only few reports are available [1, 2, 3]. Pediatric pancreatic tumors arise from embryonic precursor cells of ductal and acinar cells, and are termed pancreatoblastoma [3, 4]. Due to the stem cell origin of the tumor cells, pancreatoblastomas can subsequently differentiate to various histologic tumor cell types.
Patients with pancreatic tumors often present with weight loss, jaundice, and varying abdominal symptoms due to the tumor mass effect [5].
The treatment algorithm chosen usually depends on the type and stage of the respective tumor. An aggressive approach with complete tumor resection whenever possible seems to be the best primary option [2, 6, 7]. Patients with advanced disease may benefit from multimodality treatment [4, 8]. Primary systemic chemotherapy may be useful to reduce the tumor mass and allow subsequent secondary resection [9].
Case Report
A 15-year-old boy presented in November 2009 with a 3.2-cm tumor of the head of the pancreas and multiple diffuse liver metastases (fig. 1a, b). Liver and kidney function tests were within normal limits, serum alpha-fetoprotein (AFP) was elevated (400 U/ml). Biopsy of the liver mass revealed a malignant tumor with acinar cell differentiation, bringing up the differential diagnosis of acinar cell carcinoma, or a mixed tumor with acinar cell differentiation including pancreatoblastoma.
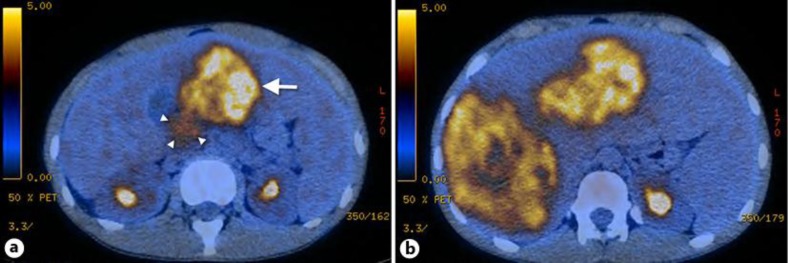
Fig. 1.
a PET/CT showing the primary tumor in the pancreatic head, 3 cm in diameter with weak fluorodeoxyglucose avidity (standard uptake value 3.8; arrowheads). In addition, a large liver metastasis can be seen in the left liver lobe (arrow). b Multiple large liver metastases are shown predominantly in the right liver lobe.
Chemotherapy was initiated with two cycles of cisplatin and doxorubicin (PLADO regimen), resulting in a partial response of the primary tumor and the liver metastases. Based on the good response, a Whipple duodenopancreatectomy and simultaneous wedge resection of the metastases of the left liver with ligation of the right portal vein were performed in March 2010. Two additional cycles of the same chemotherapy were given postoperatively.
The metastases of the right liver lobe were resected by hemihepatectomy of the right liver, any other lesions in the remnant liver were removed by wedge resection. In addition, focally infiltrated parts of the diaphragm were also resected. Altogether, this accounted for a surgical tumor reduction of about 90%. A central liver lesion could not be resected due to its vessel proximity.
Surgical pathology confirmed a mixed histology with differentiation of both, acinar cell carcinoma and ductal adenocarcinoma. Some morphologic features of the primary liver biopsy also resembled an embryonic tumor, but the pathognomonic squamoid differentiation of pancreatoblastoma was missing. One lymph node metastasis adjacent to the hepatic artery was found showing ductal differentiation. No neuroendocrine differentiation was detectable. Some tumor regression due to the preceding neoadjuvant chemotherapy was observed (fig. 2a–i). Immunostaining was focally positive for AFP in accordance with the initially elevated AFP serum levels. A diagnosis of a mixed acinar cell carcinoma/ductal adenocarcinoma was retained.
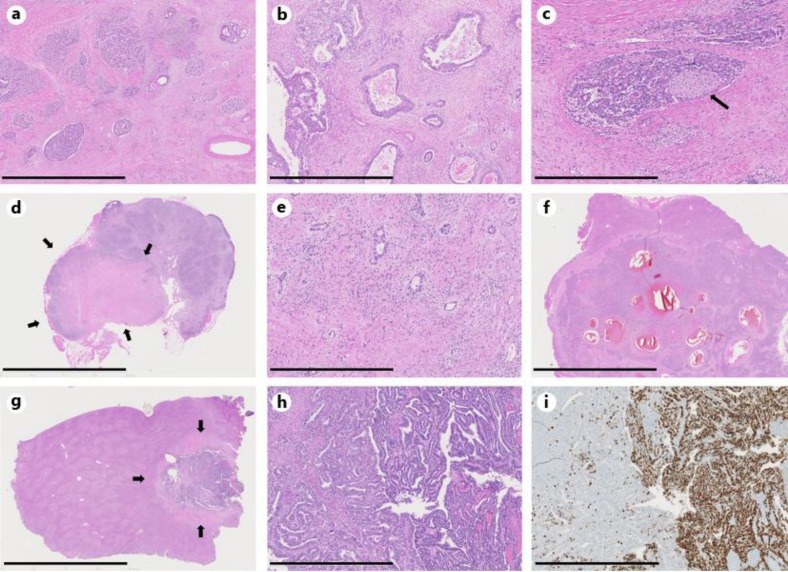
Fig. 2.
Histopathological findings of pancreas and liver resection specimens. a Overview of the pancreas; resection after chemotherapy. The tumor shows both acinar cell differentiation (left) and ductal differentiation (right), as well as regressive changes (scale bar = 2 mm). b High-power view of the former picture (scale bar = 1 mm). c Perineural infiltration by the acinar cell tumor component. The arrow points to the peripheral nerve (scale bar = 0.8 mm). d Mostly regressive perihilar lymph node metastasis (arrows); resection after chemotherapy (scale bar = 10 mm). e High-power view of the former picture showing few ductal differentiated vital tumor deposits in a regressive background (scale bar = 0.5 mm). f Overview of a liver metastasis with cystic regressive areas with hemorrhage; resection after chemotherapy (scale bar = 10 mm). g Overview of another liver metastasis (arrows); resection after chemotherapy (scale bar = 10 mm). h High-power view of the former picture shows a tumor with blastomatous features (scale bar = 1 mm). i MIB-1 staining reveals a high-proliferation rate of the blastomatous tumor component (scale bar = 0.9 mm).
Tumor regrowth in the liver was observed on positron emission tomography/computed tomography (PET/CT) scan already 1 month after the second surgery. No tumor growth was seen in the remnant pancreas after Whipple resection. After subsequent second-line chemotherapy with two cycles of ifosphamide, carboplatin and etoposide (ICE regimen), a partial remission of the liver lesions was documented.
A living donor liver transplantation (right hemiliver) from his brother was performed in November 2010. Due to a severe bile leak with abscess formation and finally hepatic artery thrombosis, the graft had to be replaced with a cadaveric liver graft after 10 days. Afterwards, the patient achieved complete recovery and was disease free. Immunosuppression with prednisone and tacrolimus was initially established. The former was subsequently tapered within 3 months, and tacrolimus was changed to everolimus.
In April 2011, multiple new lung metastases were detected while no tumor relapse was seen in the abdomen. Therefore, in May 2011 a new chemotherapy treatment with oxaliplatin, irinotecan, leucovorin and 5-fluorouracil (FOLFIRINOX regimen) was started. The new treatment was tolerated well apart from neutropenia and thrombocytopenia up to grade 4, resulting in dose reductions of the applied compounds. After six biweekly cycles, PET/CT showed a complete metabolic and partial morphologic remission of all lung lesions (fig. 3a, b).
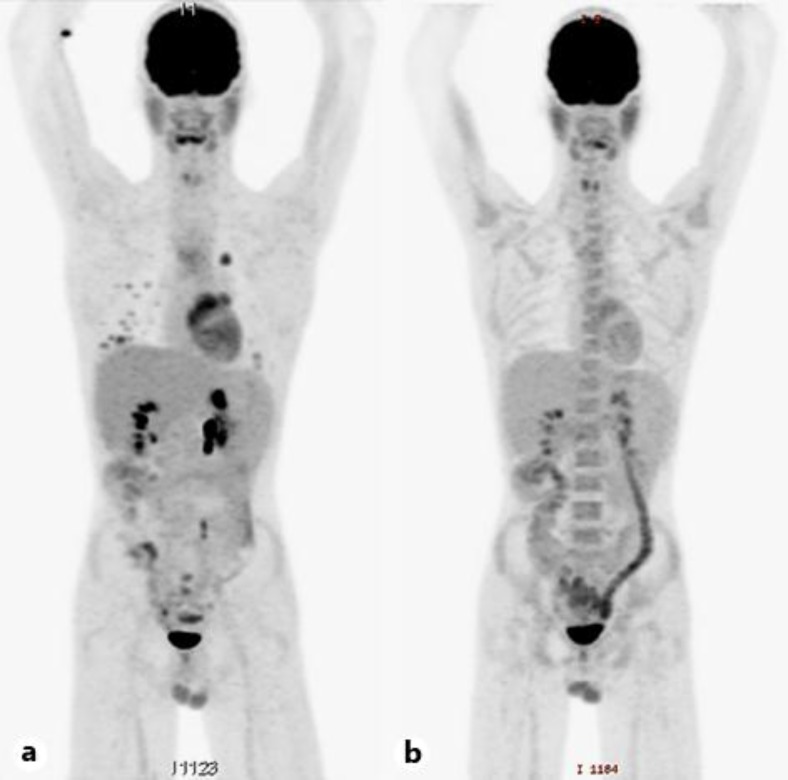
Fig. 3.
a PET/CT showing multiple new fluorodeoxyglucose avid lung metastases. No relapse could be seen in the abdomen. b Complete metabolic response of the lesions after treatment with FOLFIRINOX.
Due to the excellent response to FOLFIRINOX, we decided to consolidate with tandem high-dose chemotherapy using carboplatin, etoposide and paclitaxel as conditioning regimen with subsequent autologous stem cell transplantation (ASCT). Hematopoietic progenitor cells were successfully mobilized with filgrastim, and high-dose chemotherapy was performed in November 2011 and February 2012. Due to transiently elevated liver enzymes after the first high-dose treatment, etoposide was omitted in the second treatment to reduce the toxicity for the transplanted liver.
In August 2012, a progression of the pulmonary lesions was documented in a routine follow-up PET/CT. Treatment with FOLFIRINOX was once again initiated, resulting in a very good metabolic and morphologic remission after another six cycles until December 2012. Chemotherapy was continued for 3 additional months. While all lesions except one remained responsive to the treatment, the latter lesion was treated with stereotactic irradiation using 3 × 12 = 36 Gy.
As of today, the patient has a sustained metabolic remission of the known lung lesions and no new lesions developed with repeated use of FOLFIRINOX. He is currently in excellent general condition and has no limitations of his quality of life.
Discussion
No standard treatment for exocrine pancreatic tumors in pediatric patients has been defined due to the rarity of this disease. Accordingly, treatment decisions were based on guidelines and recommendations of the Children's Cancer and Leukaemia Group (CCLG) on the management of rare tumors like hepatoblastoma and pancreatoblastoma, as well as on treatment algorithms used in adult patients with pancreatic cancer. Surgical resection of large tumor masses seems to be the best first-line therapy in resectable tumors, even when microscopically incomplete. Recently, a successful treatment with high-dose chemotherapy and ASCT of a young patient with a pancreatoblastoma has been reported after incomplete primary resection [10]. The few reported cases of high-dose chemotherapy and ASCT in young patients suggesting a benefit from aggressive multimodality treatment prompted us to perform tandem transplantation in our patient after observing good tolerability and an excellent response to initial standard dose chemotherapy [11, 12, 13].
The tumor tissue was heterogeneous showing different lines of differentiation. Predominantly, the primary tumor in the pancreas and most of the liver metastases showed a mixed acinar and ductal cell differentiation, while some liver lesions were predominantly of acinar cell type. In addition, some morphologic features of the liver lesions resembled pancreatoblastoma, although a typical squamoid differentiation of the tumor cells was lacking. One can speculate that this heterogeneous tumor may well have been a pancreatoblastoma with differentiation along various cell types, which could finally not be diagnosed due to the regressive changes of the tumor morphology after neoadjuvant chemotherapy. Recently, the phenotype of a pancreatic carcinoma showing combined acinar cell and ductal differentiation was described and discussed as a possible distinctive tumor entity with an aggressive clinical course [14]. Irrespective of the diagnostic label, the tumor was regarded as malignant with mixed differentiation including acinar cell carcinoma, ductal adenocarcinoma, and blastomatous components, each associated with different biological behavior, respectively.
The course of our patient allows the following conclusions. Firstly, despite the fact that advanced and metastatic pancreatic tumors are generally considered to have a dismal prognosis, an aggressive multimodal treatment strategy in young and fit patients may result in improvement of quality of life and survival. In addition, treatment with high-dose chemotherapy and subsequent ASCT was possible even after orthotopic liver transplantation. Our patient is now in excellent condition without showing any clinical symptoms related to the tumor almost 4 years after the initial diagnosis. He is going to school and is not compromized in his daily living.
Secondly, the repeated use of FOLFIRINOX may be highly active also in pancreatic tumors other than ductal adenocarcinoma. In our patient, the best response to systemic chemotherapy was achieved with FOLFIRINOX, although only given as third-line chemotherapy and despite the pretreatment with various other compounds. Convincing data have been reported for the FOLFIRINOX treatment of adult patients with ductal adenocarcinoma of the pancreas in the first-line setting, but no data exist in pretreated patients with other rare tumor entities [15]. Our patient showed an excellent response even after repeated use of this treatment combination.
In conclusion, FOLFIRINOX has proven to be a highly active chemotherapy regimen in this heavily pretreated patient and may be a very good alternative to older and more toxic regimens for the treatment of pediatric patients presenting with rare pancreatic tumors.
References
- 1.Rojas Y, Warneke CL, Dhamne CA, et al. Primary malignant pancreatic neoplasms in children and adolescents: a 20 year experience. J Pediatr Surg. 2012;47:2199–2204. doi: 10.1016/j.jpedsurg.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Perez EA, Gutierrez JC, Koniaris LG, et al. Malignant pancreatic tumors: incidence and outcome in 58 pediatric patients. J Pediatr Surg. 2009;44:197–203. doi: 10.1016/j.jpedsurg.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 3.Shorter NA, Glick RD, Klimstra DS, et al. Malignant pancreatic tumors in childhood and adolescence: The Memorial Sloan-Kettering experience, 1967 to present. J Pediatr Surg. 2002;37:887–892. doi: 10.1053/jpsu.2002.32897. [DOI] [PubMed] [Google Scholar]
- 4.Bien E, Godzinski J, Dall'igna P, et al. Pancreatoblastoma: a report from the European cooperative study group for paediatric rare tumours (EXPeRT) Eur J Cancer. 2011;47:2347–2352. doi: 10.1016/j.ejca.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol. 2005;7:189–197. doi: 10.1007/BF02712816. [DOI] [PubMed] [Google Scholar]
- 6.Holen KD, Klimstra DS, Hummer A, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002;20:4673–4678. doi: 10.1200/JCO.2002.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Kitagami H, Kondo S, Hirano S, et al. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas. 2007;35:42–46. doi: 10.1097/mpa.0b013e31804bfbd3. [DOI] [PubMed] [Google Scholar]
- 8.Kleeff J, Michalski CW, Friess H, Buchler MW. Surgical treatment of pancreatic cancer: the role of adjuvant and multimodal therapies. Eur J Surg Oncol. 2007;33:817–823. doi: 10.1016/j.ejso.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Vossen S, Goretzki PE, Goebel U, Willnow U. Therapeutic management of rare malignant pancreatic tumors in children. World J Surg. 1998;22:879–882. doi: 10.1007/s002689900486. [DOI] [PubMed] [Google Scholar]
- 10.Meneses CF, Osorio CD, de Castro Junior CG, Brunetto AL. Autologous stem cell transplantation as first line treatment after incomplete excision of pancreatoblastoma. Rev Bras Hematol Hemoter. 2013;35:148–149. doi: 10.5581/1516-8484.20130038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonekura T, Kosumi T, Hokim M, et al. Aggressive surgical and chemotherapeutic treatment of advanced pancreatoblastoma associated with tumor thrombus in portal vein. J Pediatr Surg. 2006;41:596–598. doi: 10.1016/j.jpedsurg.2005.11.071. [DOI] [PubMed] [Google Scholar]
- 12.Souzaki R, Tajiri T, Kinoshita Y, et al. Successful treatment of advanced pancreatoblastoma by a pylorus-preserving pancreatoduodenectomy after radiation and high-dose chemotherapy. Pediatr Surg Int. 2010;26:1045–1048. doi: 10.1007/s00383-010-2655-9. [DOI] [PubMed] [Google Scholar]
- 13.Hamidieh AA, Jalili M, Khojasteh O, Ghavamzadeh A. Autologous stem cell transplantation as treatment modality in a patient with relapsed pancreatoblastoma. Pediatr Blood Cancer. 2010;55:573–576. doi: 10.1002/pbc.22536. [DOI] [PubMed] [Google Scholar]
- 14.Stelow EB, Shaco-Levy R, Bao F, et al. Pancreatic acinar cell carcinomas with prominent ductal differentiation: mixed acinar ductal carcinoma and mixed acinar endocrine ductal carcinoma. Am J Surg Pathol. 2010;34:510–518. doi: 10.1097/PAS.0b013e3181cfcac7. [DOI] [PubMed] [Google Scholar]
- 15.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]